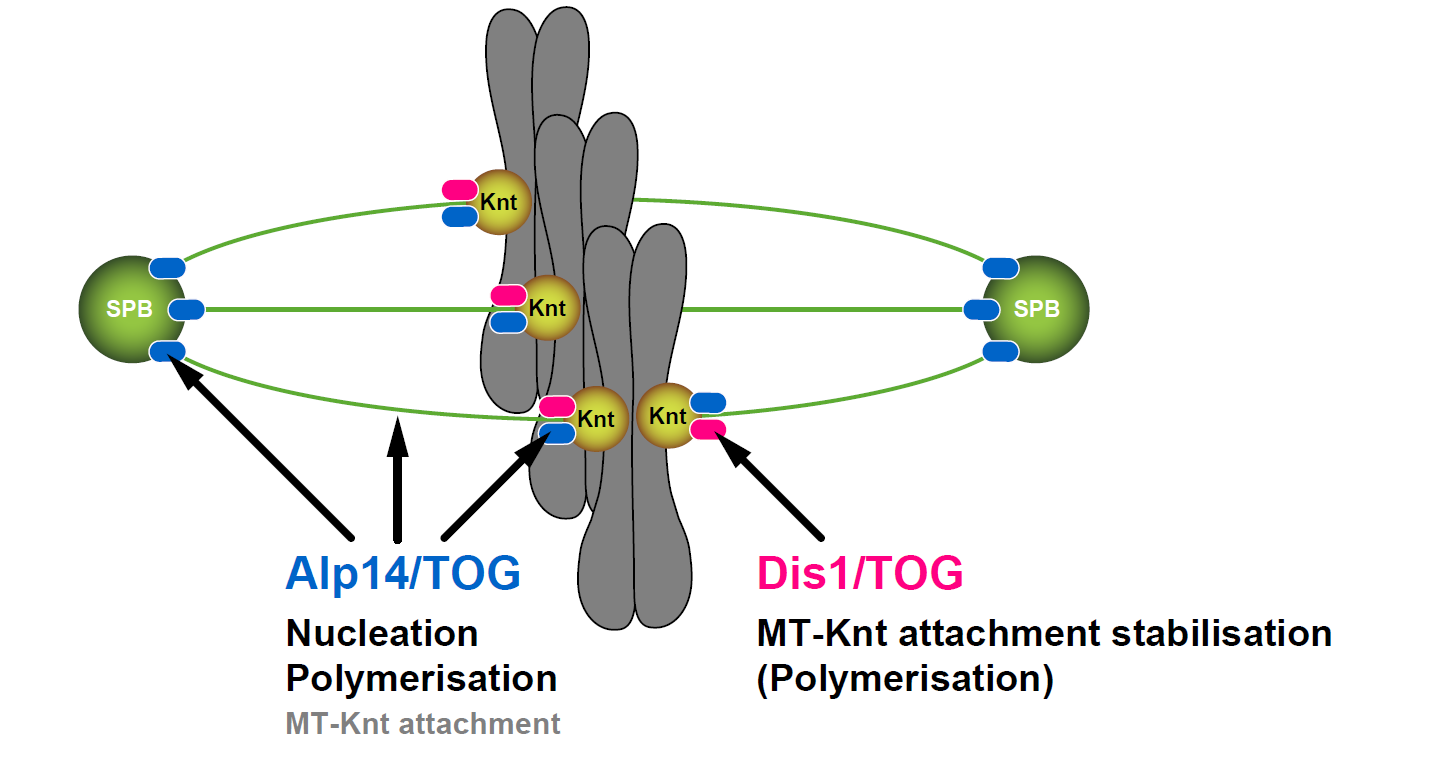
Proper bipolar spindle assembly underlies accurate chromosome segregation. A cohort of microtubule-associated proteins orchestrates spindle microtubule formation in a spatiotemporally coordinated manner. Among them, the conserved XMAP215/TOG family of microtubule polymerase plays a central role in spindle assembly. In fission yeast, two XMAP215/TOG members, Alp14 and Dis1, share essential roles in cell viability; however how these two proteins functionally collaborate remains undetermined. Here we show the functional interplay and specification of Alp14 and Dis1. Creation of new mutant alleles of alp14, which display temperature sensitivity in the absence of Dis1, enabled us to conduct detailed analyses of a double mutant. We have found that simultaneous inactivation of Alp14 and Dis1 results in early mitotic arrest with very short, fragile spindles. Intriguingly, these cells often undergo spindle collapse, leading to a lethal “cut” phenotype. By implementing an artificial targetting system, we have shown that Alp14 and Dis1 are not functionally exchangeable and as such are not merely redundant paralogues. Intriguingly, while Alp14 promotes microtubule nucleation, Dis1 does not. Our results uncover that the intrinsic specification, not the spatial regulation, between Alp14 and Dis1 underlies the collaborative actions of these two XMAP215/TOG members in mitotic progression, spindle integrity and genome stability.