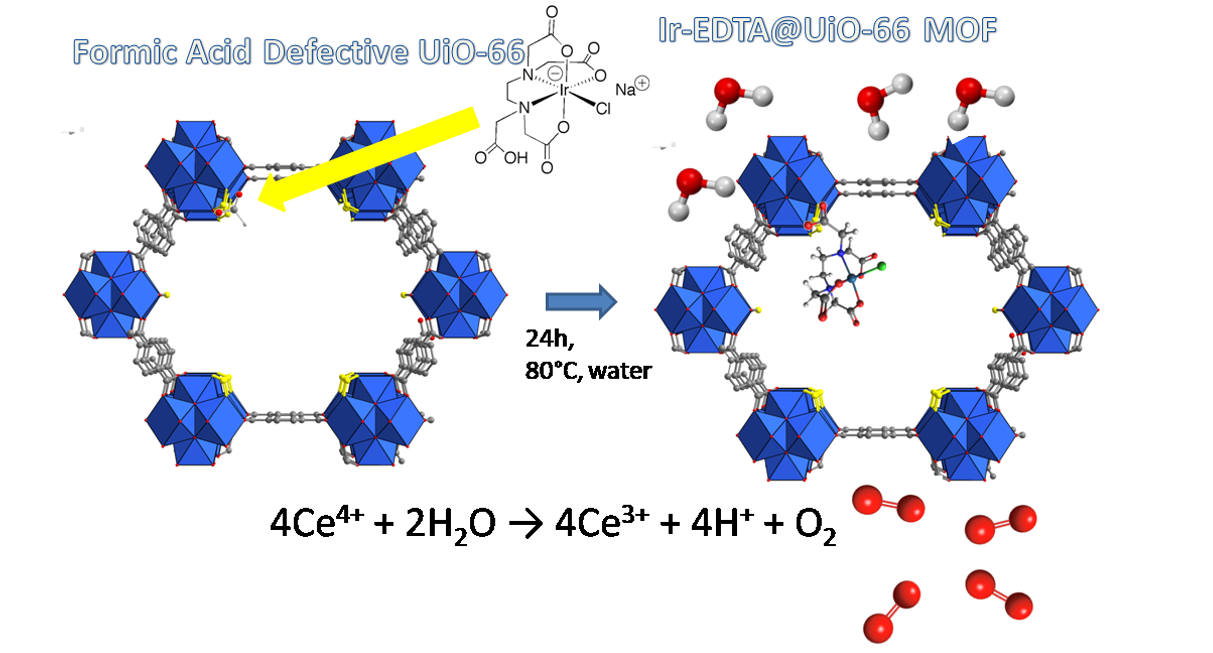
Clean production of renewable fuels is a great challenge of our scientific community. Iridium complexes have demonstrated a superior catalytic activity in the water oxidation (WO) reaction, which is a crucial step in water splitting process. Herein we have used a defective zirconium MOF with UiO-66 structure as support of a highly active Ir complex based on EDTA with formula [Ir(HEDTA)Cl]Na. The defects are induced by the partial substitution of tereftalic acid with smaller formiate groups. Anchoring of the complex occurs through a post-synthetic exchange of formiate anions, coordinated at the zirconium clusters of the MOF, with the free carboxylate group of the [Ir(HEDTA)Cl]-complex. The modified material was tested as heterogenous catalyst for the WO reaction by using Cerium Ammonium Nitrate as sacrificial agent. Although TOF and TON values are comparable to those of other iridium heterogenized catalysts, the MOF exhibits iridium leaching not limited at the first catalytic run, as usually observed, suggesting a lack of stability of the hybrid system under strong oxidative conditions.