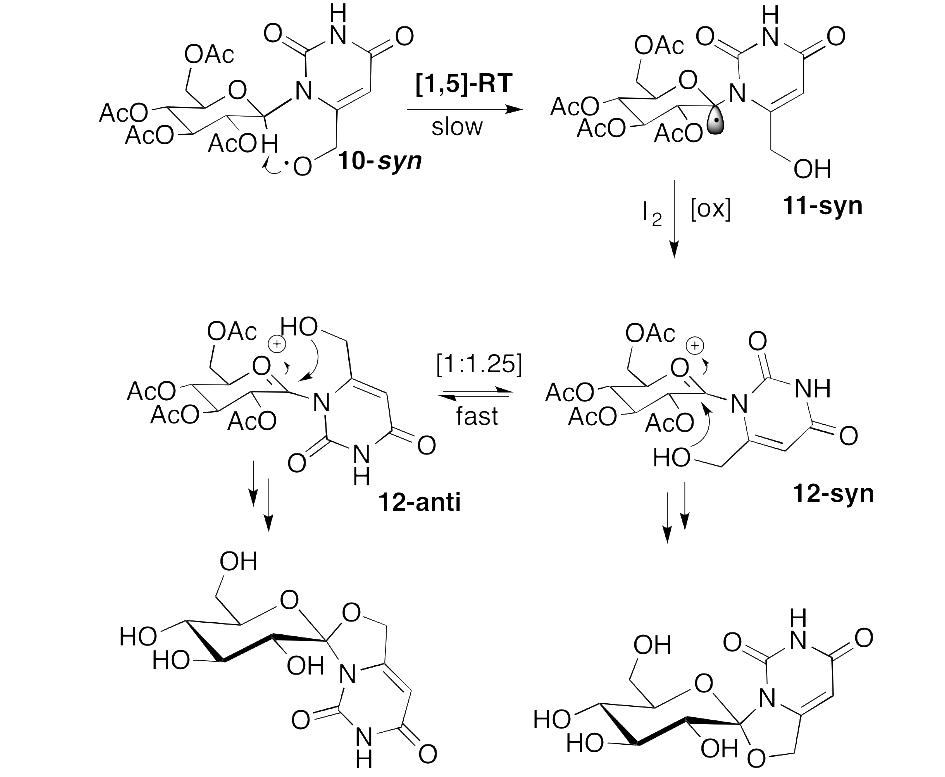
In the case of type 2 diabetes, inhibitors of glycogen phosphorylase (GP) might prevent unwanted glycogenolysis under high glucose conditions and thus aim at the reduction of excessive glucose production by the liver. Anomeric spironucleosides, such as hydantocidin, present a rich synthetic chemistry and important biological function, e.g., inhibition of GP. Herein, the Suárez radical methodology is successfully applied to synthesize the first example of a 1,6-dioxa-4-azaspiro[4.5]decane system, not been previously constructed via a radical pathway, starting from 6-hydroxymethyl-b-D-glucopyranosyluracil. It is shown that in the rigid pyranosyl conformation the required [1,5]-radical translocation is a minor process. The stereochemistry of the spirocycles obtained was unequivocally determined based on the chemical shifts of key sugar protons in the 1H NMR spectra. The two spirocycles were found to be modest inhibitors of RMGPb.