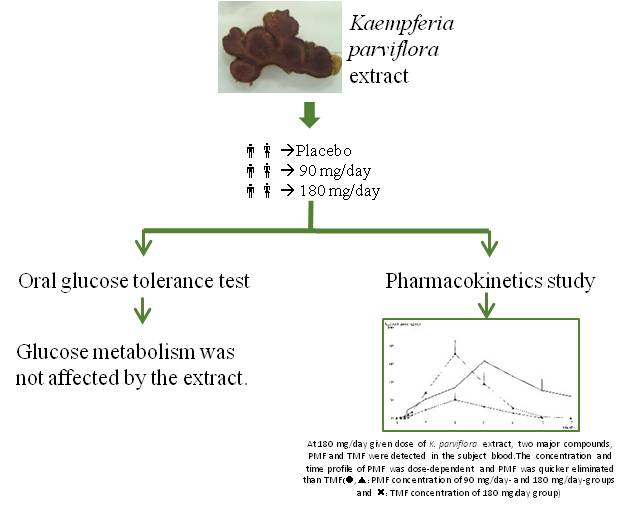
Kaempferia parviflora Wall. ex Baker (KP), Krachaidam in Thai or Thai ginseng, is an herbal medicine that has many potential pharmacological effects. This study focused on the oral glucose tolerance test and pharmacokinetic study in healthy volunteers administered with KP extract (90 and 180 mg/day, placebo). The oral glucose tolerance tests were performed at baselines and 28-days of administration. The pharmacokinetics were determined after a single dose administration of the tested products using 3,5,7,3,4-pentamethoxyflavone (PMF) and 5,7,4-trimethoxylfavone (TMF) as markers. The results showed that glucose metabolism via oral glucose tolerance test was not affected by KP extract. The results of pharmacokinetics study revealed that only TMF and PMF, but not DMF levels could be detected in human blood. The given doses of KP extract at 90 and 180 mg/day showed a linear dose-relationship of blood PMF concentration whereas blood TMF was detected only at high given dose (180 mg/day). The half-lives of PMF and TMF were 2–3 h. The Cmax, AUC and Tmax values of PMF and TMF estimated for the 180 mg/day dose were 85.3711.31, 73.2329.93 mg/ml; 291.8948.23, 412.20203.69 mg.h/ml; and 3.890.37, 4.500.96 h, respectively. PMF was quickly eliminated with higher Ke and Cl than TMF at the dose of 180 mg/day of KP extract. In conclusion, the results demonstrated that KP extract had no effect on glucose tolerance test. In addition, this is the first demonstration of the pharmacokinetic parameters of methoxyflavones of KP extract in healthy volunteers in a phase I study in drug development. The data suggest the safety of the KP extract and will be of benefit for further clinical trials using KP extract as food and sport supplements as well as a drug in health product development.