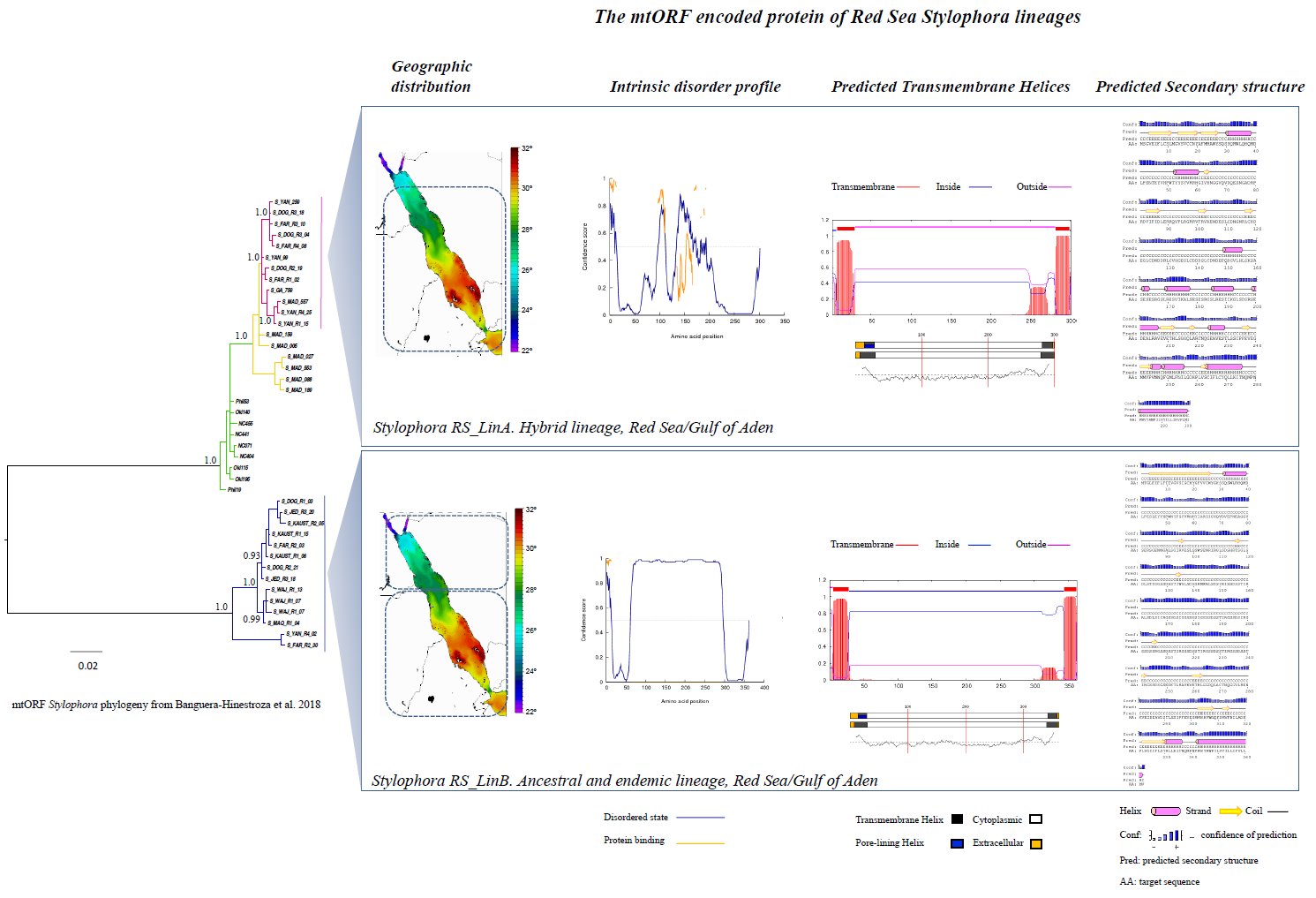
More than a decade ago, a new mitochondrial Open Reading Frame (mtORF) was discovered in corals of the family Pocilloporidae, which turn out to be an effective barcode gene for these corals. However, its function remains unknown. Recently, this gene revealed the existence of a hybrid Stylophora lineage (RS_LinA) inhabiting in sympatry along the environmental gradient of the Red Sea (18.5°C to 33.9°C) with its parental species (RS_LinB). Furthermore, in RS_LinB, the mtORF uncovered phylogeographic patterns that were strongly correlated with environmental variations. This was similar to the patterns unraveled by hsp70, suggesting that mtORF too might be involved in thermal adaptation. Here we used computational approaches to characterize the mtORF and to identify its potential role. Results showed that this gene encodes a transmembrane protein (0.97<P< 1.00) involved in transport (0.80<P< 0.87), regulation of metabolic processes (0.70<P<0.85), and likely in the cell-surface receptor signaling pathway (0.56<P<0.80). Predicted protein functions differed among Stylophora lineages and interestingly, in RS_LinB only, the protein was intrinsically disordered and displayed domains involved in cellular complexes and stress response (0.0001< P <0.001). These characteristics, exclusive of an endemic lineage adapted to extreme environmental fluctuations, support a role of the mtORF in stress response, speciation and adaptation.