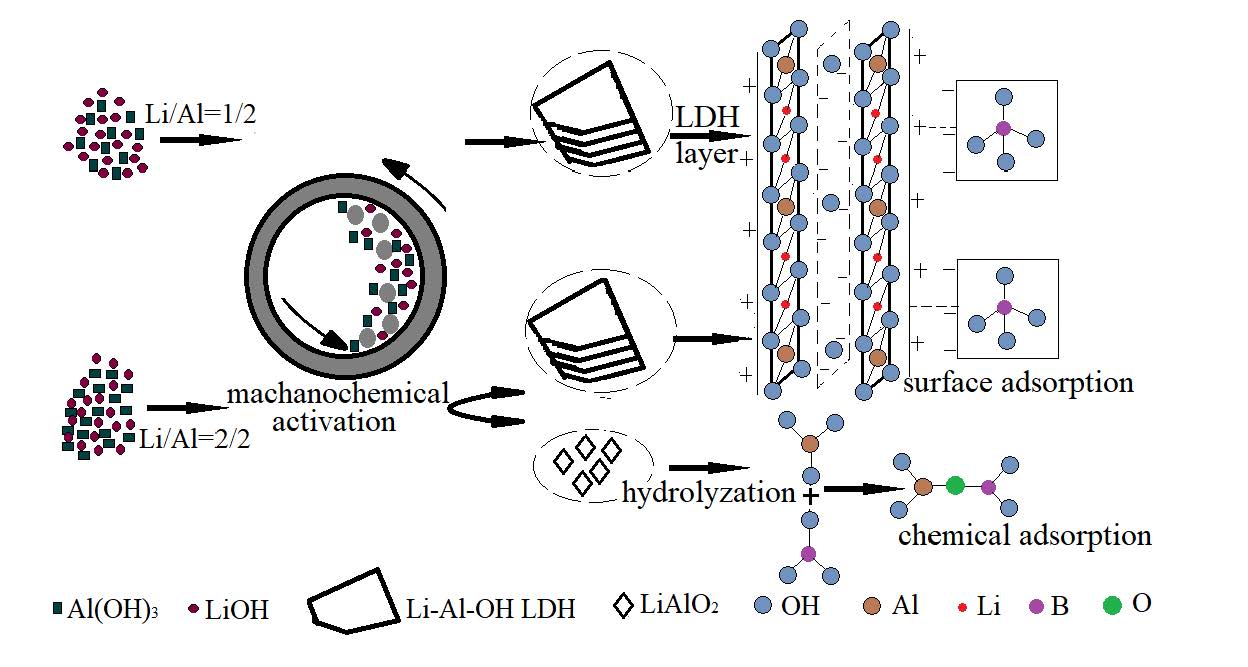
In this study, Li-Al-OH layered double hydroxide (LDH), which was prepared by solvent-free one-step mechanochemical reaction of LiOH and Al(OH)3, was applied to remove boron from aqueous solution. Dry-grinding for 3 h at a rotational speed of 500 rpm, Li/Al molar 1/2 was the optimum condition to prepare highly crystalline of Li-Al LDH phase with no evident impure phases. Two milling products with Li/Al molar ratio at 1/2 and 2/2 were evaluated for boron adsorption. The results confirmed that Li/Al molar ratio 2/2 sample showed high boron adsorption capacity due to the physical adsorption of Li-Al-OH LDH and chemical synergism of phase gel Al(OH)3. The adsorption isotherms, described by the Langmuir model, indicated maximum monolayer boron uptake capacity 45.45 mg/g, implying competitive adsorption capacity of the material in our experiment.