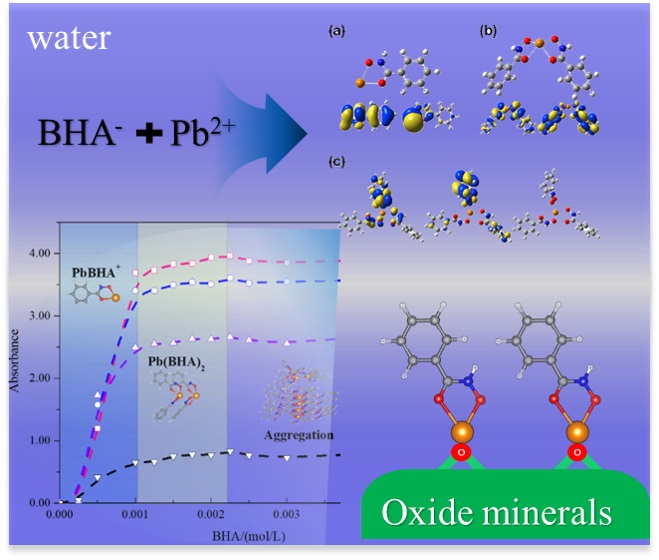
Novel collector lead(II)-benzohydroxamic acid (Pb [II]-BHA) complexes in aqueous solution were characterized by using experimental approaches, including Fourier-transform infrared spectroscopy (FTIR), powder X-ray diffraction spectroscopy (PXRD), Ultraviolet-visible (UV-Vis) spectroscopy, and electrospray ionization–mass spectrometry (ESI-MS), and first-principle density functional theory (DFT) calculations with consideration of solvation effects. The Job plot delineated that a single coordinated Pb(BHA)+ should be formed first, and the binary structures of Pb(BHA)2 can be formed subsequently. Moreover, the Pb(II)-BHA species aggregated with each other to form highly complicated structures. ESI-MS results validated the existence of Pb-(BHA)n=1,2. The well-consistent infrared spectra from the DFT calculations and FTIR measurements indicated that the cis-amide (Za)-type BHA conformer may be dominant in the solid-state crystals of BHA. The first-principle calculations suggested that Pb(BHA)2 should be the most stable structure, and the Pb atom in Pb(BHA)+ will play as an active site to attack nucleophiles. These findings are meaningful to further illustrate the adsorption mechanism of Pb(II)-BHA complexes in mineral processing.