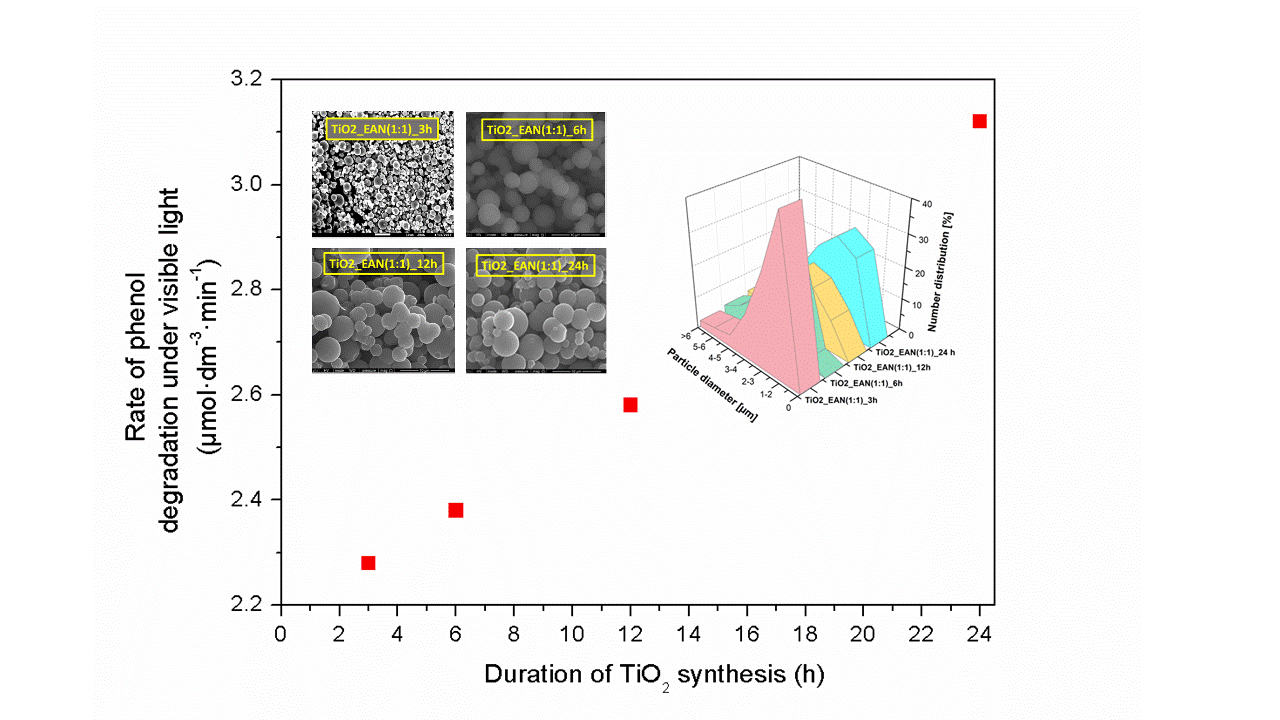
Spherical microparticles of TiO2 were synthesized by the ionic liquid-assisted solvothermal method at different reaction time (3, 6, 12 and 24h). The properties of the prepared photocatalysts were investigated by means of UV-vis diffuse-reflectance spectroscopy (DRS), BET surface area measurements, scanning electron microscopy (SEM), X-ray diffraction analysis (XRD), and X-ray photoelectron spectroscopy (XPS). The results indicated that the efficiency of phenol degradation was related with a time of the solvothermal synthesis as determined for TiO2_EAN(1:1)_24h sample. Microparticles of TiO2_EAN(1:1)_3h formed during the only 3h of synthesis time revealed really high photoactivity under visible irradiation – 75%. This value increased to 80% and 82% after 12h and 24h, respectively. The photoactivity increase was accompanied by the increase of the specific surface area thus pores size, as well as ability to absorb UV-vis irradiation. The high efficiency of phenol degradation of IL-TiO2 photocatalysts was ascribed to the interaction between the surface of TiO2 and ionic liquid components (carbon and nitrogen).