Submitted:
28 January 2026
Posted:
29 January 2026
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. The Global Burden and Evolving Landscape of Breast Cancer
1.2. 2025: A Watershed Year in Breast Oncology
1.3. The Conference Landscape as a Catalyst for Practice Change
1.4. Rationale and Objectives of This Systematic Review
- To systematically identify and evaluate pivotal breast cancer trials presented at major oncology conferences and published in high-impact journals during 2025.
- To synthesize efficacy and safety data across disease subtypes and clinical settings, with particular attention to practice-changing advances.
- To extract immediate clinical implications for practice while identifying knowledge gaps requiring further investigation.
- To identify unifying themes, persistent challenges, and future research priorities that will shape the next phase of breast oncology innovation.
2. Methods
2.1. Review Protocol and Framework
2.2. Eligibility Criteria
2.2.1. Study Designs
2.2.2. Population
2.2.3. Interventions
2.2.4. Outcomes
2.2.5. Timeframe and Sources
- Presentations at the ASCO 2025 Annual Meeting (Chicago, May 30–June 3), including plenary sessions, oral abstract presentations, and late-breaking abstracts.
- Presentations at the ESMO 2025 Congress (Berlin, October 17–21), including Presidential, Proffered Paper, and late-breaking sessions.
- Presentations at the SABCS 2025 (San Antonio, TX, December 9–12), including general session, spotlight session, and poster presentations.
- Peer-reviewed publications in journals with an impact factor ≥10 (New England Journal of Medicine, The Lancet Oncology, JAMA Oncology, Journal of Clinical Oncology, Annals of Oncology, Clinical Cancer Research) from January 1 to December 31, 2025.Conference abstracts subsequently published as full manuscripts were tracked to avoid duplication, with the most comprehensive version used for data extraction.
2.3. Search Strategy and Study Selection
2.3.1. Conference Proceedings
2.3.2. Electronic Database Search
2.3.3. Study Selection Process
2.4. Data Extraction and Management
2.4.1. Data Extraction Form
- Study characteristics: Trial name, NCT identifier, phase, design, funding source, publication/presentation details
- Patient population: Sample size, inclusion/exclusion criteria, molecular subtypes, disease stage, prior therapies, biomarker requirements
- Interventions: Experimental and control regimens, dosing, administration schedules, treatment duration
- Outcomes: Primary and secondary endpoints, efficacy measures with hazard ratios and confidence intervals, safety data including adverse events of special interest
- Biomarker analyses: Pre-specified and exploratory biomarker assessments, predictive and prognostic associations
- Quality assessment domains: Elements relevant to risk of bias assessment
2.4.2. Extraction Process
2.4.1. Title and Abstract Screening
2.4.2. Full-Text Review
2.4.3. Data Management and Synthesis
2.5. Risk of Bias Assessment Strategy and Tool
2.6. Evidence Grading and Clinical Relevance Assessment
3. Results
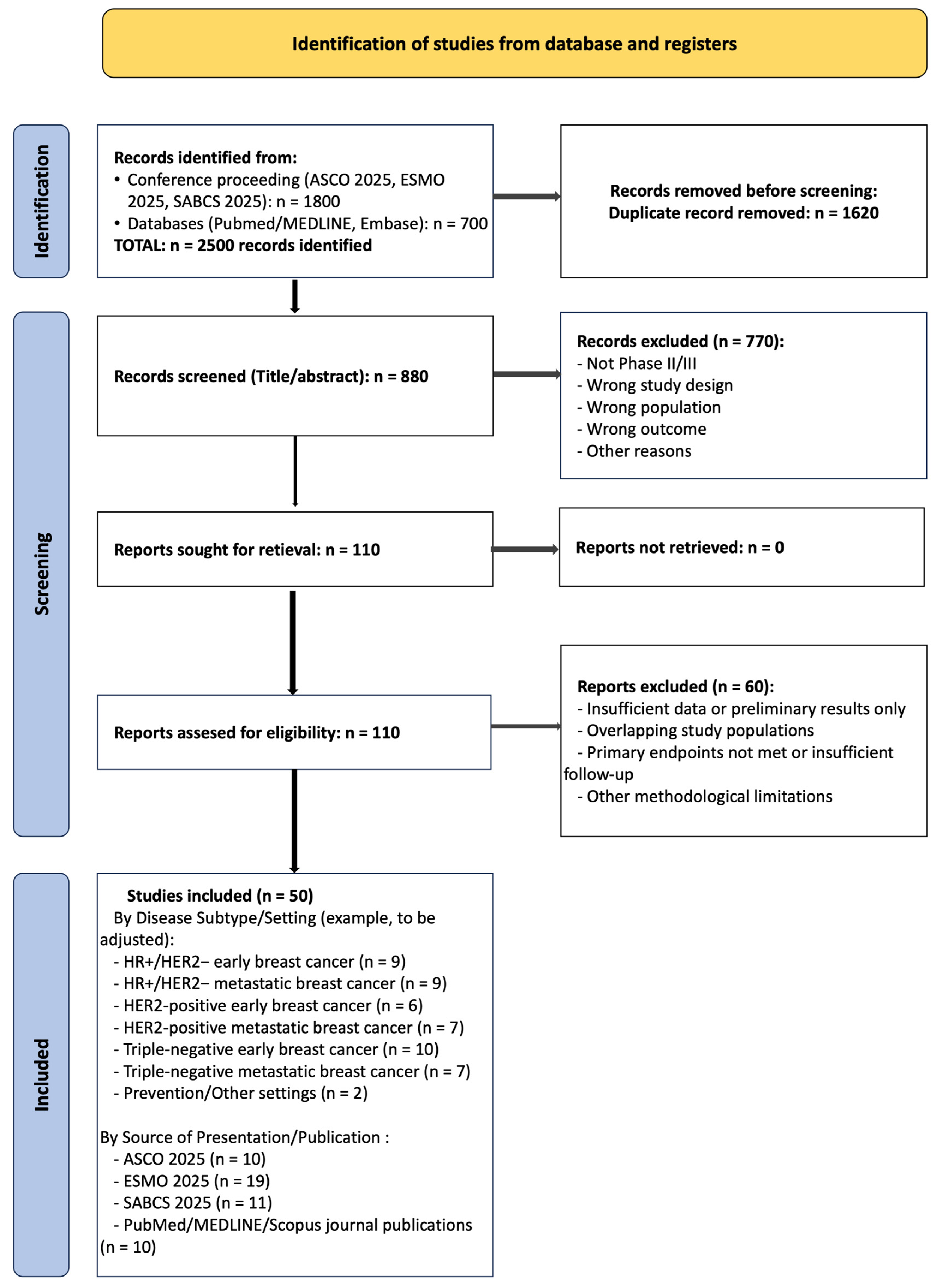
3.1. Study Selection Process
3.2. Study Selection and Characteristics
3.3. Risk of Bias Assessment
3.4. Thematic Synthesis of Findings
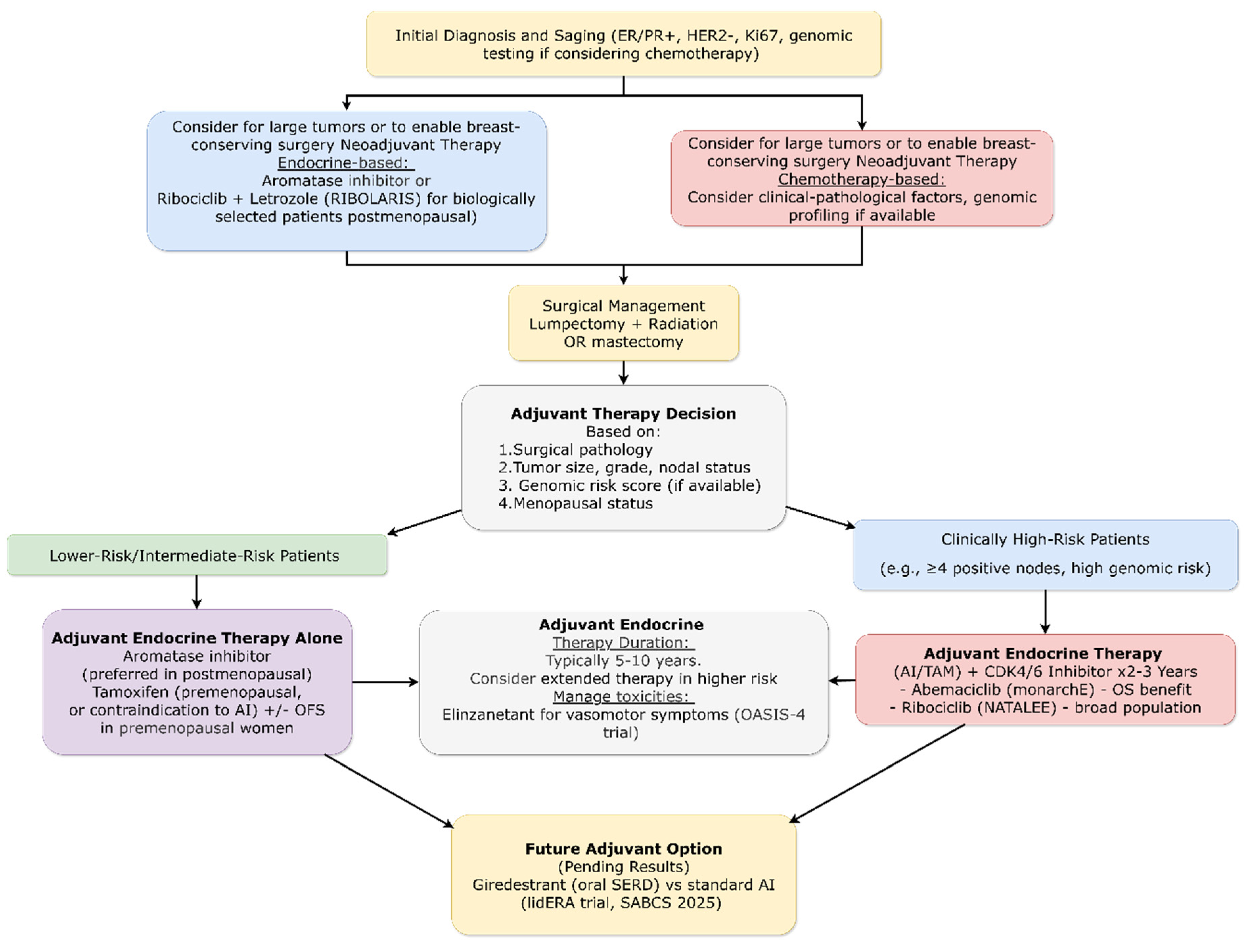
3.4.1. Early Breast Cancer: HR+/HER2− Subtype
3.4.1.1. Adjuvant CDK4/6 Inhibitors: Mature Survival Data Establishes New Standard
3.4.1.2. Biomarker-Driven De-Escalation Strategies
3.4.1.3. Fertility Preservation and Endocrine Therapy Interruption
3.4.1.4. Next-Generation Endocrine Agents: Biological Activity Validation
3.4.1.5. Long-Term Endocrine Therapy Strategies
3.4.1.6. Management of Treatment-Related Symptoms
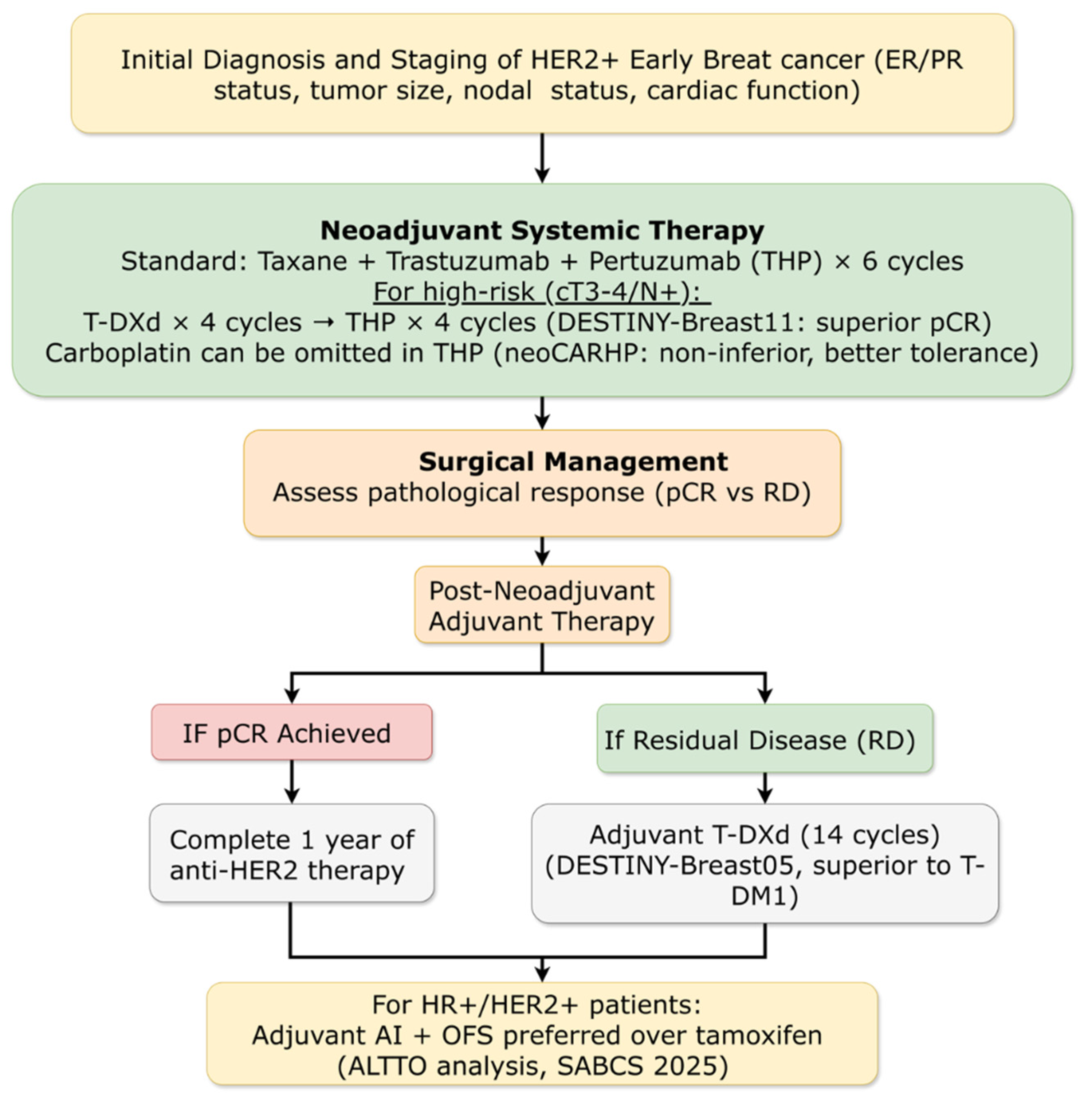
3.4.2. Early Breast Cancer: HER2-Positive Subtype
3.4.2.1. Adjuvant Therapy for High-Risk Residual Disease: New Standard Established
3.4.2.2. Neoadjuvant Strategies: Chemotherapy De-Escalation and ADC Integration
3.4.2.3. Novel HER2-Targeted Agents in Early Disease
3.4.2.4. Optimizing Adjuvant Endocrine Therapy in HER2+/HR+ Disease
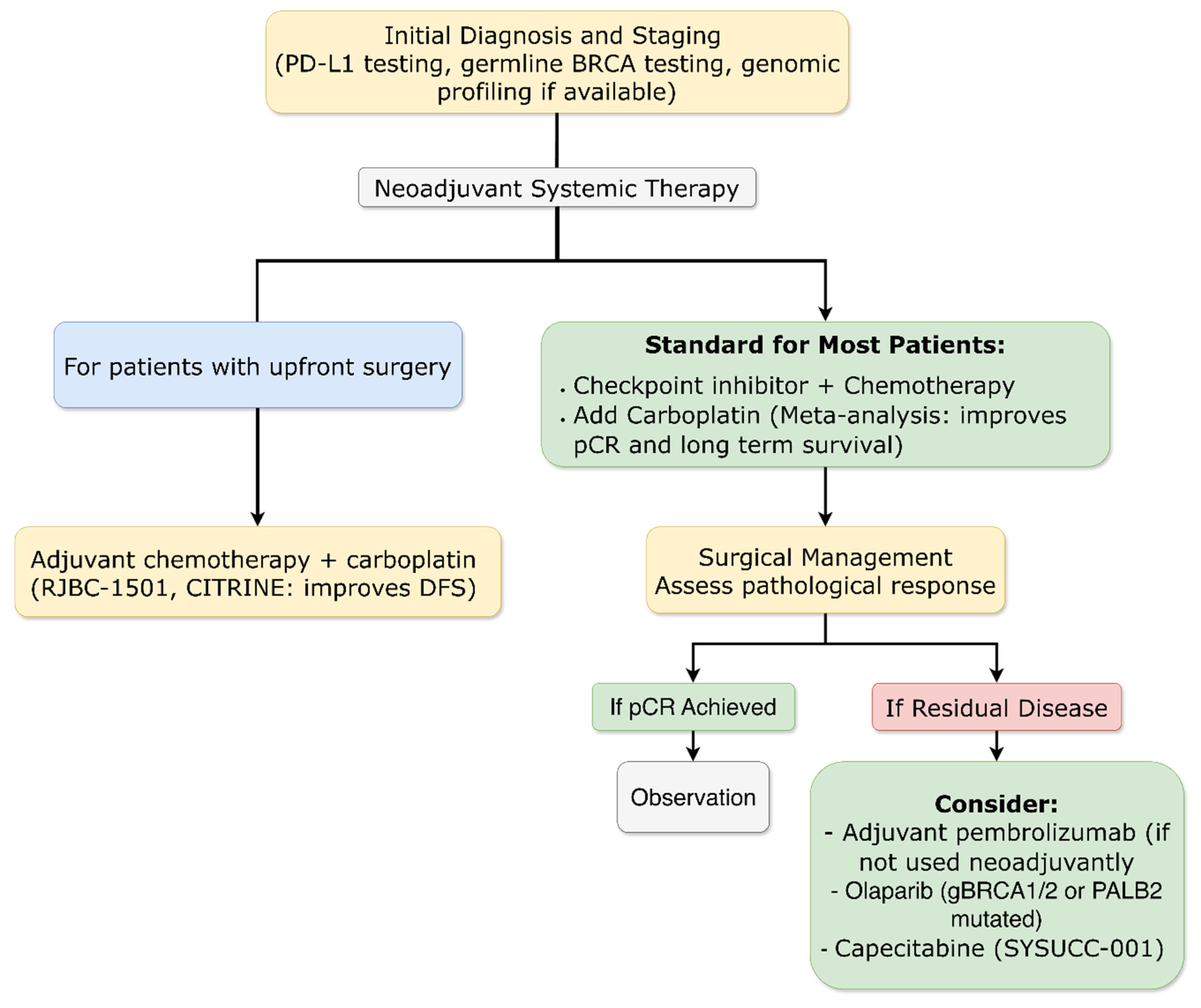
3.4.3. Early Breast Cancer: Triple-Negative Subtype
3.4.3.1. Long-Term Immunotherapy Benefits Confirmed
3.4.3.2. Biomarker Refinement for Patient Selection
3.4.3.3. Global Access Strategies
3.4.3.4. Chemotherapy Backbone Optimization: The Definitive Role of Platinum Agents
3.4.3.5. Novel Chemotherapy-Free Neoadjuvant Approaches
3.4.3.6. Novel ADC-Based Neoadjuvant Approaches
3.4.3.7. Extended Adjuvant Strategies
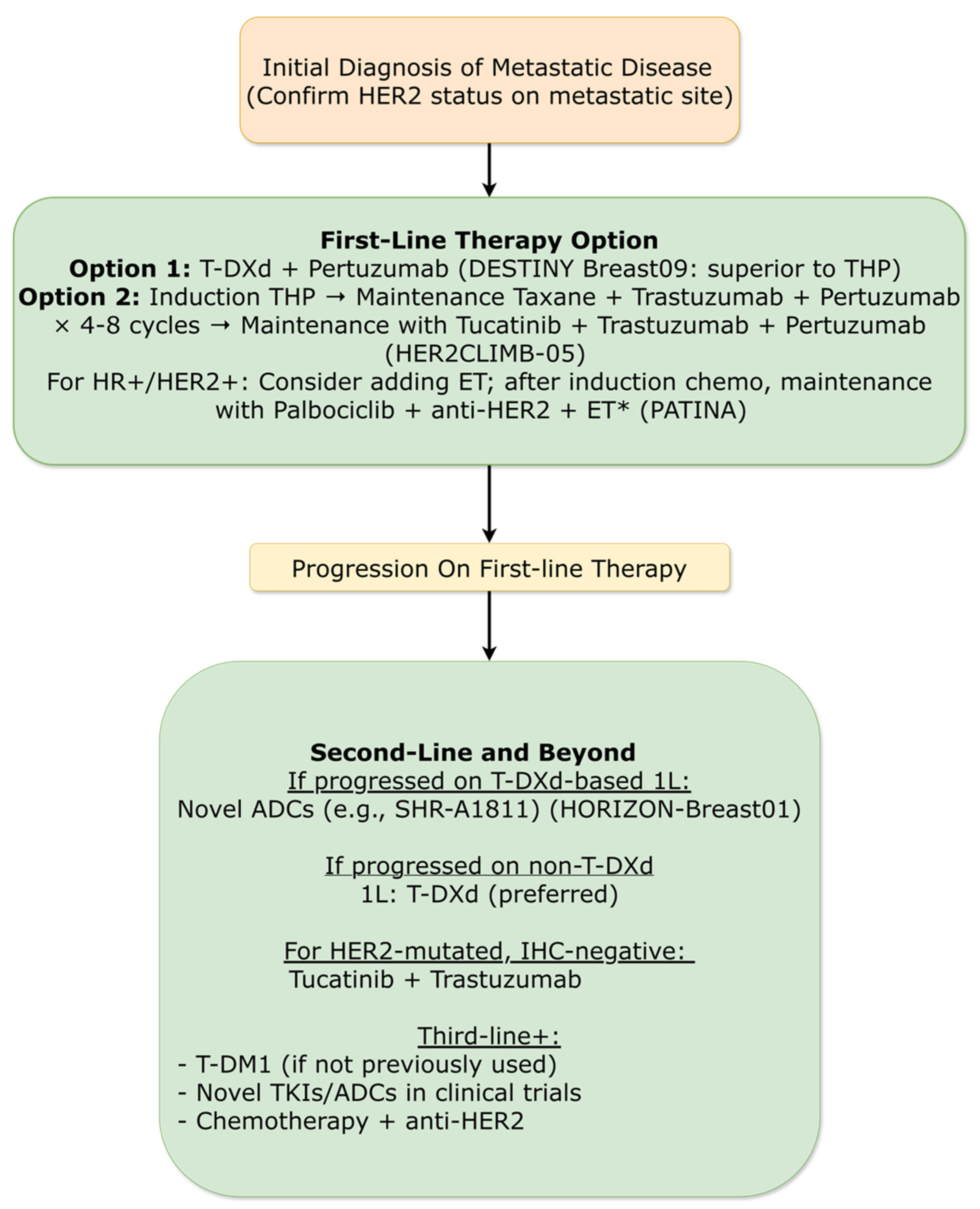
3.4.4. Metastatic Breast Cancer: HER2-Positive Subtype
3.4.4.1. First-Line Therapy Redefined
3.4.4.2. Next-Generation ADCs in Later Lines
3.4.4.3. Maintenance Strategies for HR+/HER2+ Disease
3.4.4.4. HER2-Low and HER2-Mutated Populations
3.4.4.5. Novel Combination Approaches
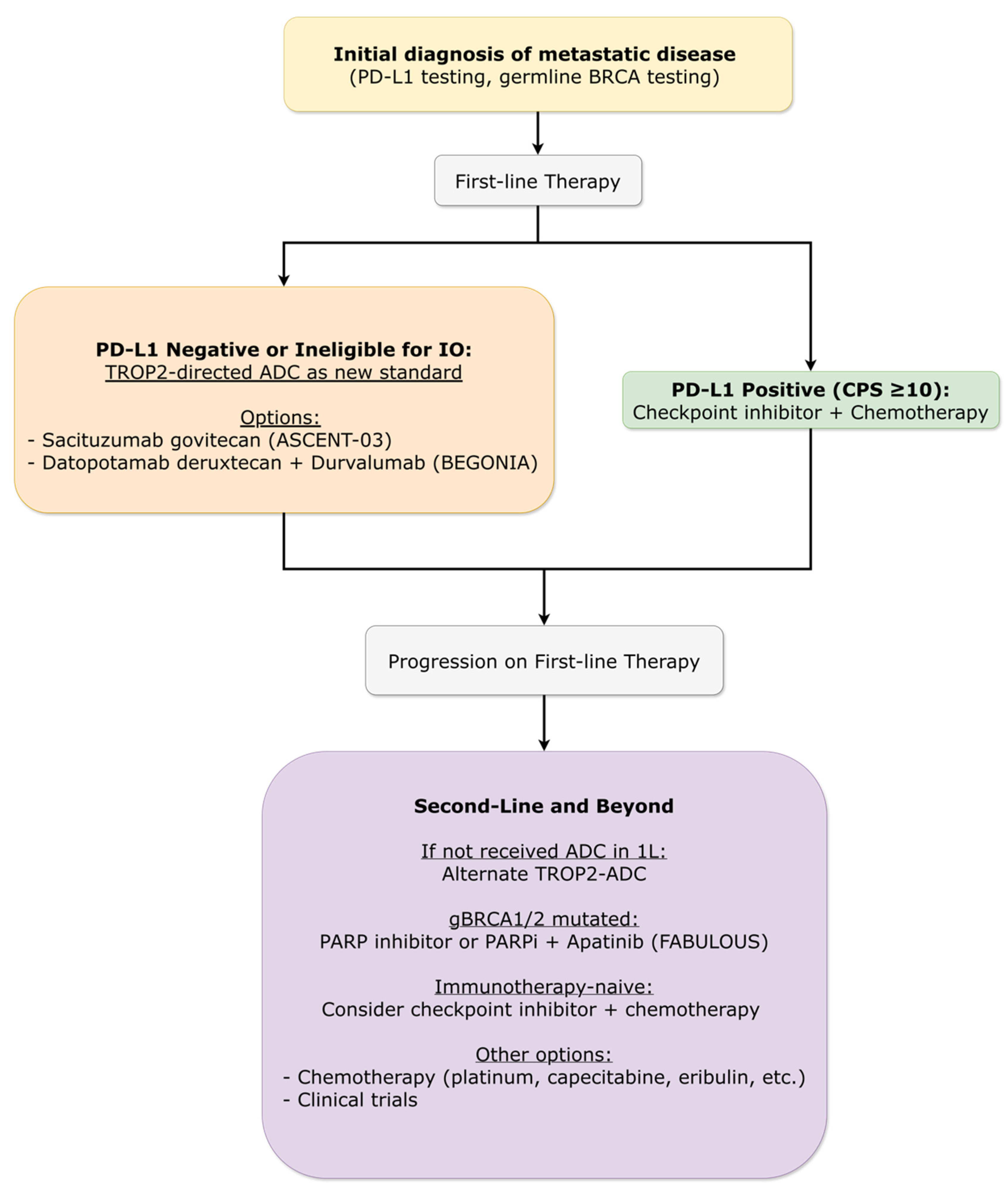
3.4.5. Metastatic Breast Cancer: Triple-Negative Subtype
3.4.5.1. First-Line Therapy Evolution
3.4.5.2. Immunotherapy Combinations Refined
3.4.5.3. Later-Line Options and Novel Targets
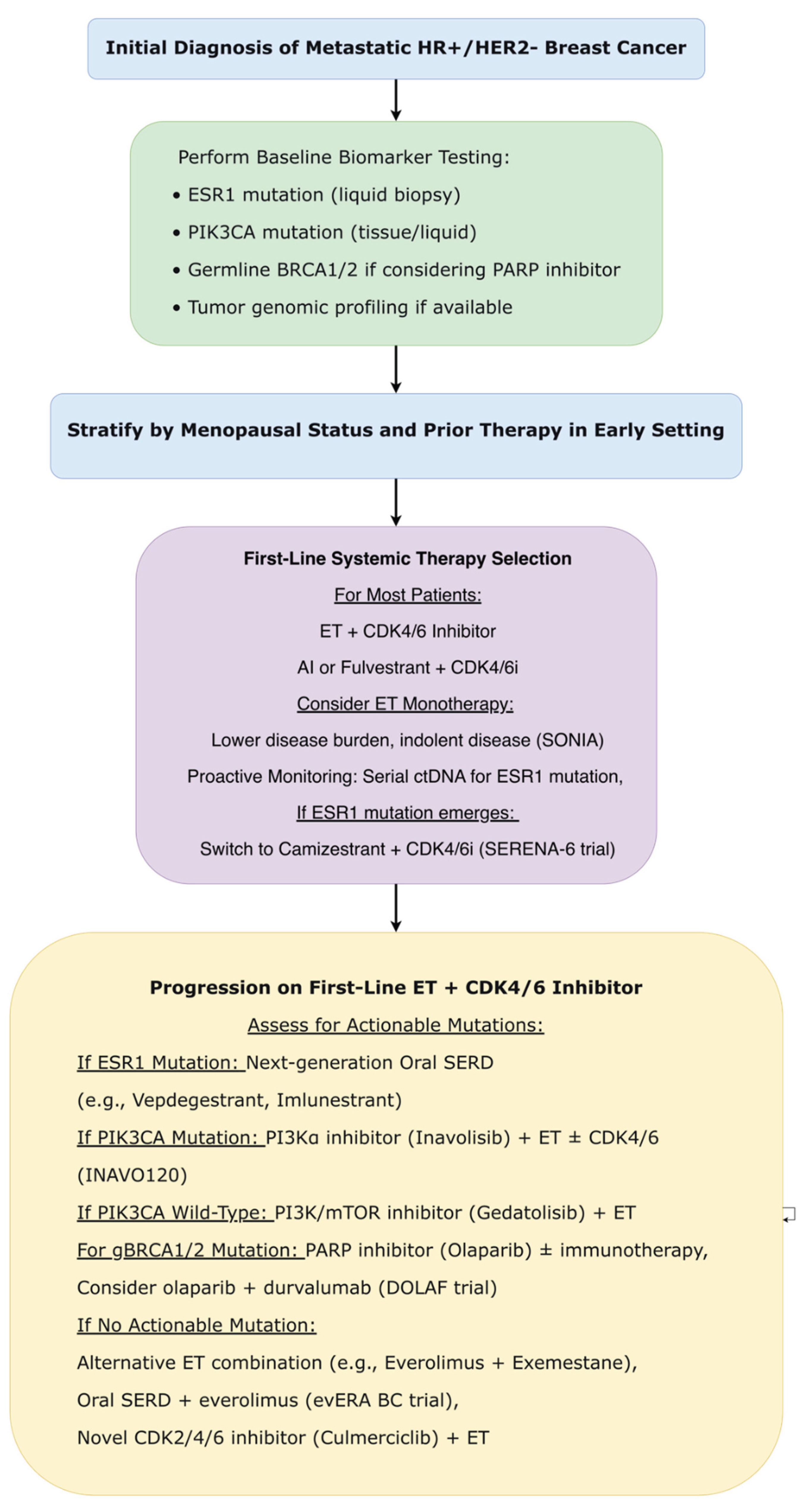
3.4.6. Metastatic Breast Cancer: HR+/HER2− Subtype
3.4.6.1. CDK4/6 Inhibitor Sequencing: De-Escalation Validated
3.4.6.2. Novel CDK Inhibition Strategies
3.4.6.3. Proactive Biomarker-Guided Strategies
3.4.6.4. Next-Generation Endocrine Agents
3.4.6.5. PI3K/AKT/mTOR Pathway Inhibition Refined
3.4.6.6. Novel Resistance Mechanisms and Targeting Strategies
3.4.7. Prevention and Special Populations
3.4.7.1. Prevention in High-Risk Populations
3.4.7.2. Special Therapeutic Scenarios
3.5. Summary of Evidence Table
4. Discussion
4.1. Integration of Major Advances by Disease Area
4.2. Overarching Themes and Implications
4.2.1. Biomarker-Driven Personalization Across the Continuum
4.2.2. Antibody-Drug Conjugates as Transformative Platform Therapeutics
4.2.3. Strategic Treatment Sequencing and Intelligent De-Escalation
4.2.4. Proactive Rather Than Reactive Management Paradigms
4.2.5. Global Equity and Access Considerations
4.2.6. Patient-Reported Outcomes as Essential Endpoints
4.3. Clinical Implementation Challenges
4.3.1. Treatment Sequencing Complexities
4.3.2. Toxicity Management Specialization
4.3.3. Biomarker Testing Infrastructure
4.3.4. Financial Toxicity and Access Barriers
4.4. Limitations of the Evidence Base
4.4.1. Interim Nature of Conference Data
4.4.2. Generalizability Concerns
4.4.3. Publication and Presentation Bias
4.4.4. Heterogeneity in Endpoints and Assessments
4.4.5. Short Follow-Up for Novel Agents
4.5. Future Research Directions
4.5.1. Biomarker Refinement and Validation
4.5.2. Treatment Sequencing and Combination Optimization
4.5.3. Overcoming Resistance Mechanisms
4.5.4. Global Health and Implementation Science
4.5.5. Patient-Reported Outcomes and Quality of Life
4.5.6. Novel Therapeutic Platforms
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Registration and Protocol
References
- Bray, F; Laversanne, M; Sung, H; Ferlay, J; Siegel, RL; Soerjomataram, I; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024, 74(3), 229–263. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S; André, F; Bachelot, T; Barrios, CH; Bergh, J; Burstein, HJ; Cardoso, MJ; Carey, LA; Dawood, S; Del Mastro, L; Denkert, C; Fallenberg, EM; Francis, PA; Gamal-Eldin, H; Gelmon, K; Geyer, CE; Gnant, M; Guarneri, V; Gupta, S; Kim, SB; Krug, D; Martin, M; Meattini, I; Morrow, M; Janni, W; Paluch-Shimon, S; Partridge, A; Poortmans, P; Pusztai, L; Regan, MM; Sparano, J; Spanic, T; Swain, S; Tjulandin, S; Toi, M; Trapani, D; Tutt, A; Xu, B; Curigliano, G; Harbeck, N. ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024, 35(2), 159–182. [Google Scholar] [CrossRef]
- Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, Dent R, Fenlon D, Gligorov J, Hurvitz SA, Im SA, Krug D, Kunz WG, Loi S, Penault-Llorca F, Ricke J, Robson M, Rugo HS, Saura C, Schmid P, Singer CF, Spanic T, Tolaney SM, Turner NC, Curigliano G, Loibl S, Paluch-Shimon S, Harbeck N; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021, 32(12), 1475–1495. [CrossRef] [PubMed]
- Johnston, S; Martin, M; O'Shaughnessy, J; Hegg, R; Tolaney, SM; Guarneri, V; Del Mastro, L; Campone, M; Sohn, J; Boyle, F; Cortes, J; Rugo, HS; Goetz, MP; Hamilton, EP; Huang, CS; Senkus, E; Cicin, I; Testa, L; Neven, P; Huober, J; Shao, Z; Wei, R; Munoz, M; San Antonio, B; Shahir, A; Rastogi, P; Harbeck, N. Overall survival with abemaciclib in early breast cancer. Ann Oncol Epub ahead of print. 2025. [Google Scholar] [CrossRef] [PubMed]
- Crown, J; Stroyakovskii, D; Yardley, DA; Huang, CS; Fasching, PA; Bardia, A; Chia, S; Im, SA; Martin, M; Xu, B; Barrios, CH; Untch, M; Moroose, R; Hurvitz, SA; Hortobagyi, GN; Slamon, DJ; Visco, F; Spera, G; Zarate, JP; Halligan, D; Li, Z; Loi, S. Adjuvant ribociclib plus nonsteroidal aromatase inhibitor therapy in patients with HR-positive/HER2-negative early breast cancer: 5-year follow-up of NATALEE efficacy outcomes and updated overall survival. ESMO Open 2025, 10(11), 105858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cottu, P; Prat, A; Pascual, T; Hu, H; Roux, E; Salvador Bofill, FJ; et al. Risk of recurrence (ROR) after neoadjuvant ribociclib plus ET in clinically high-risk ER+/HER2− BC: Preliminary analysis of the SOLTI-RIBOLARIS trial [Abstract 296O]. Ann Oncol. 2024, 35 (Suppl 1), S101–S102. [Google Scholar] [CrossRef]
- Demeestere, I; Niman, SM; Partridge, AH; Pagani, O; Azim, HA; Peccatori, FA; et al. Predictive factors of fertility in patients with breast cancer interrupting adjuvant endocrine therapy to attempt pregnancy in the POSITIVE trial [Abstract 415O]. ESMO Open. 2025, 10 (Suppl 4), 104987. [Google Scholar] [CrossRef]
- Llombart-Cussac, A; Viale, G; Ruiz-Borrego, M; Carañana Ballerini, V; López-Miranda, E; Blancas López-Barajas, I; et al. Preoperative window-of-opportunity study with giredestrant or tamoxifen (tam) in premenopausal women with estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2-) and Ki67≥10% early breast cancer (EBC): The EMPRESS study [Abstract 294MO]. Ann Oncol. 2025, 36 (Suppl 1), S101–S102. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; Lim, E.; Hamilton, E.P.; Oliveira, M.; Loi, S.; Aftimos, P.; Chandarlapaty, S.; Conlan, M.G.; Moulder, S.; Baird, R.D.; et al. Neoadjuvant vepdegestrant (ARV-471) in postmenopausal patients with ER+/HER2− early breast cancer: Primary results from the phase 2 TACTIVE-N trial. Cancer Discov. 2025, *15*, CD-25-0001. [Google Scholar]
- Bardia, A.; Schmid, P.; Martín, M.; Hurvitz, S.; Jung, K.; Rimawi, M.; Saji, S.; Werutsky, G.; Harbeck, N.; Loi, S.; et al. Giredestrant vs Standard-of-Care Endocrine Therapy as Adjuvant Treatment for Patients with Estrogen Receptor-Positive, HER2-Negative Early Breast Cancer: Results from the Global Phase III lidERA Breast Cancer Trial. In Proceedings of the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 9–12 December 2025. Abstract GS1-10. [Google Scholar]
- Pagani, O; Walley, BA; Fleming, GF; Colleoni, M; Láng, I; Gomez, HL; Tondini, C; Burstein, HJ; Goetz, MP; Ciruelos, EM; Stearns, V; Bonnefoi, HR; Martino, S; Geyer, CE, Jr.; Chini, C; Puglisi, F; Spazzapan, S; Ruhstaller, T; Winer, EP; Ruepp, B; Loi, S; Coates, AS; Gelber, RD; Goldhirsch, A; Regan, MM; Francis, PA. SOFT and TEXT Investigators and the International Breast Cancer Study Group (a division of ETOP IBCSG Partners Foundation). Adjuvant Exemestane With Ovarian Suppression in Premenopausal Breast Cancer: Long-Term Follow-Up of the Combined TEXT and SOFT Trials. J Clin Oncol. 2023, 41(7), 1376–1382. [Google Scholar]
- Francis, P. A., Fleming, G. F., Pagani, O., Walley, B., Loi, S., Colleoni, M., ... & SOFT and TEXT Investigators, IBCSG, BIG and North American BC Groups. 15-year outcomes for women with premenopausal hormone receptor-positive early breast cancer (BC) in the SOFT and TEXT trials assessing benefits from adjuvant exemestane (E)+ ovarian function suppression (OFS) or tamoxifen (T)+ OFS. 2025.
- Cardoso, F; Parke, S; Brennan, DJ; Briggs, P; Donders, G; Panay, N; Haseli-Mashhadi, N; Block, M; Caetano, C; Francuski, M; Haberland, C; Laapas, K; Seitz, C; Zuurman, L. Elinzanetant for Vasomotor Symptoms from Endocrine Therapy for Breast Cancer. N Engl J Med. 2025, 393(8), 753–763. [Google Scholar] [CrossRef] [PubMed]
- Geyer, CE, Jr.; Park, YH; Shao, Z; Huang, CS; Barrios, C; Abraham, J; et al. Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients with high-risk human epidermal growth factor receptor 2–positive (HER2+) primary breast cancer with residual invasive disease after neoadjuvant therapy: Interim analysis of DESTINY-Breast05 [Abstract LBA1]. Ann Oncol. 2025, 36 (Suppl 1), S1–S2. [Google Scholar] [CrossRef]
- Harbeck, N; Modi, S; Pusztai, L; Ohno, S; Wu, J; Kim, SB; et al. DESTINY-Breast11: neoadjuvant trastuzumab deruxtecan alone or followed by paclitaxel + trastuzumab + pertuzumab vs ddAC-THP for high-risk HER2+ early breast cancer [Abstract 291O]. Ann Oncol. 2025, 36 (Suppl 1), S98–S99. [Google Scholar] [CrossRef]
- Gao, H.-F.; Li, W.; Wu, Z.; Dong, J.; Cao, Y.; Zhao, Y.; Chen, Q.-J.; et al. De-Escalated Neoadjuvant Taxane Plus Trastuzumab and Pertuzumab With or Without Carboplatin in HER2-Positive Early Breast Cancer (neoCARHP): A Multicentre, Open-Label, Randomised, Phase 3 Trial. J. Clin. Oncol. 2025, *43* (Suppl. 17), LBA500. [Google Scholar] [CrossRef]
- Li, JJ; Zhang, WJ; Zeng, XH; et al. Efficacy and Safety of Neoadjuvant TQB2102 in Locally Advanced or Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: A Randomized, Open-Label, Multicenter, Phase II Trial. J Clin Oncol. 2026, 44(1), 20–30. [Google Scholar] [CrossRef]
- Li, JJ; Wang, ZH; Chen, L; Zhang, WJ; Ma, LXX; Wu, J; Liu, GY; Hou, YF; Yu, KD; Di, GH; Fan, L; Jiang, YZ; Jiang, SH; Liang, QN; Shen, Y; Shao, ZM. Efficacy and safety of neoadjuvant SHR-A1811 with or without pyrotinib in women with locally advanced or early HER2-positive breast cancer: a randomized, open-label, phase II trial. Ann Oncol. 2025, 36(6), 651–659. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Samy, F.; Agostinetto, E.; Arecco, L.; Freire, P.; Sonnenblick, A.; Arpino, G.; Del Mastro, L.; Choudhury, A.; Harbeck, N.; et al. Adjuvant Aromatase Inhibitor or Tamoxifen in Patients with Hormone Receptor-Positive/HER2-Positive Early Breast Cancer: An Exploratory Analysis from the ALTTO (BIG 2-06) Trial. In Proceedings of the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 9–12 December 2025. Abstract GS1-03. [Google Scholar]
- Loibl, S; Untch, M; Huober, J; Schaser, V; Braun, M; Denkert, C; et al. Durvalumab in combination with neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC): Long-term analysis from the GeparNuevo trial [Abstract 292MO]. Ann Oncol. 2025, 36 (Suppl 1), S97. [Google Scholar] [CrossRef]
- Dieci, MV; Bisagni, G; Nicolé, L; Schmid, P; Zambelli, A; Piacentini, F; et al. Efficacy of adjuvant anti-PD-L1 avelumab by tumor infiltrating lymphocytes (TILs) for high-risk triple negative breast cancer in the phase III A-BRAVE trial [Abstract]. Ann Oncol. 2025, 36 (Suppl 1), S99–S100. [Google Scholar] [CrossRef]
- Batra, A; Arora, A; Bhaskarane, H; Mathur, S; V, S; Parshad, R; et al. Low Dose Pembrolizumab in Addition to Neoadjuvant Anthracycline and Taxane in Triple Negative Breast Cancer: A Randomized Controlled Trial (PLANeT Trial) [Abstract]. Ann Oncol. 2025, 36 (Suppl 1), S94–S95. [Google Scholar] [CrossRef]
- Geyer, C.E.; Sikov, W.M.; Huober, J.; Rugo, H.S.; Wolmark, N.; O'Shaughnessy, J.; Maag, D.; Untch, M.; Golshan, M.; McIntyre, K.; et al. Long-term outcomes of carboplatin added to adjuvant dose-dense chemotherapy for high-risk triple-negative breast cancer (NRG-BR003): A randomized, phase 3 trial. J. Clin. Oncol. 2025, *43* JCO.2025.12350. [Google Scholar]
- Felsheim, B.M.; Nekljudova, V.; Carey, L.A.; Huober, J.; Schneeweiss, A.; Tolaney, S.M.; Untch, M.; Kuzma, C.S.; Fernandez-Martinez, A.; Rachakonda, S.; et al. Pooled Analysis of the BrighTNess, CALGB 40603 (Alliance), and GeparSixto Clinical Trials Identifies the Impact of Neoadjuvant Carboplatin on pCR and Survival in Early-Stage Triple-Negative Breast Cancer. In Proceedings of the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 9–12 December 2025. Abstract RF2-02. [Google Scholar]
- Chen, X.; Huang, J.; Shi, H.; Zhu, J.; Wu, W.; Ye, G.; He, Q.; Shi, Y.; Zhang, A.; Xie, X.; et al. Adjuvant Epirubicin Plus Cyclophosphamide Followed by Taxanes With or Without Carboplatin for Early Stage Triple-Negative Breast Cancer (RJBC 1501): A Randomized Controlled Phase III Trial. In Proceedings of the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 9–12 December 2025. Abstract RF2-03. [Google Scholar]
- Liu, Y.; Gong, Y.; Zhu, X.; Liu, G.; Yu, K.; Yang, F.; Chen, L.; He, M.; Hu, Z.; Chen, C.; et al. Effect of Adjuvant Carboplatin Intensified Chemotherapy Versus Standard Chemotherapy on Survival in Women with High-Risk Early-Stage Triple-Negative Breast Cancer (CITRINE): A Phase 3 Randomized Trial. In Proceedings of the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 9–12 December 2025. Abstract RF2-04. [Google Scholar]
- Mayer, E.L.; Timms, K.M.; Abramson, V.G.; Tung, N.M.; Patel, T.; Burgess, E.F.; DeMeo, M.K.; DiNome, M.L.; Elias, A.D.; Gatti-Mays, M.E.; et al. TBCRC-056: Phase II Study of Neoadjuvant Niraparib + Dostarlimab in Germline *BRCA1/2* or PALB2 Mutated, HER2-Negative Breast Cancer. Presented at the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 9–12 December 2025. Abstract RF5-02. [Google Scholar]
- Tung, N.; Stradella, A.; Brufsky, A.; Fasching, P.A.; García-Sáenz, J.A.; Paluch-Shimon, S.; Henao Carrasco, F.M.; Marquez Aragones, A.; Park-Simon, T.-W.; Antolin Novoa, S.; et al. OlympiAN: A Phase 2, Multicenter, Open-Label Study to Assess the Efficacy and Safety of Neoadjuvant Olaparib Monotherapy and Olaparib Plus Durvalumab in Patients with BRCA Mutations and Early-Stage HER2-Negative Breast Cancer. In Proceedings of the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 9–12 December 2025. Abstract RF5-03. [Google Scholar]
- Abelman, R.O.; McLaughlin, S.; Fell, G.G.; Garrido-Castro, A.C.; Lynce, F.; Trippa, L.; Poorvu, P.D.; et al. A Phase 2 Study of Response-Guided Neoadjuvant Sacituzumab Govitecan and Pembrolizumab (SG/P) in Patients with Early-Stage Triple-Negative Breast Cancer: Results from the NeoSTAR Trial. J. Clin. Oncol. 2025, *43* (Suppl. 16), 511. [Google Scholar] [CrossRef]
- Yuan, J; Bi, XW; Hua, X; et al. Metronomic capecitabine as extended adjuvant chemotherapy for early triple-negative breast cancer (SYSUCC-001): updated 10-year outcomes and post-hoc exploratory biomarker analysis from a randomised, phase 3 trial. Lancet Oncol. 2025, 26(12), 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Jiang, Z.; Zhang, Q.; Barroso-Sousa, R.; Park, Y.H.; Rimawi, M.F.; Saura Manich, C.; et al. Trastuzumab Deruxtecan (T-DXd) + Pertuzumab (P) vs Taxane + Trastuzumab + Pertuzumab (THP) for First-Line (1L) Treatment of Patients (pts) with Human Epidermal Growth Factor Receptor 2–Positive (HER2+) Advanced/Metastatic Breast Cancer (a/mBC): Interim Results from DESTINY-Breast09. J. Clin. Oncol. 2025, *43* (Suppl. 17), LBA1008. [Google Scholar] [CrossRef]
- Tolaney, SM; Jiang, Z; Zhang, Q; Barroso-Sousa, R; Park, YH; Rimawi, MF; Saura, C; Schneeweiss, A; Toi, M; Chae, YS; Kemal, Y; Chaudhari, M; Şendur, MAN; Yamashita, T; Casalnuovo, M; Danso, MA; Liu, J; Shetty, J; Herbolsheimer, P; Loibl, S. DESTINY-Breast09 Trial Investigators. Trastuzumab Deruxtecan plus Pertuzumab for HER2-Positive Metastatic Breast Cancer. N Engl J Med Epub ahead of print. 2025. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Curigliano, G.; Martin, M.; Lerebours, F.; Tsurutani, J.; Savard, M.; Jerzak, K.; Hu, X.; de Aquino Pimentel, L.M.; O’Sullivan, C.; et al. HER2CLIMB-05: A Randomized, Double-Blind, Phase 3 Study of Tucatinib Versus Placebo in Combination with Trastuzumab and Pertuzumab as Maintenance Therapy for HER2+ Metastatic Breast Cancer. In Proceedings of the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 9–12 December 2025. Abstract GS1-01. [Google Scholar]
- Song, E.; Yao, H.; Li, H.; Yin, Y.; Zhang, Q.; Wang, S.; Ouyang, Q.; Sun, T.; Wang, X.; Xie, W.; et al. SHR-A1811 Versus Pyrotinib Plus Capecitabine in Human Epidermal Growth Factor Receptor 2-Positive (HER2+) Advanced/Metastatic Breast Cancer (BC): A Multicenter, Open-Label, Randomized, Phase III Study (HORIZON-Breast01). In Proceedings of the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 9–12 December 2025. Abstract LBA19. [Google Scholar]
- Zhang, T; Xu, J; Yin, J; Gao, Y; Zheng, H; Fu, B; Sun, J; Xu, Z; Tu, S; Mao, Y; Wen, W; Qu, B; You, L; Xue, Z; Sun, X; Cao, D; Feng, J; Hu, M; He, F. SHR-A1811, a novel anti-HER2 antibody-drug conjugate with optimal drug-to-antibody ratio, efficient tumor killing potency, and favorable safety profiles. PLoS One 2025, 20(6), e0326691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vaz-Luis, I; Mazza, G; Gligorov, J; Lim, E; Ciruelos, E; Loibl, S; et al. Health-Related Quality of Life From the PATINA Trial (AFT-38): Impact of Adding Palbociclib to HER2 and Endocrine Therapy After Induction in HR+/HER2+ Metastatic Breast Cancer [Abstract]. Ann Oncol. 2025, 36 (Suppl 1), S103–S104. [Google Scholar] [CrossRef]
- Modi, S; Jacot, W; Iwata, H; Park, YH; Vidal Losada, M; Li, W; Tsurutani, J; Ueno, NT; Zaman, K; Prat, A; Papazisis, K; Rugo, HS; Yamashita, T; Harbeck, N; Im, SA; De Laurentiis, M; Pierga, JY; Wang, X; Gombos, A; Tokunaga, E; Orbegoso Aguilar, C; Yung, L; Xiao, F; Cheng, Y; Cameron, D. Trastuzumab deruxtecan in HER2-low metastatic breast cancer: long-term survival analysis of the randomized, phase 3 DESTINY-Breast04 trial. Nat Med. 2025, 31(12), 4205–4213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okines, AFC; Curigliano, G; Mizuno, N; Oh, DY; Rorive, A; Soliman, H; Takahashi, S; Bekaii-Saab, T; Burkard, ME; Chung, KY; Debruyne, PR; Fox, JR; Gambardella, V; Gil-Martin, M; Hamilton, EP; Monk, BJ; Nakamura, Y; Nguyen, D; O'Malley, DM; Olawaiye, AB; Pothuri, B; Reck, M; Sudo, K; Sunakawa, Y; Van Marcke, C; Yu, EY; Ramos, J; Tan, S; Bieda, M; Stinchcombe, TE; Pohlmann, PR. Tucatinib and trastuzumab in HER2-mutated metastatic breast cancer: a phase 2 basket trial. Nat Med. 2025, 31(3), 909–916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X; Lee, KS; Zeng, X; et al. Zanidatamab in combination with docetaxel in first-line HER2-positive breast cancer: results from an open-label, multicenter, phase Ib/II study. ESMO Open. 2025, 10(11), 105852. [Google Scholar] [CrossRef]
- Cortés, J; Bardia, A; Punie, K; Barrios, C; Hurvitz, S; Schneeweiss, A; et al. Primary Results From ASCENT-03: A Randomized Phase 3 Study of Sacituzumab Govitecan vs Chemotherapy in Patients With Previously Untreated Metastatic Triple-Negative Breast Cancer Who Are Unable to Receive PD-(L)1 Inhibitors [Abstract]. Ann Oncol. 2025, 36 (Suppl 1), S3–S4. [Google Scholar] [CrossRef]
- Punie, K.; Tolaney, S.M.; Bardia, A.; Hurvitz, S.A.; Barrios, C.; Schneeweiss, A.; Sohn, J.; et al. Patient-Reported Outcomes (PROs) with Sacituzumab Govitecan (SG) vs Chemotherapy in Patients with Previously Untreated Advanced Triple-Negative Breast Cancer (TNBC) Who Are Not Candidates for PD-(L)1 Inhibitors in the Phase 3 ASCENT-03 Study. In Proceedings of the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 9–12 December 2025. Abstract RF6-05. [Google Scholar]
- Schmid, P; Wang, HC; Lynce, F; Park, YH; Ma, CX; Jung, KH; et al. Datopotamab deruxtecan (Dato-DXd) in combination with durvalumab as first-line treatment for unresectable locally advanced/metastatic triple-negative breast cancer: Results from arms 7 and 8 of the phase 1b/2 BEGONIA study [Abstract]. Ann Oncol. 2025, 36 (Suppl 1), S96–S97. [Google Scholar] [CrossRef]
- Pistilli, B; Jhaveri, K; Im, SA; et al. Datopotamab deruxtecan versus chemotherapy in previously treated inoperable/metastatic hormone receptor-positive, HER2-negative breast cancer: final overall survival analysis of the phase III TROPION-Breast01 study. Ann Oncol. Published online. 23 December 2025. [CrossRef]
- Tolaney, SM; de Azambuja, E; Kalinsky, K; et al. Sacituzumab Govitecan plus Pembrolizumab for Advanced Triple-Negative Breast Cancer. N Engl J Med. 2026, 394(4), 354–366. [Google Scholar] [CrossRef]
- Yin, Y; Fan, Y; Ouyang, Q; et al. Sacituzumab tirumotecan in previously treated metastatic triple-negative breast cancer: a randomized phase 3 trial. Nat Med. 2025, 31(6), 1969–1975. [Google Scholar] [CrossRef]
- Li, H; Liu, J; Ouyang, Q; et al. Fuzuloparib with or without apatinib in patients with HER2-negative metastatic breast cancer with germline BRCA1/2 mutations (FABULOUS): interim analysis of a multicentre, three-arm, open-label, randomised, phase 3 trial. Lancet Oncol. 2025, 26(12), 1563–1574. [Google Scholar] [CrossRef]
- Wortelboer, N; van Ommen-Nijhof, A; Konings, I; van der Noort, V; van den Pol, E; Guerrero Páez, C; et al. Overall survival with first versus second-line use of CDK4/6 inhibitors in HR+/HER2- advanced breast cancer: Results from the phase 3 SONIA trial (BOOG 2017-03) [Abstract]. Ann Oncol. 2025, 36 (Suppl 1), S4–S5. [Google Scholar] [CrossRef]
- Song, E.; Yin, Y.; Zhao, J.; et al. LBA25 Culmerciclib plus fulvestrant as first-line treatment for HR+/HER2-advanced breast cancer: A phase III trial (CULMINATE-2). Annals of Oncology 2025, 36, S1684–S1685. [Google Scholar] [CrossRef]
- Bidard, FC; Mayer, EL; Park, YH; et al. First-Line Camizestrant for Emerging ESR1-Mutated Advanced Breast Cancer. N Engl J Med. 2025, 393(6), 569–580. [Google Scholar] [CrossRef]
- Mayer, E., Bidard, F. C., Park, Y. H., Janni, W., Ma, C. X., Cristofanilli, M., ... & Turner, N. C. 486MO Patient-reported outcomes (PROs) from the SERENA-6 trial of camizestrant (CAMI)+ CDK4/6 inhibitor (CDK4/6i) for emergent ESR1m during first-line (1L) endocrine-based therapy and ahead of disease progression in patients (pts) with HR+/HER2–advanced breast cancer (ABC). Annals of Oncology 2025, 36, S402.
- Campone, M; De Laurentiis, M; Jhaveri, K; et al. Vepdegestrant, a PROTAC Estrogen Receptor Degrader, in Advanced Breast Cancer. N Engl J Med. 2025, 393(6), 556–568. [Google Scholar] [CrossRef] [PubMed]
- Campone, M.; De Laurentiis, M.; Hu, X.; et al. 489MO Patient-reported outcomes (PROs) with vepdegestrant (VEP) vs fulvestrant (FUL) in patients (pts) with estrogen receptor (ER) 1 gene mutated (ESR1m) ER+/human epidermal growth factor receptor 2 (HER2): Advanced breast cancer (aBC) in the phase 3 VERITAC-2 trial. Annals of Oncology 2025, 36, S403–S404. [Google Scholar]
- Mayer, EL; Tolaney, SM; Martin, M; Vidal, GA; Moscetti, L; Jhaveri, KL; et al. Giredestrant (GIRE), an oral selective oestrogen receptor (ER) antagonist and degrader, + everolimus (E) in patients (pts) with ER-positive, HER2-negative advanced breast cancer (ER+, HER2– aBC) previously treated with a CDK4/6 inhibitor (i): Primary results of the Phase III evERA BC trial [Abstract]. Ann Oncol. 2025, 36 (Suppl 1), S110–S111. [Google Scholar] [CrossRef]
- Jhaveri, KL; Neven, P; Casalnuovo, ML; et al. Imlunestrant with or without abemaciclib in advanced breast cancer: updated efficacy results from the phase III EMBER-3 trial. Ann Oncol. Published online. 12 December 2025. [CrossRef]
- Jhaveri, KL; Im, SA; Saura, C; et al. Overall Survival with Inavolisib in PIK3CA-Mutated Advanced Breast Cancer. N Engl J Med. 2025, 393(2), 151–161. [Google Scholar] [CrossRef] [PubMed]
- Pistilli, B.; Layman, R.; Curigliano, G.; André, F.; Cristofanilli, M.; Martín, M.; Wesolowski, R.; Kim, S.-B.; Kim, G.; Richardet, M.; et al. Gedatolisib, a Multi-Target PI3K/AKT/mTOR (PAM) Inhibitor, Plus Fulvestrant With or Without Palbociclib for Second-Line (2L) Treatment of Patients with HR+/HER2-/PIK3CA-Wild Type (WT) Advanced Breast Cancer (ABC): Updated Results from the Randomized, Phase 3 VIKTORIA-1 Trial. In Proceedings of the San Antonio Breast Cancer Symposium (SABCS), San Antonio, TX, USA, 9–12 December 2025. Abstract RF7-04. [Google Scholar]
- Giugliano, F; De Angelis, C; Pistilli, B; Viale, G; Bianchini, G; Giuliano, M; Malorni, L; Taurelli Salimbeni, B; Esposito, A; Giordano, A; Yap, TA; Curigliano, G; Criscitiello, C. Overcoming Resistance to CDK4/6 inhibitors in Hormone Receptor positive, HER2 negative breast cancer: Innovative Combinations and Emerging Strategies. Cancer Treat Rev 2025, 139, 102980. [Google Scholar] [CrossRef] [PubMed]
- Yap, TA; Goldman, JW; Vinayak, S; et al. First-in-Human Phase I/IIa Study of the First-in-Class CDK2/4/6 Inhibitor PF-06873600 Alone or with Endocrine Therapy in Patients with Breast Cancer. Clin Cancer Res. 2025, 31(14), 2899–2909. [Google Scholar] [CrossRef] [PubMed]
- Pujol, P; Roca, L; Lortholary, A; et al. Letrozole to prevent breast cancer in postmenopausal women with BRCA1/2 mutations (LIBER study). Eur J Cancer 2025, 231, 116101. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Oliva, M.; Puntoni, M.; et al. 350P Long-term gynaecological safety and benign breast disease on low-dose tamoxifen: 10-year results from the TAM-01 phase III trial. Annals of Oncology 2025, 36, S349. [Google Scholar] [CrossRef]
- Guiu, S; Balmaña, J; Lemercier, P; et al. Combination of Olaparib, Durvalumab, and Fulvestrant in Patients with Advanced ER+/HER2- Breast Cancer and Selected Genomic Alterations: Results of the DOLAF Trial. Clin Cancer Res. 2025, 31(22), 4633–4643. [Google Scholar] [CrossRef]
| Element | Inclusion Criteria |
| Population | Adult patients (≥18 years) with histologically confirmed breast cancer of any stage (early or metastatic). |
| Intervention | Systemic therapies including: chemotherapy, endocrine therapy, targeted agents (e.g., HER2, TROP2, CDK4/6, PI3K, PARP, mTOR inhibitors), immunotherapy, ADCs, and novel mechanism agents (e.g., PROTAC degraders, oral SERDs, bispecific antibodies). |
| Comparison | Standard of care treatment, placebo, best supportive care, or active comparator (e.g., different drug or combination). Single-arm studies were considered only if they represented the only available evidence for a novel agent in a specific context. |
| Outcome | Primary efficacy endpoints: Overall Survival (OS), Progression-Free Survival (PFS), Invasive Disease-Free Survival (IDFS), Pathological Complete Response (pCR), Objective Response Rate (ORR). Secondary endpoints included safety, duration of response, and patient-reported outcomes (PROs). |
| Step | Number of Studies | Criteria |
|---|---|---|
| Database identification | 2500 | Combined searches of ASCO 2025, ESMO 2025, SABCS 2025 conference proceedings, and 2025 publications from PubMed/MEDLINE/Scopus. |
| Duplicates removed | 880 | Automatic and manual removal of duplicate records. |
| Titles/abstracts screened | 1620 | Initial screening based on inclusion criteria (Phase II/III trials in breast cancer). |
| Excluded after title/abstract | 1510 | Not Phase II/III, Wrong study design, Wrong population, Wrong outcome. |
| Full-text articles assessed | 110 | Complete evaluation of eligibility criteria. |
| Excluded after full-text | 60 | Insufficient data or preliminary results only; Overlapping study populations; Primary endpoints not met or insufficient follow-up; Other methodological limitations. |
| Studies included in the review | 50 | All inclusion criteria met. |
| Trial Name / Reference | Phase | Population (Subtype, Stage) | Experimental Arm | Control Arm | Primary Endpoint(s) | Key Efficacy Results | Key Safety/Tolerability Results | |
|---|---|---|---|---|---|---|---|---|
| HORMONE RECEPTOR-POSITIVE (HR+), HER2-NEGATIVE (18) | ||||||||
| monarchE (ESMO 2025) [4] | III | HR+/HER2- Early BC, node-positive, high-risk | Abemaciclib (2y) + ET | ET alone | iDFS, OS | 6.3-yr OS: HR 0.842 (95% CI: 0.722–0.981; p=0.0273). 5-yr OS: 86.8% vs 85%. iDFS: HR 0.734. | Diarrhea, neutropenia. No new delayed toxicity signals. | |
| NATALEE (ESMO 2025) [5] | III | HR+/HER2- Early BC (Stage II/III) | Ribociclib (400 mg, 3y) + NSAI (5y) | NSAI alone (5y) | iDFS | 5-yr iDFS: 85.5% vs 81% (delta 4.5, HR 0.716). OS trend favorable. | Consistent with known ribociclib profile. | |
| RIBOLARIS (ESMO 2025) [6] | II | HR+/HER2- Early BC, clinically high-risk | Neoadj. Ribociclib + Letrozole → ROR-guided adj. therapy | - | DMFS in ROR-low | 52.6% achieved low ROR, omitting chemo. Neoadj. progression: 2.19%. | Neutropenia, liver enzyme elevations. | |
| POSITIVE (ESMO 2025) [7] | Cohort | HR+/HER2- Early BC, ≤42 yrs, desire for pregnancy | Temporary ET interruption (≤2y) for pregnancy attempt | Matched cohort (SOFT/TEXT) | BCFI | 5-yr BCFI: 87.7% vs 86.8%, HR vs SOFT/TEXT: 0.88 (0.66–1.18). Pregnancy rate: 76%. | Safe interruption; 82% resumed ET. | |
| EMPRESS (ESMO 2025) [8] | II | HR+/HER2- Early BC, premenopausal, Ki67≥10% | Giredestrant (15d pre-op) | Tamoxifen (15d pre-op) | Ki67 change | Relative Ki67 reduction: -73% vs -51% (p<0.001). Cell cycle arrest: 17.5% vs 4.5% (p=0.074). | Fatigue, hot flush. Low TRAEs (31.0% vs 38.6%). | |
| TACTIVE-N (ESMO 2025) [9] | II | HR+/HER2- Early BC, postmenopausal, treatment-naïve | Vepdegestrant (neoadjuvant) | Anastrozole (neoadjuvant) | Ki67 change at D15 | Ki67 reduction: -71.4% vs -72.9%. mPEPI 0: 21% vs 20%. BCS rate: 70% vs 54%. | Hot flashes, asthenia. Low discontinuation (3% vs 8%). | |
| lidERA (SABCS 2025) [10] | III | ER+/HER2- Early BC, higher-risk (Stage I-III) | Adjuvant Giredestrant (oral, 30 mg QD) | Adjuvant Standard ET (Tamoxifen or AI) | IDFS (excluding second primary non-breast cancer) | IDFS HR=0.70 (95% CI: 0.57–0.87; p=0.0014). 3-year IDFS: 92.4% vs 89.6%. DRFI HR=0.69 (95% CI: 0.55–0.87). Interim OS HR=0.79 (95% CI: 0.56–1.12). |
Favorable safety profile. Lower discontinuation rate (5.3% vs 8.2%). Lower rate of discontinuations due to musculoskeletal (1.8% vs 4.4%) and vasomotor (<0.1% vs 0.9%) disorders. Comparable rates of Grade 3–4 AEs (19.8% vs 17.9%). | |
| SOFT/TEXT 15-yr Update (ASCO 2025) [11,12] | III | Premenopausal HR+ eBC | SOFT: T+OFS or E+OFS vs T; TEXT: E+OFS vs T+OFS | SOFT: T alone; TEXT: T+OFS | BCFI, DRFI, OS | SOFT: BCFI E+OFS vs T: 78.6% vs 72.1% (HR 0.70). TEXT/Combined: DRFI benefit for E+OFS vs T+OFS (87.6% vs. 83.7% HR 0.75). OS benefit in high-risk (79.4% vs. 75.6%). | Long-term safety as expected. | |
| OASIS-4 (ASCO 2025) [13] | III | HR+/HER2- eBC with VMS on ET | Elinzanetant 120 mg daily | Placebo | Mean change in daily frequency of moderate-to-severe VMS (wks 4-12) | Mean difference vs placebo: -3.5 (p<0.0001). Significant decrease in severity. | Fatigue (9.5%), somnolence (10%), diarrhea (5.1%). Well tolerated. | |
| SONIA (ESMO 2025) [47] | III and Economic | HR+/HER2- ABC | Strategy A: CDK4/6i + AI 1L → Fulvestrant 2L | Strategy B: AI 1L → CDK4/6i + Fulvestrant 2L | PFS2, QALYs | No sig. diff in PFS2. OS: 47.9 vs 48.1 mo (HR 0.91, p=0.24). QALYs: 2.694 vs 2.644. | 74% more Gr3-4 AEs with 1L CDK4/6i. | |
| CULMINATE-2 (ESMO 2025) [48] | III | HR+/HER2- ABC, pretreated | Culmerciclib (CDK2/4/6i) + Fulvestrant | Placebo + Fulvestrant | PFS | Median PFS: NR vs 20.2 mo (HR 0.56, p=0.0004). ORR: 59.3% vs 42.3% (p=0.0009). | Gr≥3 neutropenia 20.3%, leukopenia 10.7%. Low discontinuation (3.5%). | |
| SERENA-6 (ASCO 2025) [49,50] | III | HR+/HER2- ABC with emergent ESR1m on 1L AI+CDK4/6i | Switch to Camizestrant + CDK4/6i | Continue AI + CDK4/6i | PFS | Median PFS: 16.0 vs 9.2 mo (HR 0.44, p<0.0001). | Delayed TTD in GHS/QoL (HR 0.54), pain (HR 0.57). Modest neutropenia increase. | |
| VERITAC-2 (ASCO 2025) [51,52] | III | ER+/HER2- ABC with ESR1m; post-ET+CDK4/6i | Vepdegestrant | Fulvestrant | PFS | PFS benefit met | Favorable safety; low GI AEs, low discontinuations. Superior PROs. | |
| evERA BC (ESMO 2025) [53] | III | ER+/HER2- aBC (1-3L); post-CDK4/6i; 55% ESR1m | Giredestrant + Everolimus | SOC ET + Everolimus | INV-PFS (ESR1m & ITT) | ESR1m: PFS 9.99 vs 5.45 mo (HR 0.38, p<0.0001). ITT: 8.77 vs 5.49 mo (HR 0.56, p<0.0001). | Stomatitis, diarrhea, anemia. Manageable profile. | |
| EMBER-3 (SABCS 2025) [54] | III | ER+/HER2- ABC post-ET | 1. Imlunestrant mono 2. Imlunestrant + Abemaciclib |
SOC ET (Fulv/Exe) | PFS, OS (key updates) | ESR1m: OS Δ +11.4 mo (HR=0.60, p=0.0043*); PFS HR=0.62. All: Imlunestrant+Abema PFS HR=0.58 (10.9 vs 5.5 mo); OS trend HR=0.82. |
Favorable profile. Combo: Low D/C rate (6%). No new oral SERD-specific signals. | |
| INAVO120 (ASCO 2025) [55] | III | HR+/HER2- ABC with PIK3CA mutation, post-CDK4/6i | Inavolisib + Palbociclib + Fulvestrant | Placebo + Palbociclib + Fulvestrant | PFS | Median PFS: 15.0 vs 7.3 mo (HR 0.43). ORR: 58% vs 25%. Final OS: 34.0 vs 27.0 mo (HR 0.67, p=0.019). | Hyperglycemia, stomatitis, diarrhea, rash. | |
| VIKTORIA-1 (ESMO2025) [56] | III | HR+/HER2- ABC, PIK3CA-WT, post-CDK4/6i | Gedatolisib + Fulvestrant ± Palbociclib | Fulvestrant | PFS | Triplet: PFS 9.3 vs 2.0 mo (HR 0.24). Doublet: 7.4 vs 2.0 mo (HR 0.33). ORR: 31.5%/28.3% vs 1.0%. | Stomatitis, neutropenia, nausea. Low Gr3 hyperglycemia (2.3%). | |
| DOLAF (SABCS 2025) [61] | II | ER+/HER2- mBC with genomic alterations (e.g., gBRCAm), 2nd/3rd line | Durvalumab + Olaparib + Fulvestrant | - | 24-week PFS rate | 24-wk PFS rate: 66.7% (ITT), 76.3% in gBRCAm. Median PFS: 9.3 mo (ITT). | Nausea (59%), asthenia (43%). Acceptable. | |
| HER2-POSITIVE (13) | ||||||||
| DESTINY-Breast05 (ESMO 2025) [10] | III | HER2+ Early BC with residual disease post-neoadjuvant | Adjuvant T-DXd (14 cycles) | Adjuvant T-DM1 (14 cycles) | IDFS | 3-yr IDFS: 92.4% vs 83.7% (HR 0.47, p<0.0001). Benefit across subgroups. | ILD: 9.6% (any grade) vs 1.6%. Includes 0.9% Gr≥3, 0.2% fatal. | |
| DESTINY-Breast11 (ESMO 2025) [11] | III | High-risk HER2+ Early BC (cT3-4/N+) | T-DXd → THP (neoadjuvant) | ddAC-THP (neoadjuvant) | pCR | pCR: 67.3% vs 56.3% (Δ11.2%, p=0.003). Benefit in HR- (Δ16.1%). | Fewer Gr≥3 AEs (37.5% vs 55.8%), serious AEs, LV dysfunction (1.3% vs 6.1%). ILD ~4-5%. | |
| neoCARHP (ASCO 2025) [12] | III | HER2+ Early BC (Stage II-III) | THP (6 cycles) | TCbHP (6 cycles) | pCR | pCR: 64.1% vs 65.9% (non-inferior). | Lower Gr3/4 neutropenia (6.8% vs 16.4%), anemia (2.1% vs 6.6%), nausea/vomiting. | |
| TQB2102 (PUBMED 2025) [17] | II (Rand) | HER2+ Stage II-III BC (Neoadjuvant) | TQB2102 (bispecific ADC) | Historical control (40% tpCR threshold) | tpCR rate | tpCR rates: 57.7% to 76.9% across cohorts. All exceeded 40% threshold. | Gr≥3 TRAEs: 23.1%-30.8%. No treatment-related deaths. | |
| SHR-A1811 ± Pyrotinib (SABCS 2025) [18] | II | Stage II-III HER2+ BC (Neoadjuvant) | SHR-A1811 (ADC) mono or + Pyrotinib vs. PChHP | - | pCR | pCR: 63.2% (mono), 62.5% (combo), 64.4% (PChHP). Robust ADC activity. | Gr≥3 AEs: 44.8% (mono), 71.6% (combo). One G2 ILD in ADC arm. | |
| ALTTO Analysis (SABCS 2025) [19] | Explor. (III) | HER2+/HR+ Early BC post-chemo & 1y anti-HER2 | Adjuvant AI (+OFS if premenopausal) | Adjuvant Tamoxifen (± OFS) | DFS (exploratory) | AI ± OFS vs Tamoxifen: DFS HR=0.65. Premenopausal: AI+OFS vs Tamoxifen HR=0.44 (10-yr DFS 90.0% vs 77.6%). | As expected for AI vs Tamoxifen. | |
| DESTINY-Breast09 (ASCO 2025) [31,32] | III | HER2+ Metastatic BC, first-line | T-DXd ± Pertuzumab | Taxane + Trastuzumab + Pertuzumab | PFS, OS PROs (PGI-TT) |
PFS: 40.7 vs 26.9 mo (HR 0.58, p<0.00001). ORR: 89.9% vs 80.3%. OS interim: HR 0.74. PROs: Similar overall tolerability burden between T-DXd+P and THP per patient report (PGI-TT). | ILD: ~10% in T-DXd arms (majority low grade). Higher GI toxicity vs THP. Different toxicity profiles (ILD/nausea vs chemo side effects) but comparable patient-reported bother. |
|
| HER2CLIMB-05 (SABCS 2025) [33] | III | HER2+ MBC, no progression after 4-8 cycles of 1L THP | Maintenance: Tucatinib + Trastuzumab + Pertuzumab | Maintenance: Placebo + Trastuzumab + Pertuzumab | PFS | Median PFS: 24.9 vs 16.3 mo (HR 0.58, p<0.0001). Benefit across subgroups (HR- & HR+). | Safety consistent with known tucatinib profile (diarrhea, hepatotoxicity). | |
| HORIZON-Breast01 (ESMO 2025) [34,35] | III | HER2+ ABC, post-taxane & trastuzumab | SHR-A1811 | Pyrotinib + Capecitabine | PFS | Median PFS: 30.6 vs 8.3 mo (HR 0.22, p<0.0001). ORR: 81.7% vs 55.9%. OS interim: HR 0.31. | Hematologic toxicities predominant; ILD 2.8% (0.7% Gr≥3). | |
| PATINA (ESMO 2025) [36] | III | HR+/HER2+ MBC, post-induction chemo | Palbociclib + anti-HER2 + ET | anti-HER2 + ET | PFS | Median PFS: 44.3 vs 29.1 mo (HR 0.74, p=0.0109). | Higher Gr≥3 neutropenia, diarrhea, fatigue. HRQoL preserved. | |
| DESTINY-Breast04 (PUBMED 2025) [37] | III | HER2-low (IHC 1+ or 2+/ISH-) mBC, after 1-2 prior chemos | Trastuzumab Deruxtecan (T-DXd) | Physician's Choice Chemotherapy | PFS (BICR) in HR+ cohort | Median OS: 22.9 vs 16.8 mo (HR 0.69). Median PFS: 9.9 vs 5.1 mo (HR 0.50). | ILD/pneumonitis: 12.1% (Gr≥3: 0.8%). Nausea (73%), fatigue (48%). | |
| SGNTUC-019 (PUBMED 2025) [38] | II Basket | HER2-mutated mBC (HER2-negative by IHC) | Tucatinib + Trastuzumab (± Fulvestrant if HR+) | - | ORR | ORR: 41.9%. Median PFS: 9.5 mo. Active in HER2-mutated, IHC-negative disease. | No new safety signals. | |
| Zanidatamab + Docetaxel PUBMED 2025) [39] | Ib/II | HER2+ ABC (first-line) | Zanidatamab + Docetaxel | - | ORR, Safety | Confirmed ORR: 90.9%. Median PFS: 22.1 mo; Median OS: 36.9 mo. | Gr≥3 TEAEs: 71.1% (neutropenia 34%, diarrhea 13%). | |
| TRIPLE-NEGATIVE BREAST CANCER (TNBC) (17) | ||||||||
| GeparNuevo (Long-term) (ESMO 2025) [20] | II | Early TNBC (Stage II-III) | Durvalumab + NACT (no carbo) | NACT alone | pCR / iDFS | 7-yr iDFS: 73.7% vs 60.7% (HR 0.56). 7-yr OS: 91.6% vs 74.7% (HR 0.33). Benefit irrespective of pCR. | Acceptable tolerance, no new safety signals. | |
| PLANET Trial (ESMO 2025) [22] | II | Stage II-III TNBC | Neoadj. CT + ultra-low-dose Pembro (50mg q6w, 3 doses) | Neoadj. CT alone | pCR | pCR: 53.8% vs 40.5% (p=0.047). Absolute Δ +13.3%. RCB 0/1: 71.6% vs 61.0%. | Lower Gr≥3 AEs (50.0% vs 59.5%). One treatment-related death (toxic epidermal necrolysis) in Pembro arm. | |
| NRG-BR003 (ASCO 2025) [23] | III | Early TNBC (node+ or high-risk node-) | DD AC → WP + Carboplatin | DD AC → WP | IDFS | 5-yr IDFS: 82.7% vs 77.8% (HR 0.78, p=0.12). OS: 84.4% vs 87.7% (HR 0.81, p=0.16). | Higher Gr3/4 AEs, hematologic toxicity, anemia, cytopenia with carbo. | |
| Carboplatin Meta-Analysis (SABCS 2025) [24] | Metaalalysis | Early TNBC (Neoadjuvant) | CT + Carboplatin | CT alone | pCR, EFS | pCR: +16.1% (55.0% vs 38.9%). EFS: 5-yr +7% (74% vs 67%, HR=0.70). Benefit regardless of BRCA status. | Increased hematologic toxicity. | |
| RJBC-1501 (SABCS 2025) [25] | III | Stage I-III TNBC (Adjuvant, post-surgery) | EC-TCb | EC-T | DFS | 5-yr DFS: 93.1% vs 89.8% (HR=0.66, p=0.03). 5-yr Distant DFS: 92.0% vs 87.8% (HR=0.66). | Manageable toxicity profile. | |
| CITRINE (SABCS 2025) [26] | III | Node+/high-risk node-negative TNBC (Adjuvant) | DD EC → WP + Carbo | DD EC → WP | DFS | 3-yr DFS: 92.3% vs 85.8% (HR=0.64). Benefit strongest in 1st year (HR=0.31). | Increased but manageable hematologic toxicity. | |
| TBCRC-056 (SABCS 2025) [27] | II | gBRCA1/2 or PALB2m, HER2- Early BC (Neoadjuvant) | Niraparib + Dostarlimab (chemo-free) | - | pCR | TNBC Cohort: pCR rate 50% (23/46). RCB 0/1 rate: 60%. No difference with niraparib lead-in. | Safety consistent with known profiles of each agent. | |
| OlympiaN (SABCS 2025) [28] | II | gBRCA, ER-≤10%, HER2- Early BC | Olaparib ± Durvalumab (chemo-free, risk-adapted) | - | pCR | Trial ongoing; design presented at SABCS 2025. | Aims to validate a chemotherapy-sparing, risk-adapted approach. | |
| NeoSTAR (ASCO 2025) [29] | II | Early TNBC (≥T2 and/or N+) | SG + Pembro (4 cycles) → response-guided therapy | - | pCR after 4 cycles SG+Pembro | pCR after 4 cycles: 32% (60% in mBRCA). pCR after SG+Pembro ± additional CT: 50%. | 18-mo EFS 90.6%. Radiological RR 66% (30% CR, 36% PR). | |
| SYSUCC-001 (PUBMED 2025) [30] | III | Early-stage TNBC post-standard adjuvant therapy | Metronomic Capecitabine (1 year) | Observation | DFS | 10-yr DFS: 78.1% vs 66.6% (HR 0.61). FOXC1-high tumors derived significant OS benefit. | Long-term safety consistent with known capecitabine profile. | |
| BEGONIA (Arms 7 and 8) (ESMO 2025) [42] | Ib/II | 1L a/mTNBC (any PD-L1) | Dato-DXd + Durvalumab | - | Safety and ORR | Arm 7 (any PD-L1): ORR 79.0%; mPFS 14.0 mo; mDOR 17.6 mo. | Stomatitis (64-82%), nausea, alopecia, dry eye. ILD low (5-9%, no Gr≥3). | |
| TROPION-Breast01 (PUBMED 2025) [43] | III | HR+/HER2- mBC, post-ET and chemo | Datopotamab Deruxtecan (Dato-DXd) | Investigator's Choice Chemotherapy | PFS (BICR), OS | Median PFS: 6.9 vs 4.9 mo (HR 0.63). No OS difference (18.6 vs 18.3 mo). | Stomatitis, nausea, fatigue, alopecia. | |
| A-BRAVE (ESMO 2025) [21] | III | Early, high-risk TNBC after (neo)adjuvant chemo | Avelumab (1 year) | Observation | DFS | No significant DFS improvement (HR 0.81). Descriptive OS benefit (HR 0.66). | Not detailed. | |
| ASCENT-03 (ESMO 2025) [40,42] | III | Untreated advanced TNBC, not candidates for PD-1/L1 inhibitors | Sacituzumab Govitecan (SG) | Chemotherapy (paclitaxel, nab-paclitaxel, or gem/carbo) | PFS (BICR) | Median PFS: 9.7 vs 6.9 mo (HR 0.62). ORR: 48% vs 46%. | Gr≥3 AEs: 66% vs 62% (neutropenia 43% vs 41%). | |
| ASCENT-4 / KEYNOTE-D19 (ASCO 2025) [44] | III | Previously untreated PD-L1-positive advanced triple-negative breast cancer (TNBC) | Sacituzumab Govitecan + Pembrolizumab | Chemotherapy (paclitaxel, nab-paclitaxel, or gemcitabine/carboplatin) + Pembrolizumab | PFS by BICR | Median PFS: 11.2 vs 7.8 months (HR = 0.65; 95% CI: 0.52-0.81; p < 0.001). Objective Response Rate (ORR): 55% vs 47%. | Grade ≥3 adverse events: 68% vs 65%. Neutropenia: 45% vs 38%; Diarrhea: 12% vs 5%. Treatment discontinuation due to adverse events: 8% vs 6%. | |
| OptiTROP-Breast01 (PUBMED 2025) [45] | III | mTNBC, ≥2 prior lines | Sacituzumab Tirumotecan (TROP2-ADC) | Chemotherapy | PFS (BICR) | Median PFS: 6.7 vs 2.5 mo (HR 0.32, p<0.00001). OS improved. | Hematologic toxicity frequent. | |
| FABULOUS (PUBMED 2025) [46] | III | HER2- mBC with germline BRCA1/2 mutations | A: Fuzuloparib + Apatinib; B: Fuzuloparib | C: Chemotherapy | PFS (BICR) | Median PFS: A: 11.0, B: 6.7, C: 3.0 mo. A vs C: HR 0.27. | Gr3-4: neutropenia, anemia, hypertension. 1 treatment-related death (B). | |
| PREVENTION / OTHER (2) | ||||||||
| LIBER (PUBMED 2025) [59] | III | Postmenopausal women with gBRCA1/2 mutations | Letrozole (5 years) | Placebo | 5-year incidence of invasive BC | Non-significant trend favoring letrozole (7.8% vs 13.1%; HR 0.70, p=0.416). | Safety and QoL did not differ statistically. | |
| Tam-01 (PUBMED 2025) [61] | III | Breast intraepithelial neoplasia | Low-dose Tamoxifen (5 mg/d) | Placebo | Reduction of breast events | 42% reduction of breast events (HR 0.58). | Limited toxicity. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.




