1. Introduction
Broiler with ascites syndrome (AS) is a non-infectious disease caused by multiple pathogenic factors, which is common in fast-growing broilers. Such a disease has become one of the three most serious nutritional and metabolic diseases in the world poultry industry, severely restricting the healthy development of global broiler feeding [
1]. Chinese herbal medicine can reduce the morbidity and mortality of AS through different pathways [
2].Tissue fibrosis is a pathological phenomenon of abnormal proliferation of fibrous connective tissue in multiple organs, with a reduction in parenchymal cells, proliferation of fibroblasts, deposition of extracellular matrix (ECM), and an increase in collagen production in organs. The continuous progression of fibrosis can lead to structural damage and functional decline of tissues and organs. The various factors including inflammation, oxidative stress, and autoimmunity can lead to fibrosis [
3,
4]. Besides, fibrosis often occurs in a variety of organs. Additionally, some chronic diseases, such as cardiomyopathy, and pulmonary hypertension, are closely related to fibrosis. The processes of fibrosis in different tissues have the same pathogenic mechanisms in various aspects including cytokines and immunology [
5].
2. Materials and Methods
2.1. Experimental Animals
A total of 120 1-day-old Ross broilers were purchased from Shanxi Daxiang Agriculture and Animal Husbandry Group Co., Ltd.
2.2. Experimental Drugs
The main experimental drugs include Astragali radix, Poria, Angelica sinensis, Arecae pericarpium, Salvia miltiorrhiza, and Glycyrrhiza uralensis.
2.3. Main Reagents
The tumor necrosis factor alpha (TNF-α), interleukin (IL)-4, IL-1β, IL-10 enzyme-linked immunosorbent assay kits were obtained from Shanghai Enzyme-linked Biotechnology Co., Ltd. Superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), malondialdehyde (MDA) detection kits were purchased from Nanjing Jiancheng Bioengineering Institute.
2.4. Animal Grouping and Modeling
The healthy broilers were fed normally for 8 days and randomly divided into six groups: the Blank group (B), the Model group (M), The High dose group of compound traditional Chinese medicine (H), the Low dose group of compound traditional Chinese medicine (L), the Positive group (P), and the L-arginine group (L-Arg). The AS model was replicated by the multifactorial method involving low temperature, high sodium, high fat, and high protein, the high- and low-dose groups were given 2.0 and 1.0 g/kg compound Chinese medicinal decoctions per day, respectively.which was used by our research group in the early stage.
2.5. Preparation of Qi Ling Gui Fu Prescription
Astragali radix, Angelica sinensis, Salvia miltiorrhiza, Poria, and Arecae pericarpium (3:2:2:2:1) were prepared, as well as Astragali radix, Poria, Radix lithospermi, Gynostemma pentaphyllum, and Alisma plantago were formulated in a ratio of 4:3:3:7:3. Then, 1 g/mL decoction was obtained by water decoction and vacuum distillation, and L-arg mixed with lard and fish meal was added to the feed at the ratio of 1:100.
2.6. Sample Collection
Blood was collected from the wing vein of broilers at 35 days of age, followed by separation to obtain the serum. The separation of liver, heart, jejunum, lung, and kidney tissues was placed in an Eppendorf tube and stored in a refrigerator at -80 °C. Then, the right and whole ventricles were weighed. The part of the lung tissue was fixed with 4% paraformaldehyde for morphological observation.
2.7. Index Detection and Methods
2.7.1. Detection of Ascites Heart Index in Broilers
The ratio of right ventricle weight to total ventricle weight, ascites heart index (AHI), was used to assess the progress of AS in broilers.
2.7.2. Pathomorphological Observation of Lung Masson’s Trichrome Stain
The lung tissues were embedded in paraffin and cut into 5 μm sections. Then, the Masson’s trichrome stain was utilized to observe the deposition of collagen fibers in the lung tissues.
2.7.3. Measurement of Oxidative Stress in Serum
The contents of SOD, GSH-Px, MDA were measured according to kit instructions.
2.7.4. Assessment of Target Protein Contents in Different Tissues by ELISA
The liver, heart, jejunum, lungs, and kidneys (0.1–0.2 g) were washed in cold physiological saline, dried, and weighted. Next, the physiological saline was added at a mass-to-volume ratio of 1:9, and the mixture was homogenized in an ice-water bath. Then, after centrifugation at 5,000 r/min for 5 min, the supernatant was collected. Subsequently, the supernatant was diluted and mixed for later use. Enzyme-linked immunosorbent assay was employed to determine the contents of TNF-α, IL-4, IL-10, and IL-1β in the liver, heart, jejunum, lung and kidney of broilers.
2.8. Data Statistics and Analysis
Graph Pad Prizm 10 software was used for data statistics and analysis. One-way analysis of variance and least significant difference tests were utilized to analyze the data. The results were presented as mean ± standard deviation.
3. Results
3.1. Effects of Qi Ling Gui Fu Prescription on AHI in Broilers with AS
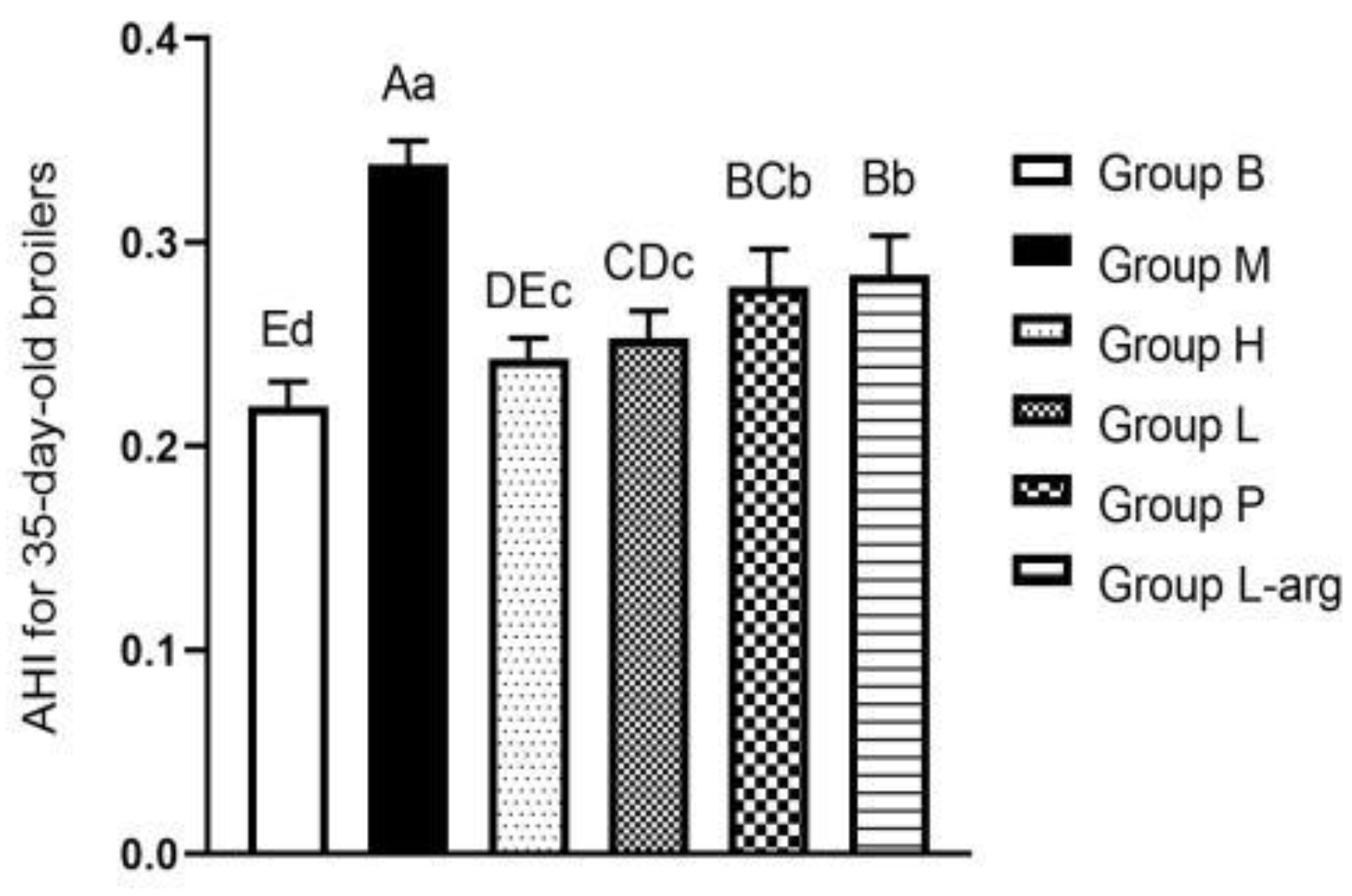
As shown in
Figure 1, the AHI of 35-day-old broilers in the M group was significantly increased compared to the B group (P < 0.01). Relative to M group, the treatment groups exhibited a marked decrease in AHI (P < 0.01). Among the treatment groups, there was a remarkable reduction in AHI in the H and L groups in comparison with the L-arg group (P < 0.01), with no significant difference in the P group (P > 0.05). As opposed to the P group, the AHI was remarkably dropped in the H group (P < 0.01) and the L group (P < 0.05). There was no significant difference in the H group in contrast to the L group, with a downward trend (P > 0.05).
3.2. Effects of Qi Ling Gui Fu Prescription on Lung Lesions in Broilers with AS
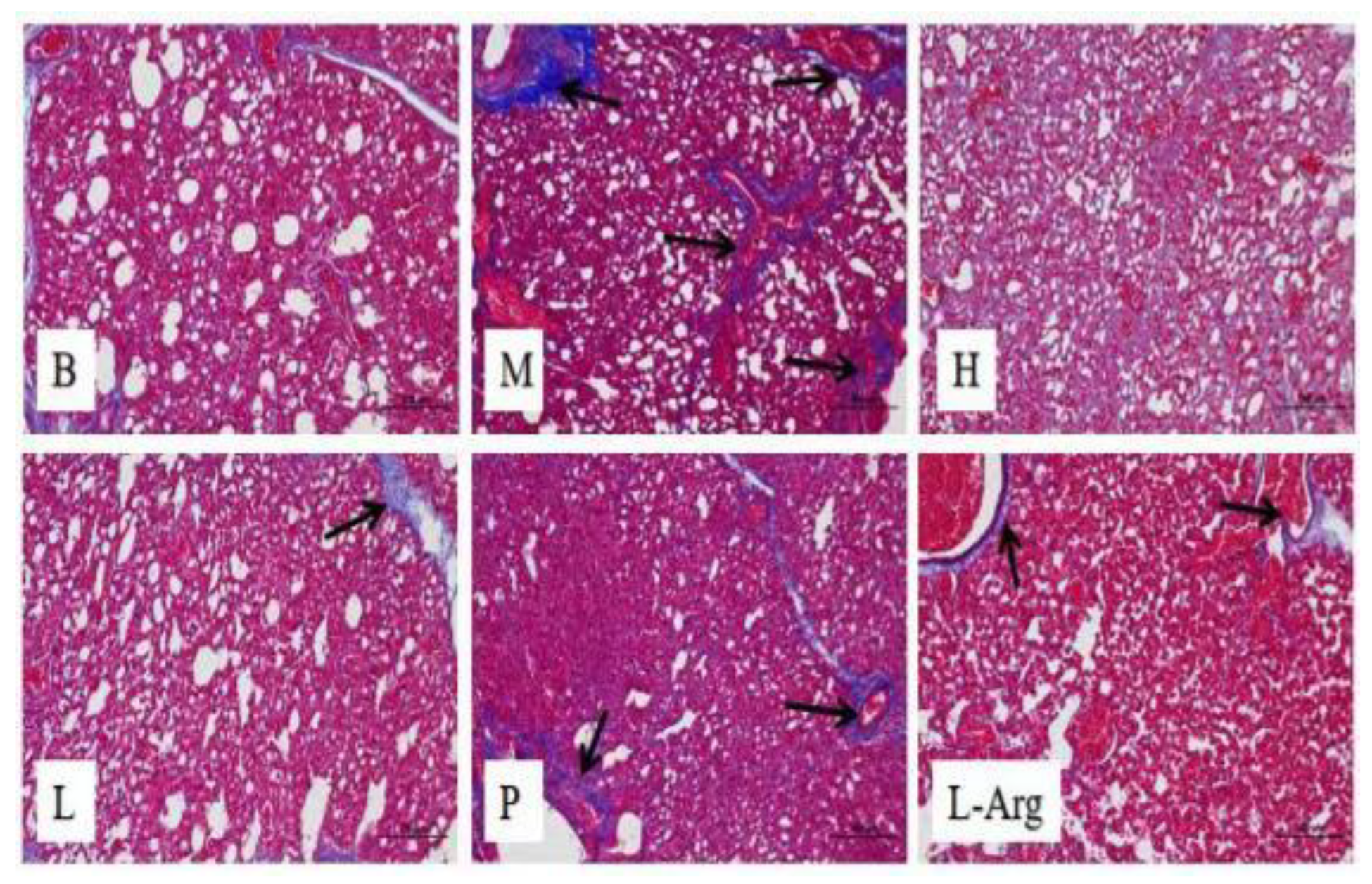
The Masson’s trichrome stain results of the lung tissues of 35-day-old broilers displayed that compared to the B group, obvious accumulation of collagen fibers and fibrosis were observed in the M group. As opposed to the M group, there was a slight distribution of collagen fibers in the interstitial space of lung tissue in the treatment groups, and the deposition of collagen fibers was significantly improved (
Figure 2).
3.3. Effects of Qi Ling Gui Fu Prescription on Oxidative Stress in Broilers with AS
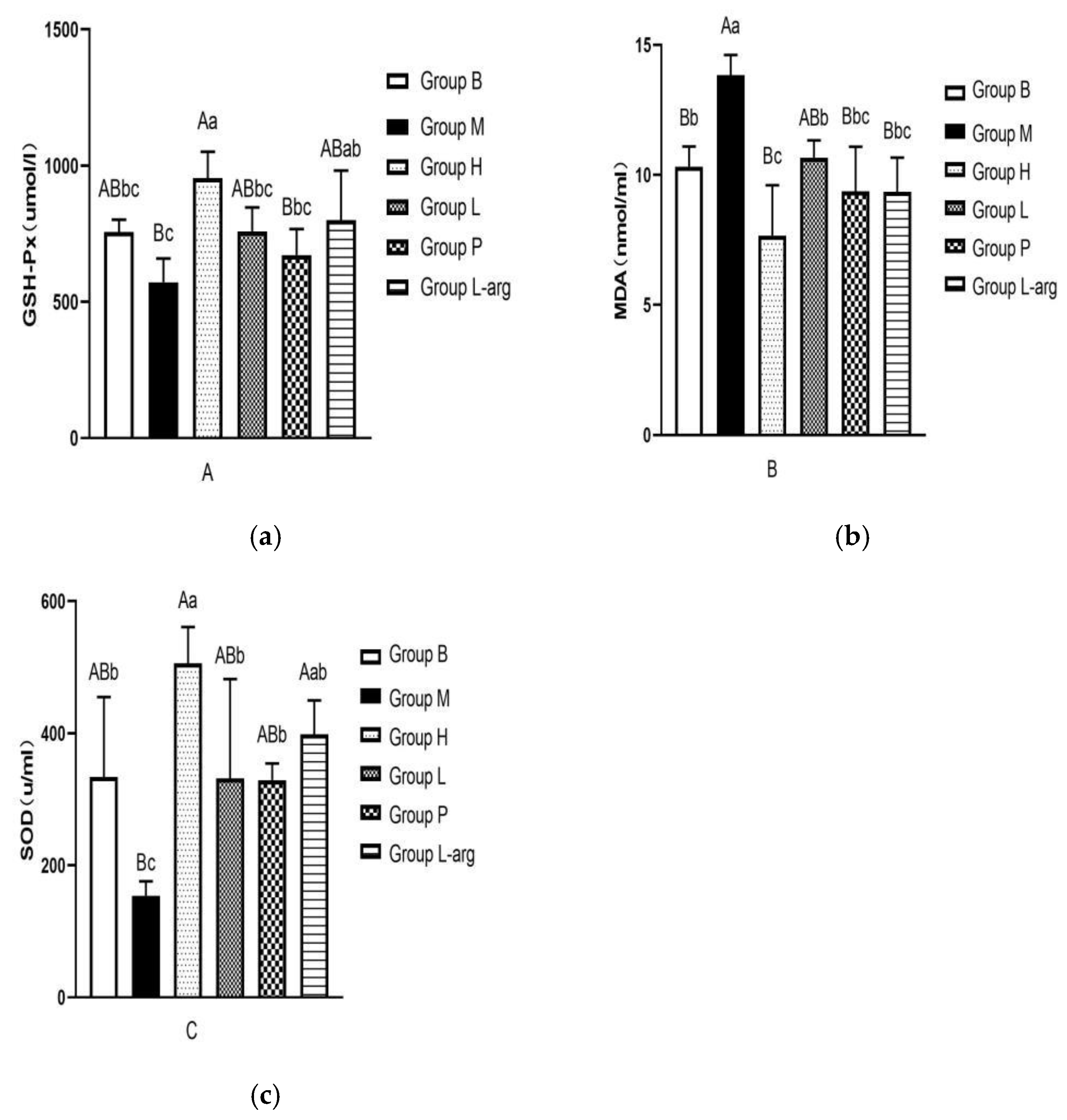
The results of GSH-Px concentration were shown in .
Figure 3a. Compared with the B group, there was no significant difference in GSH-Px concentration in the M group, with a decreasing trend (P > 0.05). In comparison with the M group, the GSH-Px concentration was markedly increased in the H group (P < 0.01) and the L-arg group (P < 0.05), with no significant difference in the L group and the P group (P > 0.05). Among the treatment groups, the GSH-Px concentration was considerably higher in the H group (P < 0.01,P < 0.05).
The measurement results of MDA (
Figure 3b) exhibited that compared to the B group, the MDA concentration was notably elevated in the M group (P < 0.01). Besides, in comparison with the M group, a marked reduction was observed in the MDA concentration in the treatment groups (P < 0.01,P < 0.05). Among the treatment groups, the H group showed the best effect.
Figure 3c displayed the results of the SOD concentration. The SOD concentration was notably dropped in the M group than that in the B group (P < 0.05). Compared to the M group, the treatment groups presented a remarkable elevation in the SOD concentration (P < 0.01,P < 0.05). Among the treatment groups, the H group showed the best effect.
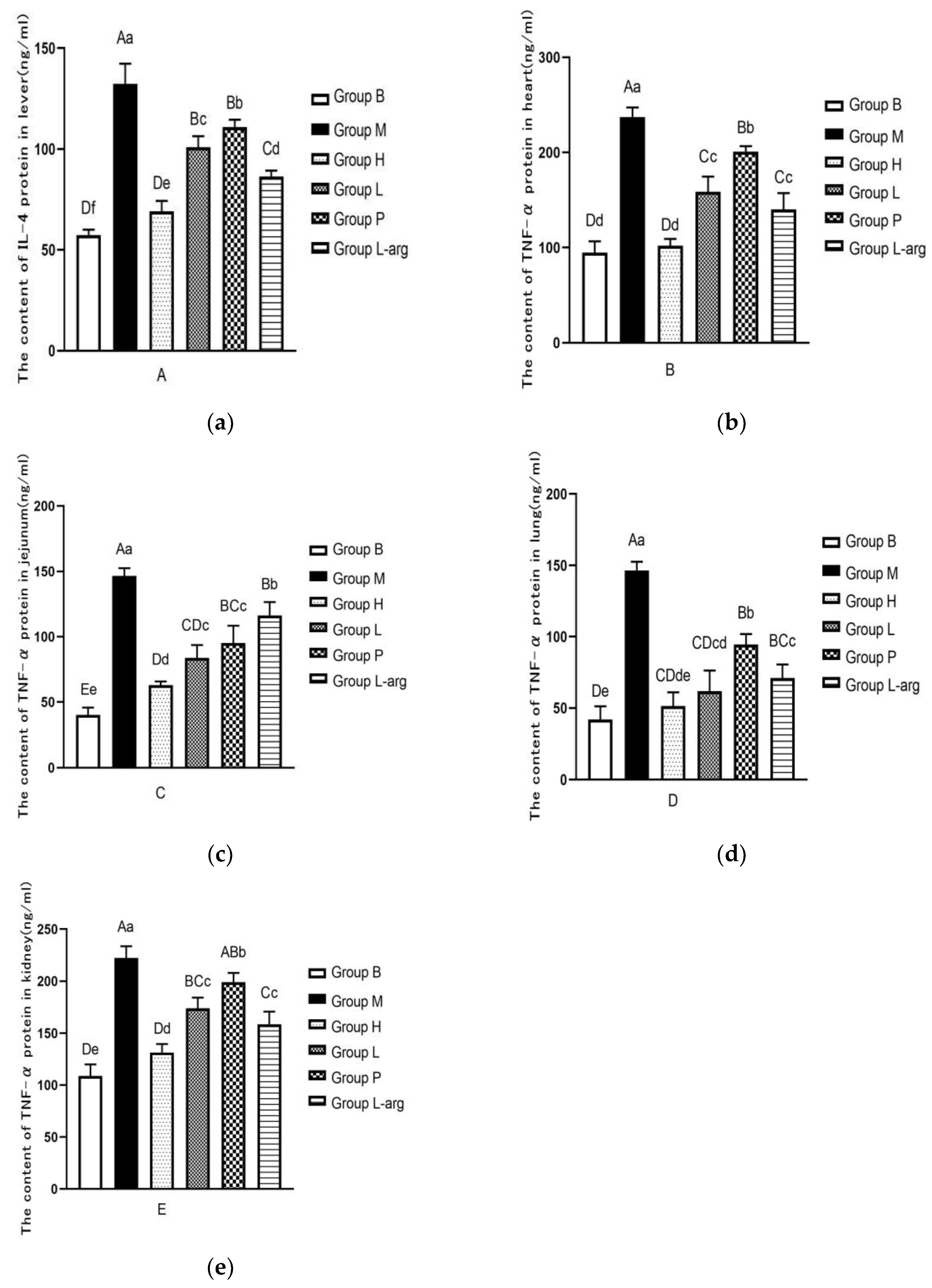
3.4. Effects of Qi Ling Gui Fu Prescription on TNF-α Protein in Different Tissues
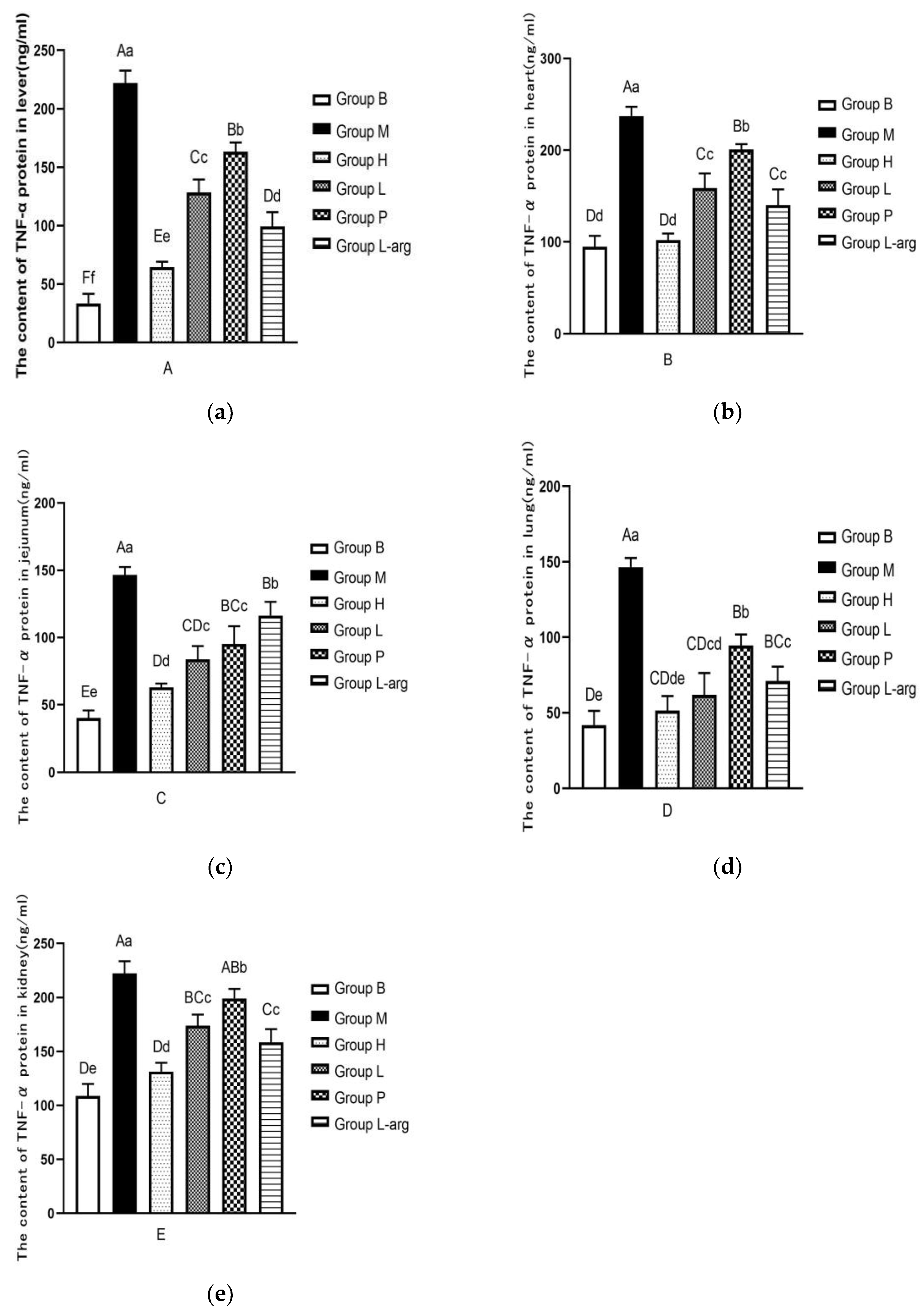
The results of TNF-α protein content in liver tissue were shown in
Figure 4a. Compared with the B group, the TNF-α protein content was significantly increased in the M group (P < 0.01). As opposed to the M group, there was a notable reduction in the TNF-α protein content in each treatment group (P < 0.01). Among the treatment groups, the TNF-α protein content was particularly lower in the H groups (P < 0.01).
The TNF-α protein content in heart tissues was detected (Figure. 4b). Compared to the B group, the TNF-α protein content was significantly increased in the M group(P < 0.01). The TNF-α protein content was markedly decreased in the treatment groups than that in the M group (P < 0.01). Among the treatment groups, the TNF-α protein content was obviously lower in the H group (P < 0.01).
The TNF-α protein content in jejunum tissues was measured (Figure. 4c). The TNF-α protein content was higher in the M group than that in the B group (P < 0.01). Compared with the M group, the TNF-α protein content was significantly decreased in the treatment groups (P < 0.01). Among the treatment groups, as opposed to the P and L-arg group, the TNF-α protein content was remarkably lower in the H and L groups (P < 0.01), the H group performed better than the L group(P < 0.05).
Further, the TNF-α protein content in lung tissues was determined (Figure. 4d). Compared with the B group, the TNF-α protein content was apparently up-regulated in the M group (P < 0.01). As opposed to the M group, the TNF-α protein content was considerably reduced in the treatment groups (P < 0.01). Among the treatment groups, relative to the P and L-arg group, the H and L groups presented a marked reduction in the TNF-α protein content (P < 0.01); there was no significant difference between the L group and the H group, but there was a decreasing trend (P > 0.05).
Ultimately, the evaluation of theTNF-α protein content in kidney tissues were carried out (
Figure 4e). The TNF-α protein content was higher in the M group than that in the B group (P < 0.01). Compared with the M group, the TNF-α protein content was particularly decreased in the treatment groups (P < 0.01,P < 0.05). Among the treatment groups, the TNF-α protein content was obviously lower in the H group (P < 0.01).
3.5. Effects of Qi Ling Gui Fu Prescription on IL-4 Protein in Different Tissues
As exhibited in
Figure 5a, the IL-4 protein content in liver tissues was detected. Compared with the B group, the IL-4 protein content was significantly increased in the M group (P < 0.01). As opposed to the M group, the IL-4 protein content was markedly decreased in the treatment groups (P < 0.01). Among the treatment groups, the IL-4 protein content was obviously lower in the H group (P < 0.01).
Besides, the IL-4 protein content in heart tissues was measured (
Figure 5b). Compared with the B group, the IL-4 protein content was significantly increased in the M group (P < 0.01). As opposed to the M group, the IL-4 protein content was considerably declined in the treatment groups (P < 0.01). Among the treatment groups, the IL-4 protein content was obviously lower in the H group (P < 0.01).
In addition, the IL-4 protein content in jejunum tissues was evaluated (
Figure 5c) The IL-4 protein content was considerably higher in the M than that in the B group (P < 0.01). Compared with the M group, the IL-4 protein content was significantly decreased in the treatment groups (P < 0.01). Among the treatment groups, the IL-4 protein content was notably declined in the H, and L groups as opposed to the P and L-arg group (P < 0.01,P < 0.05);there was no significant difference between the L group and the H group, but there was a decreasing trend (P > 0.05).
Moreover, the assessment of IL-4 protein content in lung tissues was performed (
Figure 5d). The IL-4 protein content was particularly increased in the M group than that in the B group (P < 0.01). Compared with the M group, the IL-4 protein content was apparently decreased in the treatment groups(P < 0.01). Among the treatment groups, a marked reduction was displayed in the IL-4 protein content in the H, L, and L-arg groups as opposed to the P group (P < 0.01); relative to the L and L-arg group, no significant difference was observed in the H group, but there was a decreasing trend (P > 0.05).
Ultimately, the evaluation of IL-4 protein content in kidney tissues was conducted (
Figure 5e). Compared with the B group, the IL-4 protein content was considerably elevated in the M group (P < 0.01). In contrast to the M group, the IL-4 protein content in the treatment groups was remarkably lower (P < 0.01). Among the treatment groups, the IL-4 protein content was obviously lower in the H group (P < 0.01).
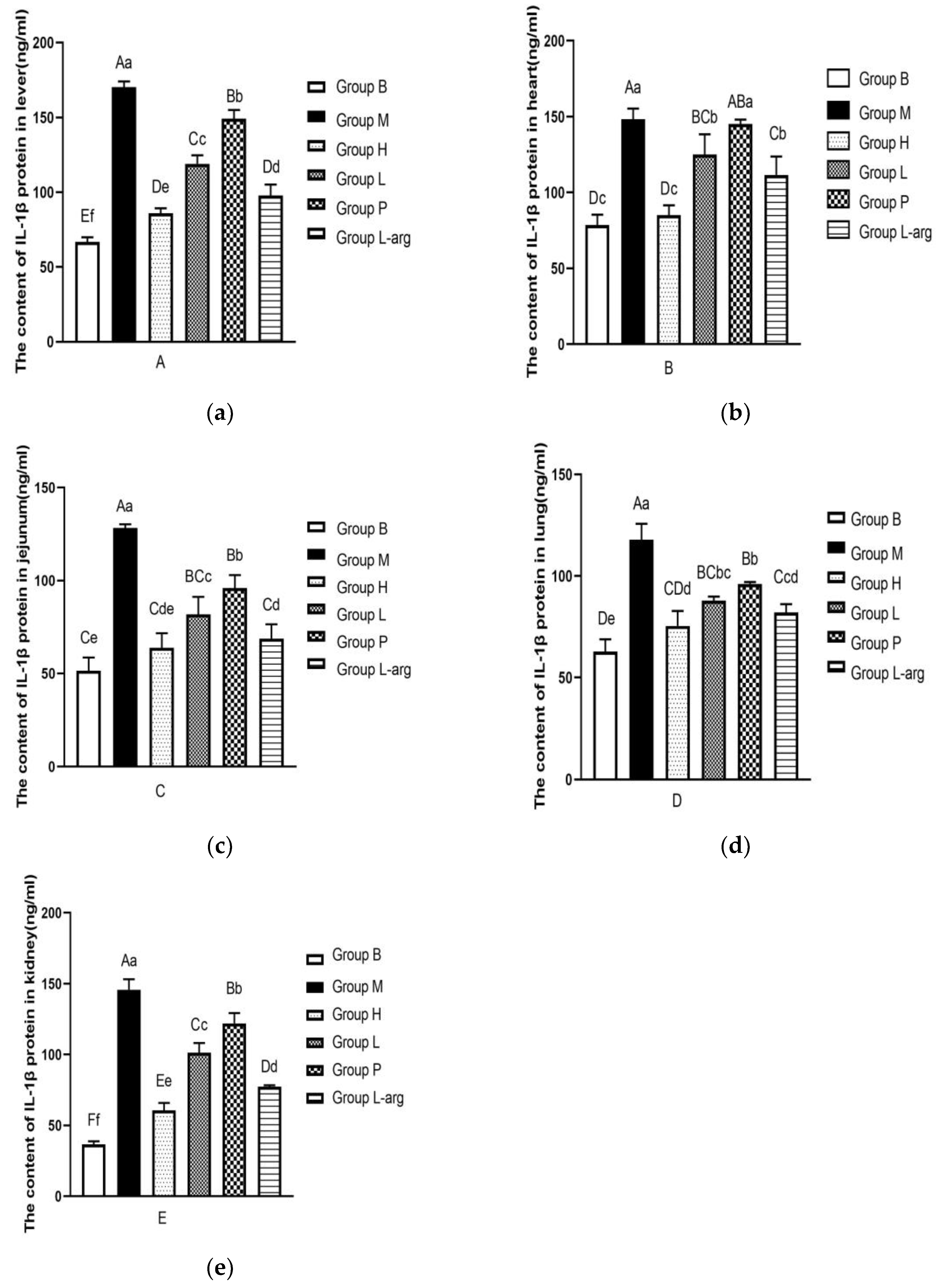
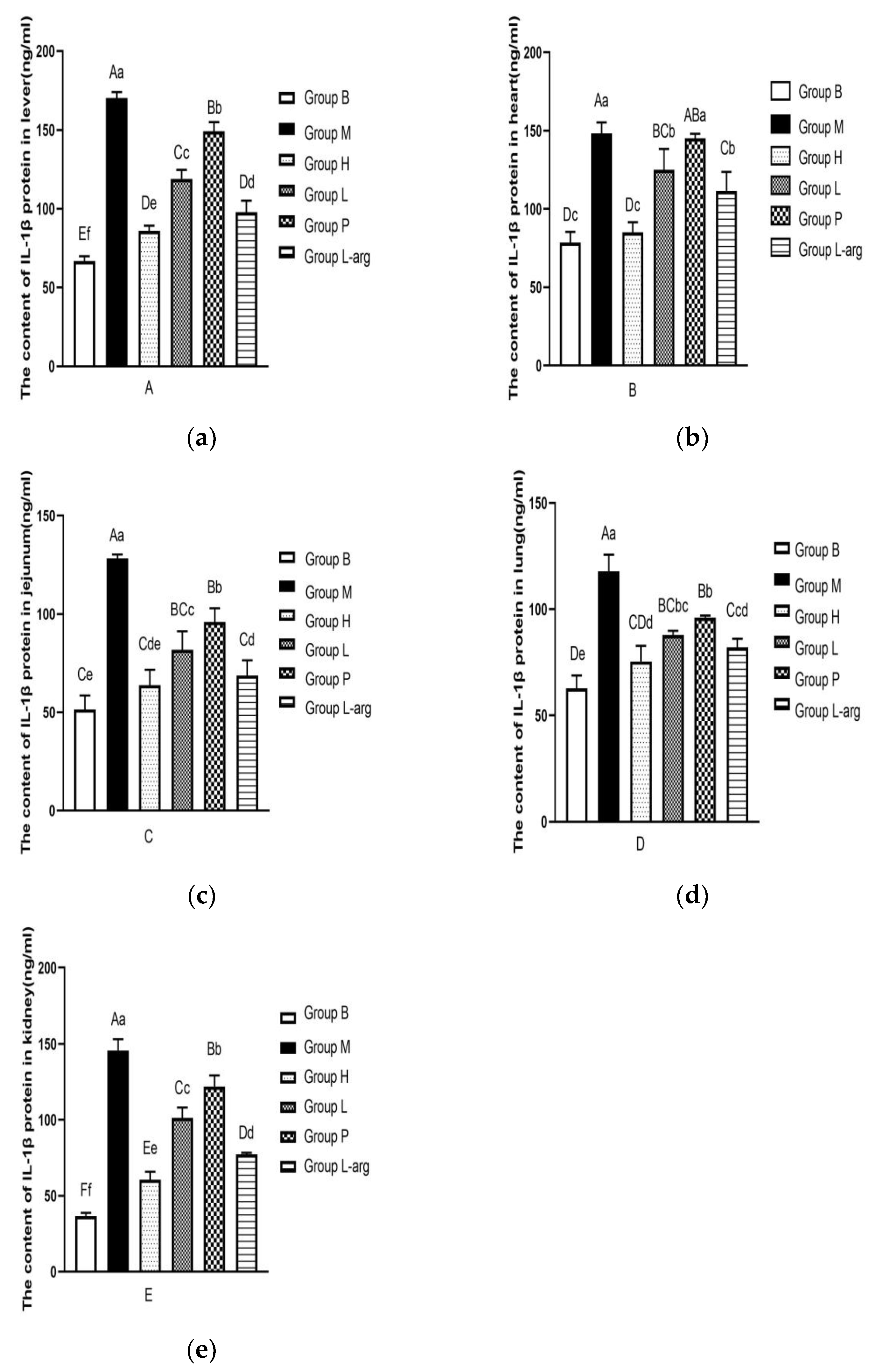
3.6. Effects of Qi Ling Gui Fu Prescription on IL-1β Protein in Different Tissues
According to the
Figure 6a, the IL-1β protein content in liver tissues was evaluated. Compared with the B group, the IL-1β protein content was significantly increased in the M group (P < 0.01). As opposed to the M group, the IL-1β protein content was remarkably reduced in the treatment groups (P < 0.01). Among the treatment groups, the IL-1β protein content was obviously lower in the H group (P < 0.01,P < 0.05).
As shown in
Figure 6b, the IL-1β protein content in heart tissues was determined. Compared with the B group, the IL-1β protein content was apparently raised in the M group (P < 0.01). The IL-1β protein content was obviously lower in the H, L, and L-arg groups than that in the M group (P < 0.01), with no significant difference in the P group (P > 0.05). Among the treatment groups, the IL-1β protein content was obviously lower in the H group (P < 0.01).
The IL-1β protein content in jejunum tissues was detected (
Figure 6c). As opposed to the B group, a marked elevation was found in the IL-1β protein content in the M group (P < 0.01). Compared with the M group, the IL-1β protein content in the treatment groups was particularly decreased (P < 0.01). Among the treatment groups, relative to the P group, the IL-1β protein content was considerably lower in the H and L-arg groups (P < 0.01), as well as in the L group (P < 0.05); the IL-1β protein content was apparently lower in the H and L-arg groups than that in the L group (P < 0.05); in contrast to the L-arg group, there was no significant difference in the H group, but there was a decreasing trend (P > 0.05).
The detection of IL-1β protein content in lung tissues was performed (
Figure 6d). Compared with the B group, the IL-1β protein content was markedly increased in the M group (P < 0.01). In contrast to the M group, the IL-1β protein content was remarkably lower in the treatment groups (P < 0.01). Among the treatment groups, the IL-1β protein content was considerably decreased in the H and L-arg groups relative to the P group (P < 0.01), with no significant difference in the L group (P > 0.05); relative to the L group, the IL-1β protein content was obviously lower in the H group (P < 0.05), with no significant difference in group L-arg (P > 0.05); as opposed to the L-arg group, there was no significant difference in the H group, showing a decreasing trend (P > 0.05).
The measurement of IL-1β protein content in kidney tissues was carried out (Figure. 6e). Compared with the B group, the IL-1β protein content was notably higher in the M group (P < 0.01). In comparison with the M group, the IL-1β protein content was markedly dropped in the treatment groups (P < 0.01). Among the treatment groups, the IL-1β protein content was obviously lower in the H group (P < 0.01).
3.7. Effects of Qi Ling Gui Fu Prescription on IL-10 Protein in Different Tissues
The IL-10 protein content in liver tissues was determined (
Figure 7a). Compared with the B group, there was a marked decrease in the IL-10 protein content in the M group (P < 0.05). Relative to the M group, the IL-10 protein content was markedly up-regulated in the treatment groups (P < 0.01). Among the treatment groups, the IL-10 protein content was obviously higher in the H group (P < 0.01).
The detection of IL-10 protein content in heart tissues was conducted (
Figure 7b). Compared with the B group, the IL-10 protein content in the M group M was particularly decreased (P < 0.01). In contrast to the M group, the treatment groups exhibited an obvious elevation in the IL-10 protein content (P < 0.01). Among the treatment groups, the IL-10 protein content was obviously higher in the H group (P < 0.01).
The IL-10 protein content in jejunum tissues was measured (
Figure 7c). The IL-10 protein content was significantly lower in the M group than that in the B group (P < 0.01). Compared with the M group, the IL-10 protein content was notably increased in the treatment group (P < 0.01). Among the treatment groups, the IL-10 protein content was obviously higher in the H group (P < 0.01).
According to the
Figure 7d, the IL-10 protein content in lung tissues was evaluated. Compared with the B group, the IL-10 protein content was particularly reduced in the M group (P < 0.01). Relative to the M group, the IL-10 protein content was apparently increased in the treatment groups (P < 0.01). Among the treatment groups, the IL-10 protein content was obviously higher in the H group (P < 0.01).
The assessment of IL-10 protein content in kidney tissues was performed (
Figure 7e). Compared with the B group, the IL-10 protein content in the M group was particularly lower (P < 0.01). the IL-10 protein content was considerably higher in the treatment groups than that in the M group (P < 0.01). Among the treatment groups, the IL-10 protein content was obviously higher in the H group (P < 0.01).
4. Discussion
The multi-factor methods, such as low temperature, high sodium, and high energy, were utilized to reflect the multiplicity of AS inducements [
6]. AHI is considered a critical pathological parameter for the occurrence of AS in broilers [
7], and is commonly used as an evaluation index for the status of the AS process. In this study, the model group exhibited obvious ascites and the AHI was greater than 0.25, indicating that the experimental model was successfully replicated.
4.1. Hypoxia and Oxidative Stress
The initiating factor for AS in broilers is hypoxia [
8]. Hypoxia leads to oxidative stress in the body, causing the development of fibrosis. The excessive ROS was generated by rapid metabolism in broilers. However, ROS can damage the vascular endothelium, resulting in the production of larger amounts of the unsaturated fatty acid metabolite, MDA. MDA, a lipid free radical with high toxicity, is involved in the progression of various diseases. SOD is an important metal antioxidant enzyme for the body to scavenge oxygen free radicals. SOD can catalyze the dismutation of ROS, thus eliminating oxygen free radicals and reducing damage to the vascular endothelium [
9]. A study has demonstrated that GSH-Px has a strong antioxidant effect, and its reduction or excessive consumption exacerbates the oxidative stress in the body [
10]. Herbal formulations such as Astragali radix and Poria, can enhance the activity of SOD and GSH-Px in broilers, reduce the content of MDA, and strengthen the antioxidant capacity of lungs to reduce lung damage, thereby effectively preventing and treating broilers with AS [
11]. In this study, all the treatment groups increased the levels of SOD and GSH-Px in the serum of broilers and reduced the content of MDA, with the best effect in the H group.
4.2. Oxidative Stress and Inflammatory Response
Oxidative stress stimulates a rapid induction of inflammatory factors. TNF-α secreted by macrophages is considered the initiating factor of inflammation, which plays an important role in the development of inflammatory diseases. Additionally, TNF-α can induce the key inflammatory factors of cardiovascular diseases caused by myocardial injury [
12]. TNF-α plays a role in myocardial cell injury, myocardial fibrosis, inflammatory bowel disease, and nephrotic syndrome [
13,
14,
15]. IL-4 is involved in the progression of various diseases, such as kidney injury and inflammatory bowel disease [
16,
17]. Astragaloside IV can alleviate the inflammatory reaction of PH rats [
18]. Poria cocos effectively reduces myocardial necrosis areas and cardiomyocyte apoptosis in rats with myocardial hypoxia, and its anti-inflammatory and antioxidant effects protect the function of myocardial tissues [
19]. Angelica sinensis polysaccharide reduces rat liver injury induced by high doses of acetaminophen, regulating and improving lipid oxidative stress and inflammatory response [
20]. In this study, the TNF-α and IL-4 contents were notably elevated in the M group of each tissue, indicating that they interact with each other and play an important role in the occurrence and development of tissue inflammation. Besides, the high expression of pro-inflammatory factor TNF-α indicates the aggravation of tissue inflammation and severe local tissue damage. The anti-inflammatory factor IL-4 was highly expressed, but the increase of its expression is not as obvious as that of TNF-α. Therefore, the anti-inflammatory and pro-inflammatory responses are imbalanced. Notably, the rapid expression of pro-inflammatory factors suggested the continuous development of inflammation. IL-4, as a major cytokine of Type 2 helper T, plays a protective and pathogenic role in the body due to its mediated immunity. The excessive production of IL-4 indicated the occurrence and development of allergic and fibrotic diseases Both Qi Ling Gui Fu Prescription and L-arg reduced the expression of TNF-α and IL-4, with the high-dose self-formulated traditional Chinese medicine showing the best effect.
4.3. Inflammation and Tissue/Organ Fibrosis
Various cytokines, such as tumor necrosis factors, and inflammatory factors, play major roles in the process of inflammatory response and fibrosis. TNF-α is a key inflammatory signaling molecule in the fibrosis of the liver, heart, kidneys, and lungs. Besides, TNF-α participates in the occurrence and development of fibrosis by inducing cell activation, differentiation, apoptosis, and cytokine production [
21]. Interleukins are important cytokines in the process of organ fibrosis. Of them, IL-1, IL-4, and IL-6 play a role in promoting fibrosis, while IL-10 mainly exerts an anti-fibrotic effect.
The differentiation of fibroblasts treated with IL-1β into myofibroblasts leads to an increase in ECM deposition and promotes the transformation of fibroblasts into myofibroblasts [
22,
23]. The anti-fibrotic effect of IL-10 can be achieved not only by directly acting on fibroblasts to inhibit the accumulation of ECM, but also by inhibiting the production of the pro-fibrotic factor TNF-α. According to a study, multiple organs in IL-10-deficient mice exhibit more severe inflammation and fibrosis. However, the expression of IL-10 can reduce the levels of TNF-α, type I collagen, and cell adhesion molecules, thereby alleviating fibrosis [
24]. IL-10 can reduce or repair tissue and organ damage by inhibiting inflammatory responses in various tissues and organs, thereby exerting its anti-fibrotic effect.
In the self-formulated compound, Astragalus polysaccharides and Astragaloside IV can significantly reduce the serum IL-1 level of rats with heart failure, thus decreasing rupture and defect of myocardial fibers and inhibiting myocardial fibrosis in rats with heart failure [
25]. Levistilide A can regulate the expression of inflammatory factors, intervene in the process of EMT, reduce cell degeneration and inflammatory necrosis, and improve fibrosis [
26]. Poria cocos can inhibit lipid peroxidation and improve fibrosis [
27]. Glycyrrhizic acid can activate autophagy in fibroblasts, inhibit the activation of fibroblasts, reduce tissue inflammation and collagen deposition, and improve lung tissue injury [
28]. Lithospermum can reduce the expression of IL-1β and TNF-α, while increasing the expression of IL-10 [
29,
30].
In this study, compared with the P and L-arg groups, the self-formulated Qi Ling Gui Fu Prescription can more effectively improve the fibrosis of organs and tissues, protect the organs of broilers, reduce the contents of IL-1β, IL-4 and TNF-α in various tissues, increase the expression of anti-inflammatory factor IL-10, reduce the inflammatory reaction and slow down the fibrosis. Besides, the effect of a high dose of self-formulated compound was more obvious, providing better prevention and treatment of AS. Masson’s trichrome stain was used to observe the fibrosis in lung tissues. The high dose group of the self-formulated compound showed a clearer lung tissue structure and significantly reduced fibrosis. These results demonstrated a more evident effect in slowing down fibrosis.
5. Conclusions
The Qi Ling Gui Fu Prescription and L-Arg can improve fibrosis in broilers with AS by mediating oxidative stress and modulating the inflammatory response. The self-formulated compound in the high dose group can significantly enhance the antioxidant capacity of broilers, improve the disorder of lipid metabolism in broilers with AS, alleviate inflammatory responses in various tissues, and slow down fibrosis, thereby better playing the role of prevention and treatment of AS.
Author Contributions
Conceptualization, Duan Zhibian. and Deng Ruiqiang.; methodology, Deng Ruiqiang and Kang Jie ,formal analysis, Wang Keyao.; investigation, Wang Huimin.; resources, Han Yufeng.; data curation, Deng Ruiqiang.; writing—review and editing, Deng Ruiqiang and Kang Jie. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was funded by the Natural Science Foundation of Shanxi Province (201901D111235) and “The APC was funded by Natural Science Foundation of Shanxi Province”.
Institutional Review Board Statement
This study was carried out in compliance with the regulations set by the State Science and Technology Commission of the People’s Republic of China on the management of experimental animals. All procedures and chicken rearing followed the guidelines provided by the Animal Ethics Committee of Shanxi Agricultural University.
Data Availability Statement
All data are available.
Acknowledgments
We would like to acknowledge the support provided by Shanxi Agricultural University. The authors wish to express their gratitude to Huaijun Zhou from the Department of Poultry Science at Texas A&M University College Station, USA, for offering a clear research concept that greatly contributed to this paper.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AS |
Ascites syndrome |
| AHI |
Ascites heart index |
| TNF-α |
Tumor necrosis factor-α |
| ECM |
Extracellular matrix |
| MDA |
Malondialdehyde |
| IL-4 |
Interleukin-4 |
| HE |
Haematoxylin and eosin |
| ELISA |
Ezyme-linked immunosorbent assay |
| ROS |
Robot Operating System |
| GSH-Px |
Glutathione Peroxidase |
| SOD |
Superoxide Dismutase |
References
- Qi, YH; Wang, WK. The Progress of Study on Ascites Syndrome in Broilers. Progress In Veterinary Medicine 2003, 24(3), 54–57. [Google Scholar]
- Xu, HZ. Research Advances on Prevention and Cure Effects of Chinese Herbal Medicine against Broiler Ascites Syndrome. China Animal Husbandry & Veterinary Medicine 2018, 34(1), 249–250. [Google Scholar]
- Li, N; Sun, WY; Sun, JC. Advances in role of inflammasome in fibrotic diseases. Chinese Pharmacological Bulletin 2020, 36(4), 479–483. [Google Scholar]
- Phan, THG; Paliogiannis, P; Nasrallah, GK; et al. Emergging cellular and molecular determinants of idiopathic pulmonary fibrosis. Cell Mol Life Sci 2021, 78, 2031–2057. [Google Scholar] [CrossRef]
- Sun, SJ; Xiang, Y; Huang, SL. Progress in studies of mechanism of anti-fibrosis traditional Chinese drugs and effective components. China Journal of Chinese Materia Medica 2008, 33(24), 2882. [Google Scholar] [PubMed]
- Guo, YR; Cui, L; Su, XF; et al. Establishment of Ascites Syndrome Model in Broiler Induced by Multi-factor andIts Effect on Diastolic-systolic Factors of Vascular Endothelium in Lung Tissue. China Animal Husbandry & Veterinary Medicine 2020, 47(05), 1350–1359. [Google Scholar]
- Pakdel, A; Bijma, P; Ducro, BJ; et al. Selection strategies for body weight and reduced ascites susceptibility in broilers[J]. Poult Sci. 2005, 84(4), 528–535. [Google Scholar] [CrossRef] [PubMed]
- Balog, J M. Ascites syndrome (pulmonary hypertension syndrome) in broiler chickens: Are we seeing the light at the end of the tunnel. Avian and Poultry Biology Reviews 2003, 14(3), 99–126. [Google Scholar] [CrossRef]
- Byeon, E; Park, J C; Hagiwara, A. Two antidepressantfluoxetine and sertraline cause growth retardation andoxidative stress in the marine rotifer Brachionuskoreanus. Ayuatic Toxicology 2019, 218, 105337. [Google Scholar]
- An, P; Gao, Z G; Sun, K. Photothermal-enhancedinactivation of glutathione peroxidase for ferroptosis sensitized by an autophagy promotor. ACS Applied Materials & Interfaces 2019, 11(46), 42988–42997. [Google Scholar]
- Gu, QY; Jin, HY; Li, D. Effects of Qi Ling Gui Fu Prescription on Blood Indexes and Antioxidant Properties of Broilers with Ascites Syndrome at High Altitude. Heilongjiang Animal Science And veterinary Medicine 2019, (18), 145–148. [Google Scholar]
- Chen, HD; Zhan, ZH; Kang, L. The Curative Effect of Linezolid on the Severe Pneumonia and the Influence on Serum IL-1β, TGF-β, TNF-α Levels. Progress in Modern Biomedicine 2017, 17(17), 3313–3316. [Google Scholar]
- Zhang, ZH; Liu, X; Zhu, YC. TNF-α-induced cardiomyocyte injury improved by microRNA-9 through SIRT1/NF-kB pathway: a study on mechanism. Chinese Journal of Evidence-Based Cardiovascular Medicine 2021, 13(7), 803–806. [Google Scholar]
- ASHMEETHA, M; LEBOGANG, M; SULE, G. Inflammation- induced left vent ricular fibrosis is partially mediated by tumor necrosis factor-a. Physiol Rep 2021, 9(21), 15062–15072. [Google Scholar]
- Wang, S; Zhang, Z; Wang, J; et al. MiR- 107 Induces TNF-a Secretionin Endothelial Cells Causing Tubular Cell Injury in Patients With Septic Acute Kidney Injury [J]. Biochem Biophys Res Commun 2017, 483(1), 45–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, MZ; Wang, X; Wang, Y. IL-4/IL-13-mediated Polarization of Renal Macrophages/Dendritic Cells to an M2a Phenotype Is.Essential for Recovery From Acute Kidney Injury. Kidney Int 2017, 91(2), 375–386. [Google Scholar] [CrossRef]
- West, GA; Matsuura, T; Levine, AD; et al. Interleukin 4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology 1996, 110(6), 16834 695. [Google Scholar] [CrossRef]
- Sun, Y; Wang, HX. Astragaloside IV attenuates inflammatory response in rats with pulmonary arterial hypertension via NF-kB/NLRP3 signaling pathway. Chinese Traditional Patent Medicine 2023, 45(2), 578–582. [Google Scholar]
- Wang, Q; Zhao, MJ. Poria cocos polysaccharide reduces tissue injury in rats with myocardial infarction by NF-κB pathway. Chongqing medicine 2022, 51(19), 3247–3252. [Google Scholar]
- Cao, P; Sun, J; Sullivan, MA; et al. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int J Biol Macromol 2018, 111(8), 1133–9. [Google Scholar] [CrossRef]
- Phan, THG; Paliogiannis, P; Nasrallah, GK; et al. Emerging cellular and molecular determinants of idiopathic pulmonary fibrosis[J]. Cell Mol Life Sci 2021, 78, 2031–2057. [Google Scholar] [CrossRef]
- Lodyga, M; Hinz, B. TGF-B1-a truly transforming growth factor in fibrosis and immunity. Semin Cell Dev Biol 2020, 101, 123–139. [Google Scholar]
- Minagawa, S; Araya, J; Numata, T; et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-B-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2011, 300, L391–401. [Google Scholar] [CrossRef]
- Kurosaki, F; Uchibori, R; Sehara, Y; et al. AAV6-Mediated IL-10 Expression in the Lung Ameliorates Bleomycin-Induced Pulmonary Fibrosis in Mice. Human Gene Therapy 2018, 29(11), 1242–1251. [Google Scholar] [CrossRef]
- Wang, SF; Chen, YS; Wang, N. Experimental Study on the Effect of Astragaloside Ⅳ on Serum Inflammatory Factors and Myocardial Remodeling in Rats with Heart Failure. World Journal of Integrated Traditional and Western Medicine 2020, 15(9), 1661–1665. [Google Scholar]
- Zhao, ZM; Huang, K; Shen, L. Effect of Levistilide A on NO and Endothelial Cell Function in Experimental Fibrosis. World Chinese Medicine 2020, 15(19), 2850–2856. [Google Scholar]
- Jiang, ZK; Wang, XF. Improvement Effect of Poria cocos Peels Water Extract on Liver Fibrosis in Rats Induced by Carbon Tetrachloride. China Pharmacy 2017, 28(22), 3065–3068. [Google Scholar]
- Cai, FL. Experimental study on the inhibition of silicosis fibrosis by isoliquiritin regulating EMT through ERK1/2 pathway. Journal of Hubei University of Medicine 2021. [Google Scholar]
- Dai, QS; Li, J; Wang, JW. Study on the effect of Lithospermum on the expression of IL-18 and IL-10 in collagen-induced arthritis. Lishizhen Medicine and Materia Medica Research 2013, 24(7), 1608–1609. [Google Scholar]
- Xu, Q.H.; Bauer, R.; Hendry, B.M.; Fan, T.P.; Zhao, Z.Z.; Duez, P.; Simmonds, M.S.J.; Witt, C.M.; Lu, A.P.; Robinson, N.; et al. The quest for modernisation of traditional Chinese medicine. Bmc Complem Altern M 2013, 13. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).