Submitted:
29 December 2025
Posted:
30 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients and Pathological Evaluation
2.2. Immunohistochemistry
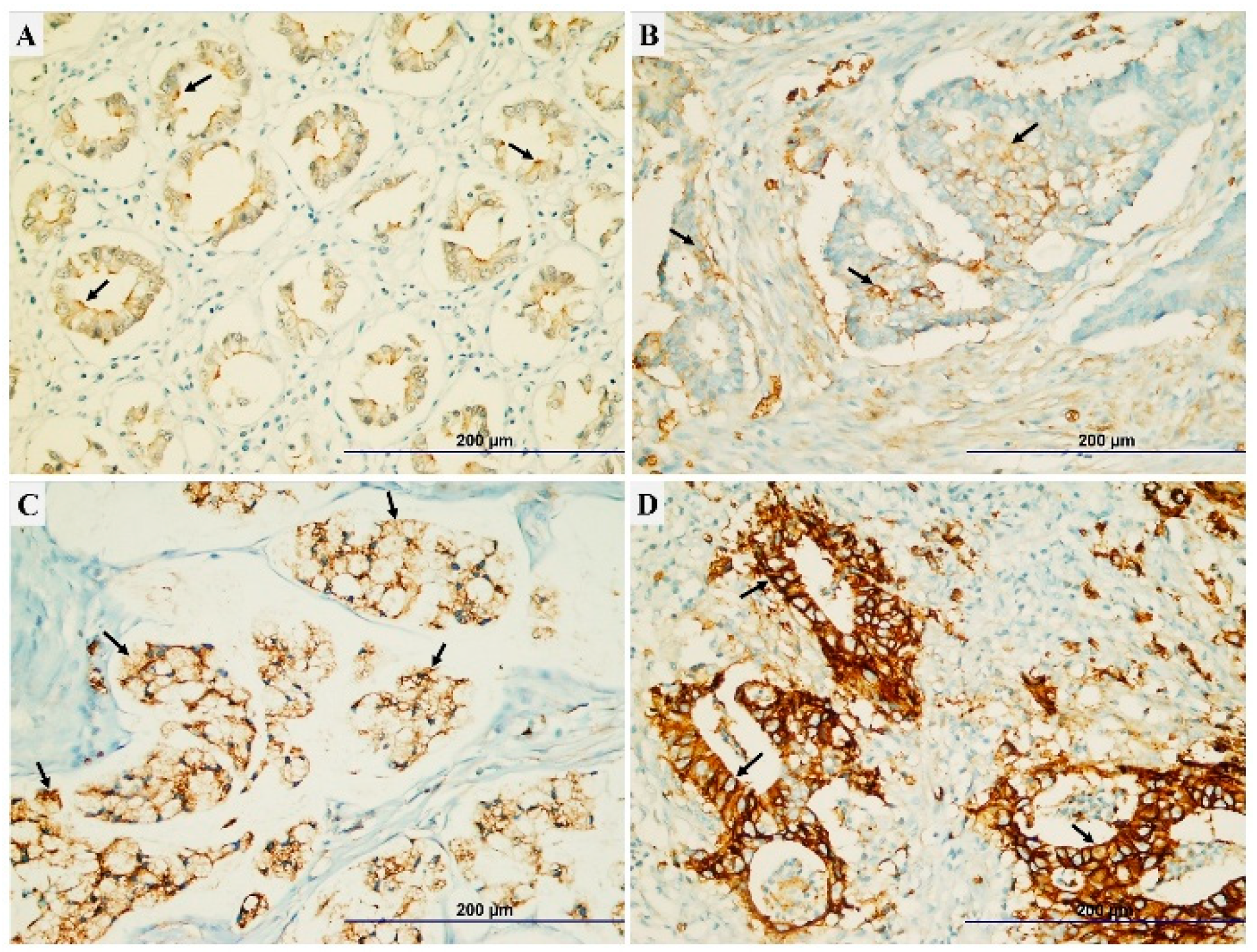
2.3. The Evaluating of TRPM7 Expression Score
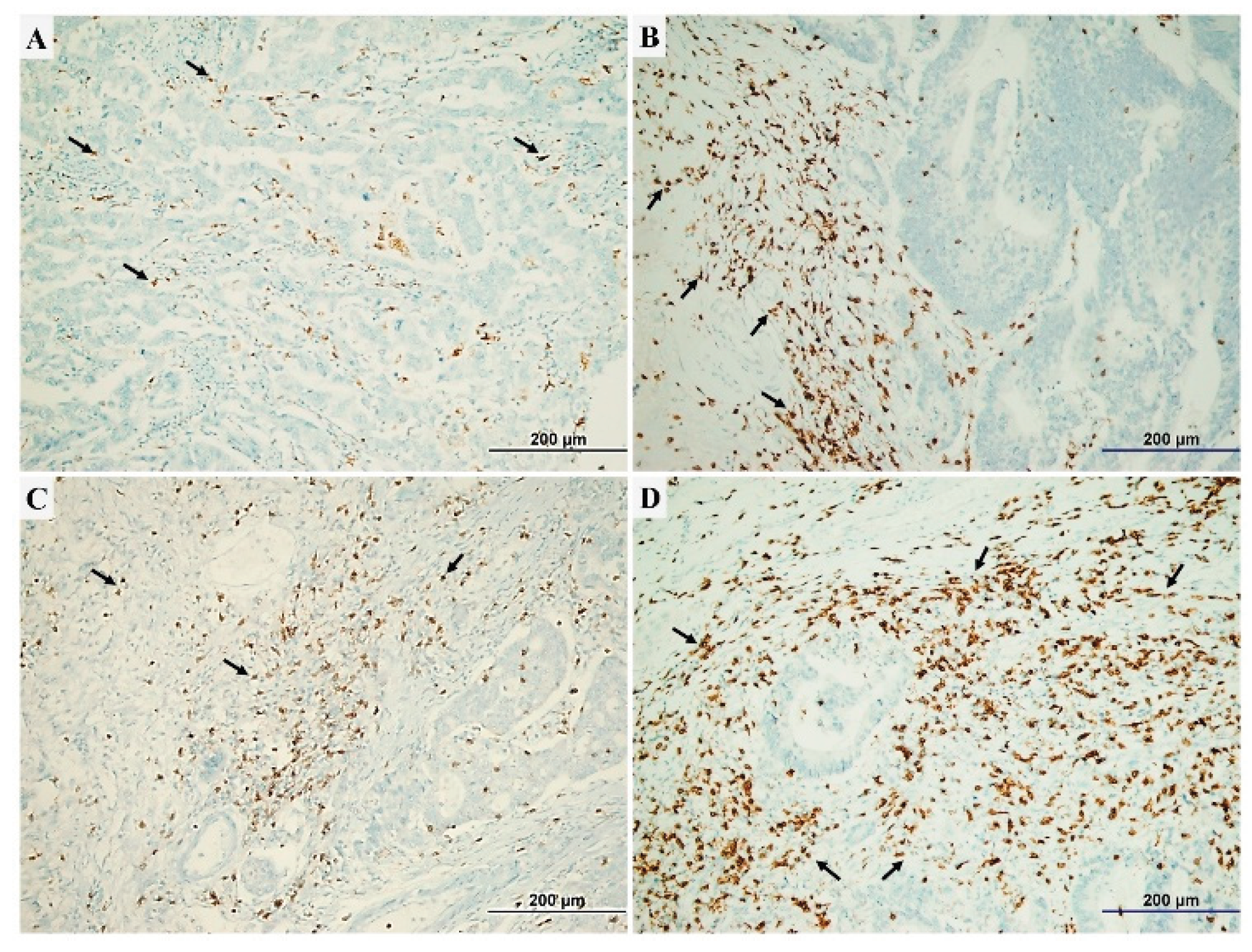
2.4. Assessment of TILs Intensity and CD4+/CD8+ TILs Ratio
2.5. Survival and Statistical Analysis
3. Results
3.1. The Correlation Between Clinicopathological Parameters and Survival
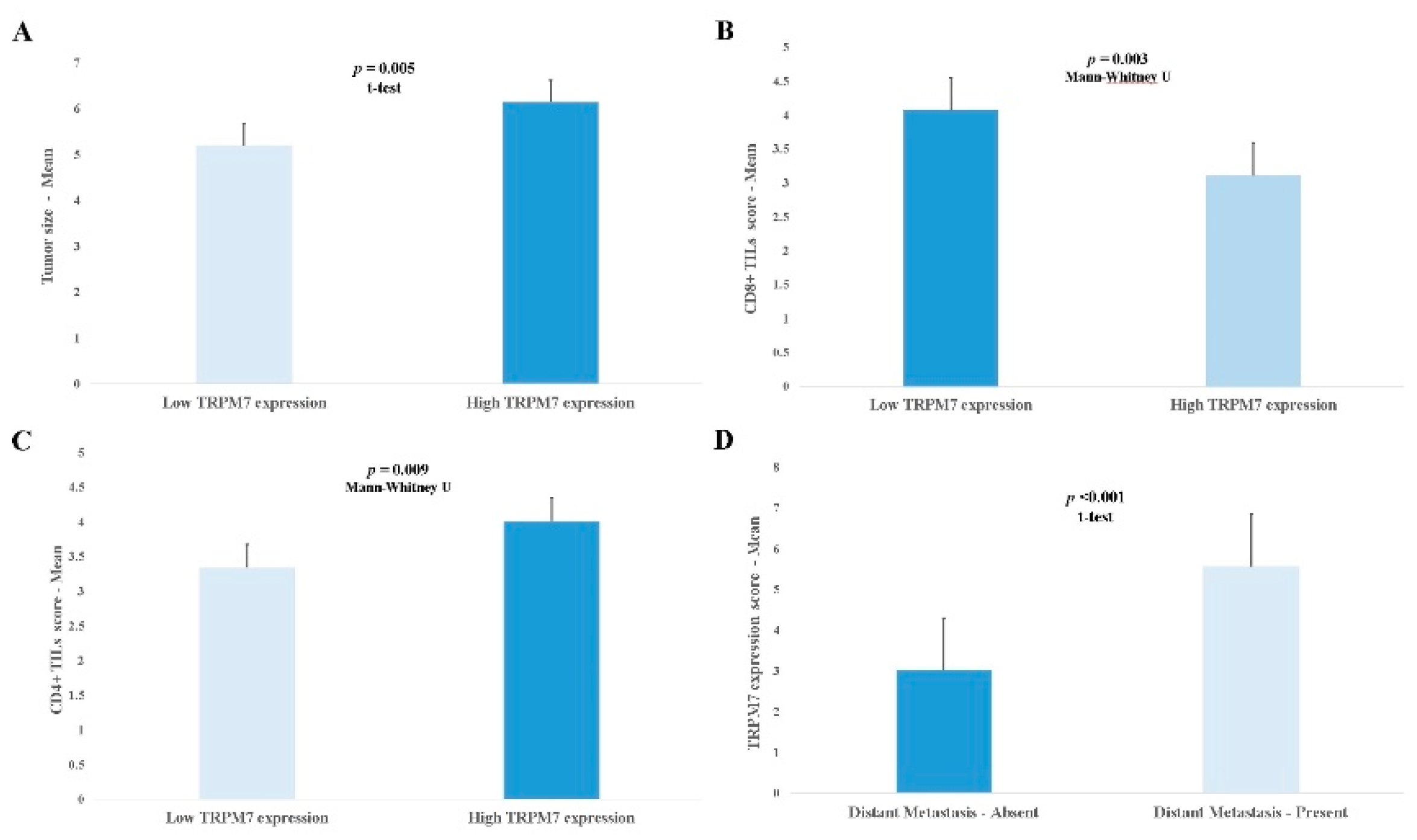
3.2. Relationship Between TRPM7 Expression and Clinicopathological Parameters
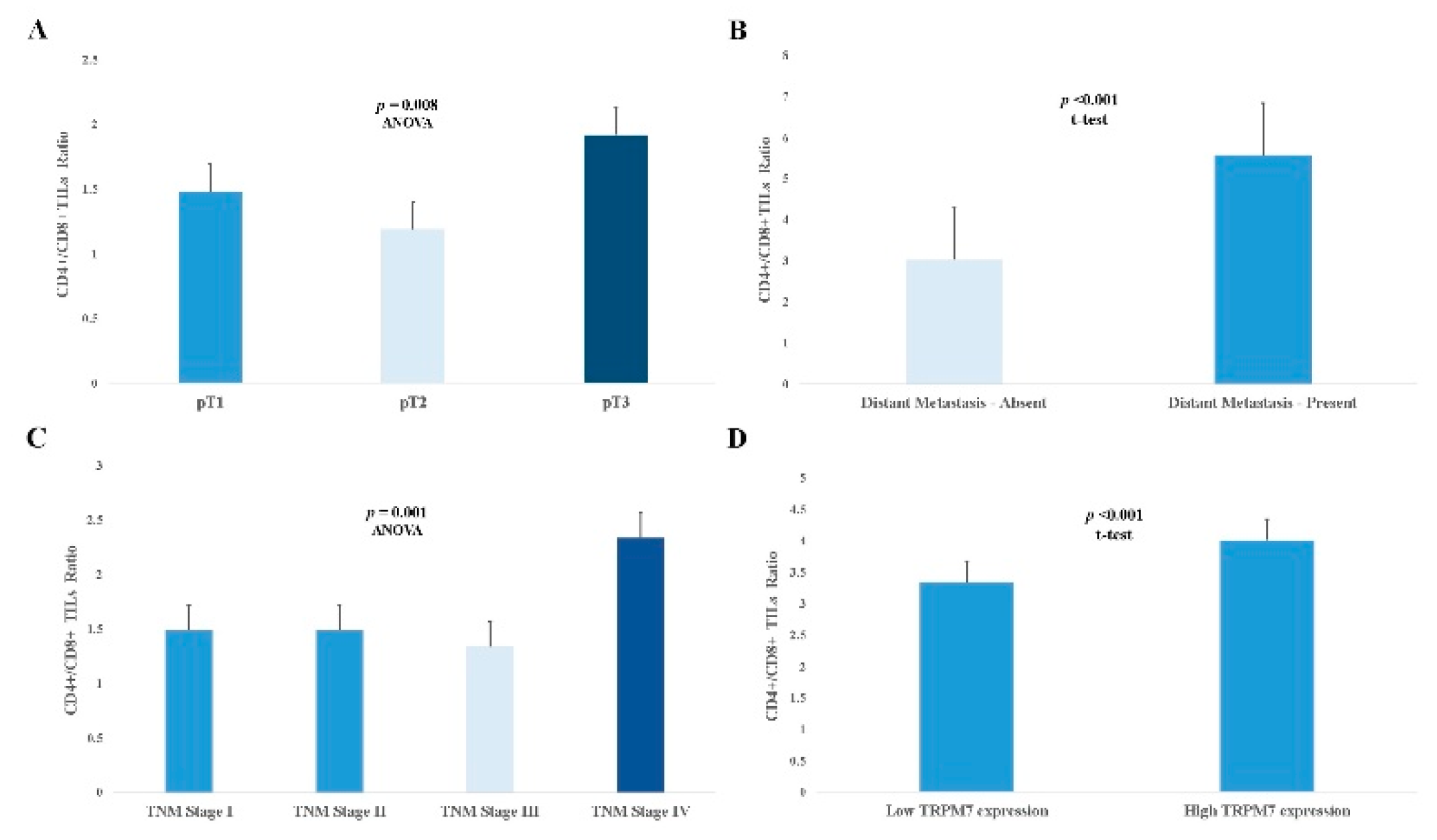
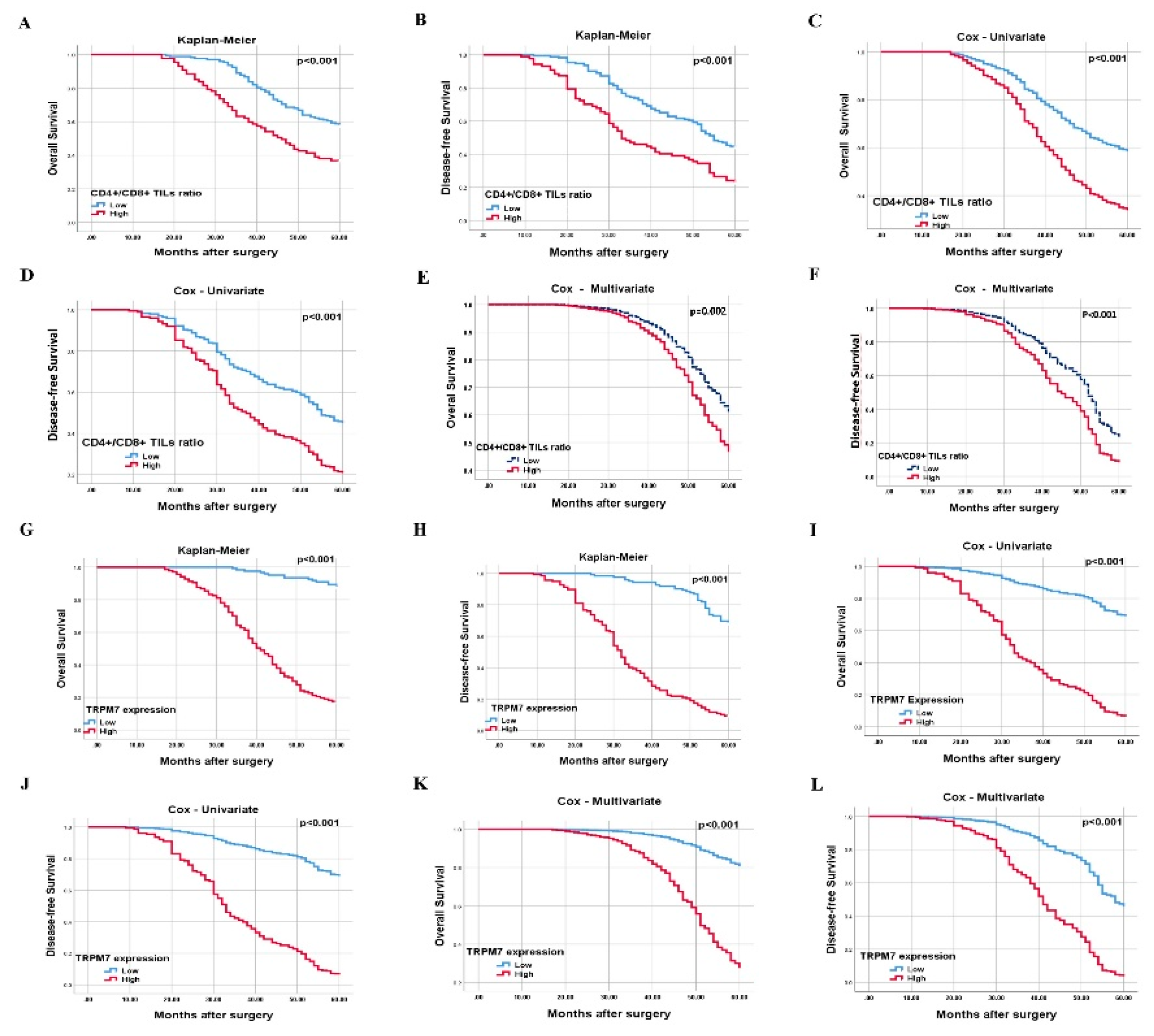
3.3. The Effect of High CD4+/CD8+ TILs Ratio on Prognosis
3.4. TRPM7 Overexpression Can Be Used as an Independent Poor Prognostic Biomarker in CRC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kokoropoulos, P.; Christodoulou, S.; Tsiakanikas, P.; Poulios, E.; Vassiliu, P.; Kontos, C.K.; Arkadopoulos, N. A Retrospective Study in Colorectal Adenocarcinoma Uncovers the Potential of Circ-CCT3 as a Predictor of Tumor Recurrence. Biomedicines 2025, 13, 2432. [Google Scholar] [CrossRef]
- Xue, Y.; Jiang, Z.; Gu, J.; Deng, S.; Cai, K.; Wu, K. Analysis of Immune Cell Infiltration Distribution and Prognostic Value in Obstructive Colorectal Cancer. Biomedicines 2025, 13, 2596. [Google Scholar] [CrossRef] [PubMed]
- Fagarasan, G.; Gheban, B.A.; Fagarasan, V.; Crisan, D.; Telecan, T.; Bintintan, V.V.; Seicean, R.L.; Caziuc, A.; Dindelegan, G.C. B and T Tumor-Infiltrating Lymphocyte Subtypes According to Subsite: A Colon Cancer Immunophenotyping Map. Biomedicines 2025, 13, 2856. [Google Scholar] [CrossRef] [PubMed]
- Betge, J.; Langner, C. Vascular Invasion, Perineural Invasion, and Tumour Budding: Predictors of Outcome in Colorectal Cancer. Acta. Gastroenterol. Belg 2011, 74, 516–529. [Google Scholar] [PubMed]
- Park, HS.; Hong, C.; Kim, B.J.; So, I. The Pathophysiologic Roles of TRPM7 Channel. Korean. J Physiol. Pharmacol. 2014, 18, 15–23. [Google Scholar] [CrossRef]
- Liu, H.; Dilger, J.P.; Lin, J. Lidocaine Suppresses Viability and Migration of Human Breast Cancer Cells: TRPM7 as a Target for Some Breast Cancer Cell Lines. Cancers 2021, 13, 234. [Google Scholar] [CrossRef]
- Sanchez, M.G.; Sanchez, A.M.; Collado, B.; Malagarie-Cazenave, S.; Olea, N.; Carmena, M.J.; Prieto, J.C.; Diaz-Laviada, I. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur. J. Pharmacol. 2005, 515, 20–27. [Google Scholar] [CrossRef]
- Su, F.; Wang, B.F.; Zhang, T.; Hou, X.M.; Feng, MH. TRPM7 deficiency suppresses cell proliferation, migration, and invasion in human colorectal cancer via regulation of epithelial-mesenchymal transition. Cancer Biomark. 2019, 26, 451–460. [Google Scholar] [CrossRef]
- Yee, N.S.; Chan, A.S.; Yee, J.D.; Yee, R.K. TRPM7 and TRPM8 ion channels in pancreatic adenocarcinoma: potential roles as cancer biomarkers and targets. Scientifica. (Cairo). 2012; p. 415158. [Google Scholar]
- Anderson, K.J.; Cormier, R.T.; Scott, P.M. Role of ion channels in gastrointestinal cancer. World J. Gastroenterol. 2019, 25, 5732–5772. [Google Scholar] [CrossRef]
- Dai, Q.; Shrubsole, M.J.; Ness, R.M.; Schlundt, D.; Cai, Q.; Smalley, W.E.; Li, M.; Shyr, Y.; Zheng, W. The relation of magnesium and calcium intakes and a genetic polymorphism in the magnesium transporter to colorectal neoplasia risk. Am. J. Clin. Nutr. 2007, 86, 743–751. [Google Scholar] [CrossRef]
- Kim, B.J.; Hong, C. Role of transient receptor potential melastatin type 7 channel in gastric cancer. Integr. Med. Res. 2016, 5, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Furuya, H.; Faouzi, M.; Zhang, Z.; Monteilh-Zoller, M.; Kawabata, K.G.; Horgen, F.D.; Kawamori, T.; Penner, R.; Fleig, A. Inhibition of TRPM7 suppresses cell proliferation of colon adenocarcinoma in vitro and induces hypomagnesemia in vivo without affecting azoxymethane-induced early colon cancer in mice. Cell. Commun. Signal. 2017, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Fleig, A.; Chubanov, V. TRPM7. Handb. Exp. Pharmacol. 2014, 222, 521–546. [Google Scholar] [PubMed]
- Dhennin-Duthille, I.; Gautier, M.; Faouzi, M.; Guilbert, A.; Brevet, M.; Vaudry, D.; Ahidouch, A.; Sevestre, H.; Ouadid-Ahidouch, H. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell. Physiol. Biochem. 2011, 28, 813–822. [Google Scholar] [CrossRef]
- Hanano, T.; Hara, Y.; Shi, J.; Morita, H.; Umebayashi, C.; Mori, E.; Sumimoto, H.; Ito, Y.; Mori, Y.; Inoue, R. Involvement of TRPM7 in cell growth as a spontaneously activated Ca2+ entry pathway in human retinoblastoma cells. J. Pharmacol. Sci. 2004, 95, 403–419. [Google Scholar] [CrossRef]
- Sterea, A.M.; Egom, E.E.; El Hiani, Y. TRP channels in gastric cancer: New hopes and clinical perspectives. Cell. Calcium 2019, 82, 102053. [Google Scholar] [CrossRef]
- Middelbeek, J.; Kuipers, A.J.; Henneman, L.; Visser, D.; Eidhof, I.; van-Horssen, R.; Wieringa, B.; Canisius, S.V.; Zwart, W.; Wessels, L.F.; et al. TRPM7 is required for breast tumor cell metastasis. Cancer. Res. 2012, 72, 4250–4261. [Google Scholar] [CrossRef]
- Qin, Y.; Liao, Z.W.; Luo, J.Y.; Wu, W.Z.; Lu, A.S.; Su, P.X.; Lai, B.Q.; Wang, X.X. Functional characterization of TRPM7 in nasopharyngeal carcinoma and its knockdown effects on tumorigenesis. Tumour. Biol. 2016, 37, 9273–9283. [Google Scholar] [CrossRef]
- Castiglioni, S.; Cazzaniga, A.; Trapani, V.; Cappadone, C.; Farruggia, G.; Merolle, L.; Wolf, F.I.; Iotti, S.; Maier, J.A.M. Magnesium homeostasis in colon carcinoma LoVo cells sensitive or resistant to doxorubicin. Sci. Rep. 2015, 5, 16538. [Google Scholar] [CrossRef]
- Idos, G.E.; Kwok, J.; Bonthala, N.; Kysh, L.; Gruber, S.B.; Qu, C. The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 3360. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Wu, Y.; Huang, X.Y.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Li, P.; Zheng, C.H.; Huang, A.M.; et al. Tumor infiltrating CD8+ T cells combined with tumor-associated CD68+ macrophages predict postoperative prognosis and adjuvant chemotherapy benefit in resected gastric cancer. BMC. Cancer 2019, 19, 920. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, Q.; Liu, J.; Xiao, Y.; Xiao, C.; Chen, K.; Xie, D.; Zhang, X. CD8+ tumor-infiltrating lymphocytes as a novel prognostic biomarker in lung sarcomatoid carcinoma, a rare subtype of lung cancer. Cancer. Manag. Res. 2018, 10, 3505–3511. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.L.; Kong, C.Z.; Zhang, Z.; Li, Z.L.; Bi, J.B.; Liu, X.K. TRPM7 is overexpressed in bladder cancer and promotes proliferation, migration, invasion and tumor growth. Oncol. Rep. 2017, 38, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, M.; Duan, X.; Chen, Y.; Li, E.; Luo, L.; Wu, W.; Peng, Z.; Qiu, H.; Zeng, G. TRPM7 Regulates AKT/FOXO1-Dependent Tumor Growth and Is an Independent Prognostic Indicator in Renal Cell Carcinoma. Mol. Cancer. Res. 2018, 16, 1013–1023. [Google Scholar] [CrossRef]
- Lee, E.H.; Chun, S.Y.; Kim, B.; Yoon, B.H.; Lee, J.N.; Kim, B.S.; Yoo, E.S.; Lee, S.; Song, P.H.; Kwon, T.G.; et al. Knockdown of TRPM7 prevents tumor growth, migration, and invasion through the Src, Akt, and JNK pathway in bladder cancer. BMC. Urol. 2020, 20, 145. [Google Scholar] [CrossRef]
- Liu, H.; Dilger, J.P.; Lin, J. The Role of Transient Receptor Potential Melastatin 7 (TRPM7) in Cell Viability: A Potential Target to Suppress Breast Cancer Cell Cycle. Cancers. (Basel) 2020, 12, 131. [Google Scholar] [CrossRef]
- Yee, N.S. Role of TRPM7 in Cancer: Potential as Molecular Biomarker and Therapeutic Target. Pharmaceuticals. (Basel) 2017, 10, 39. [Google Scholar] [CrossRef]
- Demeuse, P.; Penner, R.; Fleig, A. TRPM7 channel is regulated by magnesium nucleotides via its kinase domain. J Gen. Physiol. 2006, 127, 421–434. [Google Scholar] [CrossRef]
- Nakashima, S.; Shiozaki, A.; Ichikawa, D.; Hikami, S.; Kosuga, T.; Konishi, H.; Komatsu, S.; Fujiwara, H.; Okamoto, K.; Kishimoto, M.; et al. Transient Receptor Potential Melastatin 7 as an Independent Prognostic Factor in Human Esophageal Squamous Cell Carcinoma. Anticancer. Res. 2017, 37, 1161–1167. [Google Scholar] [CrossRef]
- Liu, K.; Xu, S.H.; Chen, Z.; Zeng, Q.X.; Li, Z.J.; Chen, Z.M. TRPM7 overexpression enhances the cancer stem cell-like and metastatic phenotypes of lung cancer through modulation of the Hsp90α/uPA/MMP2 signaling pathway. BMC. Cancer 2018, 26, 1167. [Google Scholar] [CrossRef]
- Yee, N.S.; Zhou, W.; Liang, I.C. Transient receptor potential ion channel TRPM7 regulates exocrine pancreatic epithelial proliferation by Mg2+-sensitive Socs3a signaling in development and cancer. Dis. Mod. Mech. 2011, 4, 240–254. [Google Scholar] [CrossRef]
- Yee, N.S.; Kazi, A.A.; Li, Q.; Yang, Z.; Berg, A.; Yee, R.K. Aberrant over-expression of TRPM7 ion channels in pancreatic cancer: Required for cancer cell invasion and implicated in tumor growth and metastasis. Biol. Open. 2015, 4, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, A.; Gautier, M.; Dhennin-Duthille, I.; Haren, N.; Sevestre, H.; Ouadid-Ahidouch, H. Evidence that TRPM7 is required for breast cancer cell proliferation. Am. J. Physiol. Cell. Physiol. 2009, 297, C493–C502. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Guo, A.A.; Chowdhury, I.; Guo, S.; Hibbert, J.; Wang, G.; Liu, M. TRPM7 Induces Mechanistic Target of Rap1b Through the Downregulation of miR-28-5p in Glioma Proliferation and Invasion. Front. Oncol. 2019, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, D.; Armuzzi, A.; Castri, F.; Benvenuto, R.; Mangoni, A.; Guidi, L.; Gasbarrini, A.; Rapaccini, G.L.; Wolf, F.I.; Trapani, V. TRPM7 is overexpressed in human IBD-related and sporadic colorectal cancer and correlates with tumor grade. Dig. Liver. Dis. 2020, 52, 1188–1194. [Google Scholar] [CrossRef]
- Davis, F.M.; Azimi, I.; Faville, R.A.; Peters, A.A.; Jalink, K.; Putney, J.W.; Goodhill, G.J., Jr.; Thompson, E.W.; Roberts-Thomson, S.J.; Monteith, G.R. Induction of epitheial-mesenchymal transition (EMT) in breast cancer cells is calcium signal dependent. Oncogene 2014, 33, 2307–2316. [Google Scholar] [CrossRef]
- Rybarczyk, P.; Gautier, M.; Hague, F.; Dhennin-Duthille, I.; Chatelain, D.; Kerr-Conte, J.; Pattou, F.; Regimbeau, J.M.; Sevestre, H.; Ouadid-Ahidouch, H. Transient receptor potential melastatinrelated 7 channel is overexpressed in human pancreatic ductal adenocarcinomas and regulates human pancreatic cancer cell migration. Int. J Cancer 2012, 131, E851-861. [Google Scholar] [CrossRef]
- Lee, J.C.; Bae, A.N.; Lee, H.J.; Lee, J.H. Clinical and Prognostic Values of TRPM7 in Colon and Rectal Cancers. Medicina [Kaunas] 2022, 58, 1582. [Google Scholar] [CrossRef]
- Kuwahara, T.; Hazama, S.; Suzuki, N.; Yoshida, S.; Tomochika, S.; Nakagami, Y.; Matsui, H.; Shindo, Y.; Kanekiyo, S.; Tokumitsu, Y.; et al. Intratumoural-infiltrating CD4 + and FOXP3 + T cells as strong positive predictive markers for the prognosis of resectable colorectal cancer. Br. J Cancer 2019, 121, 659–665. [Google Scholar] [CrossRef]
- Diederichsen, A.C.; Hjelmborg, J.V.; Christensen, P.B.; Zeuthen, J.; Fenger, C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer. Immunol. Immunother. 2003, 52, 423–428. [Google Scholar] [CrossRef]
- Droeser, R.; Zlobec, I.; Kilic, E.; Güth, U.; Heberer, M.; Spagnoli, G.; Oertli, D.; Tapia, C. Differential pattern and prognostic significance of CD4 +, FOXP3 + and IL-17 + tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC. Cancer 2012, 12, 134. [Google Scholar] [CrossRef]
- Kinoshita, T.; Muramatsu, R.; Fujita, T.; Nagumo, H.; Sakurai, T.; Noji, S.; Takahata, E.; Yaguchi, T.; Tsukamoto, N.; Kudo-Saito, C.; et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann. Oncol. 2016, 27, 2117–2123. [Google Scholar] [CrossRef]
- Lavotshkin, S.; Jalas, J.R.; Torisu-Itakura, H.; Ozao-Choy, J.; Lee, J.H.; Sim, M.S.; Stojadinovic, A.; Wainberg, Z.; Bifulco, C.B.; Fox, B.A.; et al. Immunoprofiling for prognostic assessment of colon cancer: a novel complement to ultrastaging. J Gastrointest Surg. 2015, 19, 999–1006. [Google Scholar] [CrossRef]
| Parameters | Overall survival [Univariate] | ||
|---|---|---|---|
|
N [%] |
HR [95% CI] | P value | |
| Gender Female Male |
114 [44.0] 145 [56.0] |
1.13 [0.79-1,6] |
0.491 |
| Age 19-44 45-54 55 ≥ |
34 [13.1] 86 [33.2] 139 [53.7] |
1.23 [0.95-1.59] |
0.109 |
| Tumor size [cm] < 5 ≥ 5 |
105 [40.5] 154 [59,5] |
1.39 [0.97-2.31] | 0.070 |
| Tumor site Left Right |
157 [60.6] 105 [39.4] |
0.64 [0.45-0.92] |
0.015 |
| Histopathologic type Adenocarcinoma Mucinous Signet-ring |
154 [59.5] 82 [31.7] 23 [8.9] |
1.27 [0.98-1.66] |
0.075 |
| Histologic grade Well Moderate Poor |
44 [17.0] 173 [66.8] 42 [16.2] |
2.16 [1.56-2.99] |
< 0.001 |
| CD8+ TILs density Low High |
131 [50.6] 128 [49.4] |
0.58 [0.41-0.83] |
0.003 |
| CD4+ TILs density Low High |
129 [49.8] 130 [50.2] |
0.61 [0.43-0.87] |
0.007 |
| CD4+/CD8+ TILs ratio Low High |
172 [66.4] 87 [33.6] |
2.02 [1.42-2.86] |
<0.001 |
| Depth of invasion Submucosa Muscularis propria Pericolorectal tissues |
34 [3.1] 68 [26.3] 157 [60.6] |
2.80 [1.98-3.97] |
< 0.001 |
| Lymph node status Absent 1-3 ≥ 4 |
113 [43.6] 97 [37.5] 49 [18.9] |
3.65 [2.90-4.60] |
< 0.001 |
| Distant Metastasis Absent Present |
186 [71.8] 73 [28.2] |
12.93 [8.67-19.28] |
< 0.001 |
| TNM staging Stage I Stage II Stage III Stage IV |
59 [22.8] 54 [20.8] 78 [30.1] 68 [26.3] |
5.92 [4.45-7.88] |
< 0.001 |
| Cox regression analyses [Multivariate] | ||||
|---|---|---|---|---|
| Parameters | Disease-free survival | Overall survival | ||
| HR [95% CI] | P value | HR [95% CI] | P value | |
| Gender [male/female] | 0.90 [0.63-1.27] | 0.553 | 1.00 [0.67-1.49] | 0.964 |
| Age [19-44/45-54/≥ 55] | 1.08 [0.86-1.36] | 0.500 | 1.18 [0.91-1.53] | 0.205 |
| Site [left/right] | 0.63 [0.45-0.88] | 0.007 | 0.72 [0.49-1.07] | 0.107 |
| Type [adeno/mucinous/signet ring] | 1.06 [0.83-1.35] | 0.626 | 1.05 [0.81-1.37] | 0.677 |
| Grade [well/moderate/poor] | 1.24 [0.92-1.69] | 0.154 | 1.12 [0.81-1.55] | 0.478 |
| CD8+ TILs density [low/high] | 0.47 [0.34-0.66] | <0.001 | 0.59 [0.40-0.85] | 0.006 |
| CD4+ TILs density [low/high] | 0.90 [0.62-1.32] | 0.619 | 1.22 [0.79-1.88] | 0.351 |
| CD4+/CD8+ TILs ratio [low/high] | 1.89 [1.35-2.64] | <0.001 | 1.77 [1.22-2.56] | 0.002 |
| pT [pT1/pT2/pT3] | 0.89 [0.62-1.26] | 0.517 | 1.00 [0.63-1.59] | 0.978 |
| pN [absent/1-3/≥ 4] | 1.37 [0.96-1.95] | 0.077 | 1.42 [0.98-2.07] | 0.063 |
| TNM stage [I/II/III/IV] | 2.32 [1.49-3.62] | <0.001 | 3.30 [1.78-6.10] | <0.001 |
| TRPM7 expression [low/high] | 4.08 [2.65-6.28] | <0.001 | 5,81 [3.16-10.65] | <0.001 |
| Parameters | TRPM7 expression | P value |
|---|---|---|
| Mean ± SD | ||
| Histopathologic type Adenocarcinoma Mucinous Signet-ring |
3.79±2.26 3.30±2.15 4.91±2.42 |
0.010 * |
| Histologic grade Well Moderate Poor |
2.86±1.98 3.74±2.30 4.64±2.15 |
0.001 * |
| CD4+ TILs density Low High |
3.39±2.27 4.08±2.23 |
0.009 ** |
| CD8+ TILs density Low High |
4.14±2.30 3.32±2.18 |
0.003 ** |
| CD4+/CD8+ TILs ratio Low High |
3.33±2.23 4.54±2.17 |
<0.001** |
| Depth of invasion Submucosa Muscularis propria Pericolorectal tissues |
2.05±1.04 3.39±2.09 4.25±2.35 |
<0.001 * |
| Lymph node status Absent 1-3 ≥ 4 |
2.31±1.40 4.65±2.21 5.20±2.19 |
<0.001 * |
| Distant Metastasis Absent Present |
3.02±2.01 5.57±1.85 |
<0.001 *** |
| TNM staging Stage I Stage II Stage III Stage IV |
2.35±1.42 2.22±1.38 4.24±2.30 5.57±1.83 |
<0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





