Submitted:
26 December 2025
Posted:
29 December 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2.1. Biological Interpretation of Restricted Diffusion in Imaging Studies
2.2. Properties of Diffusion Imaging That Affect Visualization and Sensitivity
3.1. Factors Associated with Increased Probability of a Negative Initial DWI
3.2. Safety and Efficacy of Acute Reperfusion Therapies in DWI-Negative Stroke
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| DWI | Diffusion-weighted imaging |
| AIS | Acute ischemic stroke |
| DWI-negative | DWI-negative stroke |
| IVT | Intravenous thrombolysis |
| MT | Mechanical thrombectomy |
| NCCT | Non-contrast computed tomography |
| MR | Magnetic resonance |
| ADC | Apparent diffusion coefficient |
| FLAIR | Fluid attenuated inversion recovery |
| BBB | Blood-brain barrier |
| GM | Gray matter |
| WM | White matter |
| EPI | Echo-planar imaging |
| SNR | Signal-to-noise |
| T | Tesla |
| INO | Intranuclear ophthalmoplegia |
| NIHSS | National Institutes of Health Stroke Scale |
| TIA | Transient ischemic attack |
| LVO | Large vessel occlusion |
| DWIR | DWI-Reversal |
| ICH | Intracerebral hemorrhage |
| ASPECTS | Alberta Stroke Program Early CT Score |
| CTP | Computed tomography perfusion |
| CTA | Computed tomography angiography |
| SWI | Susceptibility-weighted imaging |
| PWI | Perfusion-weighted imaging |
| ASL | Arterial spin labeling |
| AI | Artificial intelligence |
| Afib | Atrial fibrillation |
References
- Feigin, VL; Abate, MD; Abate, YH; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2021: a systematic analysis for the Global Burden of Disease Study 2021. The Lancet Neurology 2024, 23(10), 973–1003. [Google Scholar] [CrossRef]
- Rapillo, CM; Dunet, V; Pistocchi, S; et al. Moving From CT to MRI Paradigm in Acute Ischemic Stroke: Feasibility, Effects on Stroke Diagnosis and Long-Term Outcomes. Stroke 2024, 55(5), 1329–1338. [Google Scholar] [CrossRef]
- Simonsen, CZ; Madsen, MH; Schmitz, ML; Mikkelsen, IK; Fisher, M; Andersen, G. Sensitivity of diffusion- and perfusion-weighted imaging for diagnosing acute ischemic stroke is 97.5%. Stroke 2015, 46(1), 98–101. [Google Scholar] [CrossRef]
- Welch, KM; Windham, J; Knight, RA; et al. A model to predict the histopathology of human stroke using diffusion and T2-weighted magnetic resonance imaging. Stroke 1995, 26(11), 1983–1989. [Google Scholar] [CrossRef]
- Nagaraja, N. Diffusion weighted imaging in acute ischemic stroke: A review of its interpretation pitfalls and advanced diffusion imaging application. Journal of the Neurological Sciences 2021, 425, 117435. [Google Scholar] [CrossRef] [PubMed]
- Edlow, BL; Hurwitz, S; Edlow, JA. Diagnosis of DWI-negative acute ischemic stroke. Neurology 2017, 89(3), 256–262. [Google Scholar] [CrossRef]
- Sylaja, PN; Coutts, SB; Krol, A; Hill, MD; Demchuk, AM. When to Expect Negative Diffusion-Weighted Images in Stroke and Transient Ischemic Attack. Stroke 2008, 39(6), 1898–1900. [Google Scholar] [CrossRef]
- Alkhiri, A; Alturki, F; Alansari, NM; et al. Prognosis and distribution of ischemic stroke with negative diffusion-weighted imaging: a systematic review and meta-analysis. Front Neurol 2024, 15. [Google Scholar] [CrossRef]
- Panni, P; Lapergue, B; Maïer, B; et al. Clinical Impact and Predictors of Diffusion Weighted Imaging (DWI) Reversal in Stroke Patients with Diffusion Weighted Imaging Alberta Stroke Program Early CT Score 0–5 Treated by Thrombectomy. Clin Neuroradiol. 2022, 32(4), 939–950. [Google Scholar] [CrossRef] [PubMed]
- Makin, SDJ; Doubal, FN; Dennis, MS; Wardlaw, JM. Clinically Confirmed Stroke With Negative Diffusion-Weighted Imaging Magnetic Resonance Imaging. Stroke 2015, 46(11), 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D. Diffusion MRI: what water tells us about the brain. EMBO Mol Med. 2014, 6(5), 569–573. [Google Scholar] [CrossRef]
- Baliyan, V; Das, CJ; Sharma, R; Gupta, AK. Diffusion weighted imaging: Technique and applications. World J Radiol. 2016, 8(9), 785–798. [Google Scholar] [CrossRef]
- Gu, Y; Zhou, C; Piao, Z; et al. Cerebral edema after ischemic stroke: Pathophysiology and underlying mechanisms. Front Neurosci. 2022, 16, 988283. [Google Scholar] [CrossRef]
- Thomalla, G; Rossbach, P; Rosenkranz, M; et al. Negative fluid-attenuated inversion recovery imaging identifies acute ischemic stroke at 3 hours or less. Annals of Neurology 2009, 65(6), 724–732. [Google Scholar] [CrossRef]
- Bachtiar, NA; Murtala, B; Muis, M; et al. Non-Contrast MRI Sequences for Ischemic Stroke: A Concise Overview for Clinical Radiologists. Vasc Health Risk Manag 2024, 20, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, AK; Mehrotra, G; Bhargava, SK; Agarwal, S; Tripathi, RP. Studies on the time course of apparent diffusion coefficient and signal intensities on T2- and diffusion-weighted MR Imaging in acute cerebral ischemic stroke. J Med Phys. 2008, 33(4), 162–170. [Google Scholar] [CrossRef] [PubMed]
- Asdaghi, N; Campbell, BCV; Butcher, KS; et al. DWI Reversal Is Associated with Small Infarct Volume in Patients with TIA and Minor Stroke. American Journal of Neuroradiology 2014, 35(4), 660–666. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M; Ospel, JM; Menon, B; et al. Challenging the Ischemic Core Concept in Acute Ischemic Stroke Imaging. Stroke 2020, 51(10), 3147–3155. [Google Scholar] [CrossRef]
- Gosch, V; Villringer, K; Galinovic, I; et al. Automated acute ischemic stroke lesion delineation based on apparent diffusion coefficient thresholds. Front Neurol 2023, 14. [Google Scholar] [CrossRef]
- Skattør, TH; Offersen, CM; Nome, T; et al. Mapping DWI signal reversal and long-term tissue outcomes following endovascular therapy in acute ischemic stroke. Eur Radiol. Published online. 1 September 2025. [CrossRef]
- Dang, HQ; Nguyen, TQ; Chiem, DN; et al. Near-Complete Reversal of Large Diffusion-Weighted Imaging Lesion after Thrombectomy: A Case Report and Literature Review. Cerebrovasc Dis Extra 2024, 14(1), 185–192. [Google Scholar] [CrossRef]
- Navi, BB; Bach, I; Czap, AL; et al. Strokes Averted by Intravenous Thrombolysis: A Secondary Analysis of a Prospective, Multicenter, Controlled Trial of Mobile Stroke Units. Ann Neurol. 2024, 95(2), 347–361. [Google Scholar] [CrossRef]
- Rocha, M; Desai, SM; Jadhav, AP; Jovin, TG. Prevalence and Temporal Distribution of Fast and Slow Progressors of Infarct Growth in Large Vessel Occlusion Stroke. Stroke 2019, 50(8), 2238–2240. [Google Scholar] [CrossRef]
- Kim, HJ; Choi, CG; Lee, DH; Lee, JH; Kim, SJ; Suh, DC. High-b-Value Diffusion-Weighted MR Imaging of Hyperacute Ischemic Stroke at 1.5T. AJNR Am J Neuroradiol. 2005, 26(2), 208–215. [Google Scholar]
- Cihangiroglu, M; Citci, B; Kilickesmez, O; et al. The utility of high b-value DWI in evaluation of ischemic stroke at 3 T. European Journal of Radiology 2011, 78(1), 75–81. [Google Scholar] [CrossRef] [PubMed]
- Rosso, C; Drier, A; Lacroix, D; et al. Diffusion-weighted MRI in acute stroke within the first 6 hours. Neurology 2010, 74(24), 1946–1953. [Google Scholar] [CrossRef]
- Zuo, L; Zhang, Y; Xu, X; et al. A retrospective analysis of negative diffusion-weighted image results in patients with acute cerebral infarction. Sci Rep. 2015, 5(1), 8910. [Google Scholar] [CrossRef]
- Ract, I; Ferré, JC; Ronzière, T; Leray, E; Carsin-Nicol, B; Gauvrit, JY. Improving detection of ischemic lesions at 3 Tesla with optimized diffusion-weighted magnetic resonance imaging. Journal of Neuroradiology 2014, 41(1), 45–51. [Google Scholar] [CrossRef] [PubMed]
- Burdette, JH; Elster, AD; Ricci, PE. Acute cerebral infarction: quantification of spin-density and T2 shine-through phenomena on diffusion-weighted MR images. Radiology 1999, 212(2), 333–339. [Google Scholar] [CrossRef]
- Le Bihan, D; Poupon, C; Amadon, A; Lethimonnier, F. Artifacts and pitfalls in diffusion MRI. Journal of Magnetic Resonance Imaging 2006, 24(3), 478–488. [Google Scholar] [CrossRef] [PubMed]
- Gaddamanugu, S; Shafaat, O; Sotoudeh, H; et al. Clinical applications of diffusion-weighted sequence in brain imaging: beyond stroke. Neuroradiology 2022, 64(1), 15–30. [Google Scholar] [CrossRef]
- Silvera, S; Oppenheim, C; Touzé, E; et al. Spontaneous Intracerebral Hematoma on Diffusion-weighted Images: Influence of T2-shine-through and T2-blackout Effects. American Journal of Neuroradiology 2005, 26(2), 236–241. [Google Scholar]
- Brunser, AM; Hoppe, A; Illanes, S; et al. Accuracy of Diffusion-Weighted Imaging in the Diagnosis of Stroke in Patients With Suspected Cerebral Infarct. Stroke 2013, 44(4), 1169–1171. [Google Scholar] [CrossRef]
- Oppenheim, C; Stanescu, R; Dormont, D; et al. False-negative Diffusion-weighted MR Findings in Acute Ischemic Stroke. AJNR Am J Neuroradiol. 2000, 21(8), 1434–1440. [Google Scholar] [PubMed]
- Brunser, AM; Cavada, G; Venturelli, PM; et al. Diffusion-weighted imaging determinants for acute ischemic stroke diagnosis in the emergency room. Neuroradiology 2018, 60(7), 687–692. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C; Qin, W; Xu, J; Hu, W. Perfusion deficits in thrombolysis-treated acute ischemic stroke patients with negative or positive diffusion-weighted imaging. BMC Neurology 2023, 23(1), 380. [Google Scholar] [CrossRef]
- Pektezel, MY; Arsava, EM; Göçmen, R; Oğuz, KK; Topçuoğlu, MA. Diffusion-Weighted-Imaging Negative Stroke Syndromes. Turk J Neurol. 2021, 27(2), 151–157. [Google Scholar] [CrossRef]
- Wang, LL; Khatri, P; Prabhakaran, S; et al. DWI Positivity in Mild, Nondisabling Acute Cerebral Ischemia: Data From the PRISMS Trial. Stroke: Vascular and Interventional Neurology 2025, 5(2), e001613. [Google Scholar] [CrossRef]
- Kim, K; Kim, BJ; Huh, J; et al. Delayed Lesions on Diffusion-Weighted Imaging in Initially Lesion-Negative Stroke Patients. J Stroke 2021, 23(1), 69–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y; Jing, J; Pan, Y; Wang, M; Meng, X; Wang, Y. Distribution and prognosis of acute ischaemic stroke with negative diffusion-weighted imaging. Stroke Vasc Neurol. 2022, 7(6). [Google Scholar] [CrossRef]
- Fustier, A; Naggara, O; Tisserand, M; et al. Total mismatch in anterior circulation stroke patients before thrombolysis. J Neuroradiol. 2013, 40(3), 158–163. [Google Scholar] [CrossRef]
- Mekue Fotso, V; Leibinger, F; Rivas Lamelo, S; et al. Management of patients with diffusion-weighted imaging-negative acute ischemic stroke: Retrospective analysis of 47 consecutive patients. Journal of Stroke and Cerebrovascular Diseases 2024, 33(11), 107924. [Google Scholar] [CrossRef] [PubMed]
- Li, J; Huang, C; Liu, Y; et al. DWI and Clinical Characteristics Correlations in Acute Ischemic Stroke After Thrombolysis. medRxiv. Preprint posted online 2025, 2025.06.03.25328925. [Google Scholar] [CrossRef]
- Saur, D; Kucinski, T; Grzyska, U; et al. Sensitivity and interrater agreement of CT and diffusion-weighted MR imaging in hyperacute stroke. AJNR Am J Neuroradiol. 2003, 24(5), 878–885. [Google Scholar]
- Chalela, JA; Kidwell, CS; Nentwich, LM; et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. The Lancet 2007, 369(9558), 293–298. [Google Scholar] [CrossRef] [PubMed]
- Bulut, HT; Yildirim, A; Ekmekci, B; Eskut, N; Gunbey, HP. False-Negative Diffusion-Weighted Imaging in Acute Stroke and its Frequency in Anterior and Posterior Circulation Ischemia. Journal of Computer Assisted Tomography 2014, 38(5), 627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y; Fu, S; Hu, S; et al. Efficacy and safety of intravenous tenecteplase thrombolysis in diffusion-weighted imaging-negative posterior circulation ischemic stroke. Front Neurol 2025, 16. [Google Scholar] [CrossRef]
- Seyhan, M; Mackenrodt, D; Gunreben, I; et al. Should IV Thrombolysis be given in Patients with Suspected Ischemic Stroke but Unknown Symptom Onset and Without Diffusion-Weighted Imaging Lesion? – Results of a Case-Control Study. Journal of Stroke and Cerebrovascular Diseases 2020, 29(2). [Google Scholar] [CrossRef]
- Launhardt, N; Jesser, J; Hasan, D; et al. DWI Reversibility in Acute Ischemic Stroke Due to Basilar Artery Occlusion Following Successful Recanalization. Clin Neuroradiol. 2025, 35(3), 551–558. [Google Scholar] [CrossRef]
- Freeman, JW; Luby, M; Merino, JG; et al. Negative Diffusion Weighted Imaging After IV tPA is Rare and Unlikely To Indicate Averted Infarction. Stroke 2013, 44(6), 1629–1634. [Google Scholar] [CrossRef]
- Scheldeman, L; Wouters, A; Bertels, J; et al. Reversibility of Diffusion-Weighted Imaging Lesions in Patients With Ischemic Stroke in the WAKE-UP Trial. Stroke 2023, 54(6), 1560–1568. [Google Scholar] [CrossRef]
- Nagaraja, N; Forder, JR; Warach, S; Merino, JG. Reversible diffusion-weighted imaging lesions in acute ischemic stroke. Neurology 2020, 94(13), 571–587. [Google Scholar] [CrossRef] [PubMed]
- Skattør, TH; Bakke, KM; Nome, T; et al. Significance of Subtle Diffusion Weighted Imaging Lesion Dynamics: A Comparative Analysis of Methods for Detecting Diffusion Weighted Imaging Lesion Reversal in Endovascular Stroke Treatment. Stroke: Vascular and Interventional Neurology 2025, 0(0), e001835. [Google Scholar] [CrossRef]
- Skattør, TH; Bjørnerud, A; Nome, T; et al. Predicting lesion reversal in acute cerebral ischaemia via apparent diffusion coefficient threshold on diffusion-weighted MRI. Eur Radiol. Published online. 29 September 2025. [CrossRef]
- Berrada, K; Ouali, IE; Aouadi, SE; Fikri, M; Jiddane, M; Touarsa, F. When stroke hides: A case of negative diffusion MRI. Radiol Case Rep. 2025, 20(10), 5375–5381. [Google Scholar] [CrossRef]
- Li, M; Fu, W; Zhang, K; Hou, Z; Yu, Y; Ma, N. Risk factors and outcomes of patients with early spontaneous recanalization after acute middle cerebral artery occlusion. Quant Imaging Med Surg. 2025, 15(11), 10459–10469. [Google Scholar] [CrossRef]
- Gomis, M; Dávalos, A. Recanalization and Reperfusion Therapies of Acute Ischemic Stroke: What have We Learned, What are the Major Research Questions, and Where are We Headed? Front Neurol 2014, 5. [Google Scholar] [CrossRef]
- Li, G; Feng, X; Wang, C; et al. In-hospital clinical outcomes in diffusion weighted imaging-negative stroke treated with intravenous thrombolysis. BMC Neurol. 2022, 22, 349. [Google Scholar] [CrossRef]
- Uchino, K; Massaro, L; Hammer, MD. Transient Ischemic Attack after Tissue Plasminogen Activator: Aborted Stroke or Unnecessary Stroke Therapy? Cerebrovasc Dis 2009, 29(1), 57–61. [Google Scholar] [CrossRef]
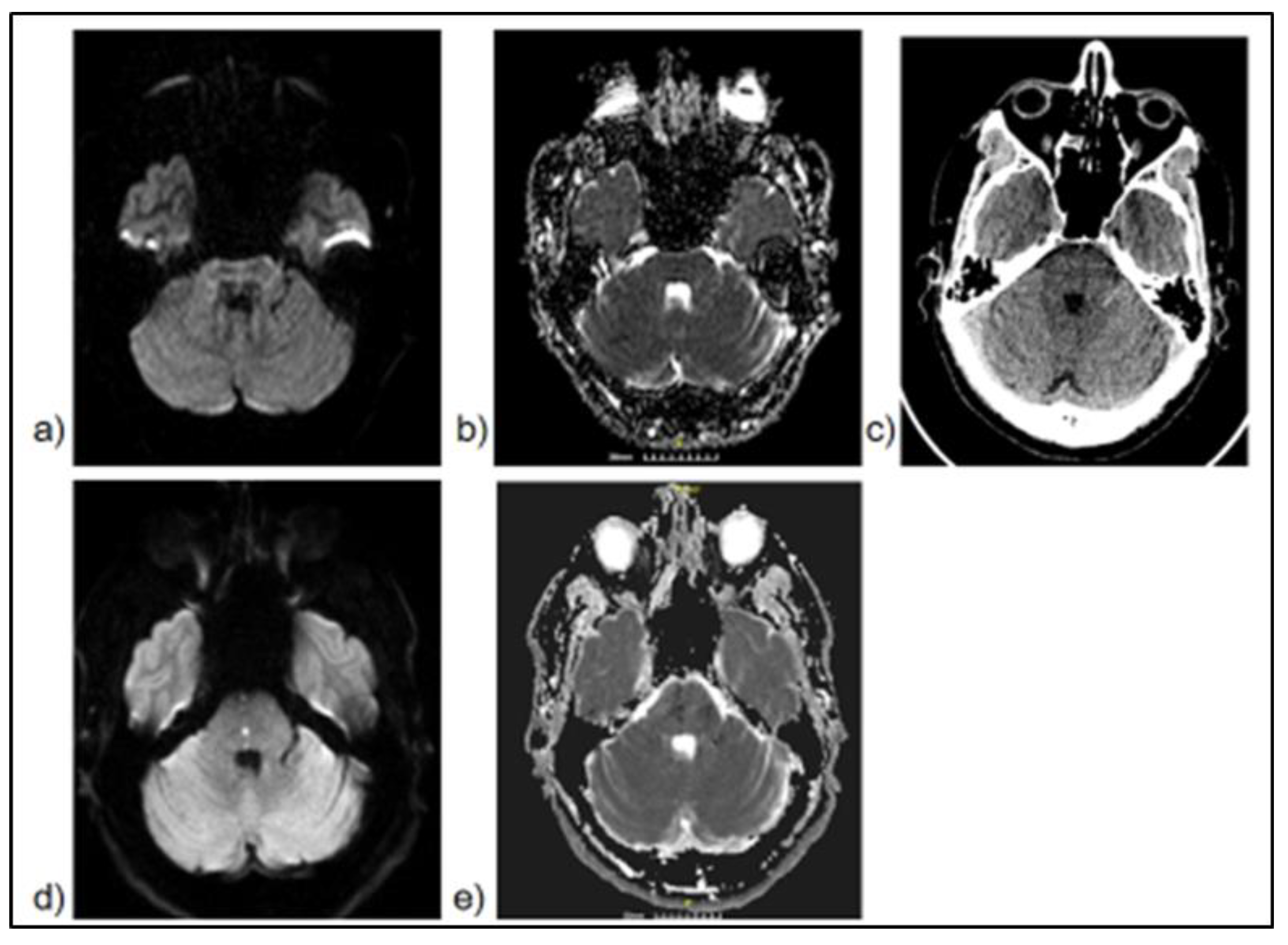
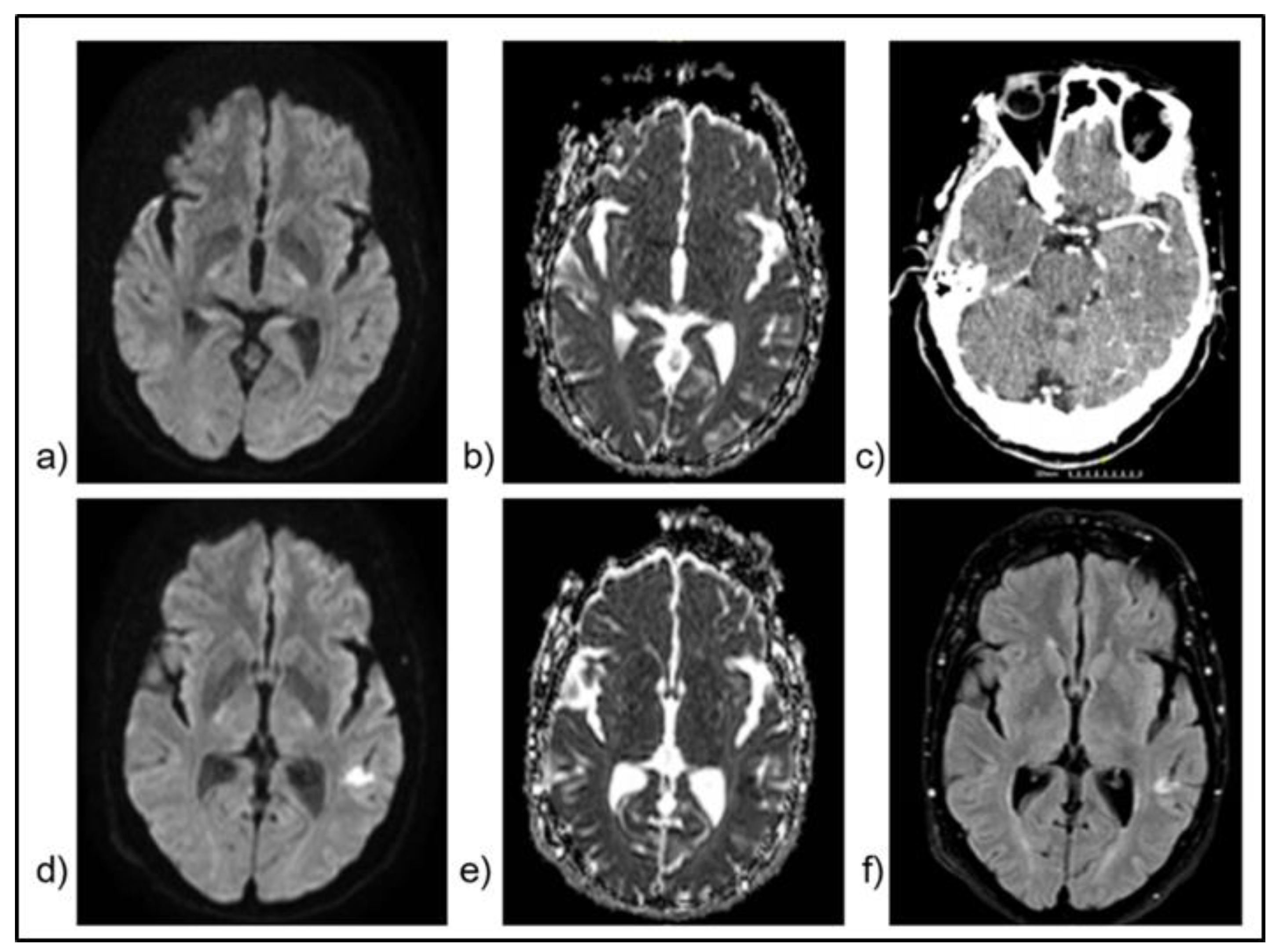
- Alhaj Omar, O; Yenigün, M; Alchayah, F; et al. Combining Coronal and Axial DWI for Accurate Diagnosis of Brainstem Ischemic Strokes: Volume-Based Correlation with Stroke Severity. Brain Sciences 2025, 15(8), 823. [Google Scholar] [CrossRef] [PubMed]
- Lozano, RJ; Shareef, F; Neupane, A; et al. Detectability of acute ischemic stroke with thin (3 mm) axial versus thin (3 mm) coronal diffusion-weighted imaging in patients presenting to the emergency department with acute dizziness. Emerg Radiol. 2025, 32(2), 225–231. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Jilwan, M; Wintermark, M. Automated Brain Perfusion Imaging in Acute Ischemic Stroke: Interpretation Pearls and Pitfalls. Stroke 2021, 52(11), 3728–3738. [Google Scholar] [CrossRef]
- ElBeheiry, AA; Hanora, MA; Youssef, AF; Al Neikedy, AAM; Elhabashy, A; Khater, HM. Role of arterial spin labeling magnetic resonance perfusion in acute ischemic stroke. Egyptian Journal of Radiology and Nuclear Medicine 2023, 54(1), 43. [Google Scholar] [CrossRef]
- Zimmermann, J; Reolon, B; Michels, L; et al. Intravoxel incoherent motion imaging in stroke infarct core and penumbra is related to long-term clinical outcome. Sci Rep. 2024, 14(1), 29631. [Google Scholar] [CrossRef]
- Alis, C; Ay, E; Genc, G; Bulut, S. Evaluating Deep Learning-Based Commercial Software for Detecting Ischemic Lesions on DWI in Stroke Patients. Diagnostics 2025, 15(18), 2357. [Google Scholar] [CrossRef]
- Namgung, E; Kim, YS; Lee, EJ; et al. Deep learning to identify stroke within 4.5 h using DWI and FLAIR in a prospective multicenter study. Sci Rep. 2025, 15(1), 26262. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, AH; Psychogios, K; Turc, G; et al. Off-Label Use of Tenecteplase for the Treatment of Acute Ischemic Stroke: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022, 5(3), e224506. [Google Scholar] [CrossRef]
- Miller, SE; Warach, SJ. Evolving Thrombolytics: from Alteplase to Tenecteplase. Neurotherapeutics 2023, 20(3), 664–678. [Google Scholar] [CrossRef] [PubMed]
- Pensato, U; Singh, N; Joundi, RA; et al. Left-Sided Neurological Symptoms and Negative Diffusion-Weighted MRI in Suspected Minor Stroke Patients. Canadian Journal of Neurological Sciences Published online. 2025, 1–6. [Google Scholar] [CrossRef]
- Lansberg, MG; O’Brien, MW; Norbash, AM; Moseley, ME; Morrell, M; Albers, GW. MRI abnormalities associated with partial status epilepticus. Neurology 1999, 52(5), 1021–1021. [Google Scholar] [CrossRef] [PubMed]
- Brunser, AM; Lavados, PM; Muñoz-Venturelli, P; et al. Clinical and Radiological Differences between Patients Diagnosed with Acute Ischemic Stroke and Chameleons at the Emergency Room: Insights from a Single-Center Observational Study. Cerebrovasc Dis. 2025, 54(3), 315–322. [Google Scholar] [CrossRef]
- Thomalla, G; Simonsen, CZ; Boutitie, F; et al. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. New England Journal of Medicine 2018, 379(7), 611–622. [Google Scholar] [CrossRef] [PubMed]
- Schellinger, PD; Bryan, RN; Caplan, LR; et al. Evidence-based guideline: The role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke. Neurology 2010, 75(2), 177–185. [Google Scholar] [CrossRef] [PubMed]

| Study | DWI-negative AIS prevalence | Description/Limitations |
| Pektezel et al., 2021 [37] |
6,6% 8101 cases, 535 DWI-negative |
Combination of a single center cohort of 1506 patients [DWI-negative n=20, (1,3%)] with cases from the literature: increased heterogeneity and potential underestimation. |
| Kim et al., 2021 [39] |
13,2% 5,271 cases, 694 DWI-negative |
Retrospective single-center study that included TIA patients: potential overestimation. |
| Wang, Yu et al., 2022 [40] |
7,7% 12,026 cases, 932 DWI-negative |
Prospective multicenter cohort study, included a relatively low number of non-minor (NIHSS > 5) AIS patients. |
| Zhu et al., 2023 [36] |
23,2% 151 cases, 35 DWI-negative |
Retrospective single-center study, smaller sample size compared to the other studies. DWI status was evaluated post-IVT. |
| Fotso et al., 2024 [42] |
3,9% 1210 cases, 47 DWI-negative |
Retrospective single-center study. Only patients with anterior circulation AIS were enrolled. |
| Li et al., 2025 [43] |
22,74% 277 cases, 63 DWI- negative |
Single-center study. Included only posterior circulation strokes. DWI status was evaluated post-IVT. Small sample size and missing clinical data in a small number of patients, variability in follow-up: may have introduced biases and the risk of stroke mimics inclusion. |
| Alkhiri et al., 2024 [8] |
16%, 11% after sensitivity analysis. 16.268 cases, 2603 DWI-negative |
Meta-analysis. Significant heterogeneity between studies, I2 = 91% estimated by the authors after sensitivity analysis. Temporal heterogeneity: the earliest study was conducted in 2000 and the most recent in 2023. Due to potential overrepresentation of minor stroke patients, sensitivity analysis was performed. |
|
DWI: Diffusion-weighted imaging, AIS: Acute ischemic stroke, TIA: Transient ischemic attack, NIHSS: National Institutes of Health Stroke Scale, IVT: Intravenous thrombolysis, I2 : I2 heterogeneity index | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





