1. Introduction
Levonorgestrel, a synthetic progestogen, is widely used in various birth control options as a natural progesterone hormone, including emergency oral contraceptive pills (EOCPs), combined oral contraceptives (COCs), injectables, silicone-based long-acting reversible contraceptive (LARC) implants, and intrauterine device systems (IUDs) [
1,
2,
3,
4]. Among these formulations, LNG-releasing contraceptive implants or implantable contraceptives consisting of small, non-biodegradable and flexible two rods resembling a matchstick are inserted subdermally into the upper arm that provide a steady release of low-dose progestin, an effective hormonal treatment used to control menstrual complaints and endometriosis [
5]. LNG, a biologically active levorotatory enantiomer of norgestrel, implants primarily work by stopping ovulation, the process of egg release from the ovaries, and by thickening cervical mucus, causing sperm blockage from reaching the eggs in the ovaries [
6]. These devices can be safely placed during the immediate postpartum period, ensuring reliable contraceptive protection. The benefits of these implants include long-term contraception with low doses of highly effective hormones [
7,
8], less than 1% failure rate [
9,
10,
11,
12,
13], less or no side effects with quick restoration of fertility after removal [
14], and being innocuous for breastfeeding women [
15].
Worldwide, various types of LNG-releasing LARC subdermal implants have become increasingly available for use, providing an effective safeguard against pregnancy for up to 3-5 years in low- and middle-income countries, including Bangladesh [
16,
17,
18,
19]. Precise quantification of circulating LNG concentration is very crucial for assessing the contraceptive effectiveness, metabolic performance, and safety of the implants, especially in various population groups [
20,
21,
22]. Several analytical methods like UV-Vis spectrophotometry, reverse phase high-performance liquid chromatography (RP-HPLC), mass spectrometry (MS) as well as liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS), have been established for quantification of LNG or a combination of LNG and ethinylestradiol in various dosage forms. Out of them, LC-MS/MS has been considered as a gold standard analytical technique due to high specificity, sensitivity, and reproducibility, which allows the accurate measurement of low-concentration analytes in complex biological matrices like human plasma or serum [
23,
24,
25,
26,
27,
28,
29].
In 2020, Femiplant, a locally manufactured contraceptive implant similar to the widely used two implants, namely Jadelle of Bayer Healthcare, Germany, for five years, and Levoplant/Sino-implant (II) produced by Shanghai Dahua Pharmaceuticals Company Limited, China, for three years, approved by the Directorate General of Drug Administration (DGDA), was introduced into the national family planning program under the Government of Bangladesh for three years of contraception [
30,
31].
Although women have been using Femiplant after its introduction, to date, no study on the safety, effectiveness, and acceptability of this hormonal implant among the users has been conducted. In this perspective, we had attempted to develop and validate a very reliable, sensitive, specific, as well as rapid LC-MS/MS method to quantify LNG released from contraceptive implants in blood serum among reproductive-aged women attending various family planning settings in Bangladesh.
2. Materials and Methods
2.1. Chemicals and Reagents
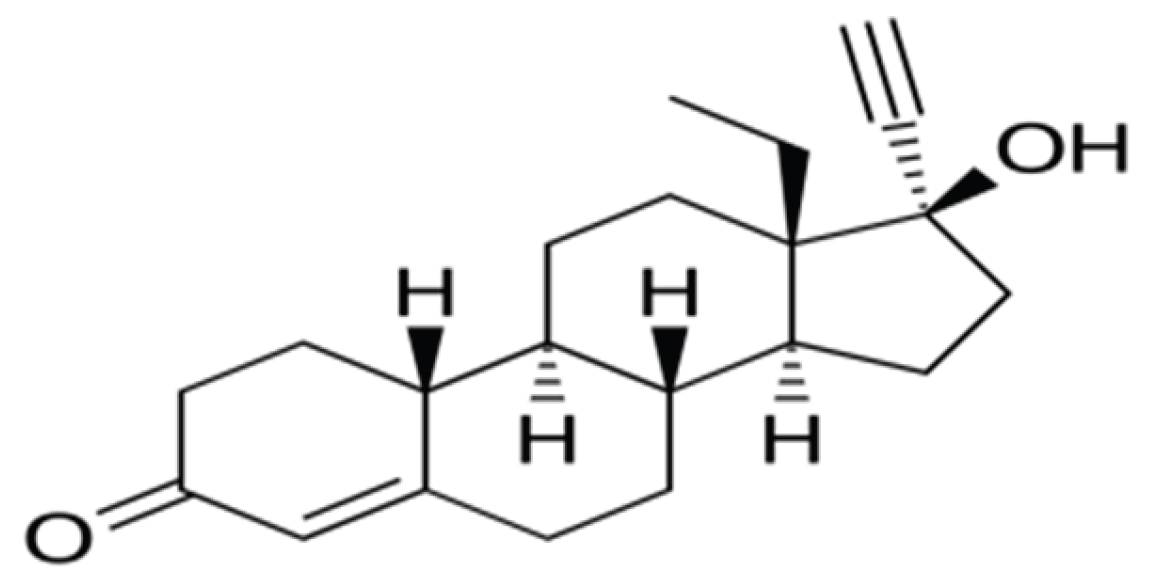
Reference standard LNG (MW: 312.45 and chemical structure shown in
Figure 1) and blank human serum were collected from Sigma-Aldrich, UK. Methanol, formic acid, acetonitrile, and water of LC-MS/MS grade had been procured from Merck, Germany. All other reagents used were of LC-MS/MS grade recommended for bioanalysis.
2.2. LC–MS/MS Instrumentation
The analysis has been performed utilizing a WatersTM XevoTM TQ Absolute triple quad mass spectrometry system operating in electrospray ionization with positive ion mode (ESI+) and multiple reaction monitoring (MRM) coupled with an Acquity H class plus UPLC. Chromatographic separation of LNG was accomplished with an Acquity UPLCTM BEH C18 reverse phase column of 2.1 × 100 mm, 1.7 μm, where the mobile phase consisting of acetonitrile: water with 0.1% formic acid (70:30, v/v) was delivered as a gradient at a 300 μL/min rate of flow with a column temperature of 40 °C and 10 °C (autosampler). Compound-dependent parameters, such as collision energy, cone voltage, MRM transitions, and instrumental parameters, like capillary voltage, source temperature, desolvation temperature, and gas flow etc., were optimized. MassLynx V4.2 was used as software for data handling of calibration and integration.
2.3. Calibration Standard and Quality Control Solution Preparation
Stock and working solutions for LNG were made in methanol at a concentration of 1 mg/mL. LNG calibration standard solutions and quality control solutions (QCs) were made through serial dilution of the stock solution with methanol to yield calibration standards ranging from 32.5-2000 pg/mL in blank human serum. QC solutions were prepared independently at three levels: LLOQ (lower limit of quantification), MQC (medium quality control), and HQC (high quality control), equivalent to concentrations of 32.5, 500, and 2000 pg/mL, respectively. Then, all stock solutions were warehoused at 4 °C. In addition, quality control and all other working calibration standard solutions in serum were preserved at -20 °C.
2.4. Sample Preparation
A widely used liquid-liquid extraction (LLE) approach was used for sample preparation in steroid hormone quantification. The gradual steps were followed for the preparation of the sample including, 500 μL of human serum placed in a glass tube of 10 mL and 2 mL of 70:30, v/v hexane–ethyl acetate added for extraction and allowed to vortex for 60 seconds for mixing, then centrifuged at 12,000 rpm for 5 minutes for separation, transferred 1 mL of the supernatant into a clean test tube of 10 mL for supernatant collection, vaporized under vacuum at 50 °C for drying, reconstituted the residue in 100 μL methanol-water (70:30, v/v) with 0.1% formic acid and vortexed for 60 seconds for further mixing, transferred the mixture to the autosampler vials, and finally 10 μL of supernatant injected into the system (LC-MS/MS) for analysis.
2.5. Chromatographic Conditions
Chromatographic separation: ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm), mobile phase: Acetonitrile: water with 0.1% formic acid (70:30, v/v), temperature: 40 °C (column) and 10 °C (autosampler), flow rate: 300 μL/minute, and run time: 3.0 minutes.
2.6. Mass Spectrometry Parameters
Mode: Positive ion mode (ESI+), multiple reaction monitoring (MRM), Gases: Nitrogen, Source temperature: 150 °C, Desolvation temperature: 500 °C, Desolvation gas flow: 800 L/hr, Capillary voltage: 3.4 kV, and Cone voltage: 72 V.
2.7. Collision-Induced Dissociation (CID)
Collision gas: Argon (cell pressure 1.2 mTorr), Collision energy: 40 V (for LNG), Transitions monitored: m/z 312.9 → 81.4, 90.9, 108.9 (LNG), and Scan time: 0.1 second per analyte.
2.8. Method Validation
As per the International Council for Harmonization (ICH) M10 guidelines on bioanalytical method, the assay method has been validated, and the validation parameters included selectivity, accuracy, precision, recovery, calibration curve range, and reproducibility [
31,
32].
2.8.1. Selectivity and Specificity
Six different blank human serum lots were analyzed to confirm whether the interfering peaks were present or not at the specific retention time for LNG. The peak area response of interference (%) remained acceptable if <20% of the average LLOQ (32.5 pg/mL) area response (n=6).
2.8.2. Calibration Curve and Linearity
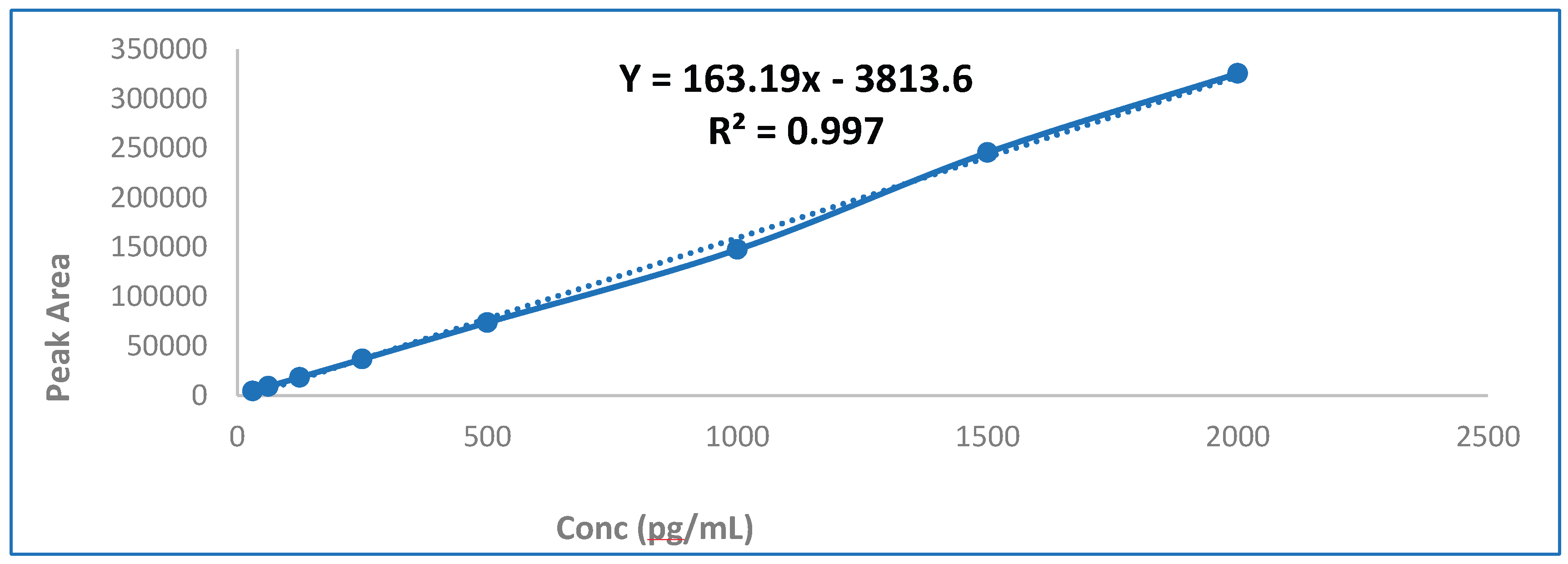
Calibration plots were constructed by using the peak area of LNG against various concentrations. A linear regression model with a weighting factor of 1/x² was utilized. The method proved linearity over the concentration ranges of 32.5–2000 pg/mL, with a correlation coefficient (R²) consistently ≥ 0.997, following FDA/EMA bioanalytical validation guidance.
2.8.3. Accuracy and Precision
Accuracy and precision were assessed at intra- and inter-day by analyzing quality control (QC) solutions at three levels of concentration, each performed in five replicates. Accuracy was expressed as relative error (RE%), while precision was stated as the coefficient of variation (%CV). The method met the acceptance criteria, with accuracy and precision values within ±15% for all QC levels and within ±20% at the lower limit of quantification (LLOQ), as recommended by FDA and EMA bioanalytical method validation guidelines.
2.9. Recovery and Matrix Effect
Recovery was evaluated by comparing the peak areas of extracted QCs to post-extracted spiked samples at equivalent concentrations. By comparing analyte responses in post-extracted spiked samples to those in neat solutions, matrix effect (ME) was assessed. Both recovery and ME were determined at low, medium, and high QCs in six different serum lots.
2.10. Stability Study
Stability of LNG in serum was evaluated under various conditions, including at room temperature for four hours, after freeze-thaw cycles, in extracted samples for twelve hours at room temperature, and during long-term storage (-0 °C for 79 days). Samples were considered stable if measured concentrations remained within ±15% of nominal values.
2.11. Clinical Application of the Validated Method
The described validated method by LC-MS/MS herein was used to calculate LNG concentrations in human blood (5 mL) collected in a clot activator tube from 8 AM to 1 PM. The serum was immediately separated from blood by centrifuging at 10,000 rpm for 10 minutes at 4 °C, which was then stowed at -20 °C for analysis, consistent with established bioanalytical stability protocols. The pharmacokinetic parameters (Cmax, Tmax, and AUC) were derived using non-compartmental analysis. Inter-individual variability and preliminary population-specific trends were assessed.
3. Results
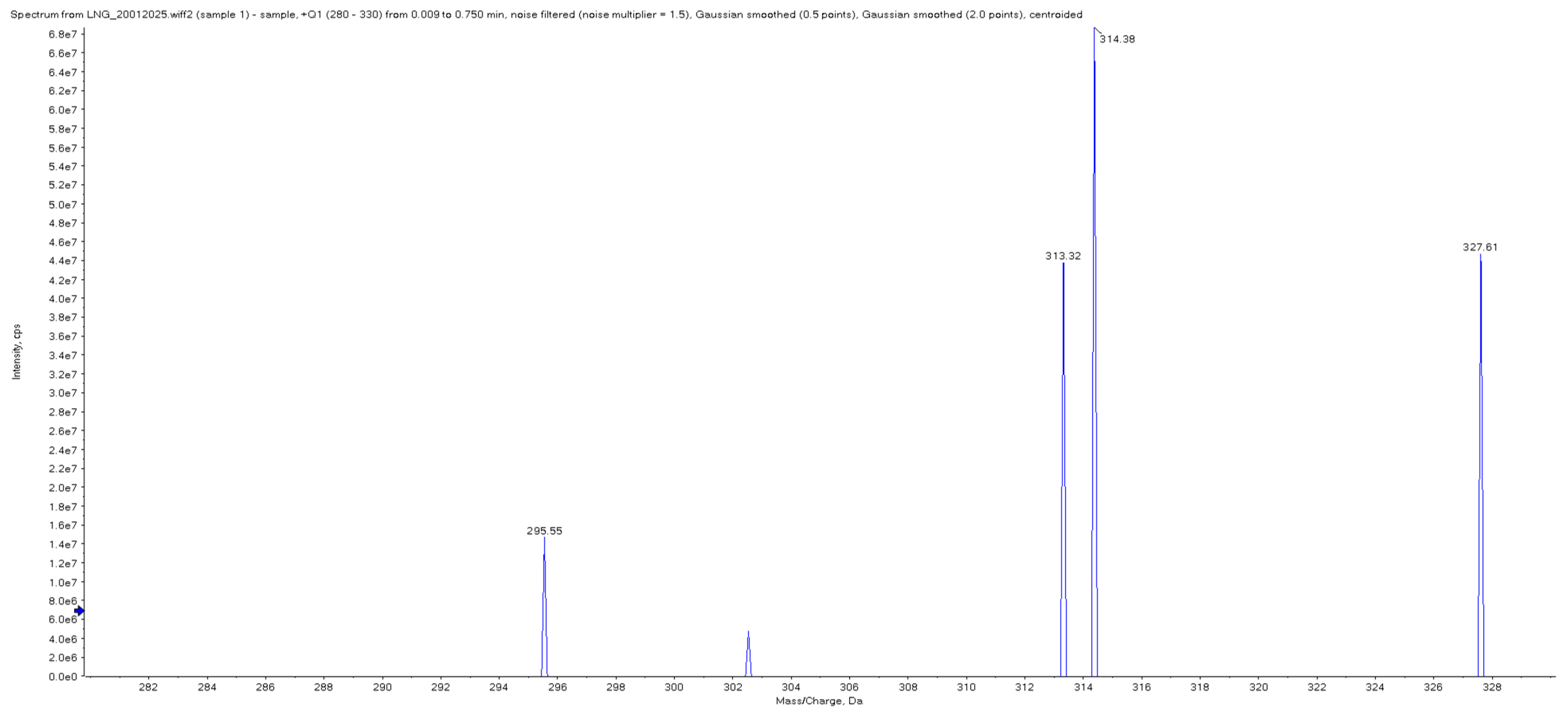
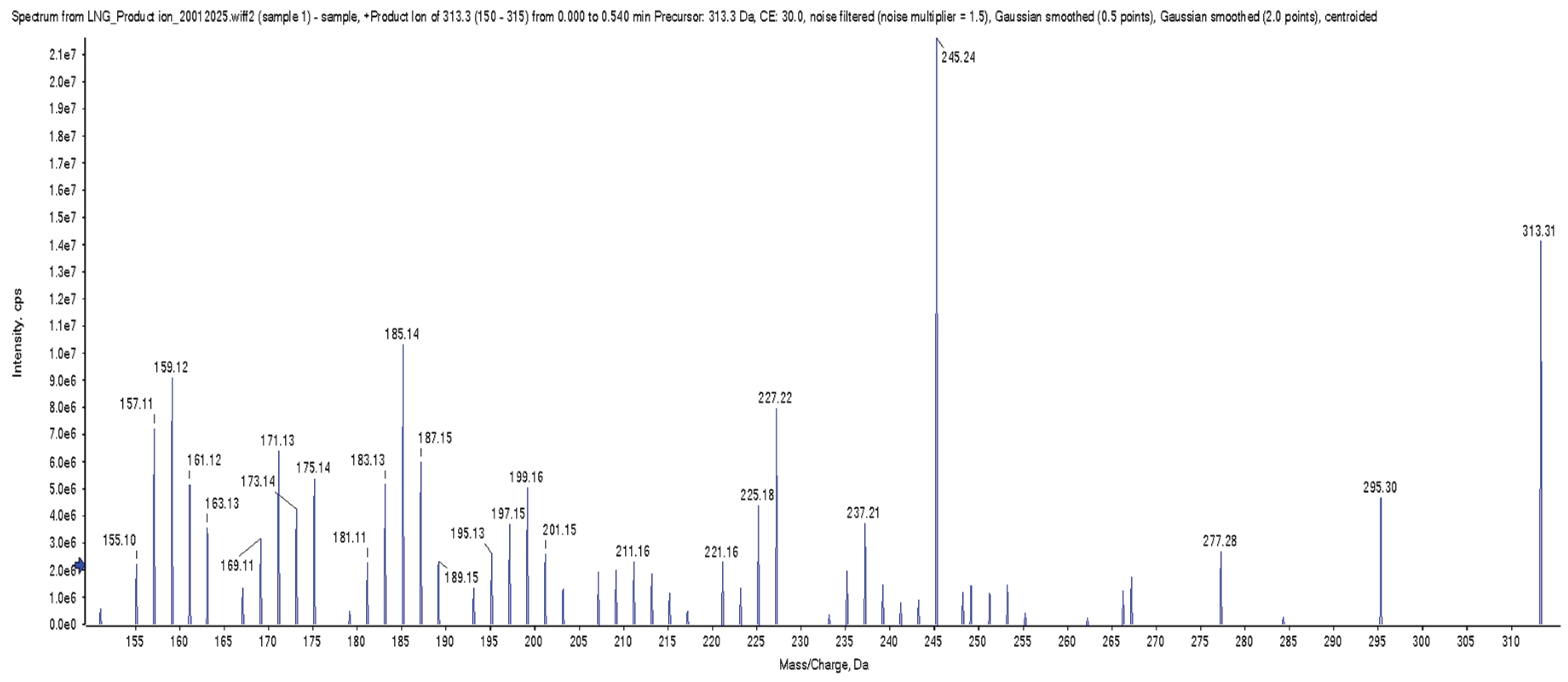
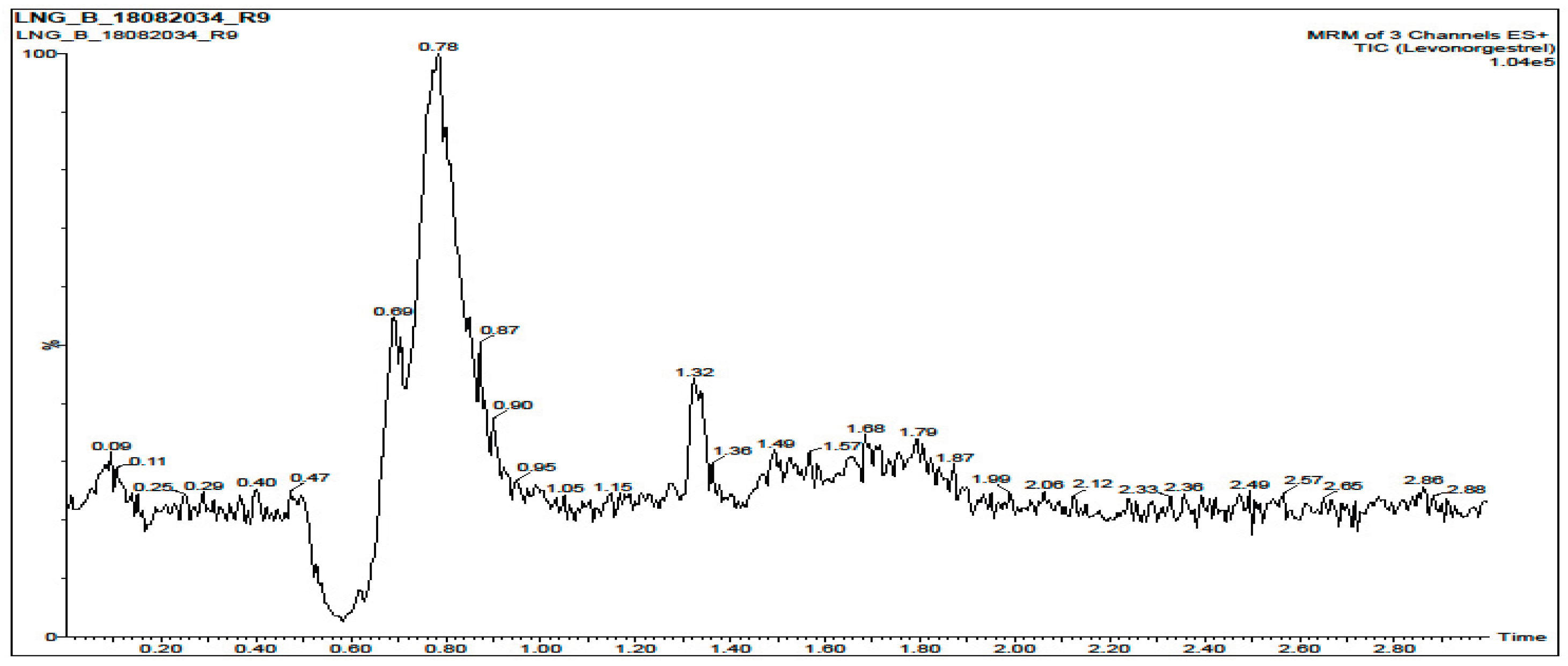
Chromatographic conditions have been optimized utilizing various mobile phase compositions. In addition, all the conditions were confirmed during the early stage, and the positive mode of ionization presented a higher degree of sensitivity. During development, an atmospheric pressure chemical ionization source vs ESI was evaluated, and ESI provided the greater signal-to-noise ratio. The monitored m/z values for parent and product ions are shown in
Table 1 and
Figure 2 and
Figure 3.
3.1. Linearity and Calibration Curve
Calibration standards were analyzed by back calculating the LNG concentration for a linear regression model with a weighting factor of 1/x², which was then compared to the value of each calibration standard, and % CV and % of deviation from the concentration values quantified for evaluating precision and accuracy, respectively. The linearity of the method was confirmed over the concentration range of 32.5 to 2000 pg/mL, with a correlation coefficient (R²) of all calibration standard curves getting R² ≥ 0.997 (
Figure 4).
3.2. Selectivity and Specificity
The LC-MS/MS method demonstrated excellent selectivity, with no significant endogenous interference observed at the minimum LNG retention time after screening six different blank serum lots, with less than 20% of the signal response at the LLOQ for LNG for five of the six samples. A blank sample chromatogram and a typical chromatogram obtained during the analysis of the LLOQ calibrator (32.5 pg/mL) are shown in
Figure 5 and
Figure 6.
3.3. Accuracy and Precision
Precision and accuracy on intra- and inter-day analysis were done by analyzing QC samples at three concentrations, and five replicates each were run on three different validation days for all QC solutions. Accuracy was expressed as relative error (RE%), and precision as coefficient of variation (%CV), which were within acceptance limits ±15% for QC samples and ±20% at the LLOQs, respectively.
3.4. Recovery and Matrix Effect
By comparing spiked serum samples, the recovery was determined at equivalent concentrations before and after liquid extraction, representing full LNG recovery. Absolute and relative matrix effect (ME) was compared and assessed the final effect for LNG spectral response, and found no significant variation in LNG peak.
3.5. Stability Study
Stability of LNG in serum samples was measured and obtained the results under various conditions, including, short-term (room temperature, 4 hours): recovery within 95–105%, long-term (–80 °C for 60 days): no significant degradation and three freeze-thaw cycles: no impact on accuracy or precision and post-preparative (autosampler stability, 24 hours at 4 °C): stable with minimal variance.
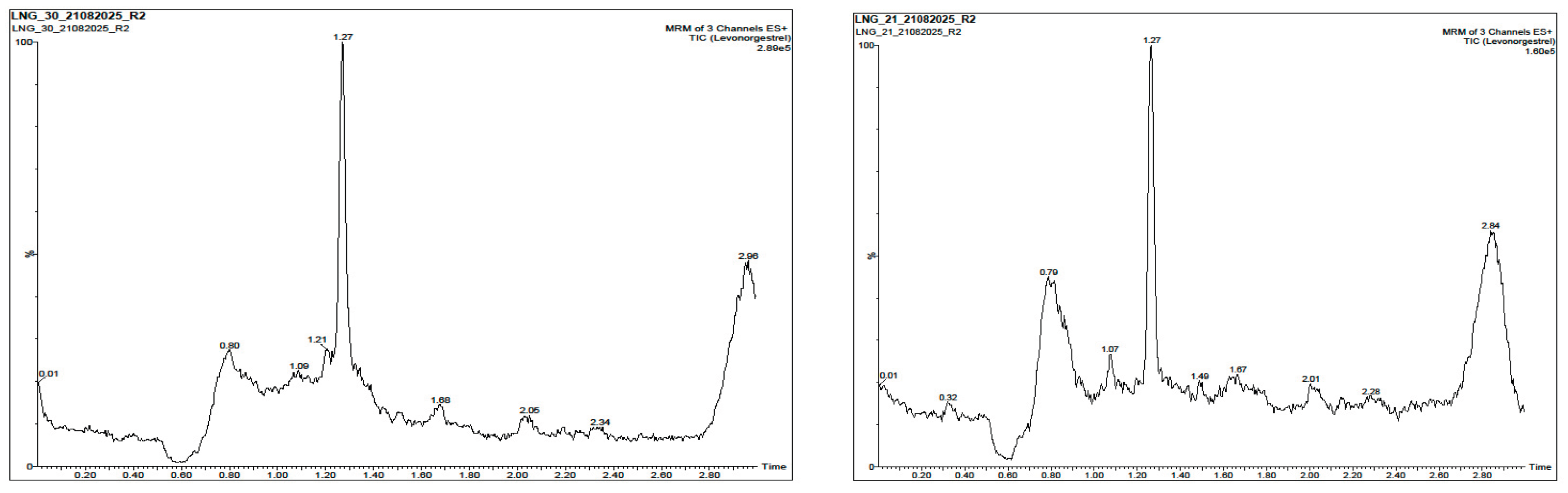
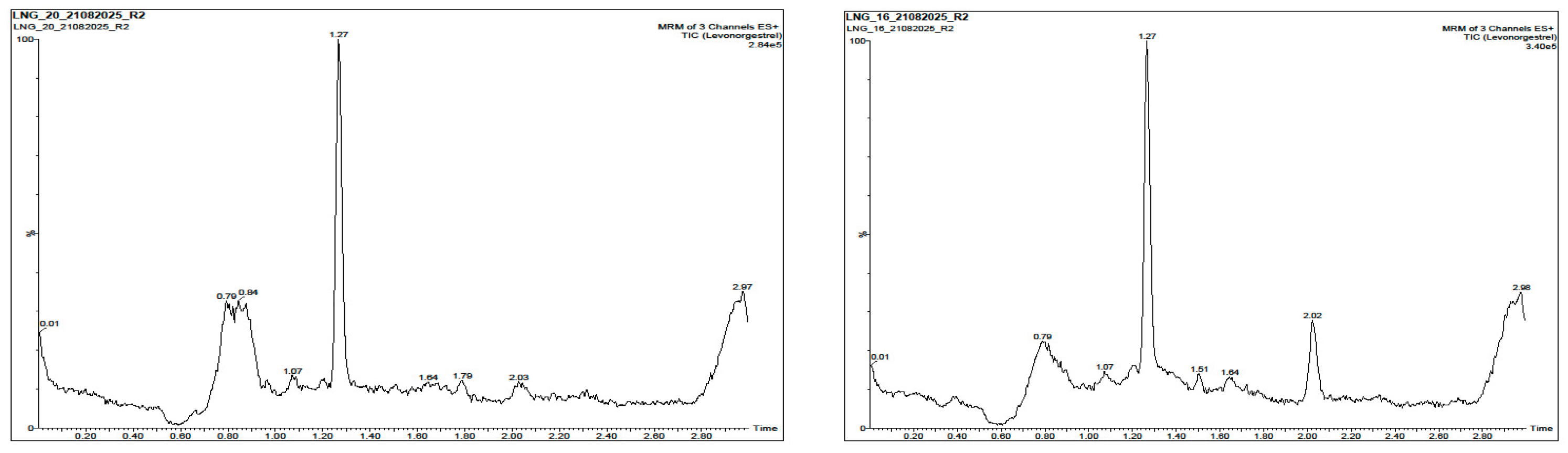
3.6. Clinical Application of the Validated Method
The validated LC-MS/MS method was utilized to calculate LNG concentrations in serum samples collected at various time intervals of 1-, 6-, 12-, 24-, and 36-month post-insertion from the women who had already been implanted with a median implant duration of 24 months. No significant adverse effects or hepatic/renal abnormalities were reported. The mean women’s age was 28.33 ± 4.5 years (95% CI: 27.50-29.16). Pharmacokinetic parameters such as C
max, T
max, and AUC have been derived using non-compartmental analysis and described in another report. Inter-individual variability and preliminary population-specific trends were assessed. The serum LNG concentrations in the study participants ranged from 457.39 to 564.63, with a mean concentration of 513.30±32.55 pg/mL after 6 months, 289.55 to 397.07, with a mean concentration of 341.88±31.53 pg/mL after 12 months, 225.91 to 274.91, with a mean concentration of 243.02±15.61 pg/mL after 24 months and 146.48 to 262.43, with a mean concentration of 205.34±24.03 pg/mL after 36 months of implant insertion shown in
Figure 7.
4. Discussion
The current study report describes the successful development and validation of a robust and sensitive LC-MS/MS method for the quantification of LNG in human blood serum. Method optimization included systematic evaluation of chromatographic conditions, mobile phase compositions, and ionization sources. Among ionization techniques, electrospray ionization (ESI) demonstrated a superior signal-to-noise ratio compared to atmospheric pressure chemical ionization (APCI), which is consistent with prior reports highlighting ESI as the preferred ionization mode for steroidal hormones and low-molecular-weight compounds due to its higher ionization efficiency and improved sensitivity [
33,
34]. The positive ionization mode enabled the detection of characteristic LNG transitions, with well-defined parent and product ions (m/z 312.9 → 81.4, 90.9, 108.9), facilitating precise and reliable quantification.
The method confirmed outstanding linearity where calibration curves showed excellent linearity across a broad dynamic concentration range (32.5–2000) pg/mL, with R² ≥ 0.99. This vibrant range is predominantly appropriate for pharmacokinetic studies of subdermal implants. Hence, circulating LNG levels persist within the low picogram to nanogram level per milliliter range. In addition, the use of a 1/x² weighting factor enhanced accuracy at lower levels of concentration, reducing bias at the LLOQ, a collective challenge in bioanalytical analysis for steroidal contraceptives [
35,
36]. At the retention time, there was no substantial endogenous interference, which confirms good selectivity and specificity, emphasizing the robustness of the method when used in diverse biological matrices. Interference levels of less than 20% of the LLOQ in blank serum lots met the international bioanalytical method validation guidelines, supporting assurance in the reliability of the assay [
37,
38]. The characteristic chromatograms for blank and LLOQ calibrator samples emphasized the capability of the assay to differentiate LNG from complex matrices, confirming precise detection of LNG at trace levels.
The accuracy and precision were within the acceptance criteria of ±15% for all QC samples and ±20% at the LLOQ for the method. Both intra- and inter-day reproducibility established the assay’s consistency, which is very critical for longitudinal pharmacokinetic assessments where frequent dimensions are needed over prolonged timeframes. Recovery rates similar to almost 100% and insignificant matrix effects show that the liquid–liquid extraction technique used was both effective and selective, which diminishes apprehensions about ion suppression or increment that can reconcile measurable presentation in LC-MS/MS analysis [
39].
Stability studies ensured the stability of LNG through a diversity of clinically related circumstances such as short-term handling, long-term frozen storage, freeze–thaw cycles, and autosampler storage. The values of 95-105% for the recovery under these situations align with well-known standards, approving the suitability of the technique for routine analysis in clinical research where the collection, storage, and analysis of the sample cannot always be instant [
40].
The validated assay has been successfully utilized in a clinical application or setting where it was used to quantify LNG concentrations in human serum obtained at 1, 6, 12, 24, and 36 months post-implant insertion. The pharmacokinetic parameters (Cmax, Tmax, and AUC) were determined using the measured concentrations, which are important for illustrating drug release and systemic exposure in Bangladeshi women already using contraceptive implants. Significantly, the method confirmed the sensitivity necessary to capture inter-personal inconsistency and primary population-specific pharmacokinetic inclinations. Such types of findings are important for understanding both the efficacy and variability in LNG release profiles, particularly in populations where demographic, reproductive, and metabolic factors may differ from the cohorts in which these implants were originally developed and tested [
41,
42].
Overall, the LC-MS/MS method validated herein meets the international regulatory standards for bioanalytical techniques and delivers a dependable stage for LNG pharmacokinetics and experimental investigations. The precision, accuracy, and robustness of the method make it an important tool for further study evaluating the performance of subdermal implants, therapeutic monitoring, and potential inter-subject variability in PK studies. Additionally, this method contributes to the wider arena of reproductive health studies by allowing exact monitoring of the concentration of serum LNG levels, thereby assisting evidence-based assessments of contraceptive options in various clinical settings.
5. Conclusions
The above-discussed LC–MS/MS validated method proved highly reliable, sensitive, and specific for quantifying LNG in human serum, supporting its use in both clinical monitoring and research applications. LNG serum concentrations herein demonstrated the expected decline with implant duration but remained within an effective contraceptive range throughout the intended duration of use. Together, these findings reinforce the efficacy, safety, and analytical reliability of LNG contraceptive implants as a cornerstone of long-acting contraception, particularly within the national family planning program in Bangladesh.
Author Contributions
Conceptualization: AKLK and EH, methodology: AKLK, EH, SCB; FAD, and SR; formal analysis, AKLK, FAD, and MNU; writing: original draft preparation, AKLK and MNU; writing review and editing, AKLK, EH, and SN. All authors have read and agreed to the published version of the manuscript.
Funding
The study was self-funded by the authors.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Faculty of Pharmacy, Dhaka University (Ref. No. Fa.Ph.E/047/2025) on May 13, 2025. Informed consent for participation was obtained from all subjects involved in the study. Verbal informed consent was obtained from the participants. Informed consent for publication was obtained from all identifiable human participants.
Acknowledgments
The authors are grateful to the study participants and the authority of the family planning centers under DGFP, GOB, for their kind and generous support in carrying out the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stefanović, O. D., 2017, 'Synergistic Activity of Antibiotics and Bioactive Plant Extracts: A Study Against Gram-Positive and Gram-Negative Bacteria', in S. Sahra (ed.), Bacterial Pathogenesis and Antibacterial Control, Intech Open, London. 10.5772/intechopen. 72026.
- Al Dalaty, A.; Gualeni, B.; Coulman, S.A.; Birchall, J.C. Models and methods to characterise levonorgestrel release from intradermally administered contraceptives. Drug Deliv. Transl. Res. 2021, 12, 335–349. [CrossRef]
- Sivin, I.; Nash, H.; Waldman, S. Jadelle® levonorgestrel rod implants: A summary of scientific data and lessons learned from programmatic experience; Population Council: New York, NY, United States, 2002; ISBN: .
- Rocca, M.L.; Palumbo, A.R.; Visconti, F.; Di Carlo, C. Safety and Benefits of Contraceptives Implants: A Systematic Review. Pharmaceuticals 2021, 14, 548. [CrossRef]
- Manoukian, O.S.; Arul, M.R.; Sardashti, N.; Stedman, T.; James, R.; Rudraiah, S.; Kumbar, S.G. Biodegradable polymeric injectable implants for long-term delivery of contraceptive drugs. J. Appl. Polym. Sci. 2017, 135. [CrossRef]
- Scarsi, K.K.; Darin, K.M.; Chappell, C.A.; Nitz, S.M.; Lamorde, M. Drug–Drug Interactions, Effectiveness, and Safety of Hormonal Contraceptives in Women Living with HIV. Drug Saf. 2016, 39, 1053–1072. [CrossRef]
- Steinauer, J.E.; Upadhyay, U.D.; Sokoloff, A.; Harper, C.C.; Diedrich, J.T.; Drey, E.A. Choice of the levonorgestrel intrauterine device, etonogestrel implant or depot medroxyprogesterone acetate for contraception after aspiration abortion. Contraception 2015, 92, 553–559. [CrossRef]
- Groskaufmanis, L.; Masho, S.W. Source of care and variation in long-acting reversible contraception use. Fertil. Steril. 2016, 105, 401–409. [CrossRef]
- Conde-Agudelo, A.; Belizán, J.M.; Lammers, C. Maternal-perinatal morbidity and mortality associated with adolescent pregnancy in Latin America: Cross-sectional study. Am. J. Obstet. Gynecol. 2005, 192, 342–349. [CrossRef]
- Gunardi. Monoplant the Indonesian implant: the overview of implant and its development. Indones J Obstet Gynecol. 2011; 35:35–45.
- Patton, G.C.; Coffey, C.; Sawyer, S.M.; Viner, R.M.; Haller, D.; Bose, K.; Vos, T.; Ferguson, J.; Mathers, C.D. Global patterns of mortality in young people: a systematic analysis of population health data. Lancet 2009, 374, 881–892. [CrossRef]
- Stover, J.; Ross, J. How Increased Contraceptive Use has Reduced Maternal Mortality. Matern. Child Heal. J. 2009, 14, 687–695. [CrossRef]
- Weisberg, E.; Bateson, D.; McGeechan, K.; Mohapatra, L. A three-year comparative study of continuation rates, bleeding patterns and satisfaction in Australian women using a subdermal contraceptive implant or progestogen releasing-intrauterine system. Eur. J. Contracept. Reprod. Heal. Care 2013, 19, 5–14. [CrossRef]
- Modesto, W.; Bahamondes, M.V.; Bahamondes, L. A randomized clinical trial of the effect of intensive versus non-intensive counselling on discontinuation rates due to bleeding disturbances of three long-acting reversible contraceptives. Hum. Reprod. 2014, 29, 1393–1399. [CrossRef]
- Phillips, S.J.; Tepper, N.K.; Kapp, N.; Nanda, K.; Temmerman, M.; Curtis, K.M. Progestogen-only contraceptive use among breastfeeding women: a systematic review. Contraception 2016, 94, 226–252. [CrossRef]
- Callahan, R.L.; Taylor, D.; Jenkins, D.W.; Owen, D.H.; Cheng, L.; Cancel, A.M.; Dorflinger, L.J.; Steiner, M.J. In vivo release of levonorgestrel from Sino-implant (II) — an innovative comparison of explant data. Contraception 2015, 92, 350–355. [CrossRef]
- Wathoni, N.; Alfauziah, T.Q.; Rantika, N. EVOLUTION OF CONTRACEPTIVE IMPLANTS: A REVIEW. Int. J. Appl. Pharm. 2018, 10, 16–22. [CrossRef]
- Fuchs, R.; Taylor, D.; Jenkins, D.W.; Brache, V.; Luo, D.; Dorflinger, L.J.; Steiner, M.J. Levonorgestrel release rates measured through analysis of two-rod contraceptive explants. Contraception: X 2020, 2. [CrossRef]
- Berzas, J.J.; Rodríguez, J.; Castañeda, G. Simultaneous Determination of Ethinylestradiol and Levonorgestrel in Oral Contraceptives by Derivative Spectrophotometry. Anal. 1997, 122, 41–44. [CrossRef]
- Theron, H.; Coetzee, C.; Sutherland, F.; Wiesner, J.; Swart, K. Selective and sensitive liquid chromatography–tandem mass spectrometry method for the determination of levonorgestrel in human plasma. J. Chromatogr. B 2004, 813, 331–336. [CrossRef]
- Wang, R.; Tian, Y.; Zhang, L.; Zhang, Z. Simultaneous Determination of Levonorgestrel and Two Endogenous Sex Hormones in Human Plasma Based on LC–MS/MS. Bioanalysis 2016, 8, 1133–1144. [CrossRef]
- Zhong, G.; Bi, H.; Zhou, S.; Chen, X.; Huang, M. Simultaneous determination of metformin and gliclazide in human plasma by liquid chromatography–tandem mass spectrometry: application to a bioequivalence study of two formulations in healthy volunteers. J. Mass Spectrom. 2005, 40, 1462–1471. [CrossRef]
- Cirrincione, L.R.; Penchala, S.D.; Scarsi, K.K.; Podany, A.T.; Winchester, L.C.; Back, D.J.; Khoo, S.H.; Fletcher, C.V.; Siccardi, M.; Else, L.J. Development, validation and utilization of a highly sensitive LC-MS/MS method for quantification of levonorgestrel released from a subdermal implant in human plasma. J. Chromatogr. B 2018, 1084, 106–112. [CrossRef]
- Veeran, M.G.; C., K.; B., B.; Painuly, D.; Aprem, A.S. RP-HPLC method validation for fast extraction and quantification of Levonorgestrel drug from silicone based intrauterine device intended for in-process and finished formulation. DARU J. Pharm. Sci. 2021, 29, 185–193. [CrossRef]
- Midhu GV, Karthikeyan C, Bakkiyaraja B, Painuly D, Abi SA. RP-HPLC method validation for fast extraction and quantification of Levonorgestrel drug from silicone based intrauterine device intended for in-process and finished formulation. DARU Journal of Pharmaceutical Sciences. 2021.185-193.
- Pal, V.K.; Pal, Y. ANALYTICAL METHOD DEVELOPMENT AND METHOD VALIDATION FOR DETERMINATION ASSAY AND CONTENT UNIFORMITY OF LEVONORGESTREL BY REVERSED-PHASE HIGHPERFORMANCE LIQUID CHROMATOGRAPHY. Asian J. Pharm. Clin. Res. 2020, 101–107. [CrossRef]
- Patel PU, Patal BM. Development and Validation of Stability Indicating Reverse Phase High Performance Liquid Chromatographic Method for Estimation of Levonorgestrel in Bulk Dosage Form. International Journal of Pharmaceutics and Drug Analysis. 2014; 2(4): 311-318.
- Chang, H.-F.; Wang, J.-Q.; Wang, B.; Deng, A.-P. An immunochromatographic assay for rapid and simultaneous detection of levonorgestrel and methylprednisolone in water samples. Chin. Chem. Lett. 2013, 24, 937–940. [CrossRef]
- Lee, J.H.; Na Park, H.; Kim, N.S.; Park, H.-J.; Park, S.; Shin, D.; Kang, H. Detection of Illegal Abortion-Induced Drugs Using Rapid and Simultaneous Method for the Determination of Abortion-Induced Compounds by LC–MS/MS. Chromatographia 2019, 82, 1365–1371. [CrossRef]
- Ezebialu, I.; Okafo, O.; Oringanje, C.; Ogbonna, U.; Udoh, E.; Odey, F.; Meremikwu, M.M. Surgical and nonsurgical interventions for vulvar and clitoral pain in girls and women living with female genital mutilation: A systematic review. Int. J. Gynecol. Obstet. 2017, 136, 34–37. [CrossRef]
- Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/bioanalytical-method-validation#current-effective-version-section (accessed on 24 February 2021).
- US Food and Drug Administration. Bioanalytical Method Validation, Guidance for Industry. US Department of Health and Human Services, Center for Drug Evaluation and Research. 2018. Available online: https://www.fda.gov/media/70858/download (accessed on).
- Smith R, Taylor PJ. Electrospray ionization in bioanalytical mass spectrometry: advances and applications. J Chromatogr B. 2014;960:1–15.
- Xu Y, Wu W, Williams TD. Atmospheric pressure ionization for steroid analysis: comparison of APCI and ESI. Rapid Commun Mass Spectrom. 2017;31(10):843–852.
- Shah, V.P.; Midha, K.K.; Findlay, J.W.A.; Hill, H.M.; Hulse, J.D.; McGilveray, I.J.; McKay, G.; Miller, K.J.; Patnaik, R.N.; Powell, M.L.; et al. Bioanalytical Method Validation—A Revisit with a Decade of Progress. Pharm. Res. 2000, 17, 1551–1557. [CrossRef]
- US FDA. Bioanalytical Method Validation Guidance for Industry. Food and Drug Administration, 2018.
- European Medicines Agency (EMA). Guideline on Bioanalytical Method Validation. EMA, 2011.
- Peters FT, Drummer OH, Musshoff F. Validation of new methods. Forensic Sci Int. 2007;165(2–3):216–224.
- Taylor, P.J. Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography–electrospray–tandem mass spectrometry. Clin. Biochem. 2005, 38, 328–334. [CrossRef]
- Viswanathan CT, Bansal S, Booth B, et al. Workshop/conference report: quantitative bioanalytical methods validation and implementation. AAPS J. 2007;9(1):E30–E42.
- Diaz S, Pavez M, Moo-Young AJ, et al. Clinical performance of levonorgestrel-releasing contraceptive implants: a pharmacokinetic and pharmacodynamic perspective. Contraception. 2006;74(1):59–67.
- Fraser IS, Weisberg E. A comprehensive review of levonorgestrel contraceptive implants. Contraception. 2011;84(5):402–417.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |