1. Introduction
Over the past few decades, dietary supplements have become increasingly integrated into personal health routines around the world. These products, which include a wide range of substances such as vitamins, minerals, amino acids, and botanical extracts, are commonly consumed to complement dietary intake and support various aspects of health. The industry surrounding these supplements has experienced rapid growth, driven by both consumer interest in preventative health measures and the marketing efforts of manufacturers.
Despite their popularity, the scientific consensus remains divided—especially when these supplements are used without medical guidance. In certain demographics, such as older adults, athletes, or individuals with dietary restrictions, supplements may serve as valuable tools for maintaining nutritional adequacy and physiological resilience. Similarly, within military populations, they are often used to sustain energy levels, concentration, and overall performance under demanding conditions [
1,
4]
Nevertheless, concerns persist regarding improper use, excessive intake, inconsistencies in labeling, and interactions with pharmaceuticals [
3,
5].
Adding to the complexity, the level of regulatory oversight differs widely between countries, leading to variable product quality and safety standards. This review seeks to critically examine the current state of knowledge surrounding dietary supplements, with a focus on both their therapeutic potential and their limitations. Special attention is given to clinical relevance, occupational health considerations, and the importance of responsible consumption practices, especially in environments with heightened physical and psychological stress.
Addressing micronutrient deficiencies
Dietary supplements are often essential in preventing or correcting micronutrient imbalances, especially among individuals with increased nutritional needs or limited dietary variety. Deficiencies in elements such as vitamin D, iron, and vitamin B12 remain prevalent in various populations, particularly among the elderly, vegans, or those with chronic illnesses[
1,
10].
Improving physical and mental performance
A variety of supplements have been investigated for their potential to enhance performance in both physical and cognitive domains. Substances like creatine, caffeine, and branched-chain amino acids (BCAAs) are widely used by athletes and active individuals to support muscle recovery, strength gains, and exercise endurance (Kreider et al., 2010). On the cognitive side, omega-3 fatty acids, particularly EPA and DHA, have been associated with improved memory, concentration, and overall brain health [
9].
Adjunctive role in clinical care
Beyond general wellness, certain supplements have demonstrated promise in clinical contexts. For example, probiotics have been studied for their beneficial effects on gut microbiota and gastrointestinal disorders. Similarly, antioxidants such as coenzyme Q10 and vitamins C and E have shown potential in reducing oxidative damage in patients with cardiovascular conditions or metabolic disorders [
13].
Risks and limitations
Taking high doses of certain vitamins or minerals—especially fat-soluble ones like vitamins A, D, E, and K—can lead to toxic effects. Symptoms range from mild digestive discomfort to serious complications involving liver or kidney damage. Over-the-counter availability of supplements often leads to unsupervised consumption, increasing the likelihood of overdose or misuse.
In contrast to pharmaceuticals, dietary supplements are often subject to less stringent oversight. Research has exposed inconsistencies between label claims and the actual contents of products, as well as the presence of harmful or unlisted ingredients[
3].
Although some supplements are supported by scientific research, many others are promoted without strong clinical evidence [
2]. Products marketed with vague promises or exaggerated claims frequently underdeliver in real-world use.
Dietary supplements can alter the way drugs are absorbed or metabolized in the body. For instance, vitamin K can reduce the efficacy of anticoagulants, while magnesium might interfere with certain antibiotics [
5].
Perspective and occupational health
Members of the armed forces often face intense physical exertion, sleep deprivation, and psychological stress. In such conditions, dietary supplements may offer perceived or real benefits in maintaining energy, focus, and endurance. Nonetheless, the unregulated nature of some supplements and their potential for containing banned substances make them a liability. Military health systems are increasingly encouraging evidence-based use and close supervision of supplement intake to mitigate health risks and preserve operational readiness [
4].
2. Results and Discussion
Regulatory approaches to dietary supplements differ widely across countries. In the U.S., the Food and Drug Administration treats supplements more like food than medicine, limiting pre-market evaluation. Meanwhile, the European Food Safety Authority provides structured but inconsistently enforced guidelines. Harmonizing global standards and introducing mandatory quality controls could improve product consistency and safety[
11].
While supplements can play a beneficial role in personal and public health, misuse and poor oversight can result in significant harm. Aggressive marketing and easy access contribute to their widespread, and sometimes inappropriate, consumption. More robust, long-term studies are necessary to better understand their effects, especially in high-risk or performance-demanding populations.
This paper explores the current legal and regulatory framework surrounding traditional plant-based food supplements and herbal medicinal products within the European Union (EU) and USA. The sale of botanical ingredients in foods and dietary supplements is governed by various EU food laws, which address issues such as safety, manufacturing standards, labeling, and product formulation—including rules on additives and limits for contaminants and residues. However, because EU-wide harmonization in this area remains incomplete, individual Member States have also implemented their own national rules. As a result, products can be marketed across different EU countries through the principle of mutual recognition, even if regulations differ [
12,
14,
15,
16].
In contrast, traditional herbal medicinal products are subject to a specific directive—Directive 2004/24/EC—which thoroughly outlines the regulatory requirements, including a simplified national registration process. By drawing a line between traditional herbal medicines and plant-based food supplements and introducing separate pathways for their authorization, the EU has encountered both challenges in enforcement and a lack of consistency in regulatory practices among Member States.
Currently, the classification and availability of botanical products—whether marketed as traditional medicines or as supplements—depend heavily on the specific interpretations and regulatory decisions of national authorities and producers in each country. This leads to considerable variation across the EU, not necessarily based on the properties of the botanical ingredients themselves, but on how rules are applied. When compared to regulatory models in other parts of the world, significant differences become apparent in how these types of products are handled.
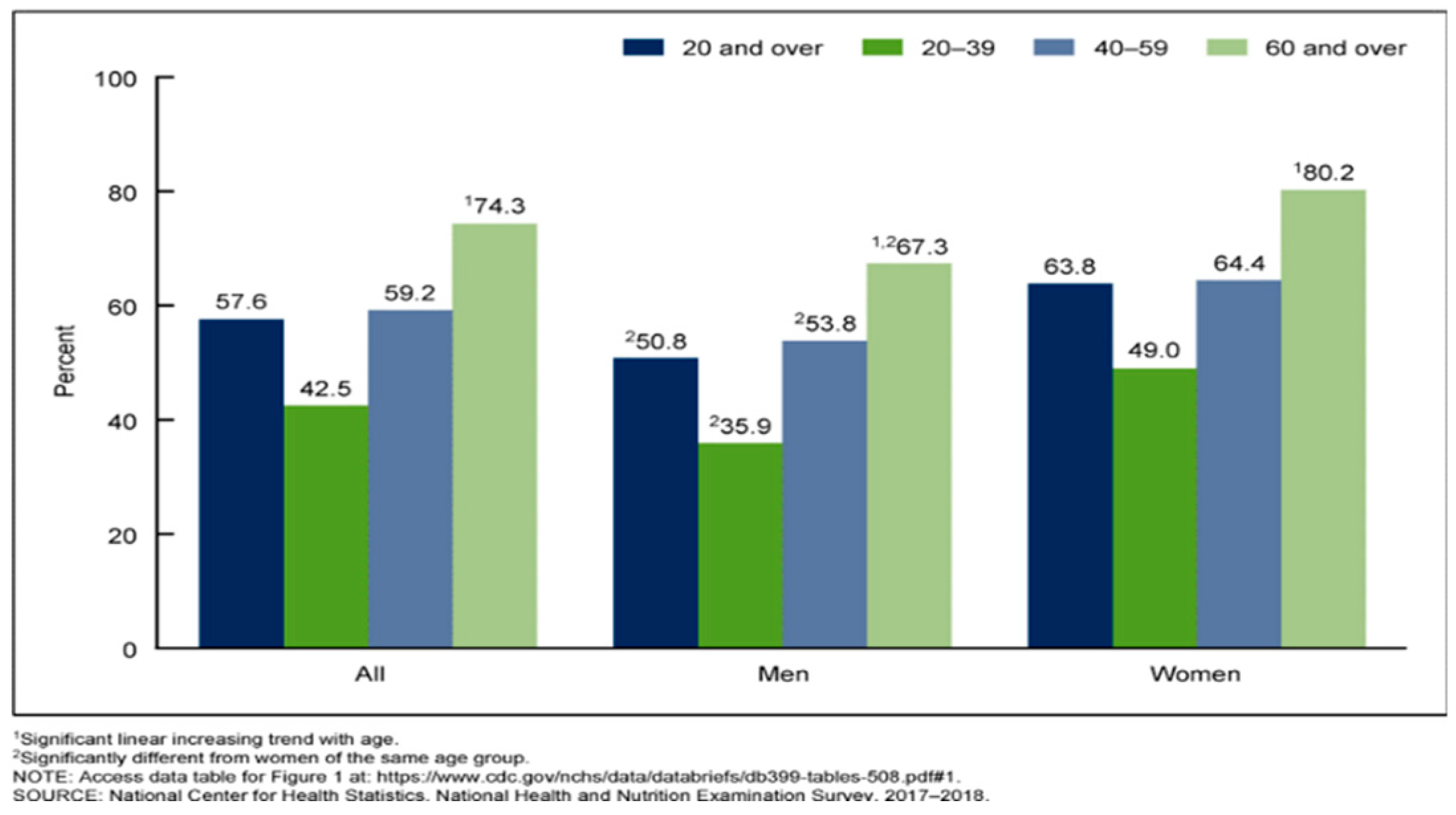
Figure 1.
Percentage of adults aged 20 and over who used any dietary supplement, by sex and age: United States, 2017–2018 [
6].
Figure 1.
Percentage of adults aged 20 and over who used any dietary supplement, by sex and age: United States, 2017–2018 [
6].
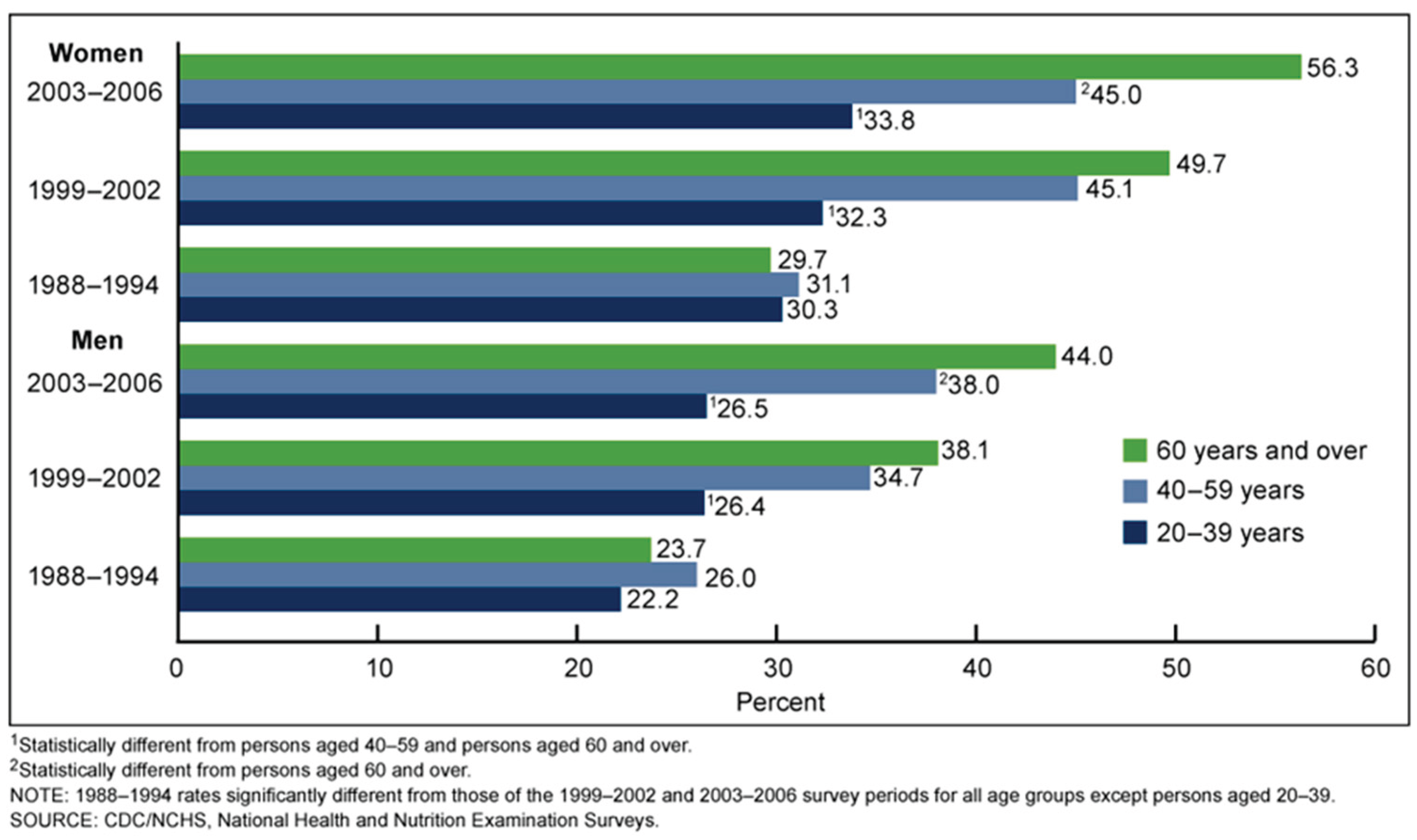
Figure 2.
Prevalence of supplemental vitamin D use in adults aged 20 and over, by age group: United States, 1988–2006 [
10].
Figure 2.
Prevalence of supplemental vitamin D use in adults aged 20 and over, by age group: United States, 1988–2006 [
10].
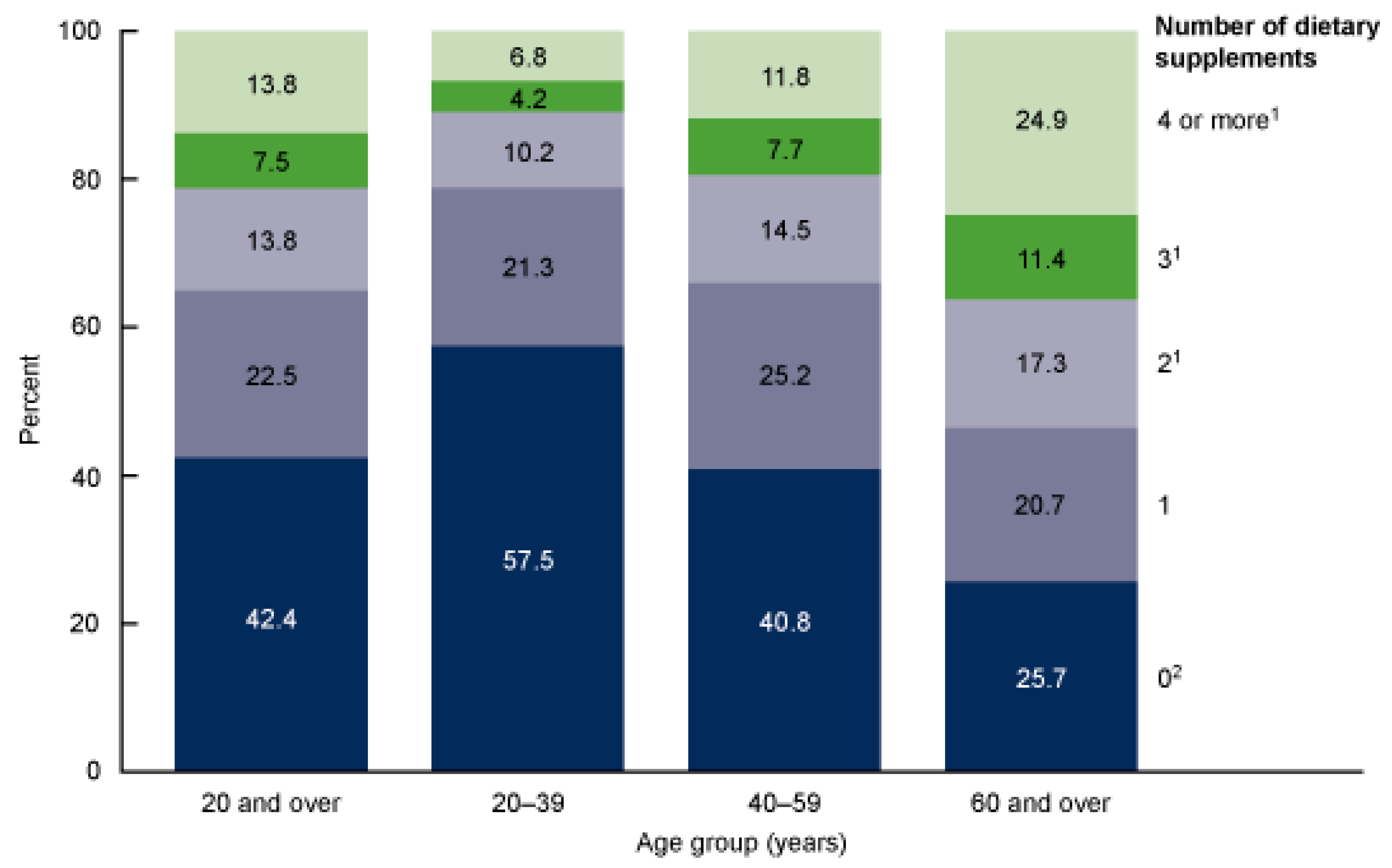
Figure 3.
Number of dietary supplements used by adults aged 20 and over, by age: United States, 2017–2018 [
8].
Figure 3.
Number of dietary supplements used by adults aged 20 and over, by age: United States, 2017–2018 [
8].
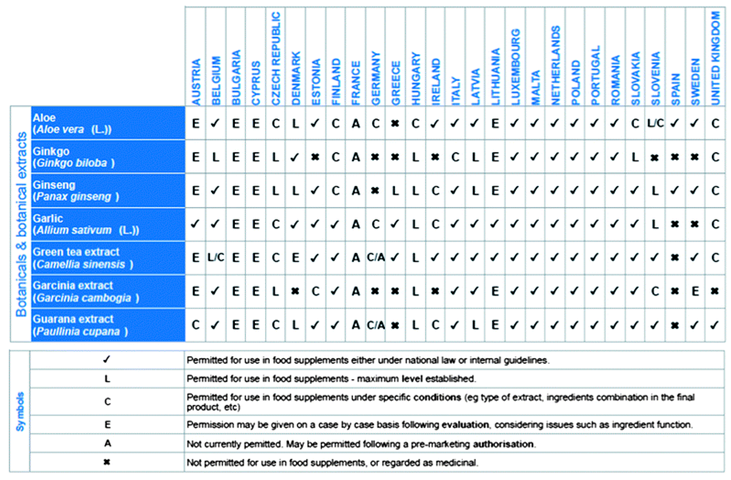
Table 1.
Examples of different national approaches for the use of selected botanicals in food supplements in the Member States of the EU [
12].
Table 1.
Examples of different national approaches for the use of selected botanicals in food supplements in the Member States of the EU [
12].
Gels as a dietary supplements
Nutritional gels represent a specialized category of dietary supplements increasingly used in endurance sports and military environments due to their rapid absorption and ease of administration under physically demanding conditions. These formulations typically contain fast-acting carbohydrates—such as maltodextrin, glucose, or fructose—often combined with electrolytes, amino acids, or bioactive compounds like caffeine, enabling rapid replenishment of muscle glycogen and supporting thermoregulation during prolonged exertion [
17,
18]. Research demonstrates that carbohydrate gels consumed at regular intervals help maintain blood glucose concentrations, delay fatigue onset, and improve endurance performance, particularly in long-duration activities or operational contexts involving heat stress or load carriage [
19].
Despite these benefits, their use requires careful individualization. Excessive intake or inappropriate timing may lead to gastrointestinal discomfort, osmotic diarrhea, or altered glycemic responses, which can impair performance rather than enhance it [
20]. Interactions with other supplements—especially caffeinated products or concentrated electrolyte solutions—may further increase risks such as dehydration or cardiovascular strain if not properly supervised. Quality assurance remains a key concern, as variability in ingredient purity, undeclared stimulants, and inconsistencies between labeling and content have been documented in certain commercial gel formulations, posing potential risks for athletes and military personnel subject to strict safety or anti-doping regulations [
21].
When used according to evidence-based guidelines and within structured nutritional strategies, energy gels can serve as effective adjuncts for sustaining physical performance, cognitive alertness, and resilience during extended operational or athletic challenges.
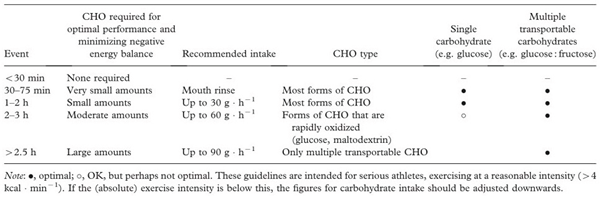
Table 2.
Recommendations for carbohydrate (CHO) intake during different endurance events[
17].
Table 2.
Recommendations for carbohydrate (CHO) intake during different endurance events[
17].
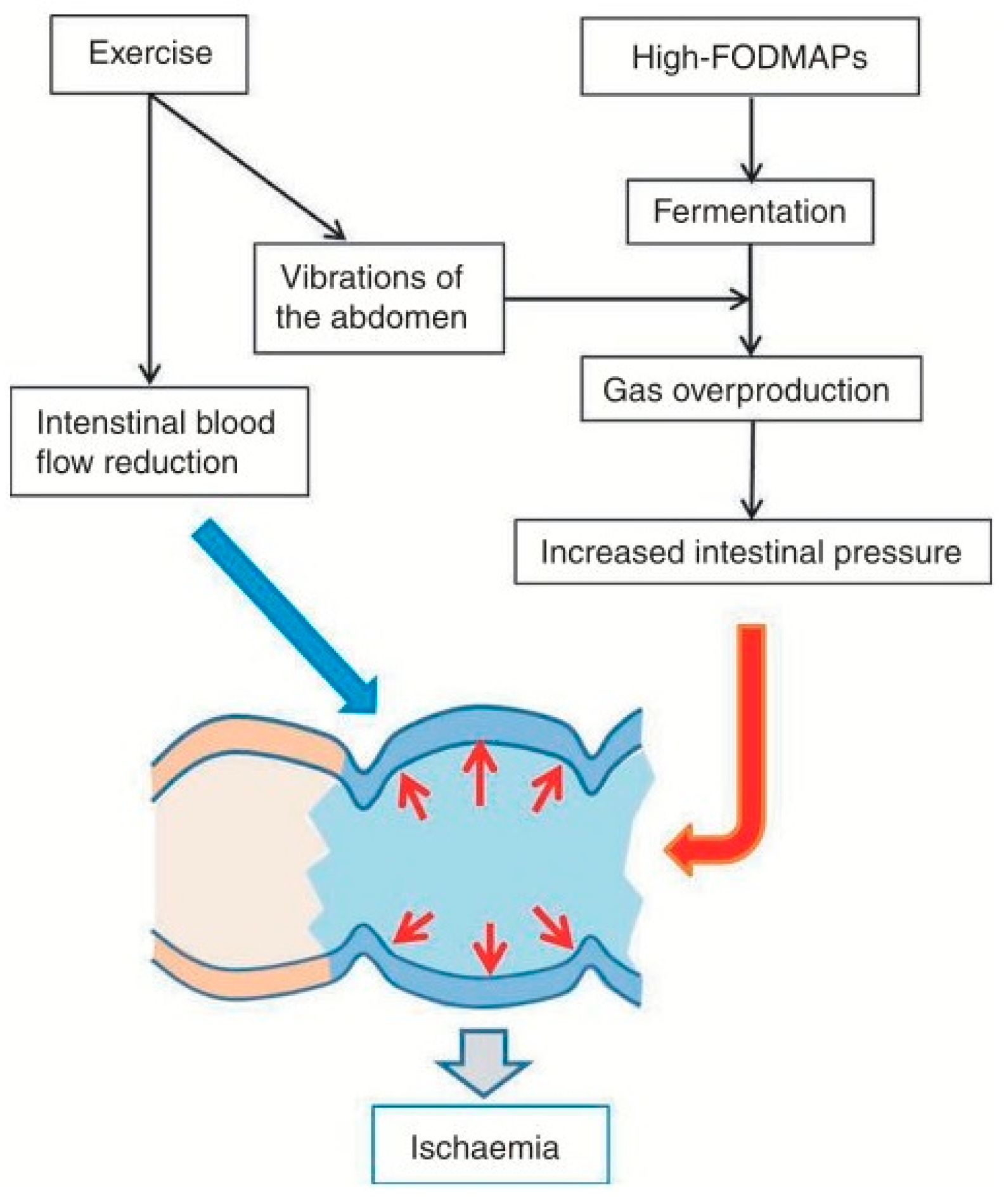
Figure 4.
Colonic ischaemia with exercise and high FODMAPs[
20].
Figure 4.
Colonic ischaemia with exercise and high FODMAPs[
20].
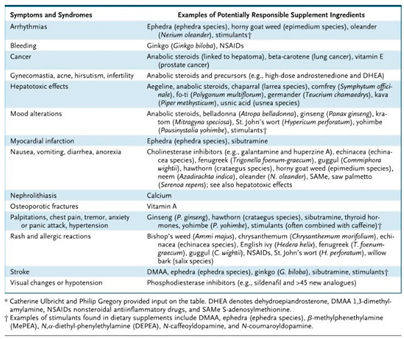
Table 3.
Examples of Potential Adverse Reactions to Legal Ingredients and Adulterants in Dietary Supplements.*[
21].
Table 3.
Examples of Potential Adverse Reactions to Legal Ingredients and Adulterants in Dietary Supplements.*[
21].
Hydrogels natural compound in therapy
Natural products derived from renewable and sustainable biological resources continue to serve as invaluable sources of structurally diverse bioactive compounds and secondary metabolites. These molecules—including polyphenols, flavonoids, terpenoids, alkaloids, and polysaccharides—exhibit significant therapeutic potential across antimicrobial, antioxidant, anti-inflammatory, and anticancer applications. However, their translation into clinical or cosmeceutical use is often restricted by physicochemical challenges such as poor water solubility, rapid enzymatic degradation, and limited membrane permeability.
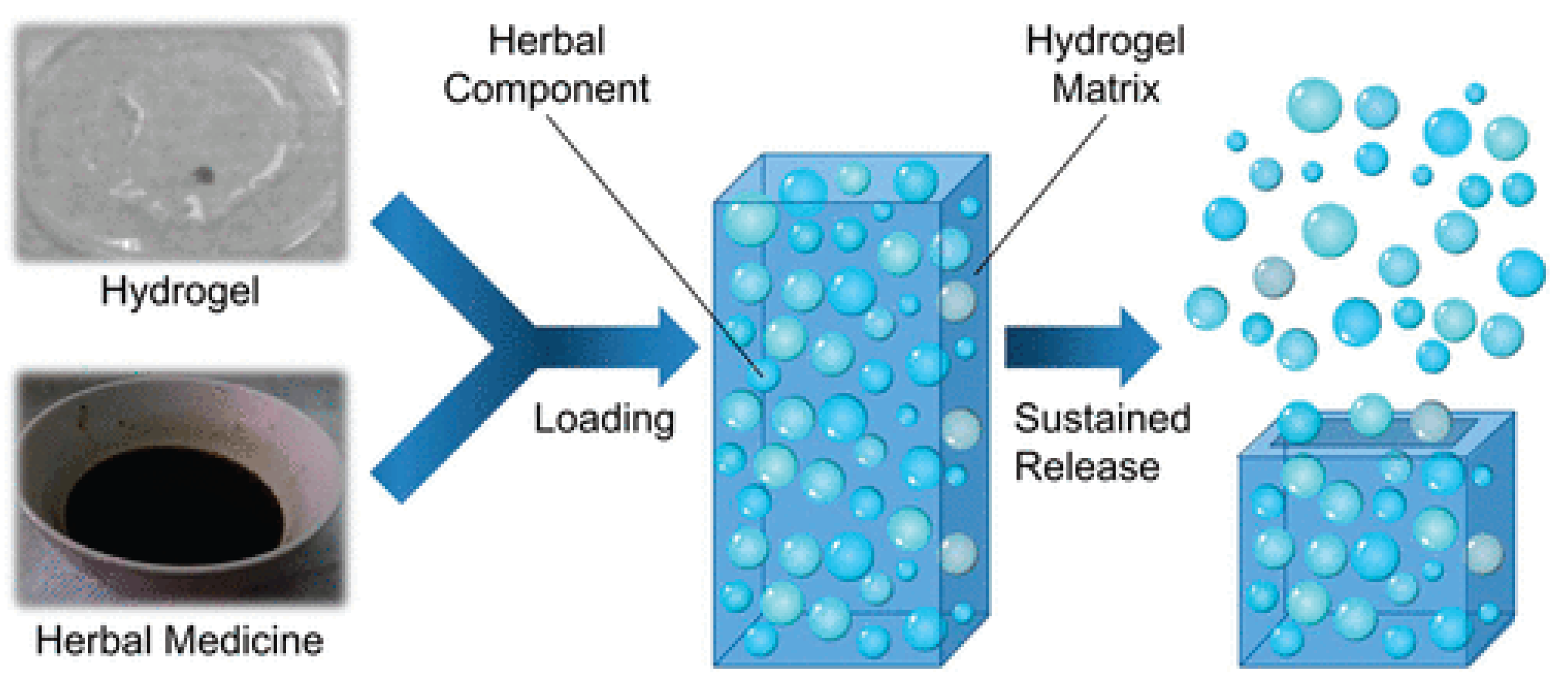
Recent pharmaceutical innovations have prompted the development of advanced hydrogel and nano-hydrogel platforms, which address many of these delivery limitations. Hydrogels—three-dimensional, crosslinked polymeric networks—create a hydrophilic microenvironment ideal for the encapsulation, stabilization, and controlled release of natural compounds. Examples of commonly used hydrogel materials include chitosan, alginate, hyaluronic acid, gelatin, polyvinyl alcohol (PVA), and polyethylene glycol (PEG). These biopolymers are valued for their biodegradability, mucoadhesiveness, and inherent biocompatibility, all of which improve their suitability for pharmaceutical and dermatological applications[
22,
23,
24].
Figure 5.
Hydrogel-based materials for delivery of herbal medicines[
22].
Figure 5.
Hydrogel-based materials for delivery of herbal medicines[
22].
Examples of hydrogels and the natural compounds encapsulated
Curcumin – Chitosan and PVA nano-hydrogels
Curcumin, a hydrophobic polyphenolic compound from
Curcuma longa, exhibits potent anti-inflammatory and antioxidant effects but suffers from poor aqueous solubility and rapid metabolic degradation. Encapsulating curcumin in chitosan nano-hydrogels enhances mucoadhesion, promotes interaction with negatively charged cellular membranes, and increases epithelial permeability. Chitosan’s pH-responsive swelling further supports controlled release in acidic environments. PVA-based nano-hydrogels improve curcumin’s dispersion and photostability while providing sustained release kinetics, making them suitable for oral, transdermal, and wound-healing applications[
25,
26]].
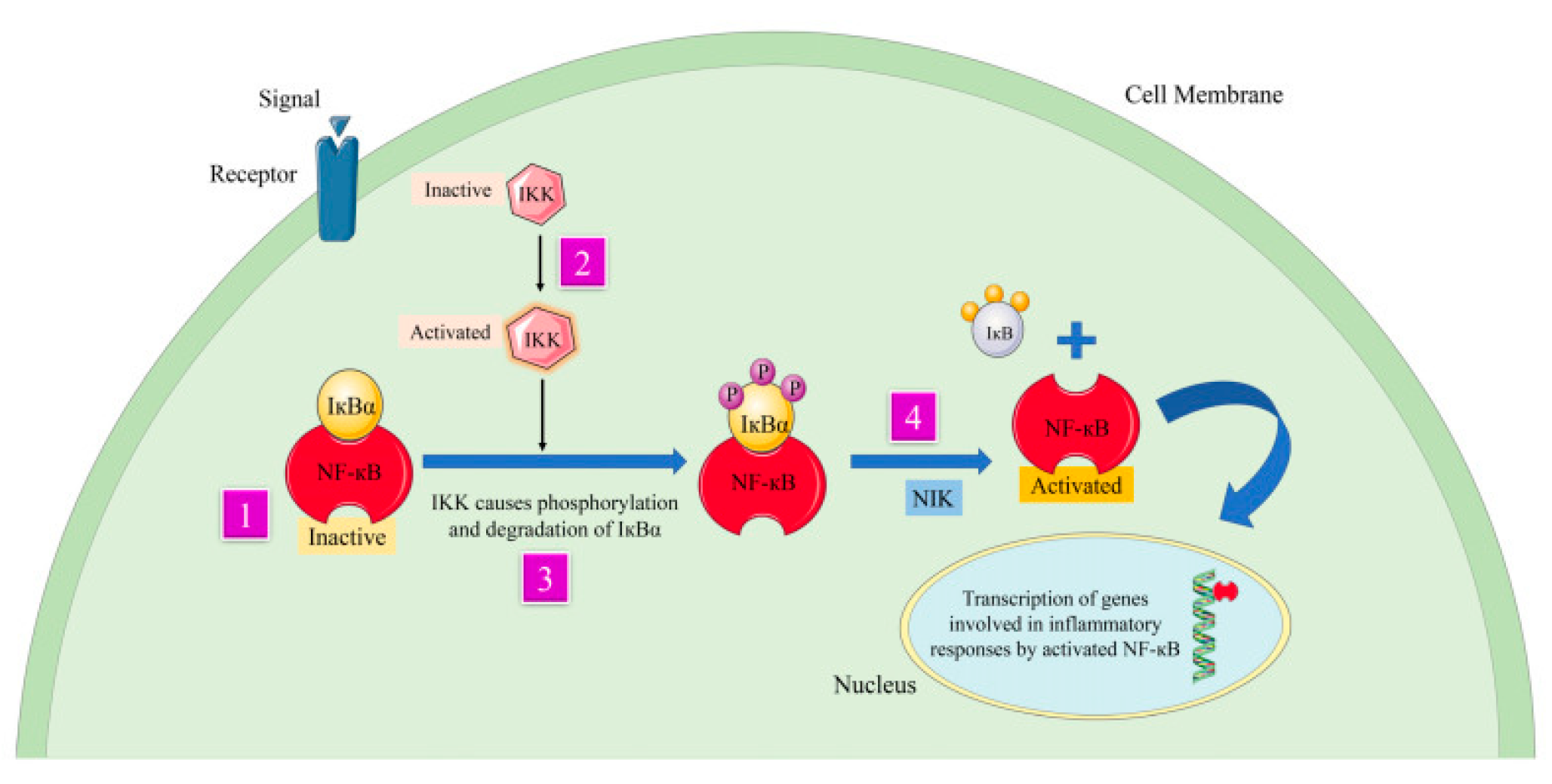
Figure 6.
Curcumindeals with the mechanism of inflammation and signaling pathways by suppressing transcription factor NFκB. The numbers 1, 2, and 3 in the figure indicate different pathways involved in the activity of the bioactive compoundthat makes up Curcumin[
26].
Figure 6.
Curcumindeals with the mechanism of inflammation and signaling pathways by suppressing transcription factor NFκB. The numbers 1, 2, and 3 in the figure indicate different pathways involved in the activity of the bioactive compoundthat makes up Curcumin[
26].
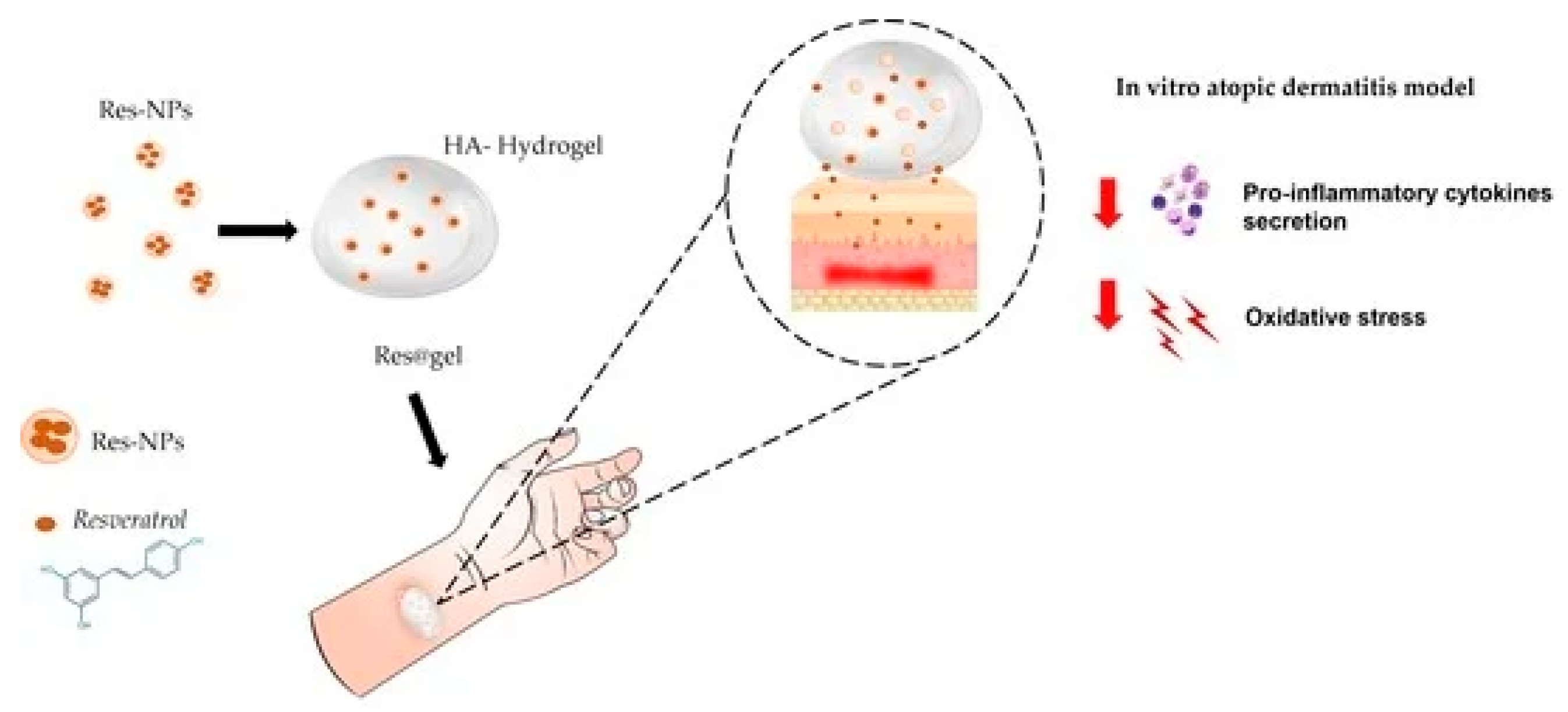
Resveratrol – Alginate and hyaluronic acid hydrogels
Resveratrol, a stilbene with well-documented antiaging and cardioprotective properties, is chemically unstable and rapidly oxidized. Alginate hydrogels, crosslinked with divalent cations such as Ca²⁺, protect resveratrol from oxidative degradation and allow mild encapsulation conditions that preserve its structural integrity. Hyaluronic acid (HA) hydrogels, widely used in dermatology, offer excellent hydration and biocompatibility, enhancing dermal penetration of resveratrol and enabling its use in cosmeceutical antiaging formulations[
27,
28].
Figure 7.
Graphical abstract[
27].
Figure 7.
Graphical abstract[
27].
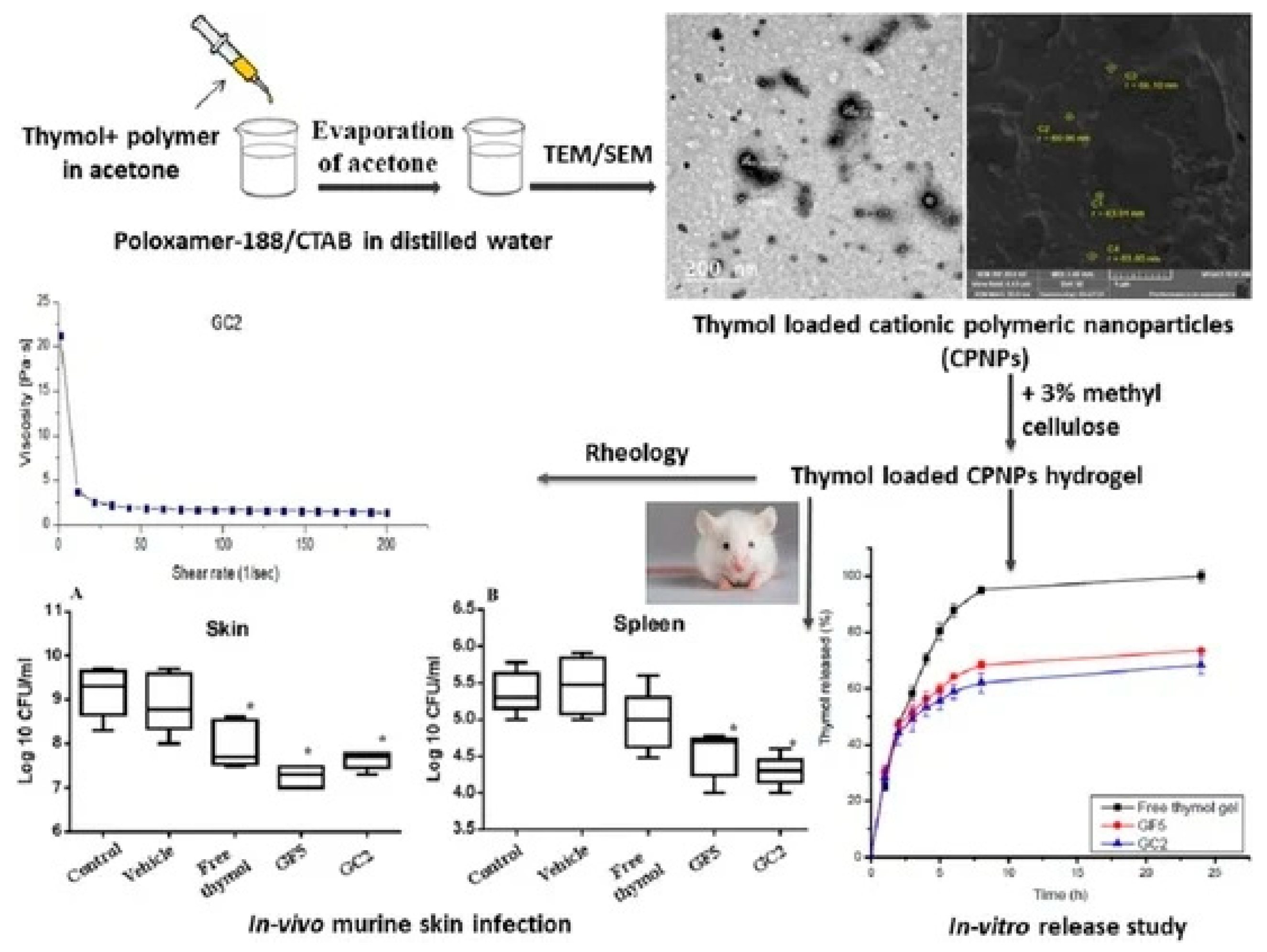
Thymol and carvacrol – PEG nano-hydrogels
Thymol and carvacrol, monoterpenoid phenols found in thyme and oregano oils, are potent antimicrobial agents but are volatile and hydrophobic. Encapsulation within PEG-based nano-hydrogels improves their solubility, reduces volatilization, and enables sustained antimicrobial activity. PEG provides a nonionic, biocompatible matrix that stabilizes these essential-oil constituents, enhancing their efficiency against bacterial and fungal pathogens while mitigating irritation associated with direct application[
29,
30,
31,
32,
33,
34].
Figure 7.
Graphical abstract[
33].
Figure 7.
Graphical abstract[
33].
Quercetin – gelatin–chitosan hydrogels
Quercetin is a flavonoid with strong antioxidant, anti-inflammatory, and cytoprotective properties but has poor water solubility and limited permeability. Gelatin–chitosan composite hydrogels combine the biocompatibility and cell-adhesive properties of gelatin with the mucoadhesive and pH-responsive characteristics of chitosan. This dual-polymer network effectively encapsulates quercetin, increases its aqueous stability, and enhances its transdermal or mucosal absorption. Such systems are promising for wound healing and anti-inflammatory therapies[
35].
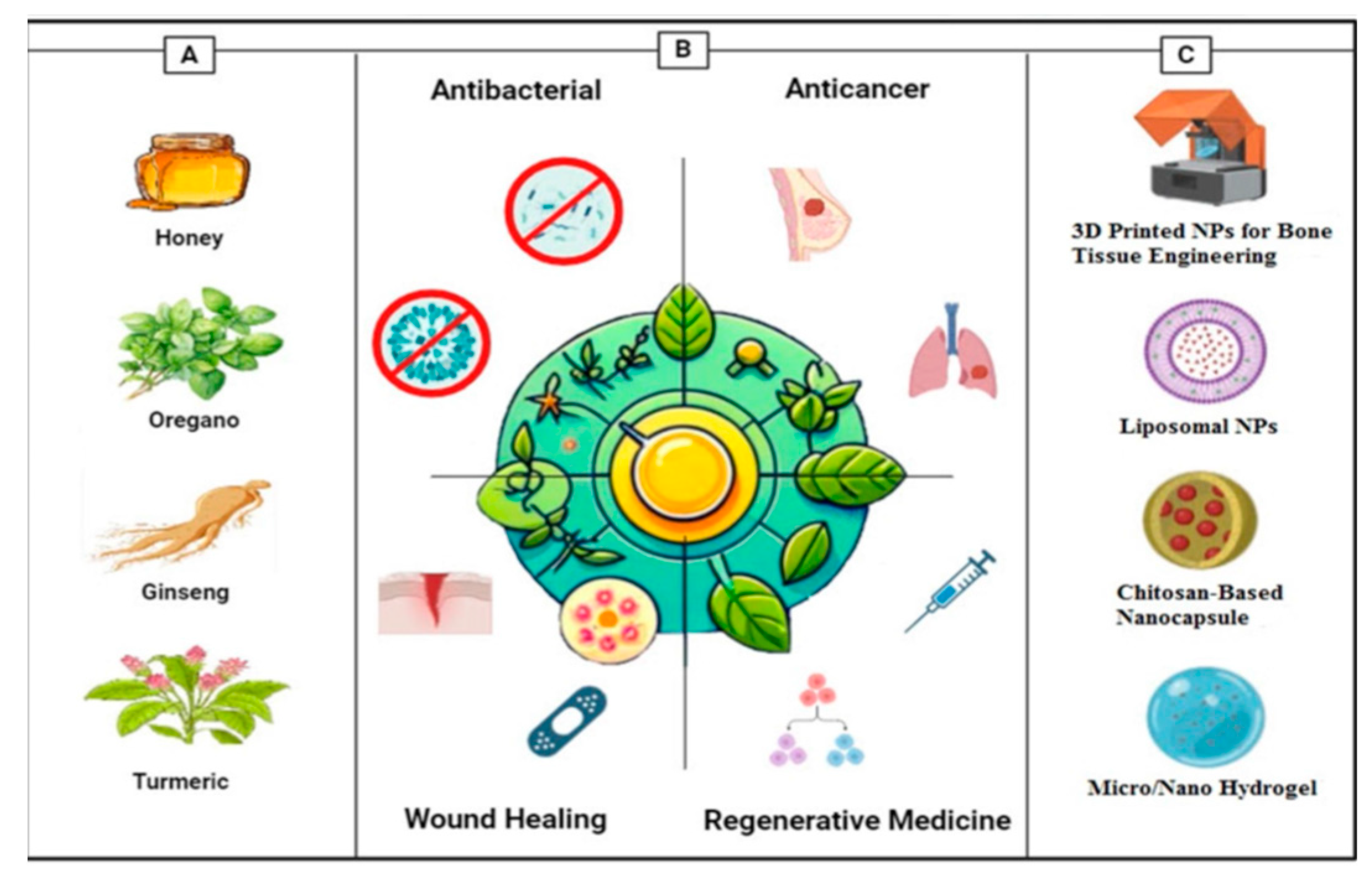
Figure 8.
Overall view of this report. A The commonly used plants and natural compounds and their bioactive compound [Honey–Turmeric–Ginseng–Oregano] B Main applications [Antibacterial–anticancer–wound healing–regenerative medicine] C Main delivery systems[
35].
Figure 8.
Overall view of this report. A The commonly used plants and natural compounds and their bioactive compound [Honey–Turmeric–Ginseng–Oregano] B Main applications [Antibacterial–anticancer–wound healing–regenerative medicine] C Main delivery systems[
35].
Aloe vera polysaccharides – natural hydrogels for wound healing
Polysaccharides extracted from
Aloe vera—including acemannan—possess intrinsic wound-healing, immunomodulatory, and moisturizing effects. When used to form natural hydrogels, these polysaccharides create a moist wound environment, promote fibroblast proliferation, and stimulate collagen synthesis. Their intrinsic bioactivity allows Aloe-based hydrogels to serve as both the matrix and the therapeutic agent, reducing the need for additional actives and supporting applications in burns, ulcers, and tissue regeneration[
36,
37,
38,
39,
40].
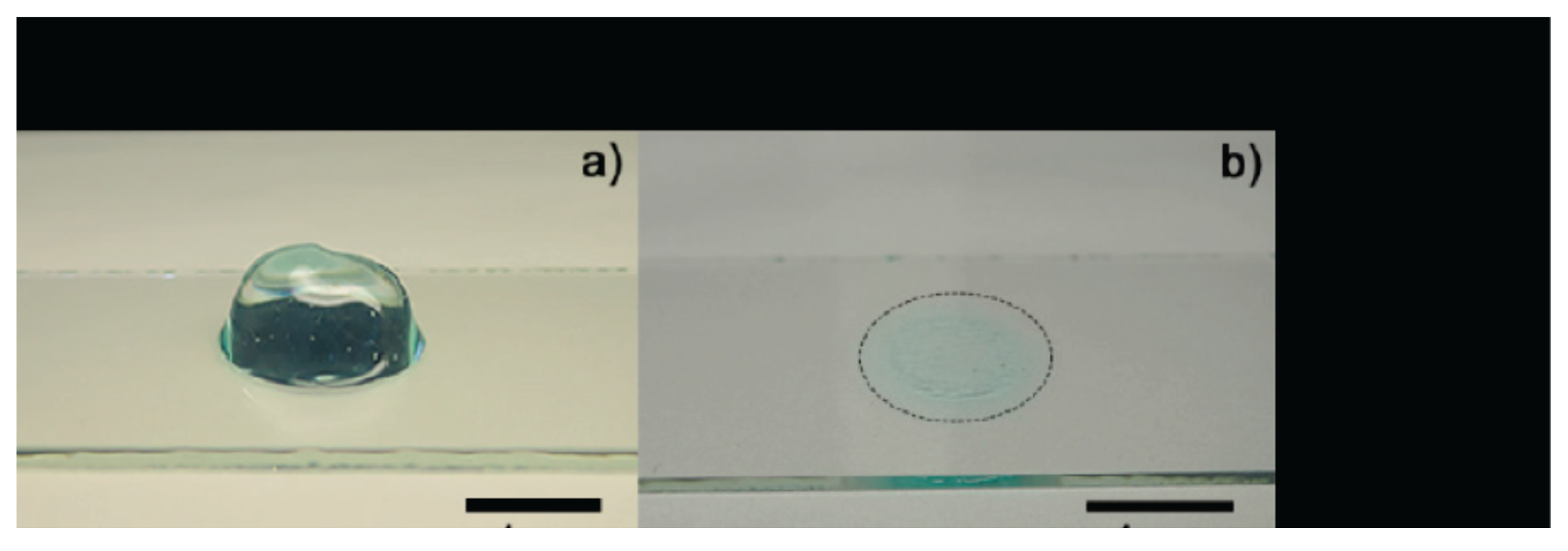
Figure 9.
Optical images of
Aloe vera hydrogel: (a) sample before drying process and (b) dried (xerogel)[
40].
Figure 9.
Optical images of
Aloe vera hydrogel: (a) sample before drying process and (b) dried (xerogel)[
40].
Ginsenosides – Hyaluronic acid (HA) hydrogels
Ginsenosides, the key bioactive compounds of
Panax ginseng, exhibit antioxidant, antitumor, and antiaging activities but are prone to degradation in biological environments. HA hydrogels provide a hydrated, viscoelastic matrix that protects ginsenosides from enzymatic breakdown and enhances their uptake by skin and tumor tissues due to HA’s natural affinity for CD44 receptors overexpressed in many cancer cells. This receptor-mediated targeting mechanism makes HA–ginsenoside hydrogels attractive for dermatological therapy and localized anticancer delivery[
41,
42,
43,
44,
45].
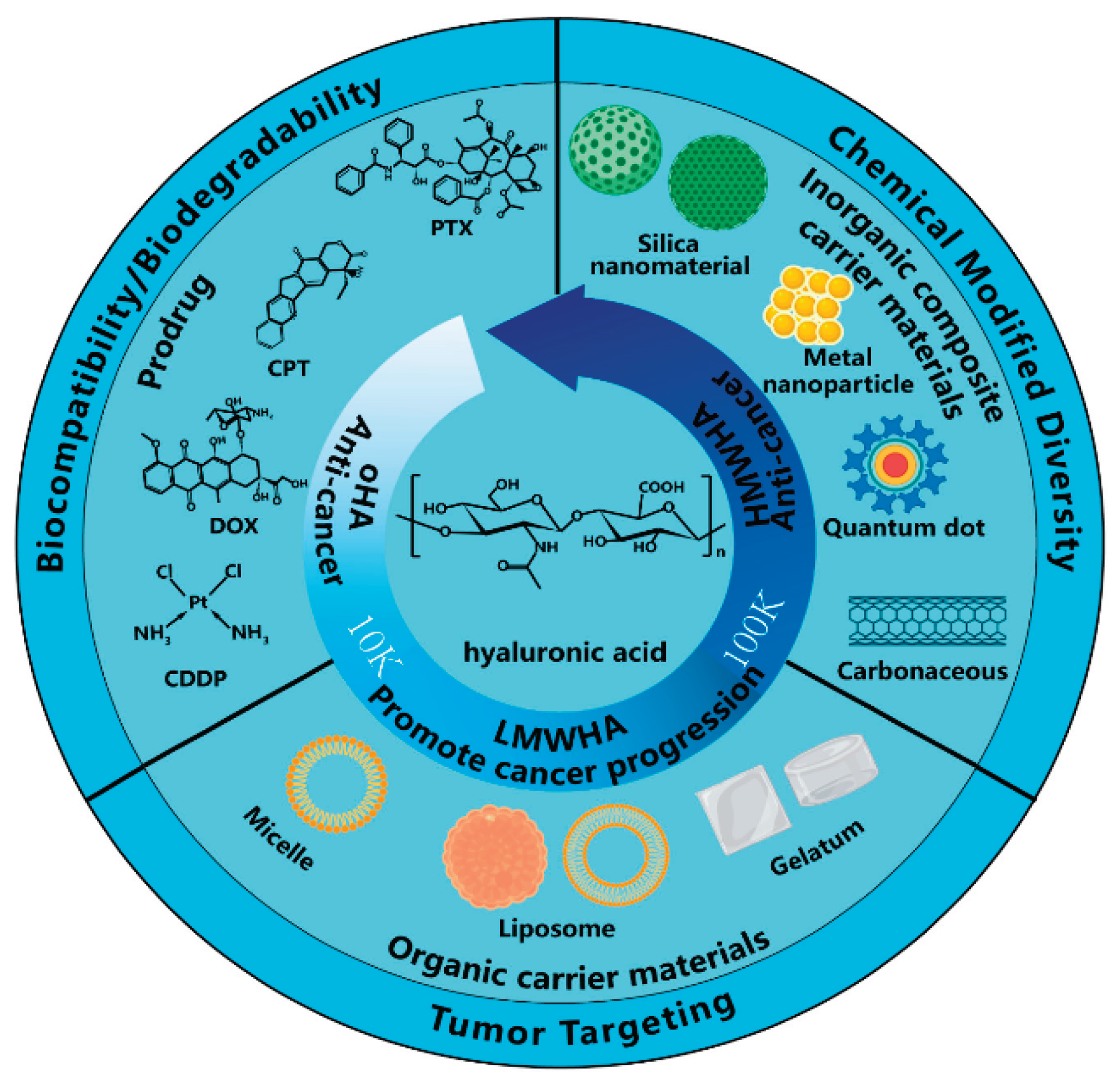
Figure 10.
Chemical structure of HA and the hypothesized procancer (promoting cancer growth) and anticancer (preventing cancer growth) activity of HA with different molecular weights and its application[
41].
Figure 10.
Chemical structure of HA and the hypothesized procancer (promoting cancer growth) and anticancer (preventing cancer growth) activity of HA with different molecular weights and its application[
41].
Mechanistic basis for improved delivery
Hydrogels enhance the bioavailability of natural molecules through several interconnected mechanisms:
1.Encapsulation and protection
-Prevents oxidation and enzymatic degradation.
-Stabilizes sensitive molecules (e.g., curcumin against light and pH instability).
2. Controlled and sustained release
-Crosslink density governs water diffusion and molecule mobility, enabling release kinetics ranging from hours to weeks.
-pH-responsive hydrogels (e.g., chitosan swelling at acidic pH) release compounds specifically at target tissues such as gastric or inflamed environments.
3. Enhanced cellular uptake
Nano-hydrogels (<200 nm) facilitate receptor-mediated endocytosis or direct membrane translocation, improving intracellular delivery of hydrophobic compounds such as limonene, thymol, and curcuminoids.
4.Improved solubility and dispersion
Hydrophilic polymer matrices increase the apparent solubility of lipophilic molecules by generating nano-dispersed or colloidal forms.
5.Targeting capabilities (in advanced nano-hydrogels)
Surface functionalization with ligands (e.g., folate, peptides) enables selective delivery to cancer cells or inflamed tissues.
Hydrophobic or hydrophilic natural compounds are trapped within the porous hydrogel network, protected from degradation.
3. Conclusions
When used appropriately, dietary supplements can play a valuable role in supporting overall health, preventing nutrient deficiencies, and enhancing physical and cognitive performance. Their benefits are most evident when they are integrated into a balanced lifestyle and guided by evidence-based medical advice. However, the uncritical or unsupervised use of supplements—particularly in high-stress environments such as military settings—carries significant risks, including adverse interactions, toxicity, and a false sense of security that may overshadow proper nutrition and medical care.
To maximize the positive impact of dietary supplements while minimizing potential harm, it is crucial to strengthen public education on their safe and effective use. Individuals must be equipped with reliable information that enables them to distinguish evidence-based products from those unsupported by research or marketed through misleading claims. At the same time, regulatory frameworks need continuous refinement to improve product quality, transparency, and consumer protection, ensuring that supplements on the market meet rigorous standards for safety and efficacy.
Equally important is the development of clear clinical guidelines tailored to both civilian and military populations. Healthcare professionals should be empowered to provide informed recommendations, monitor usage, and address specific nutritional needs arising in different operational contexts. Through coordinated efforts in education, regulation, and clinical oversight, dietary supplements can be integrated responsibly into health strategies, ultimately supporting well-being, resilience, and mission readiness.
In addition to conventional supplement formats such as tablets, powders, and capsules, nutritional gels and hydrogel-based formulations have emerged as increasingly popular tools for supporting health and performance, particularly in environments where rapid nutrient delivery, portability, and physiological efficiency are essential. These gel-based supplements are semi-solid matrices designed to provide concentrated sources of carbohydrates, electrolytes, amino acids, and micronutrients in a form that is easy to ingest and rapidly absorbed. Their popularity is especially notable among endurance athletes, emergency responders, and individuals operating under extreme physical or environmental stress—including military personnel, for whom sustaining energy, hydration, and cognitive alertness can be mission-critical.
Nutritional gels are formulated to deliver energy with minimal gastrointestinal burden. Their semi-fluid consistency bypasses many of the delays associated with the digestion of solid foods, allowing for rapid gastric emptying and quick entry of nutrients into the bloodstream. Typically, these gels rely on blends of simple and complex carbohydrates—such as maltodextrin, glucose, and fructose—selected to optimize simultaneous absorption through multiple intestinal transport pathways. This dual- or multi-carbohydrate strategy enables higher rates of carbohydrate oxidation during strenuous activity, supporting sustained energy output while reducing the likelihood of gastrointestinal distress that can occur when a single carbohydrate type is consumed in excess.
Beyond carbohydrate delivery, modern gel supplements often include electrolytes crucial for maintaining fluid balance, nerve transmission, and muscle function. Sodium, potassium, magnesium, and chloride are commonly incorporated to counteract sweat-related mineral losses and delay the onset of fatigue or muscle cramping. Some gel formulations further enhance performance by integrating caffeine, beta-alanine, B vitamins, or branched-chain amino acids (BCAAs), each serving a specific physiological purpose such as reducing perceived exertion, buffering lactic acid, or supporting mental focus. When used correctly, these gels can help stabilize energy levels and maintain operational readiness in scenarios where traditional meal consumption is impossible.
However, the convenience and potency of nutritional gels also underscore the importance of responsible usage. Overconsumption—particularly without adequate accompanying hydration—can lead to nausea, bloating, or diarrhea, undermining rather than supporting performance. Products containing stimulants like caffeine must be carefully monitored, as excessive intake may amplify dehydration risks, impair sleep cycles, or exacerbate anxiety in high-pressure environments. Thus, while gels offer meaningful advantages, they must be integrated thoughtfully into personalized nutrition plans guided by trained healthcare or performance professionals.
Alongside conventional gels, the rise of hydrogels represents a more advanced and scientifically sophisticated frontier in dietary supplementation. Hydrogels are three-dimensional polymer networks capable of absorbing and retaining large volumes of water while maintaining structural integrity. Their unique physicochemical properties—biocompatibility, adjustable porosity, and controlled-release functionality—make them highly attractive for both biomedical applications and emerging nutritional technologies.
In the context of dietary supplementation, hydrogels can serve as delivery platforms that encapsulate nutrients, protect them from degradation, and release them in a targeted or gradual manner. This technology is particularly promising for sensitive bioactive compounds such as probiotics, omega-3 fatty acids, antioxidants, or certain vitamins that can be damaged by heat, oxidation, or the acidic environment of the stomach. Encapsulation within a hydrogel matrix shields these molecules until they reach their intended site of absorption, improving their bioavailability and functional impact.
Hydrogel-based nutrient delivery also offers potential benefits for metabolic stability and sustained energy release. Unlike traditional gels that produce rapid spikes in blood glucose, hydrogel systems can be engineered to modulate the release rate of carbohydrates or other nutrients, minimizing glycemic fluctuations and reducing the risk of energy crashes. This characteristic is particularly relevant in prolonged military operations, long-distance endurance events, and settings where consistent cognitive performance is essential over extended periods.
From a material science perspective, the polymers used to create edible hydrogels are often derived from natural sources such as alginate, pectin, agar, carrageenan, gelatin, or modified starches. These biopolymers are recognized as safe for consumption and can be structured to create hydrogels with varying textures, viscosities, and degradation profiles. Advances in food engineering have enabled the development of hydrogels that remain stable under environmental stresses—heat, vibration, humidity—making them well suited for deployment in military rations and emergency nutrition kits.
A particularly innovative application involves hydrogel-based hydration systems, which encapsulate water and electrolytes in gel form, allowing individuals to consume hydration sources without carrying bulky containers or risking contamination. These gels could play a transformative role in desert operations, high-altitude missions, or disaster relief scenarios, where water scarcity or compromised water quality poses significant challenges. By slowly releasing water inside the digestive tract, such hydrogels may also enhance fluid retention and reduce the risk of dehydration.
Despite their promise, hydrogel supplements—like all nutritional interventions—require careful regulation and thorough scientific validation. Their complexity raises questions about optimal dosing strategies, long-term safety, and interactions with other dietary components. Additionally, the novelty of hydrogel-based consumption systems requires clear educational frameworks to ensure that users understand how these products should be integrated into larger nutritional strategies.
From a regulatory standpoint, hydrogel supplements must adhere to rigorous quality and purity standards. Ensuring the stability of encapsulated nutrients, validating release kinetics, and confirming the biodegradability of polymer materials are essential steps in safeguarding consumer health. As the market expands, regulatory agencies must adapt criteria for evaluating hydrogel-based products, addressing challenges that differ from those associated with traditional supplement forms.
Clinically, the integration of gels and hydrogels into personalized nutrition plans offers numerous opportunities for innovation. Healthcare providers can leverage these technologies to support patients with dysphagia, digestive disorders, or elevated metabolic demands. In military and athletic settings, performance specialists can tailor gel-based supplementation protocols to specific training loads, environmental stressors, and mission requirements. However, such optimization requires comprehensive research, standardized guidelines, and clear communication with end users.
Overall, the rise of nutritional gels and hydrogel-based delivery systems reflects a broader shift toward precision nutrition—solutions that are not only convenient but also biologically intelligent, adaptable, and responsive to real-world conditions. When anchored in scientific evidence, regulatory oversight, and professional guidance, these technologies hold significant potential to enhance human resilience, health, and performance across civilian and military domains.
Author Contributions
Conceptualization, A.D.R., and M.N.; methodology, A.D.R., G.T, I.D.F; softare, X.X.; validation, A.D.R., and M.C.E.; formal analysis, I.D.F.; investigation, G.T., I.D.F.; resources, C.M.; data curation, A.D.R.; writing—original draft preparation, A.D.R..; writing—review and editing, A.D.R., and M.C.E.; visualization, A.D.R.; supervision, C.M.; project administration, M.C.E.; funding acquisition, A.D.R.
Funding
This research was funded by UEFISCDI Romania, grant number 30/PED/2025 entitled Liposomal nanosystems with microbial inulinase for the prevention of metabolic and nutritional diseases" – acronym LIFHOIN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bailey, R.L.; Gahche, J.J.; Lentino, C.V.; et al. Dietary supplement use in the United States 2003–2006. J Nutr. 2011, 141, 261–266. [Google Scholar] [CrossRef]
- Kreider, R.B.; Wilborn, C.D.; Taylor, L.; et al. ISSN Exercise & Sport Nutrition Review. J Int Soc Sports Nutr. 2010, 7, 7. [Google Scholar]
- Cohen, P.A. Hazards of hindsight — monitoring the safety of nutritional supplements. N Engl J Med. 2014, 370, 1277–1280. [Google Scholar] [CrossRef]
- Austin, K.G.; Price, L.L.; McGraw, S.M.; et al. Dietary supplement use in the U.S. Military. J Acad Nutr Diet 2020, 120, 102–114. [Google Scholar]
- Sood, A.; Sood, R.; Brinker, F.J.; et al. Potential interactions between dietary supplements and prescription medications. Am Fam Physician 2008, 77, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Gahche, J.J.; Bailey, R.L.; Potischman, N.; et al. Dietary supplement use among adults: United States 2017–2018, 2021 NCHS Data Brief No. 399.
- Kantor, E.D.; Rehm, C.D.; Du, M.; et al. Trends in dietary supplement use among US adults. JAMA 2016, 316, 1464–1474. [Google Scholar] [CrossRef] [PubMed]
- NIH Office of Dietary Supplements. Vitamin D Fact Sheet for Health Professionals. https://ods.od.nih.gov.
- Yurko-Mauro, K.; Alexander, D.D.; Van Elswyk, M.E. Docosahexaenoic acid and adult memory: meta-analysis. PLoS One 2015, 10, e0120391. [Google Scholar] [CrossRef] [PubMed]
- Looker, A.C.; Johnson, C.L.; Lacher, D.A.; et al. Vitamin D status: United States, 1988–2006, 2011, NCHS Data Brief No. 59.
- Wallace, T.C. Twenty years of DSHEA. Nutr Today 2016, 51, 266–271. [Google Scholar]
- European Commission. Directive 2004/24/EC on traditional herbal medicinal products.
- Littarru, G.P.; Tiano, L. Clinical aspects of coenzyme Q10. Curr Opin Clin Nutr Metab Care 2005, 8, 641–646. [Google Scholar]
- EFSA, Compendium of botanicals. EFSA Journal 2012, 10, 2663.
- García-Cortés, M.; Lucena, M.I.; Andrade, R.J. Herbal and dietary supplement hepatotoxicity. Drug Saf. 2018, 41, 913–936. [Google Scholar]
- European Medicines Agency, Reflection paper on the quality of herbal medicinal products, 2016, EMA/HMPC.
- Jeukendrup, A.E. Nutrition for endurance sports: Marathon, triathlon, and road cycling. Journal of Sports Sciences 2011, 29, S91–S99. [Google Scholar] [CrossRef]
- Cermak, N.M.; van Loon, L.J.C. The use of carbohydrates during exercise as an ergogenic aid. Sports Medicine 2013, 43, 1139–1155. [Google Scholar] [CrossRef]
- Pfeiffer, B.; et al. Carbohydrate oxidation during a marathon: A comparison between liquid, gel, and solid carbohydrate sources. Journal of Applied Physiology 2012, 112, 806–815. [Google Scholar]
- Costa, R.J.S.; et al. Systematic review: Exercise-induced gastrointestinal syndrome—Impairments, symptoms and nutritional interventions. Sports Medicine 2017, 47, 79–100. [Google Scholar]
- Cohen, P.A. Hazards of hindsight — Monitoring the safety of nutritional supplements. New England Journal of Medicine 2014, 370, 1277–1280. [Google Scholar] [CrossRef]
- Lai, W.F.; Rogach, A.L. Hydrogel-based materials for delivery of herbal medicines. ACS Appl Mater Interfaces 2017, 9, 11309–11320. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Luo, W.; Wu, L.; Zhang, L.; Chen, Y.; Li, T.; et al. Natural products from herbal medicine self-assemble into advanced bioactive materials. Adv Sci. 2024, 11, 2403388. [Google Scholar] [CrossRef] [PubMed]
- Muslim, M.R.F.; Chabib, L.; Hamzah, H. Nano-hydrogel systems in herbal medicine: A systematic review. J Herbmed Pharmacol. 2025, 14, 153–162. [Google Scholar] [CrossRef]
- Kenawy, E.R.S.; Elbadawy AKamoun Ghaly, Z.S.; Abdel-baset, M.S.; El-Meligy, M.A. Novel physically cross-linked curcumin-loaded PVA/Aloe vera hydrogel membranes for acceleration of topical wound healing: In vitro and in vivo experiments. Arabian Journal for Science and Engineering 2023, 48, 497–514. [Google Scholar] [CrossRef]
- Chopra, H.; et al. In vitro and in silico characterization of curcumin-loaded hydrogel system: antimicrobial potential and wound healing activity. Gels 2023, 9, 394. [Google Scholar] [CrossRef]
- Conte, R.; De Luca, I.; Valentino, A.; Cerruti, P.; Pedram, P.; Cabrera-Barjas, G.; Moeini, A.; Calarco, A. Hyaluronic acid hydrogel containing resveratrol-loaded chitosan nanoparticles as an adjuvant in atopic dermatitis treatment. J Funct Biomater 2023, 14, 82. [Google Scholar] [CrossRef]
- Law, S.K.; Liu, C.W.C.; Tong, C.W.S.; Au, D.C.T. Potential of resveratrol to combine with hydrogel for photodynamic therapy against bacteria and cancer — a review. Biomedicines 2024, 12, 2095. [Google Scholar] [CrossRef]
- Folle, C.; Díaz-Garrido, N.; Mallandrich, M.; Suñer-Carbó, J; Sánchez-López, E.; Halbaut, L.; Baldoma, L. Hydrogel of Thyme-Oil-PLGA Nanoparticles Designed for Skin Inflammation Treatment. Gels 2024, 10, 149. [Google Scholar] [CrossRef]
- Moghtaderi, M.; Bazzazan, S.; Sorourian, G.; Sorourian, M.; Akhavanzanjani, Y.; Noorbazargan, H.; Ren, Q. Encapsulation of Thymol in Gelatin Methacryloyl (GelMa)-Based Nanoniosome Enables Enhanced Antibiofilm Activity and Wound Healing. Pharmaceutics 2023, 15, 1699. [Google Scholar] [CrossRef]
- Yammine, J.; Chihib, N.-E.; Gharsallaoui, A.; Ismail, A.; Karam, L. Advances in essential oils encapsulation: development, characterization and release mechanisms. Polymer Bulletin 2023, 81, 3837–3882. [Google Scholar] [CrossRef]
- Naz, S.; et al. Recent advances in polymer nanoencapsulation of essential oils for biomedical and industrial applications. Mater. Adv. 2025, 6, 2460–2476. [Google Scholar] [CrossRef]
- Mohsen, A.M.; et al. Thymol-loaded cationic polymeric nanoparticles (CPNPs) for enhanced skin retention and wound healing. Pharmaceutics 2023, 15, 19. [Google Scholar] [CrossRef]
- Fakhariha, M.; et al. Nanoencapsulation enhances stability, release behavior, and antimicrobial efficacy of essential oils including Thyme oil. Scientific Reports 2025. [Google Scholar] [CrossRef]
- Najafi, F.; et al. Advancement in nanocarrier-mediated delivery of herbal medicine: a systematic review. Natural Products and Bioprospecting 2025, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Chithra, P.; Sajithlal, G.B.; Chandrakasan, G. Influence of Aloe vera on collagen turnover in healing of dermal wounds in rats. Indian J Exp Biol. 1998, 36, 896–901. [Google Scholar]
- Zhang, L.; Tizard, I. Immunomodulatory effects of acemannan, a β-(1→4)-acetylated mannan, on macrophage functions. Int Immunopharmacol. 1996, 18, 167–171. [Google Scholar]
- Eshun, K.; He, Q. Aloe vera: A valuable ingredient for the food, pharmaceutical, and cosmetic industries—A review. Crit Rev Food Sci Nutr. 2004, 44, 91–106. [Google Scholar] [CrossRef]
- Cruz, E.D.; Pimentel, T.C.; de Souza Sant’Ana, A.; da Silva, M.V. Aloe vera–based hydrogels for wound healing applications: A review. Polymers 2022, 14, 745. [Google Scholar]
- Meza-Valle, K.Z.; Saucedo-Acuña, R.A.; Tovar-Carrillo, K.L.; Cuevas-González, J.C.; Zaragoza-Contreras, E.A.; Melgoza-Lozano, J. Characterization and Topical Study of Aloe Vera Hydrogel on Wound-Healing Process. Polymers 2021, 13, 3958. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, H.; Xia, C. Hyaluronic acid-modified nanocarriers for tumor-targeted delivery of ginsenosides: Advances and perspectives. International Journal of Biological Macromolecules 2023, 236, 124116. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Chen, J.; Zhang, R. Hyaluronic acid-based hydrogels for targeted cancer therapy: A review. Carbohydrate Polymers 2020, 246, 116605. [Google Scholar]
- Huang, J.; Teng, X.; Liu, H.; et al. Ginsenosides as anticancer agents: Mechanisms, pharmacokinetics, and future perspectives. Pharmacological Research 2019, 148, 104448. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Liu, M.; Zhang, H. Antioxidant and antiaging effects of ginsenosides: Molecular mechanisms and therapeutic potential. Biomedicine & Pharmacotherapy 2021, 134, 111076. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, J.S.; Lee, S.H. Hyaluronic acid hydrogels for tissue regeneration and targeted drug delivery. Journal of Controlled Release 2018, 278, 30–45. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).