Introduction:
Spinal cord injury (SCI) represents a severe clinical condition and is among the most complex neurological disorders to manage, often leading to long-term and permanent neurological deficits in affected individuals. Injury to nervous tissue may arise from traumatic events, such as vertebral fractures or dislocations, or may result from various underlying pathological conditions. The impact of a spinal cord injury can result in impairments in the sensory, motor, and autonomic nervous systems in the affected area [
1,
2,
3]. Spinal injuries, characterized by the severance or damage to spinal nerves, have a profound effect on both sensory and motor functions, resulting in a diminished capacity for sensation and movement in the impacted regions. The degree of paralysis, whether partial or complete, is determined by the specific location and severity of the spinal cord injury. Affected individuals may experience a loss of the ability to detect tactile stimuli, temperature variations, or pain, and may also report atypical sensations, such as burning. In more severe instances, the disruption of nerve signals from the brain to the muscles can lead to paralysis, which may present as paraplegia (involving the lower limbs) or quadriplegia (involving all four limbs). Furthermore, individuals with spinal injuries may face additional complications, including difficulties with bladder and bowel control, respiratory challenges, and psychological repercussions such as depression and anxiety [
4,
5]. At present, a conclusive cure for this disease remains elusive. Nevertheless, substantial progress in foundational research suggests that under certain conditions, nerve cells may possess the capacity for regeneration [
6,
7]. From a pathophysiological standpoint, spinal cord injuries are categorized into primary and secondary injuries. Primary injuries occur due to an abrupt force exerted on the spinal column at the moment of injury, potentially leading to vertebral fractures or dislocations, which coincide with damage to the spinal cord [
6,
8].
The severity and extent of spinal cord injury are predominantly determined by the initial level of damage sustained by the spinal cord at the moment of injury, as well as the duration of subsequent compression. Following the primary injury, secondary injuries initiate almost immediately.
Within hours of the initial trauma, a series of secondary injury mechanisms are activated, leading to ischemia, inflammation, and the degeneration of neurons and glial cells, all of which play a significant role in advancing secondary spinal cord injury [
6,
9,
10]. Among the key factors contributing to secondary damage are cytokines, various free radicals, and other substances released from damaged cells in the nervous tissue at the injury site. These factors lead to the recruitment of immune cells to the area, including innate immune cells such as neutrophils, macrophages, and microglia, as well as adaptive immune cells like lymphocytes. These immune cells release a range of inflammatory mediators, including pro-inflammatory cytokines (such as TNF-α and interleukin-6), chemokines (like CXCL1 and CXCL12), nitric oxide, and factors related to apoptosis, including members of the caspase protein family. This cascade of events results in cell death and apoptosis of the affected cells, leading to the destruction of nervous tissue and the initiation of a series of inflammatory responses. Collectively, these factors contribute to the disease's progression to other organs and tissues in the body [
11]. Clinical manifestations linked to secondary spinal cord injury encompass heightened cellular permeability, the activation of apoptosis-related pathways, ischemia, vascular damage, edema formation, the generation of free radicals, disruptions in ion homeostasis, glial scar formation, and inflammation. The cumulative effects of these injuries exacerbate inflammatory responses, resulting in a pro-inflammatory environment. This induced inflammation has the potential to propagate to adjacent tissues and organs, leading to functional impairments and deficiencies [
6,
12,
13,
14,
15].
Research has established that spinal cord injury (SCI) triggers substantial systemic inflammation, which represents a significant consequence of the injury. This inflammatory response promotes the spread of the condition to additional organs and tissues, leading to dysfunction within these systems. The inflammatory process is orchestrated by activating various mediators that initiate, propagate, and sustain inflammation. Among these mediators, cytokines such as tumor necrosis factor-alpha (TNF-α) and apoptosis-regulating proteins, including Bax and Bcl-2, are particularly significant. Numerous studies have underscored the pivotal roles of inflammation and apoptosis in the aftermath of SCI and their contributions to disease progression. It is now recognized that apoptosis is governed by an intracellular proteolytic cascade involving members of the caspase family and the Bcl-2 gene family. Specifically, Bax and Bcl-2 are critical in regulating the promotion and inhibition of apoptosis, respectively. Caspase-3, a key member of the caspase family, is integral to the apoptotic and inflammatory processes that ensue following SCI. These proteins participate in several essential cellular events, including chromatin condensation, DNA fragmentation, degradation of vital cellular proteins, clearance of damaged cells, formation of apoptotic bodies, and the terminal phases of apoptosis. Moreover, several cytokines such as interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α) are essential in the development and spread of inflammation after spinal cord injury (SCI). In particular, TNF-α, primarily produced by macrophages and natural killer cells, is associated with the onset of various inflammatory and autoimmune diseases. Additionally, it promotes the spread of inflammation to other tissues and organs, including the kidneys [
16,
17,
18,
19,
20,
21].
Kidney complications that occur during both the acute and chronic phases subsequent to spinal cord injury (SCI) pose considerable challenges and significant risks to patient health. Spinal cord injury (SCI) disrupts the autonomic nervous system, leading to dysfunction or failure of multiple organs due to the spinal cord's essential role in coordinating bodily functions. The impairment of
kidney function and the emergence of urinary disorders following SCI have been thoroughly documented in the literature, underscoring the necessity for meticulous management and ongoing monitoring of
kidney status in this patient population [
22]. The
kidneys are integral to the regulation of blood pressure, the maintenance of fluid balance, the excretion of waste products, the preservation of electrolyte homeostasis, the stabilization of acid-base equilibrium, and the management of endocrine functions. They are responsible for the synthesis of renin, erythropoietin, and the hydroxylation of vitamin D, thereby exhibiting characteristics akin to those of endocrine glands. The
kidneys receive approximately 25% of the cardiac output, highlighting their pivotal role in blood pressure regulation and overall cardiovascular health.
Kidney dysfunction can significantly disrupt blood pressure regulation and cardiovascular well-being, both directly and indirectly, thereby contributing substantially to mortality rates among individuals with SCI. Given the essential role of the kidneys in maintaining homeostasis and the fact that spinal cord injury (SCI) can lead to severe complications in renal function, it is crucial to assess kidney function as an early indicator for diagnosing complications associated with SCI. Therefore, the early detection and management of
kidney complications in this population are essential to prevent further health issues and to improve diagnostic and therapeutic approaches for prevention and treatment. Recent studies have highlighted the importance of monitoring kidney function in patients with spinal cord injuries (SCI) to enhance management strategies, implement more effective treatments, and reduce the risk of serious health complications [
23,
24,
25].
Numerous studies have shown that natural products and herbal medicines possess a variety of active biological properties, making them highly effective in treating different diseases. Research has demonstrated that the alcoholic extracts of two plants—
Melissa officinalis and
Rosmarinus officinalis—contain a range of substances with potential medicinal effects. Traditional medicine has utilized these extracts to treat a variety of illnesses. For instance,
Rosmarinus officinalis has been used to improve memory and focus, reduce headaches and migraines, treat inflammatory diseases like arthritis, and fortify the immune system.
Melissa officinalis, on the other hand, is acknowledged as a natural sedative that works well for easing stress, treating sleep difficulties, boosting immunity against viruses, and relieving gastrointestinal problems including bloating and spasms. According to recent research, these plants' alcoholic extracts may be very useful in the management, prevention, and treatment of a variety of neurological conditions, including spinal cord injuries, because of their anti-inflammatory, antioxidant, immunomodulatory, and neuroprotective qualities. This study explores the potential benefits of these extracts in improving kidney function and mitigating kidney complications associated with spinal cord injuries [
26,
27,
28].
Melissa Officinalis, commonly referred to as
Lemon balm, is esteemed for its aromatic characteristics and has a longstanding tradition of application in the treatment of diverse health issues. This herb is employed in therapeutic settings as a tonic, antispasmodic, antiseptic, sedative, and analgesic, particularly for mitigating stress-related discomfort and enhancing cognitive performance. Pharmacological investigations have revealed that
Melissa Officinalis exhibits a wide array of biological activities, encompassing antioxidant, hypoglycemic, antidepressant, antimicrobial, and analgesic properties. The essential oil and extracts obtained from this plant are abundant in bioactive compounds, including citronellal, geraniol, citral, terpineol, and rosmarinic acid, which play a significant role in its therapeutic effectiveness and the prevention of various conditions, such as cerebral ischemia, spinal injuries, and Alzheimer's disease [
29,
30].
Rosmarinus officinalis (
Rosmary) , belonging to the
Lamiaceae family, is acknowledged as an aromatic medicinal plant with a wide range of therapeutic properties. These properties encompass antimicrobial, anticancer, antidiabetic, anti-inflammatory, analgesic, antioxidant, anticoagulant, and diuretic effects. Furthermore,
Rosmarinus officinalis's natural essential oils contribute to its extensive application in the perfume industry. The presence of various bioactive compounds in
Rosmarinus officinalis extract, including carnosol, rosmarinic acid, and diterpenes, notably amplifies the therapeutic potential of its alcoholic extract in the prevention, management, and treatment of neurological disorders [
31,
32].
Recent studies indicate that the alcoholic extracts of Melissa officinalis and Rosmarinus officinalis are known as natural compounds with anti-inflammatory and anti-apoptotic properties. These extracts are capable of reducing the expression of apoptotic genes such as Bax, Bcl-2, and Caspase-3, which leads to the reduction of cellular death processes and prevents damage caused by apoptosis. In addition, these extracts can reduce inflammatory factors such as Tumor Necrosis Factor-alpha (TNF-α), helping to prevent the progression of inflammation in damaged tissues. By reducing apoptosis and inflammation, they can protect sensitive tissues such as the spinal cord and kidneys, and may be effective in treating kidney injuries in patients with spinal cord injury. The use of these plants may help reduce kidney tissue destruction and improve kidney function in these patients [
33,
34].
Research has substantiated the varied therapeutic attributes of these two botanical species. For example, a study conducted in 2012 by Mr. Bayat and his associates indicated that the alcoholic extract of
Melissa officinalis demonstrates neuroprotective effects and may be beneficial in both the prevention and treatment of neurological disorders [
30]. Furthermore, a 2017 investigation revealed that the alcoholic extract of
Rosmarinus officinalis also displays neuroprotective properties and has the potential to alleviate the adverse effects associated with spinal cord injuries in laboratory rats [
32]. Additionally, research has demonstrated that the alcoholic extract of Melissa officinalis, due to its anti-inflammatory and antioxidant properties, can aid in improving kidney function and mitigating renal damage caused by oxidative stress [
34]. Similarly, the alcoholic extract of
Rosmarinus officinalis has shown significant protective effects on kidney tissue by reducing inflammation and oxidative damage, which are crucial in preventing renal complications in various disease models [
35]. In light of the bioactive compounds identified in the alcoholic extracts of Melissa officinalis and Rosmarinus officinalis, as well as their significant therapeutic properties, we integrated these extracts into our research framework.
The principal aim of this study is to demonstrate the therapeutic effects of the coadministration of alcoholic extracts of Melissa officinalis and Rosmarinus officinalis on kidney tissue and apoptosis-related gene expression in a rat model of spinal cord injury. Although these extracts exhibit significant therapeutic effects individually, our study demonstrated that the combined therapeutic effects of the two plants are superior. This research seeks to propose a novel, innovative, and economically viable therapeutic approach for this condition, while also offering new insights for future research initiatives.
Materials and Methods:
Gathering and extracting plants
The Firouze Medicinal Plant Garden in Tehran provided the botanical specimens, which were then verified by the Islamic Azad University's Tehran Medical Branch's Faculty of Pharmaceutical Sciences. The following protocols were followed to prepare alcoholic extracts from Melissa officinalis and Rosmarinus officinalis:
The freshly harvested leaves of Melissa officinalis and Rosmarinus officinalis were initially subjected to a crushing and drying process at room temperature. Subsequently, 250 mL of 70% ethanol was added to the crushed plant material to prepare the hydroalcoholic extract. The mixture was agitated every 12 hours for a total of 48 hours to ensure thorough extraction. This extraction was conducted using a 500 mL Erlenmeyer flask equipped with a magnetic stirrer (Model IKA C-MAG HS 7) set at 300 RPM to maintain consistent agitation. After the maceration period, the mixture was filtered using a vacuum filtration apparatus (Model Buchi V-700) at a pressure of 20 inHg to separate the liquid extract from the solid residue. The solid residue was then washed with 70% ethanol and filtered again using the same filtration system. The resulting liquid extract was combined with the initial extract to produce the final hydroalcoholic solution [
30,
36,
37,
38]. A concentrated plant extract weighing 2.4 grams was obtained through vacuum distillation, which effectively removed the solvent. The extraction yield for Rosmarinus officinalis was 20%; therefore, 12 grams of Rosmarinus officinalis leaves were used to produce 2.4 grams of extract. In contrast, the extraction yield for Melissa officinalis was 15%, necessitating 16 grams of Melissa officinalis leaves to obtain the same 2.4 grams of extract. Subsequently, over the course of one day at room temperature, the extracts from Melissa officinalis and Rosmarinus officinalis were combined with 50 mL of saline solution. The experimental rats received daily doses of these solutions via intraperitoneal injection after the extracts had completely dissolved, for a duration of four weeks.
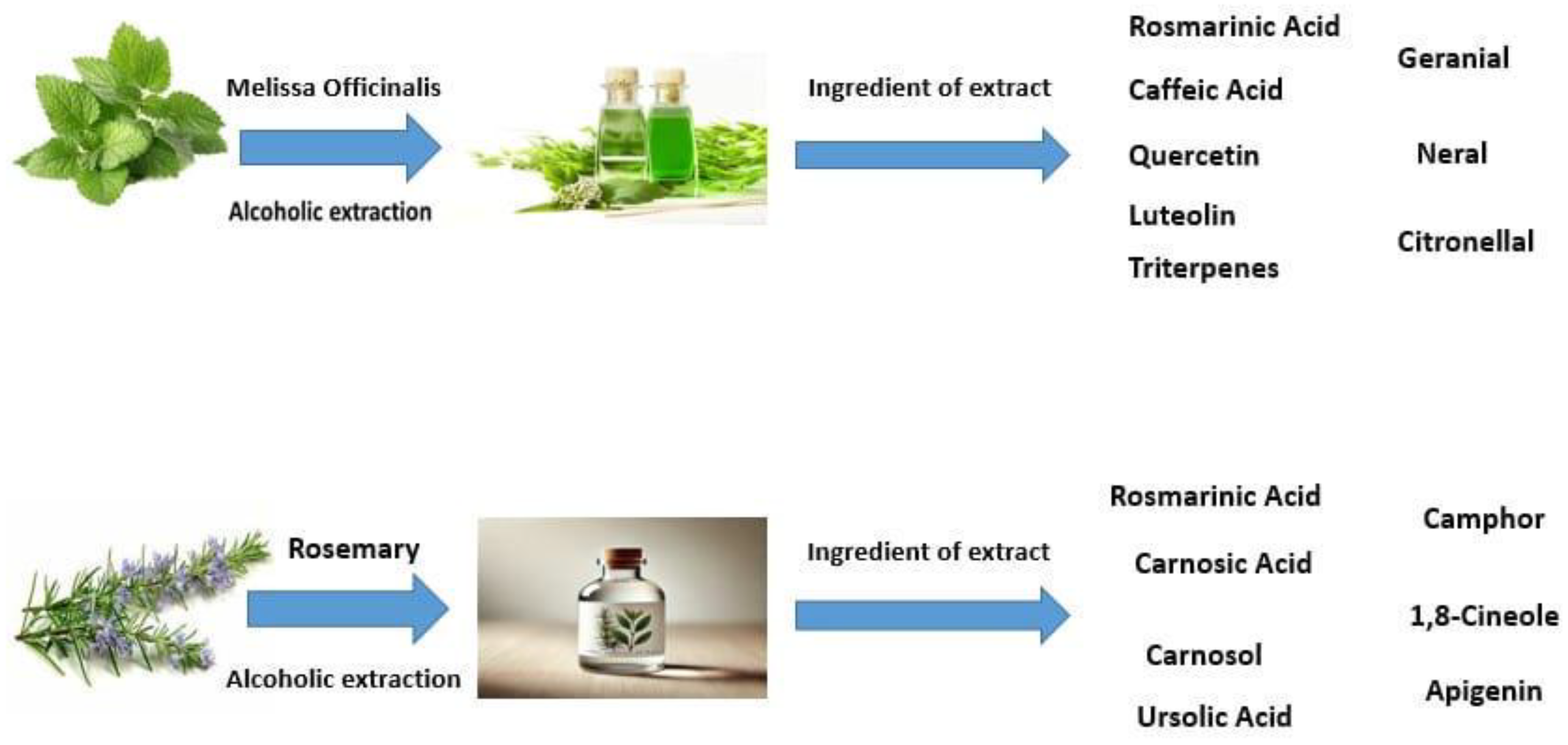
High-performance liquid chromatography (HPLC)
To assess the concentrations of the primary active constituents in the alcoholic extracts of
Rosemary and
Melissa officinalis, reverse-phase high-performance liquid chromatography (HPLC) was utilized in conjunction with data analysis techniques. The HPLC apparatus included a K-1001 pump and a K-2800 photodiode array detector, both sourced from Knauer (Berlin, Germany). Chromatographic separation was achieved using a Eurosphere C18 column characterized by a particle size of 5 micrometers and dimensions of 25 mm × 4.6 mm. Data processing was performed using ChromGate software. The mobile phase consisted of 0.01% formic acid and methanol, with a gradient transition from a ratio of 75:25 to 80:20 (v/v) over 30 minutes, a flow rate of 0.5 mL/min, and a column temperature maintained at 25 °C. The detector was calibrated to a wavelength of 330 nm. A detailed composition of each extract (see
Figure 1) is provided below:
The Rosemary extract contains 13.4 % caffeic acid, 9.52 % rosmarinic acid, 8.8 % cineole, 5.2 % α-pinene, 3.14 % borneol, 1.52 % camphor, and 1.48 % limonene.
The Melissa Officinalis extract includes 41.3 % estragole, 13.4 % limonene, 6.97 % nerol, 6.03 % geranial, 4.13 % nerallyl acetate, 3.08 % geraniol, less than 0.5% caffeic acid, and 0.75 % rosmarinic acid.
Animals
For this research, a group of 35 adult male Wistar rats, each weighing between 200 and 250 grams, was chosen and used. These rats were sourced from the Faculty of Veterinary Medicine at the University of Tehran and were kept under standard laboratory conditions at the animal facility of Tehran University of Medical Sciences. They had unlimited access to food and water. The laboratory conditions were regulated to maintain a temperature of 22 ± 2°C, relative humidity between 10% and 50%, and a 12-hour light-dark cycle. The study focused specifically on healthy rats within the specified weight range. Rats that were sick did not meet the weight criteria, or did not have spinal cord injuries were excluded from the study.
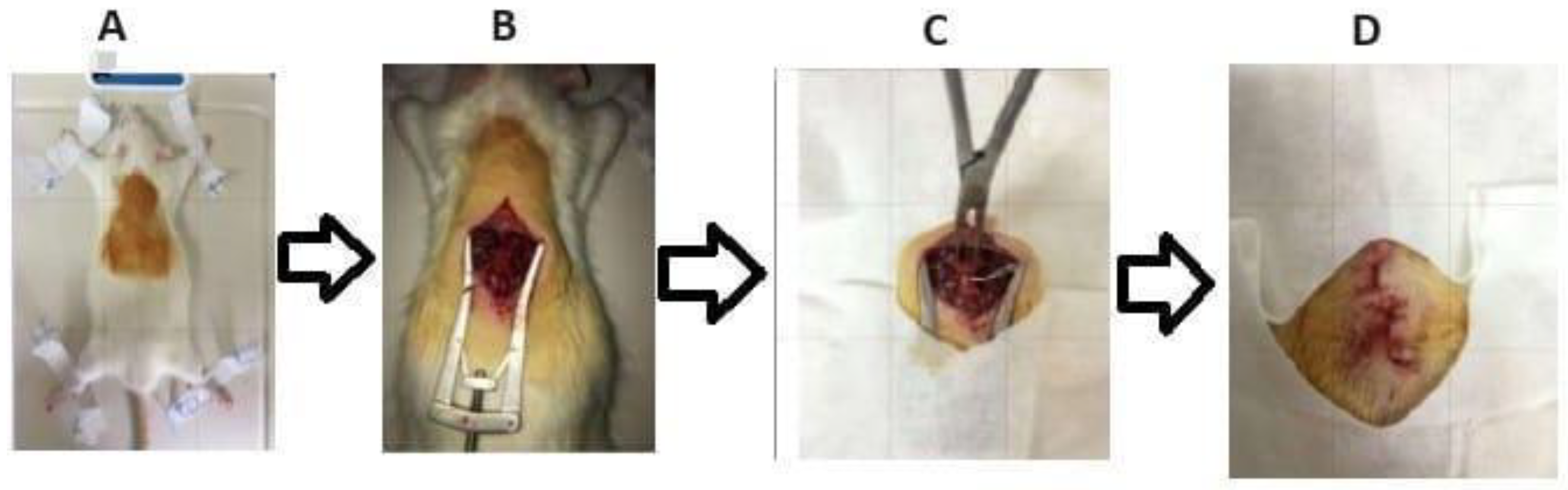
Creating a Model for Spinal Cord Injuries
The subjects were anesthetized with ketamine (80 mg/kg) and xylazine (15 mg/kg), administered through intraperitoneal injection. The dorsal region of the subjects was sterilized with alcohol and betadine, and the hair in the surgical area was removed. A longitudinal incision was made along the midline, extending from the eighth to the twelfth thoracic vertebrae. The fascia and superficial muscles were retracted to reveal the spinous and transverse processes of the vertebrae. The tenth vertebra was located using a specialized retractor, and a laminectomy was performed under a surgical microscope, ensuring the integrity of the dura mater was maintained. Following the exposure of the spinal cord, a unilateral spinal cord injury was induced through a compression technique, utilizing an aneurysm clip applied to the right side of the spinal cord for one minute. To ensure precision, the aneurysm clip was pre-calibrated by adjusting it to a consistent closing pressure, which ensured uniform application of force. This pre-calibration helped achieve controlled damage to the spinal cord, targeting the lateral side to affect the motor neurons responsible for locomotion. Following the release of the clip, visible signs of localized tissue damage, including slight discoloration and reduced blood flow, were observed. The surrounding tissue was meticulously inspected to ensure that no excessive hemorrhaging occurred [
39,
40]. To mitigate the risk of dehydration in the animals, 2-3 milliliters of normal saline were administered subcutaneously in the dorsal region of the neck. Following the infliction of the injury, the surgical site was sutured closed. To minimize the potential for infection at the surgical site, as well as within the central nervous system and urinary system, a subcutaneous injection of 15 mg/kg of gentamicin was administered in the dorsal neck area one day before the surgical procedure and was continued for two days postoperatively. The various stages of spinal cord injury are depicted in
Figure 2 [
41].
The Drug Administration and animal advocacy organizations.
For this study, the animals were randomly divided into five separate groups, each containing seven Wistar rats:
1. Control Group (L): This group of rats underwent laminectomy surgery without any resultant spinal cord damage.
2. Control Group (S): The control group (S) consisted of rats that experienced spinal cord injuries for this research. These rats were given daily subcutaneous injections of 100 mg/kg of a normal saline solution, beginning 24 hours post-injury and lasting for four weeks.
3. Treatment Group 1 (M): consisted of rats with spinal cord injuries that were administered a hydroalcoholic extract of Melissa officinalis via subcutaneous injection at a dosage of 150 mg/kg at the nape of the neck. This therapy started the day after the injury and continued every day for four weeks.
4. Treatment Group 1 (R): consisted of rats with spinal cord injuries that were administered a hydroalcoholic extract of Rosemary via subcutaneous injection at a dosage of 150 mg/kg at the nape of the neck. This therapy started the day after the injury and continued every day for four weeks.
5. Treatment Group 3 (R+M) involved treating spinal cord injured rats concurrently with a combination of hydroalcoholic extracts of Melissa officinalis and Rosemary. Every extract was injected subcutaneously into the nape of the neck at half the recommended dosage. Following the establishment of the spinal cord injury model, this treatment started right away and lasted for four weeks every day.
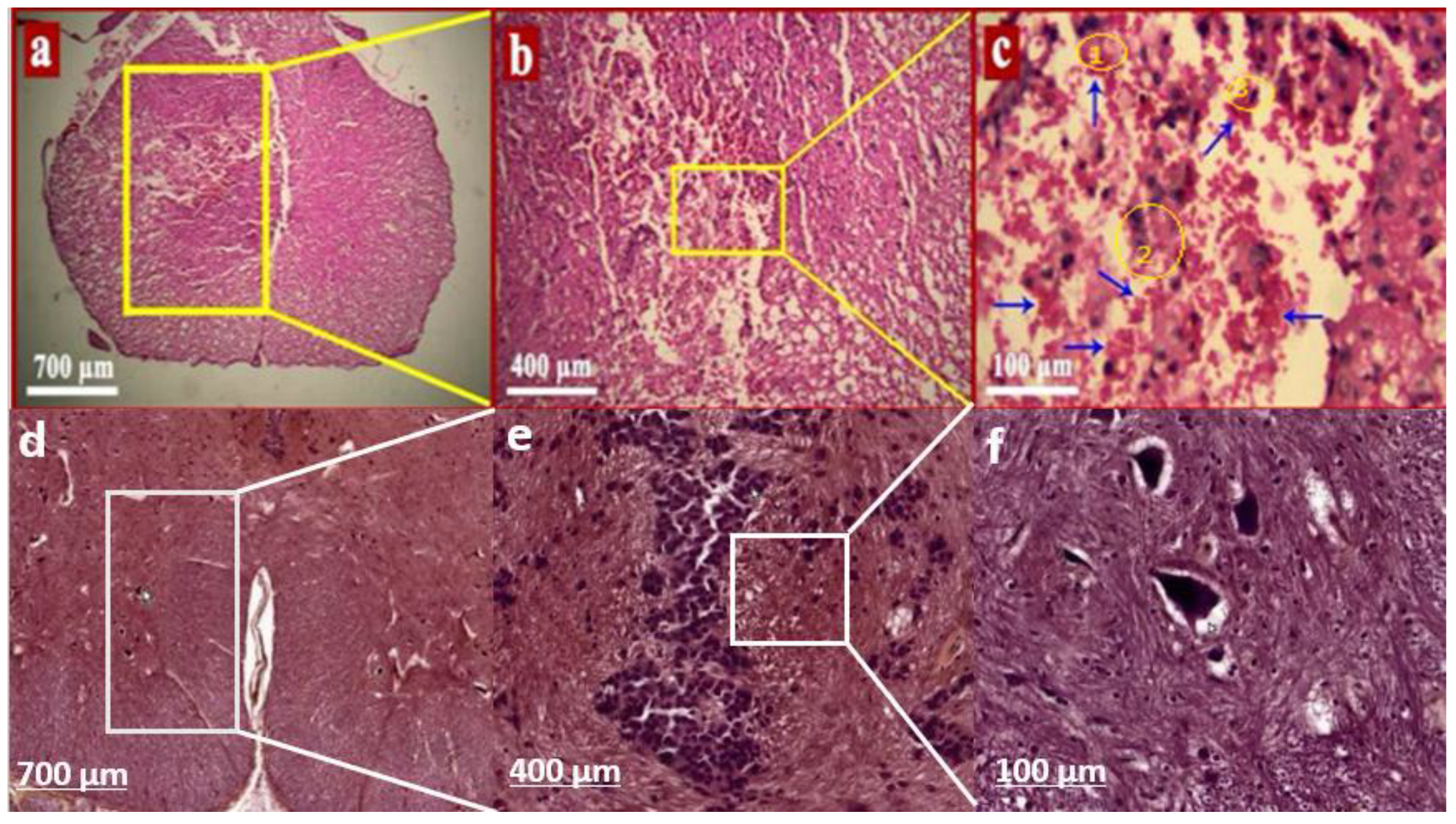
Assessment of the Spinal Cord Injury Model
A tissue sample was obtained from the injured region to confirm the spinal cord injury model. Microscopic examinations following hematoxylin-eosin staining revealed a compressed region on the right side of the spinal cord in the samples, indicating damage to this area. Furthermore, the analyzed regions showed signs of emptiness, suggesting the presence of axonal loss or degeneration. These findings support the validity of the spinal cord injury model (see
Figure 3).
Kidney Tissue Specimen Preparation
After establishing the spinal cord injury model and completing a four-week treatment period, the
rats were euthanized to collect kidney tissue samples. Sodium pentobarbital was administered intraperitoneally at a dose of 100 mg/kg to ensure deep and irreversible anesthesia. Following this,
0.9% saline solution and 10% buffered formalin were injected into the heart to perfuse and fix the tissue [
42]
. A midline incision was then made in the abdominal skin, and the right kidney was removed from four randomly chosen rats in each experimental group. The kidneys were quickly placed in liquid nitrogen for rapid freezing and preservation and later stored at -80 degrees Celsius for future biochemical analyses. The remaining three rats from each group had their kidneys preserved in 10% formalin for histopathological examination and tissue analysis.
The preparation of tissue sections is a meticulous procedure that consists of seven distinct phases. Initially, tissue specimens are fixed in a 10% formalin solution to preserve their structural integrity. Following fixation, the samples undergo a dehydration process, which involves sequential immersion in 70% ethanol, followed by 90% ethanol, and ultimately three immersions in 100% ethanol. In the next phase, the samples are subjected to three one-hour immersions in xylene to remove any residual water, thereby preparing the tissue for paraffin infiltration. The subsequent step involves placing the samples in molten paraffin to ensure thorough permeation of the tissue. After complete infiltration, the samples are carefully positioned in molds filled with molten paraffin, ensuring that any remaining voids are filled, resulting in the complete encapsulation of the tissue specimens. During the sectioning phase, thin sections approximately 5 micrometers thick are cut from the paraffin-embedded blocks using a microtome, facilitating precise examination. Finally, the tissue sections are stained using the Hematoxylin and Eosin (H&E) staining technique, which enhances contrast and highlights cellular components. This comprehensive and precise methodology is essential for the accurate preparation of kidney tissue samples and is critical for enabling subsequent biochemical and histopathological analyses.
Neurological Examination
To assess neurological function in rats, the Basso-Beattie-Bresnahan (BBB) scale was employed, which is specifically designed to evaluate motor function and mobility in an open-field environment. This scale operates on a continuum from 0 to 21 and encompasses various aspects of hind-limb motor performance, including weight support, stepping ability, coordination, and toe spread. Functional assessments were performed and recorded on days 1, 7, 14, 21, and 28 by two researchers who were blinded to the treatment conditions. The final functional score for each rat was determined by calculating the average of the scores obtained from both evaluators [
43,
44].
Pain sensitivity was evaluated through behavioral tests using the hot water method on the hind limbs of rats after spinal cord injury (SCI). Functional scores were recorded on days 1, 7, 14, 21, and 28. The response to heat was measured by timing how long it took for the rats to withdraw their hind paw from water heated to 60°C. Each rat had its paws individually placed in containers of hot water, and a total of six trials (three for each paw) were conducted for every rat. The average reaction time from these trials was noted. Rats that did not react to the heat were taken out of the hot water after 60 seconds [
45,
46].
Evaluation of Changes in the Structure of Kidney Tissue
The kidney tissue samples were analyzed using a light microscope. In the field of histology and histological research, each sample was evaluated from three different perspectives utilizing Dino Capture software (Version 2, developed by Dino-Lite Company, Netherlands), with measurements recorded in micrometers (μm). The analysis included the dimensions of various anatomical structures, such as the Bowman's capsule, glomerulus, urinary space, proximal tubule, lumen of the proximal tubule, height of the proximal tubule epithelium, diameter of the distal tubule, lumen of the distal tubule, and height of the distal tubule epithelium. These measurements were performed using the aforementioned software.
Evaluation of Gene Expression RNA Isolation:
RNA was isolated using TRIzol reagent (RNX) (Invitrogen, USA) from the kidney tissue of experimental rats, utilizing four rat kidneys per group, with approximately 100 mg of kidney tissue per rat for RNA extraction. The tissue was homogenized in 1 mL of TRIzol reagent per 100 mg of tissue to ensure complete lysis. Prior to extraction, the tissue samples were stored at -80°C to preserve RNA integrity. For transport, the samples were maintained on dry ice to prevent RNA degradation during transit. After homogenization, 200 μL of chloroform was added to the mixture, which was then thoroughly vortexed for 15 seconds and allowed to equilibrate at room temperature for 5 minutes. The sample was subsequently centrifuged at 4°C for 1 to 5 minutes at a relative centrifugal force (RCF) of 12,000 × g, resulting in phase separation. The RNA, located in the aqueous phase (upper phase), was carefully collected.
For RNA precipitation, an equal volume of isopropanol (typically 1 mL for every 1 mL of TRIzol used) was added to the collected upper phase. The sample was then incubated at -20°C for 1 hour. Following incubation, the sample was centrifuged at 4°C at 12,000 × g for 15 minutes to pellet the RNA. The supernatant was discarded, and the RNA pellet was washed with 75% ethanol prepared using nuclease-free water. One milliliter of 75% ethanol was added to the pellet, and the sample was gently vortexed before being centrifuged at 4°C for 10 minutes at 8,000 × g. The ethanol was carefully removed, and the pellet was air-dried at room temperature for 15 minutes to ensure the complete removal of ethanol.
Finally, the quantification and purity of RNA were assessed using a NanoDrop spectrophotometer.
Synthesis of cDNA from Total RNA:
The initial stage of reverse transcription polymerase chain reaction (RT-PCR) involves synthesizing complementary DNA (cDNA) from RNA using universal primers, such as dT-Oligo or hexamer random primers. Accurate quantification of mRNA levels is essential for analyzing gene expression. Prior to cDNA synthesis, the purity of the RNA was assessed using spectrophotometry with a Nanodrop spectrophotometer, measuring absorbance at 260 nm (A260) and 280 nm (A280) wavelengths to evaluate RNA purity. The A260/A280 ratio was employed to confirm RNA quality, with an optimal value ranging from 1.8 to 2.0 indicating pure RNA. Additionally, RNA integrity was verified through agarose gel electrophoresis to check for intact ribosomal RNA (rRNA) bands, ensuring the absence of RNA degradation. Only RNA samples exhibiting a clear, sharp band pattern were utilized for cDNA synthesis.
For cDNA synthesis, the Premix PCR-2xRT 2-Step Kit (Biofact, Korea) was utilized. This kit contains a reverse transcriptase enzyme along with the necessary reagents for synthesizing cDNA from RNA templates. In this study, 0.5 μg of RNA was used as the template for reverse transcription in a 20 μL reaction volume. The dT-Oligo primer was added at a final concentration of 1 μM, and the reaction mixture was incubated at 42°C for 60 minutes to ensure optimal reverse transcription. Following this incubation, the reaction was terminated by heating at 95°C for 5 minutes to inactivate the reverse transcriptase enzyme. The synthesized cDNA was subsequently employed for gene expression analysis.
Real-time PCR reaction:
In this study, quantitative PCR (qPCR) was utilized to evaluate the expression levels of key target genes, including TNF-α, Caspase-3, Bcl-2, and Bax, which were selected for their essential roles in apoptosis and inflammatory pathways. RNA was extracted from kidney tissue samples, and the expression of these genes was quantified relative to the reference gene β2M, chosen for normalization due to its stable expression across various experimental conditions. A comprehensive list of primers for the genes of interest is presented in
Table 1.
For cDNA synthesis, 0.5 μg of RNA was used as the template, and cDNA was synthesized using the Premix PCR-2xRT 2-Step Kit (Biofact, Korea) according to the manufacturer's protocol. Each qPCR reaction was performed in triplicate to ensure the reliability of the data. The reaction mixture consisted of 1 μL of cDNA, 10 μL of SYBR Green Master Mix (Biofact, Korea), 1 μL of each forward and reverse primer (final concentration of 0.5 μM), and 7 μL of nuclease-free water, resulting in a total volume of 20 μL.
The thermocycling conditions were as follows: an initial denaturation at 95°C for 5 minutes, followed by 40 cycles consisting of denaturation at 95°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 30 seconds. Fluorescence was measured at the end of each cycle to monitor the amplification process. The efficiency of the amplification was evaluated using a standard curve, which demonstrated an optimal amplification efficiency range of 90-110% and an R² value exceeding 0.99, thereby confirming the reliability of the amplification process. Melt curve analysis was conducted to verify the specificity of the amplification, with a single peak indicating the absence of non-specific products.
The relative gene expression was calculated using the ΔΔCt method. The ΔCt for each sample was determined by subtracting the Ct value of the reference gene (β2M) from the Ct value of the target gene. The ΔΔCt was then calculated by subtracting the ΔCt of the control group from that of the experimental group. Relative expression levels were determined using the formula: Relative Expression = 2^(-ΔΔCt).
Data analysis was performed using GraphPad Prism 10.0 (GraphPad Software, San Diego, CA). Statistical significance was assessed by comparing relative expression values among experimental groups, with p-values less than 0.05 deemed statistically significant.
Primer Design:
Gene sequences for Caspase-3, Bax, Bcl-2, and TNF-α were retrieved from the NCBI database. Primers for these genes were designed using R software to ensure optimal amplification efficiency and specificity. The design process was conducted with careful attention to key parameters, including primer length, melting temperature (Tm), and GC content, all of which contribute to the reliability and reproducibility of quantitative PCR (qPCR) results.
The primers were designed to ensure the specific amplification of target genes while minimizing non-specific binding and primer-dimer formation. The reference gene β2M was selected for normalization due to its stable expression under experimental conditions. The sequences of the forward and reverse primers, along with their corresponding product sizes and melting temperatures (Tm), are presented in
Table 1 below.
Statistical analysis:
Histological changes in kidney tissue were assessed using light microscopy, and image analysis was conducted with Dino Capture Software (Version 2, Dino-Lite Company, Netherlands). The sensory and motor functions of the rats were evaluated using the Basso-Beattie-Bresnahan (BBB) scale to assess locomotor recovery following spinal cord injury. The expression levels of key apoptotic and inflammatory genes were quantified through quantitative real-time PCR (qRT-PCR). The analyzed data included gene expression levels, motor function scores, and histological alterations. Results are presented as means ± standard deviation (SD) for continuous variables, with statistical significance defined as p < 0.05.
Data preparation involved normalizing gene expression data using the ΔΔCt method, with β-actin (ACTB) and β2-microglobulin (B2M) serving as internal controls. The inclusion of B2M as a normalization factor is crucial for correcting variability in RNA quantity or quality between samples, ensuring that observed changes in gene expression reflect biological differences rather than technical artifacts. The normality of the data distribution was assessed using the Shapiro-Wilk test, while the homogeneity of variances was evaluated using Levene's test; both are essential assumptions for conducting ANOVA.
Data analysis was conducted using GraphPad Prism 10.0 (GraphPad Software, San Diego, CA) and R software (version 4.4.2, R Foundation for Statistical Computing, Vienna, Austria). Between-group comparisons were performed using one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test for multiple comparisons. Significant differences were assessed by comparing relative expression levels between the experimental and control groups. Statistical significance was defined as p < 0.05.
Results
During all phases of the experiments, a substantial disparity was noted between the spinal cord injury (SCI) group and the control group. Nevertheless, this disparity was markedly reduced in the treated groups. Statistical analysis consistently demonstrated a p-value less than 0.05 (p < 0.05), highlighting the significance of the results. This difference constituted the principal criterion for confirming the establishment of the SCI model.
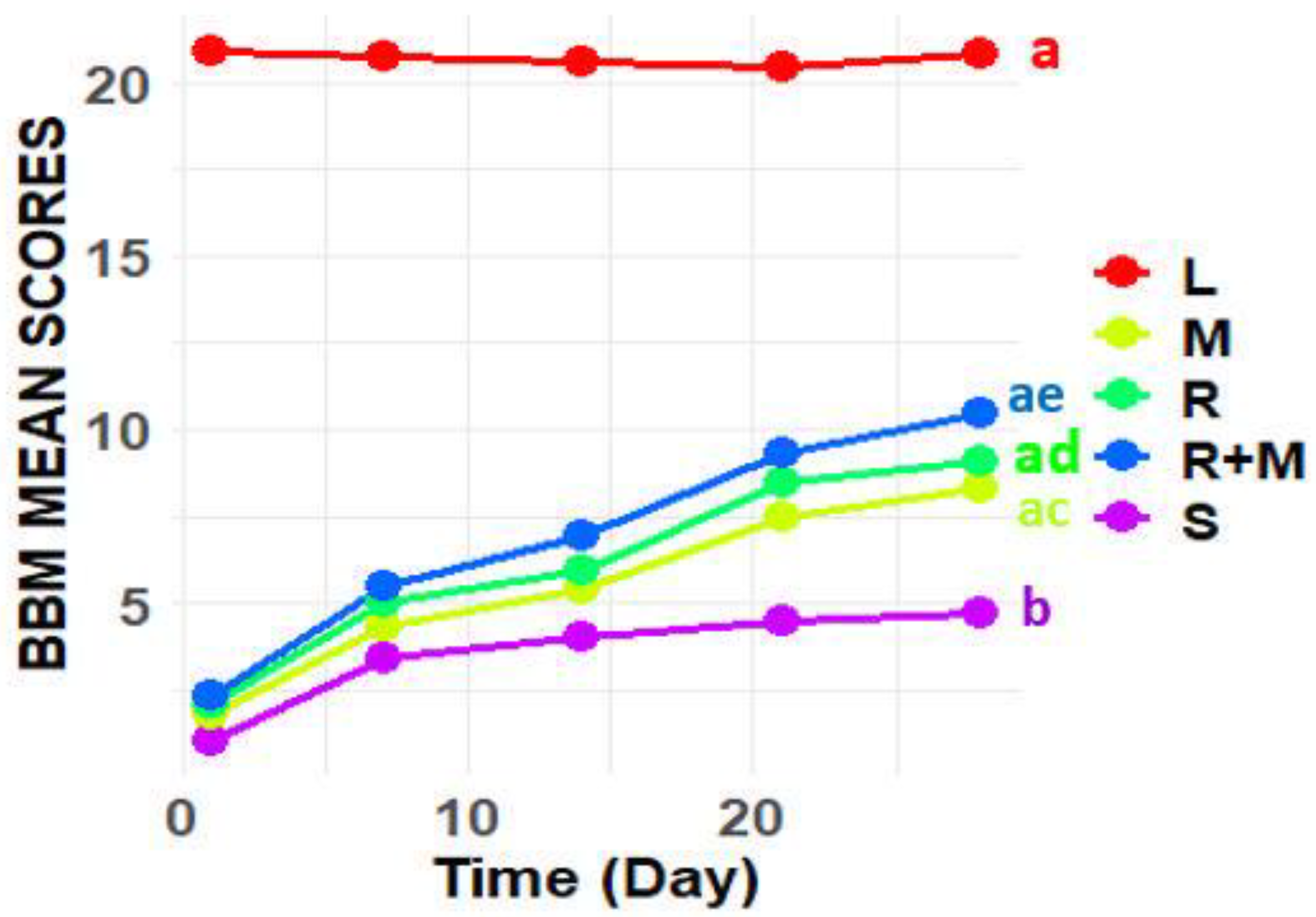
Results of Neurological Performance
The use of alcoholic extracts from
Melissa officinalis and
Rosmarinus officinalis resulted in enhanced motor function following spinal cord injury, which caused paraplegia (loss of motor function in the hind limbs). Additionally, the spinal cord injury group exhibited significant changes in motor scores compared to the control group (healthy group).
Statistical analyses, including the Shapiro-Wilk test for normality, revealed that the data were normally distributed (p > 0.05, Shapiro-Wilk test). The administration of
Melissa officinalis alcoholic extract significantly improved motor function in rats compared to the spinal cord injury group. However, when
Rosmarinus officinalis alcoholic extract was used for treatment, motor function in rats improved significantly compared to both the spinal cord injury group and the group treated with
Melissa officinalis alcoholic extract.
One-way ANOVA and Tukey’s post-hoc test revealed significant interaction effects between treatment variables and time (p < 0.01). The Bonferroni post-hoc multiple comparisons test demonstrated a significant improvement in motor function following treatment with
Melissa officinalis alcoholic extract at a dose of 150 mg/kg on days 14, 21, and 28 (p < 0.001), as well as treatment with
Rosmarinus officinalis alcoholic extract at a dose of 150 mg/kg on days 7, 14, 21, and 28 (p < 0.01). The combined use of alcoholic extracts from
Melissa officinalis and
Rosmarinus officinalis significantly improved motor function on days 14, 21, and 28 (p < 0.001; see
Figure 4).
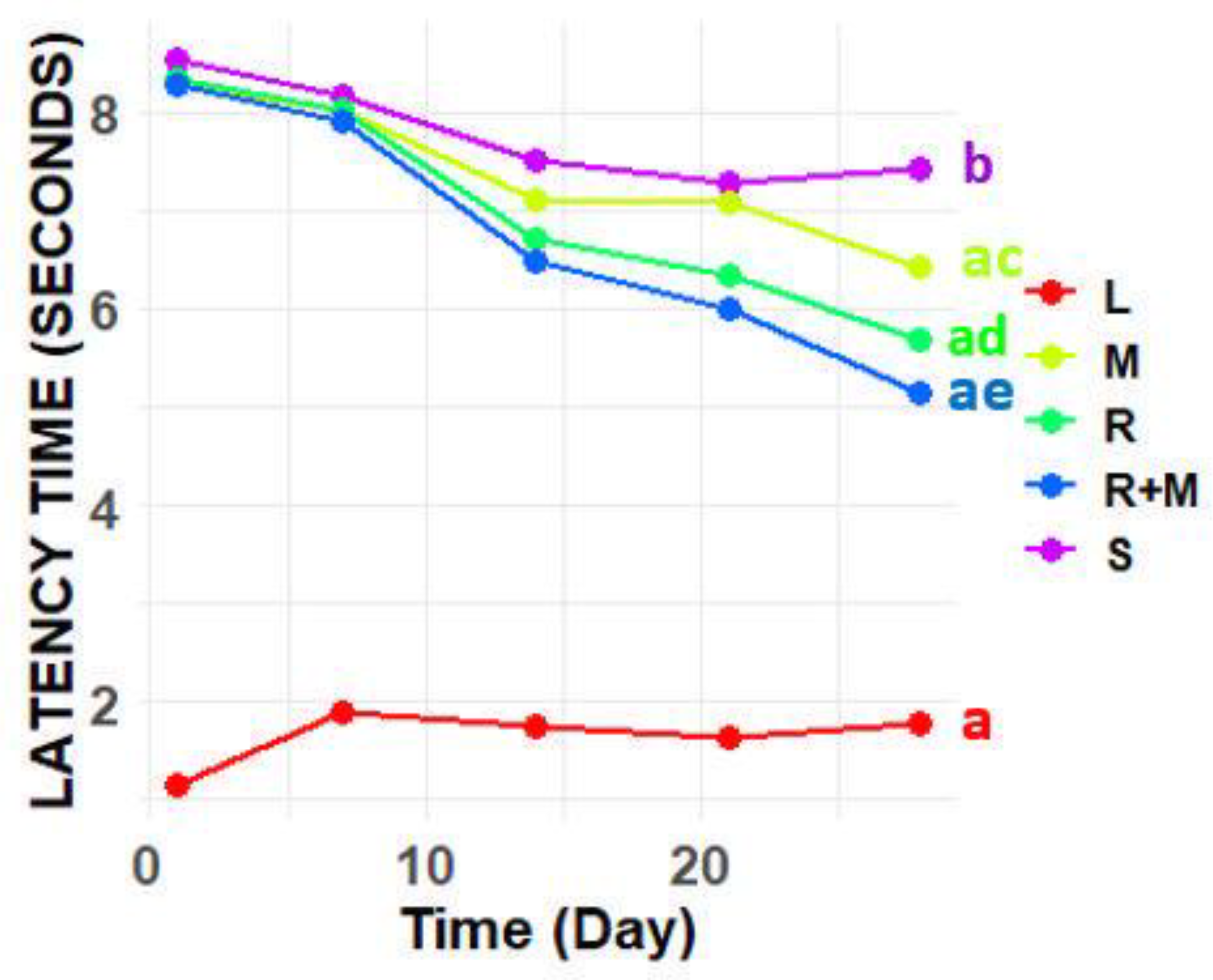
The concurrent administration of alcoholic extracts derived from
Melissa officinalis and
Rosmarinus officinalis demonstrated a notable enhancement in sensory function following spinal cord injury.
Statistical analyses, including the Shapiro-Wilk test for normality, revealed that the data were normally distributed (p > 0.05, Shapiro-Wilk test). The mean latency to respond to painful stimuli in the group receiving the
Melissa officinalis extract was significantly reduced in comparison to the spinal cord injury cohort. Furthermore, the application of
Rosmarinus officinalis alcoholic extract as a therapeutic intervention resulted in a marked improvement in sensory function in rats, surpassing the outcomes observed in both the spinal cord injury group and the group treated with
Melissa officinalis extract. Additionally, the combined administration of alcoholic extracts from
Melissa officinalis and
Rosmarinus officinalis led to a significant enhancement in sensory function relative to the other treatment groups and the spinal cord injury group.
One-way ANOVA and Tukey’s post-hoc test indicated a significant interaction effect among the variables, including treatment and time (p < 0.001). The Bonferroni post-hoc multiple comparisons test further illustrated that sensory function significantly improved following the administration of
Melissa officinalis extract compared to the spinal cord injury group on days 14, 21, and 28 (p < 0.01), post-injury. Similarly, treatment with
Rosmarinus officinalis extract also resulted in a reduction of the mean response time to painful stimuli compared to the spinal cord injury group on the same days (p < 0.01). Notably, the simultaneous administration of alcoholic extracts from both
Rosmarinus officinalis and
Melissa officinalis yielded significant improvements in performance on days 14, 21, and 28 (p < 0.001; see
Figure 5).
Outcomes of alterations in texture:.
The current investigation examined the therapeutic effects of alcoholic extracts obtained from two botanical sources, namely
Rosmarinus officinalis and
Melissa officinalis, in a rat model of spinal cord injury. The primary aim of this study was to evaluate the impact of spinal cord injury on renal tissue and the subsequent functional alterations in the affected rats. Additionally, the research sought to compare these structural and functional changes across various experimental groups in relation to a control group comprising healthy rats. Furthermore, the study aimed to assess the therapeutic efficacy of the alcoholic extracts of
Rosmarinus officinalis and
Melissa officinalis in ameliorating renal tissue damage and enhancing functional outcomes in rats with spinal cord injuries, as compared to the injury group. To facilitate this investigation, the rats were categorized into five distinct groups: a control group (healthy), a spinal cord injury group, and three spinal cord-injured groups, each receiving different alcoholic extracts. One group was administered the alcoholic extract of
Melissa officinalis, another received the alcoholic extract of
Rosmarinus officinalis, and the third group underwent a combined treatment with both extracts. The findings showed that alcoholic extracts of both
Rosmarinus officinalis and
Melissa officinalis, used individually or together, offered substantial therapeutic advantages. These extracts significantly improved changes in renal tissue caused by spinal cord injury in rat models and enhanced renal function. These findings are visually represented in
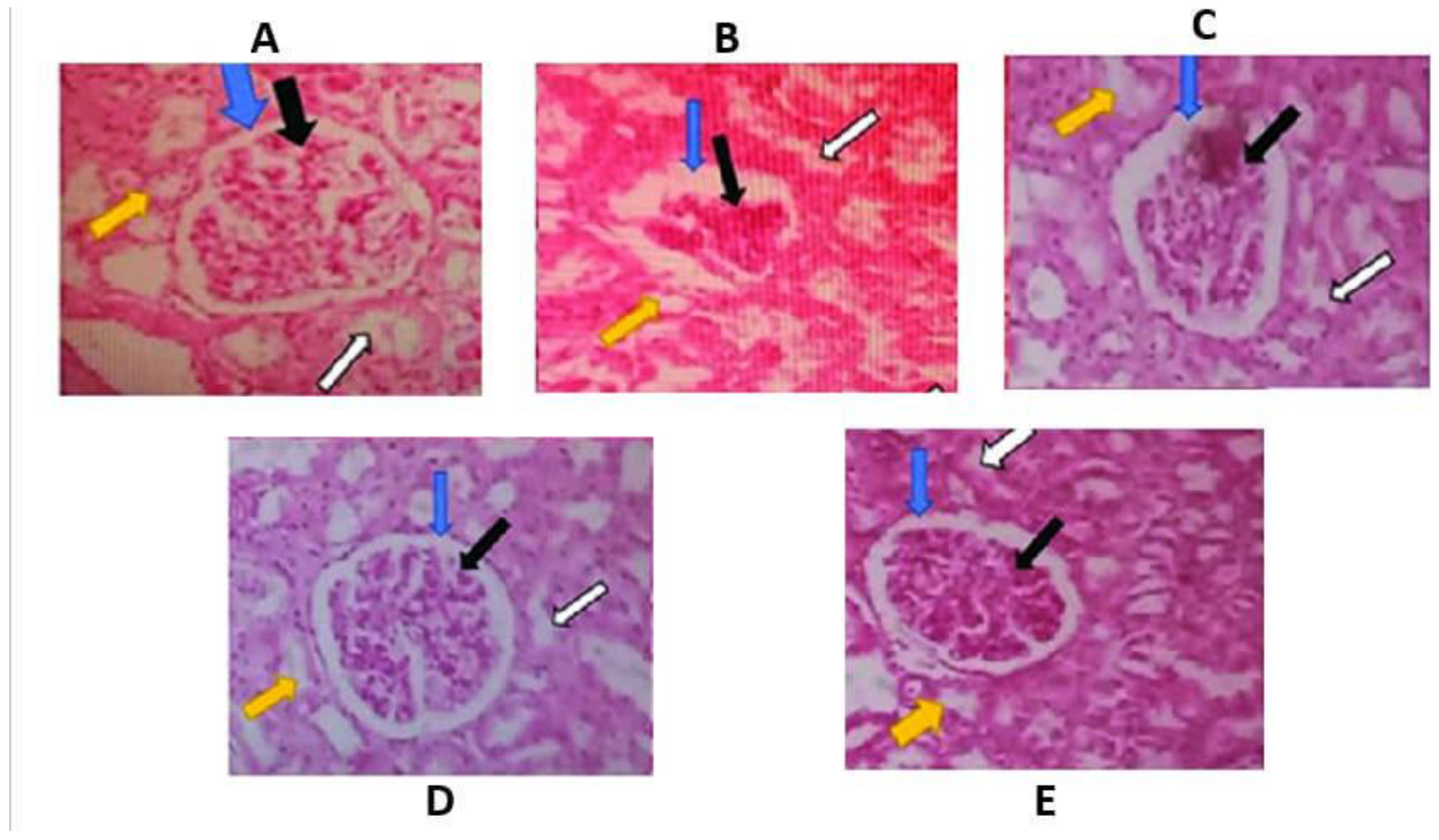
Figure 6, which presents images of kidney tissue samples from all five groups: the control group (A), the spinal cord injury group (B), the group treated with
Melissa officinalis extract (C), the group treated with
Rosmarinus officinalis extract (D), and the combined treatment group (E). The images effectively depict the observed tissue changes across the different groups.
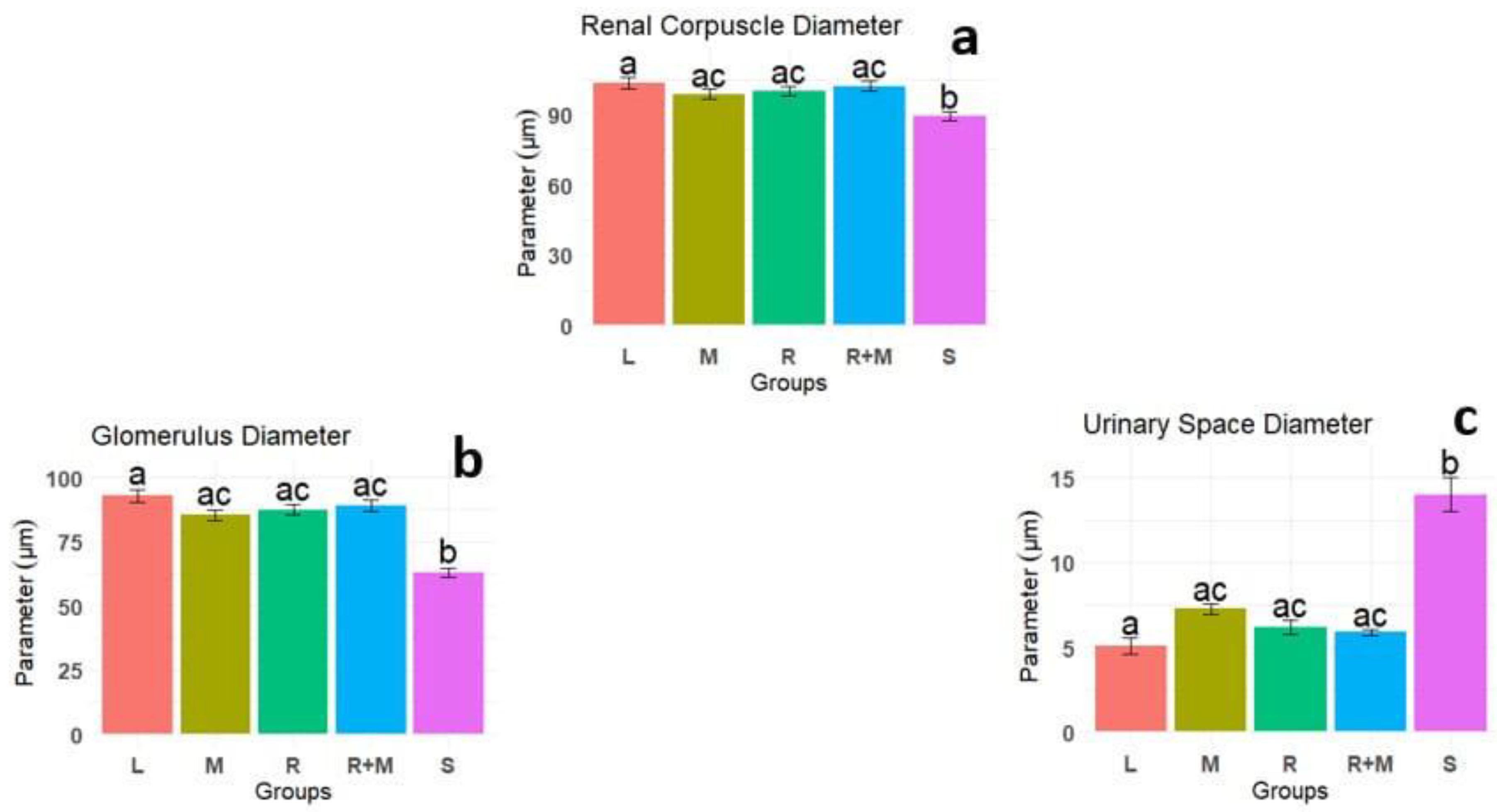
The subcutaneous administration of alcoholic extracts derived from
Rosmarinus officinalis and
Melissa officinalis resulted in an increase in the diameters of renal corpuscles and glomeruli, alongside a reduction in the diameter of the urinary space.
Histological analysis, complemented by one-way ANOVA and Tukey’s post-hoc test, and normality verified by the Shapiro-Wilk test, indicated that the kidney tissue in the control group, which received physiological saline, maintained a normal structural integrity without any discernible pathological lesions. Conversely, rats with spinal cord injury (SCI) exhibited a significant decrease in the diameters of renal corpuscles and glomeruli when compared to the control group, while the urinary space was found to be enlarged (p < 0.001). In the cohort treated with
Melissa officinalis extract, there was a significant enhancement in the diameters of renal corpuscles and glomeruli, coupled with a marked reduction in the diameter of the urinary space, relative to the SCI group (p < 0.01). Treatment with
Rosmarinus officinalis extract yielded an even greater improvement in the mean diameters of renal corpuscles, glomeruli, and urinary space compared to both the SCI group and the
Melissa officinalis-treated group. Moreover, the concurrent administration of both extracts resulted in a significant decrease in the diameters of renal corpuscles and glomeruli, as well as a substantial increase in urinary space when compared to the other treatment groups and the SCI group (p < 0.01; refer to
Figure 7). Thus, the simultaneous application of both alcoholic extracts exhibited more pronounced therapeutic effects than those recorded in the other treatment groups.
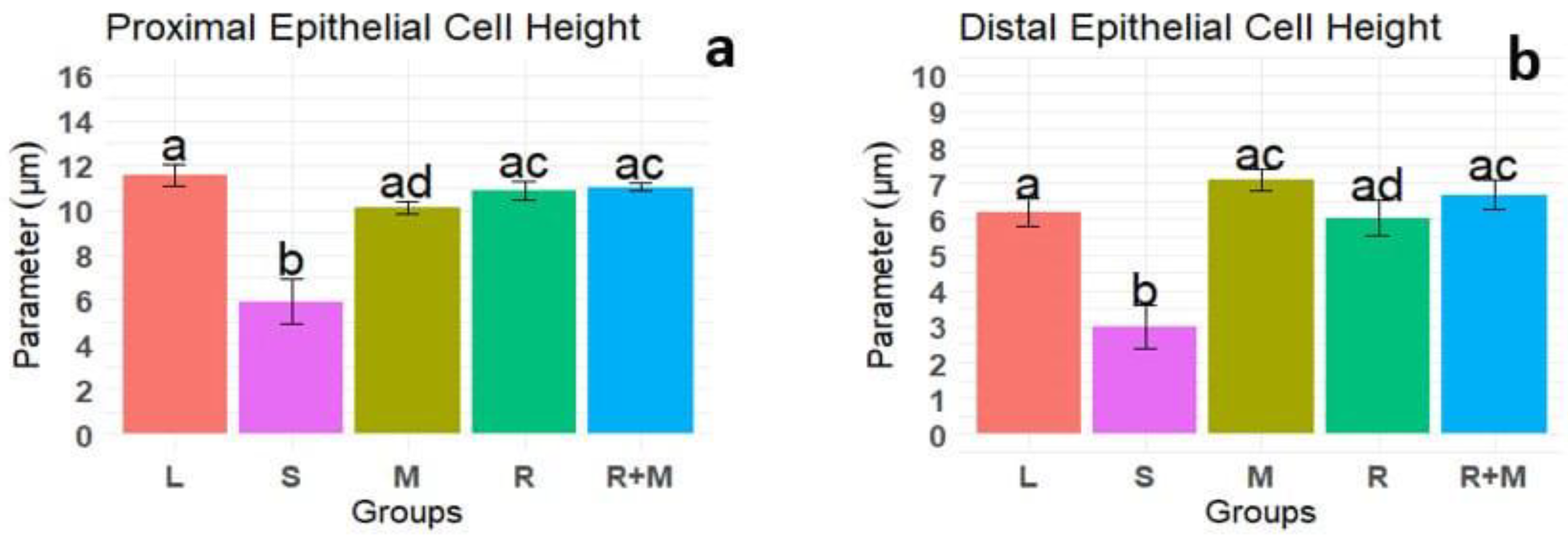
The administration of alcoholic extracts derived from
Melissa officinalis and
Rosmarinus officinalis resulted in a significant enhancement in the height of both proximal and distal epithelial cells following spinal cord injury.
Statistical analyses, including the Shapiro-Wilk test for normality, revealed that the data were normally distributed (p > 0.05, Shapiro-Wilk test). The height of these epithelial cells in the spinal cord injury (SCI) group was markedly lower than that observed in the control group (p < 0.03). One-way ANOVA and Tukey's post-hoc test results demonstrated that treatment with the alcoholic extract of
Melissa officinalis significantly increased proximal epithelial cells' height compared to the SCI group (p < 0.02). Additionally, the height of distal epithelial cells in this treatment group also exhibited a significant increase relative to other experimental groups (p < 0.02). Furthermore, the application of extracts from
Rosmarinus officinalis and
Melissa officinalis simultaneously led to a noticeably higher increase in the height of both proximal and distal epithelial cells in comparison to the control group, the group that received only
Rosmarinus officinalis extract, and the SCI group (p < 0.001). The height of the proximal and distal epithelial cells was also considerably increased by treatment with
Rosmarinus officinalis extract alone as compared to the SCI group (p < 0.01; see
Figure 8). When compared to the other treatment groups, this one showed better therapeutic results since the
Rosmarinus officinalis-treated group's epithelial cell height was closest to that of the control group (
Figure 8).
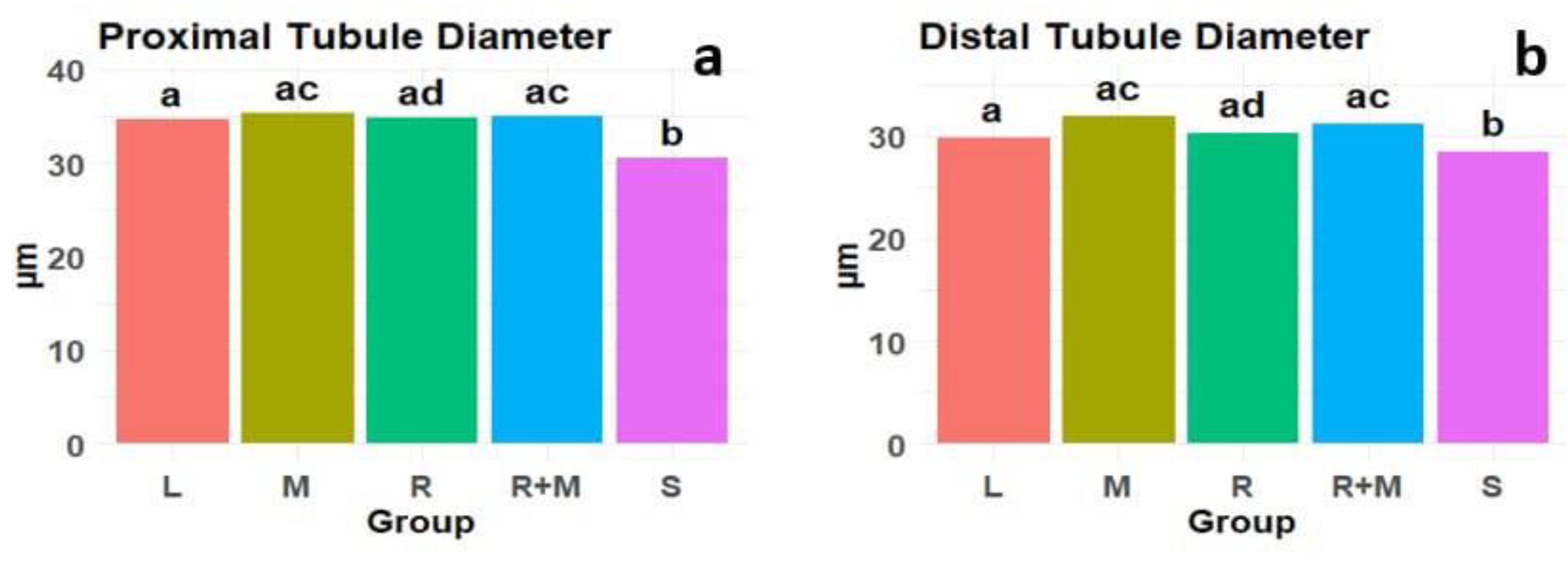
The administration of an alcoholic extract derived from
Rosmarinus officinalis exhibited notable therapeutic effects on the diameters of renal proximal and distal tubules
. Statistical analyses, including the Shapiro-Wilk test for normality, revealed that the data were normally distributed (p > 0.05, Shapiro-Wilk test). The diameters of these tubules in the spinal cord injury (SCI) group were significantly reduced compared to those in the control group (p < 0.007). A one-way ANOVA, followed by Tukey’s post-hoc test, indicated that in the group treated with the alcoholic extract of
Melissa officinalis, the diameters of both proximal and distal tubules, which had been diminished due to spinal cord injury, significantly increased in comparison to the other experimental groups (p = 0.0073). Moreover, tubule diameters were significantly increased in the group treated with a combination of alcoholic extracts from
Rosmarinus officinalis and
Melissa officinalis compared to the SCI group, the control group, and the group treated with
Rosmarinus officinalis alone (p < 0.01). When compared to the SCI group, the group treated with the
Rosmarinus officinalis extract alone showed a substantial increase in proximal tubule diameter, indicating the most marked improvement in both proximal and distal tubule diameters among the different treatment groups. As a result, this therapy demonstrated better therapeutic effectiveness (p = 0.0045; see
Figure 9).
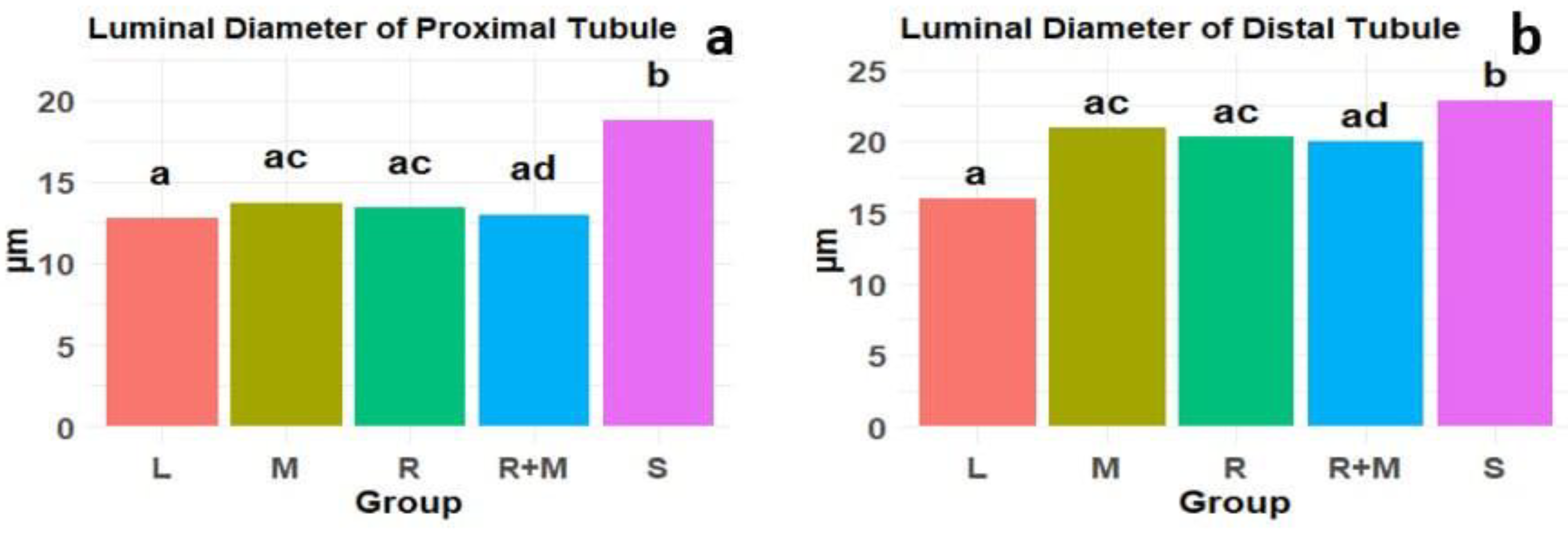
The concurrent administration of alcoholic extracts from
Melissa officinalis and
Rosmarinus officinalis resulted in a significant increase in the lumen diameter of both proximal and distal renal tubules.
Statistical analyses, including the Shapiro-Wilk test for normality, revealed that the data were normally distributed (p > 0.05, Shapiro-Wilk test). In the spinal cord injury cohort, the lumen diameter of these renal tubules was markedly greater than that observed in the control group (p < 0.03). The proximal and distal tubule lumen diameters of the
Melissa officinalis group showed a significant decrease compared to the spinal cord injury group, according to the one-way ANOVA and Tukey's test results. Additionally, the group administered with the alcoholic extract of
Rosmarinus officinalis showed a significant decrease in lumen diameter (p = 0.0065) compared to both the spinal cord injury group and the
Melissa officinalis-treated group, despite the lumen diameter having previously increased due to spinal cord injury. Additionally, the combined administration of both extracts led to a significant reduction in the proximal and distal renal tubules' lumen diameter compared to the other treatment groups and the spinal cord injury group, suggesting a notable structural improvement (p = 0.0047; see
Figure 10). These findings support the conclusion that the simultaneous application of alcoholic extracts from both plants demonstrates superior therapeutic efficacy compared to the other treatment modalities.
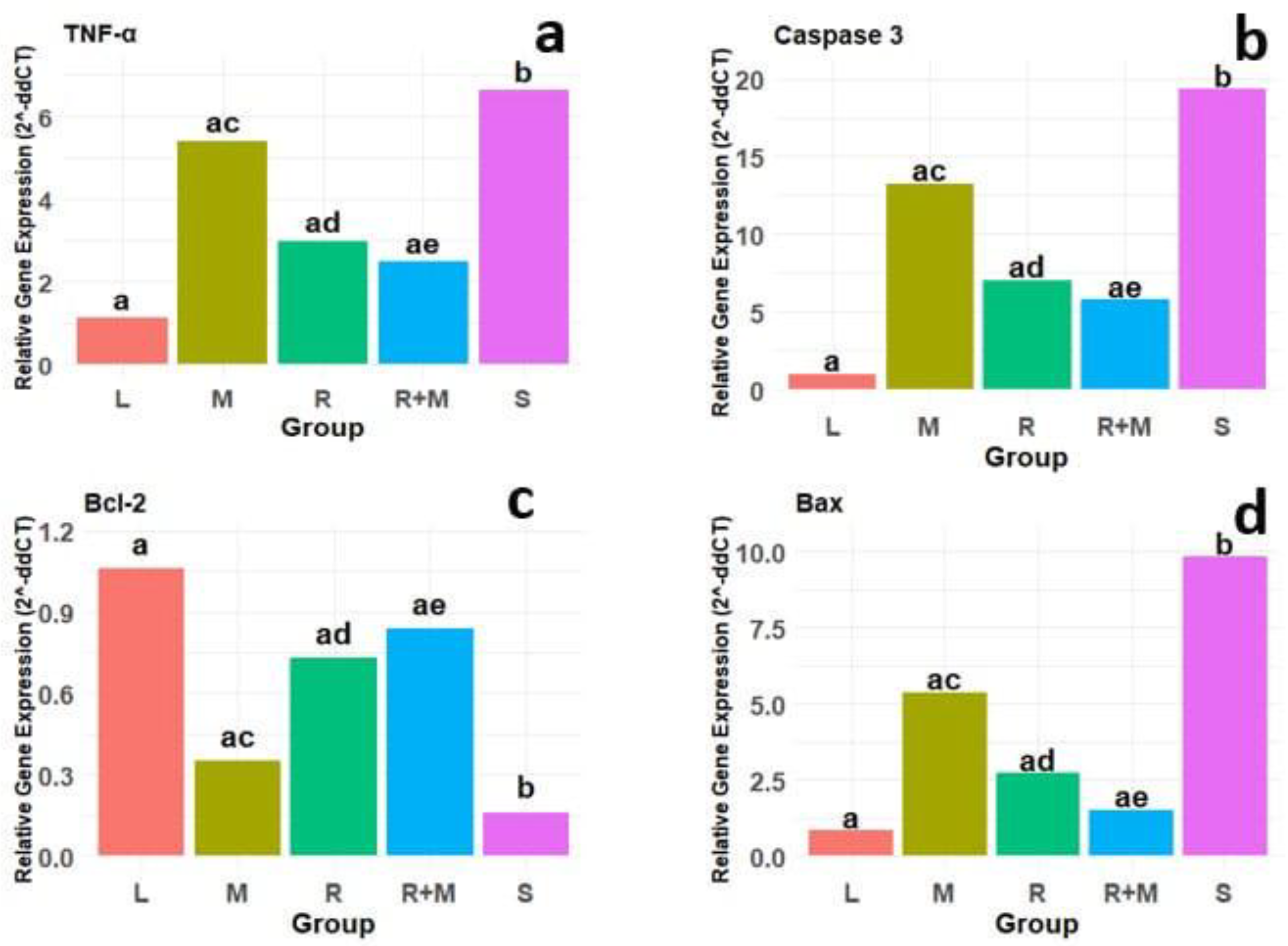
Results of gene expression assessment:
The alcoholic extracts of
Melissa officinalis and
Rosmarinus officinalis have demonstrated efficacy in the management and treatment of inflammation associated with spinal cord injuries, as evidenced by a significant reduction in the expression of inflammation-related genes, particularly tumor necrosis factor-alpha (TNF-α).
Gene expression analysis, including the Shapiro-Wilk test for normality, revealed that the data were normally distributed (p > 0.05, Shapiro-Wilk test). Quantitative reverse transcription polymerase chain reaction (RT-PCR) and subsequent statistical assessments indicated that the expression levels of inflammation-related genes, including TNF-α, were markedly elevated in rats with spinal cord injuries when compared to the control group (p < 0.03). Results from one-way analysis of variance (ANOVA) and Tukey's post hoc test revealed that the group treated with the alcoholic extract of
Melissa officinalis exhibited a significant decrease in TNF-α expression relative to the spinal cord injury group (p < 0.0001). Likewise, the group receiving the alcoholic extract of
Rosmarinus officinalis showed a significant reduction in TNF-α expression compared to the spinal cord injury group, with this reduction being more pronounced than that observed in the
Melissa officinalis treatment group (p = 0.0065). Furthermore, the group that was administered a combination of both plant extracts displayed significantly lower levels of TNF-α expression compared to the other treatment groups as well as the spinal cord injury group (p = 0.0047). These findings suggest that the concurrent application of these extracts exerts a more potent therapeutic effect in modulating and alleviating inflammation resulting from spinal cord injury (refer to
Figure 11a, p < 0.02).
The alcoholic extracts of Rosmarinus officinalis and Melissa officinalis exhibit notable therapeutic effects on the expression of genes related to inflammation and apoptosis, both when administered separately and in conjunction.
Gene expression analysis conducted through quantitative RT-PCR, accompanied by statistical assessments, revealed a significant increase in the expression level of caspase 3—a pivotal element in the apoptosis and cell death pathway—in spinal cord-injured rats when compared to the control group (p < 0.03).
Statistical analyses, including the Shapiro-Wilk test for normality, revealed that the data were normally distributed (p > 0.05, Shapiro-Wilk test). Results from one-way analysis of variance (ANOVA) and Tukey's test indicated that the expression level of caspase 3 in all three-treatment groups significantly decreased relative to the spinal cord-injured group. This finding implies a beneficial role of the alcoholic extract of
Melissa officinalis in modulating and diminishing caspase 3 expression in patients with spinal cord injuries. In the cohort receiving solely the alcoholic extract of
Melissa officinalis, there was a significant reduction in caspase 3 expression compared to the injured group (p < 0.01). Notably, the group treated with the alcoholic extract of
Rosmarinus officinalis exhibited an even more substantial decrease in expression levels when compared to both the injured group and the group treated with the alcoholic extract of
Melissa officinalis (p = 0.0005). Furthermore, the group that received a combination of both plant extracts showed an even more pronounced reduction in caspase 3 expression compared to the injured group and the other treatment groups (p < 0.005, see
Figure 11b). These findings suggest that the combined extract of the two plants is more effective in reducing the expression of this gene than the other treatment modalities, underscoring its potential as a superior therapeutic option.
The results of this investigation offer significant insights into the expression levels of the Bcl-2 protein, an essential anti-apoptotic factor integral to the mitochondrial apoptosis pathway.
Quantitative RT-PCR analysis, including the Shapiro-Wilk test for normality, revealed that the data were normally distributed (p > 0.05, Shapiro-Wilk test). Subsequent statistical assessments demonstrated a notable reduction in the expression of this gene within the spinal cord injury cohort when compared to the control group (p < 0.01). Furthermore, one-way ANOVA and Tukey’s test indicated that the expression of Bcl-2 in the group administered the alcoholic extract of
Melissa officinalis exhibited a significant increase relative to the spinal cord injury group (p < 0.03). This enhancement in gene expression was even more pronounced in the cohort treated with the alcoholic extract of
Rosmarinus officinalis (p < 0.01). Additionally, the group receiving both plant extracts concurrently showed a significant elevation in Bcl-2 gene expression compared to the other treatment groups and the spinal cord injury group, underscoring the remarkable therapeutic potential of the alcoholic extracts, which appear to surpass the efficacy of the other treatments (p < 0.001, see
Figure 11c).
The results obtained from gene expression evaluation using quantitative RT-PCR and statistical analyses indicated that the expression level of the Bax gene, a crucial apoptotic protein in the intrinsic mitochondrial pathway, significantly increased in the spinal cord injury group compared to the control group (p < 0.04). Statistical analyses, including the Shapiro-Wilk test for normality, revealed that the data were normally distributed (p > 0.05, Shapiro-Wilk test). Additionally, the results from one-way ANOVA and Tukey’s test revealed that the expression level of this gene in the group treated with the alcoholic extract of Melissa officinalis significantly decreased compared to the spinal cord injury group (p < 0.01). Nonetheless, compared to the group treated with Melissa officinalis, the group treated with the alcoholic extract of Rosmarinus officinalis showed a more marked decrease in Bax gene expression, suggesting a more successful therapeutic result. The reduction in gene expression was higher (p < 0.001) in the group that received the combination extract of Melissa officinalis and Rosmarinus officinalis compared to the other treatment groups and the spinal cord injury group (p < 0.005). Based on these findings, it is recommended to use the alcoholic extracts of these two plants to treat and prevent inflammation and apoptosis that arise from spinal cord damage. The group treated with the combined extract showed a greater degree of recovery.
Discussion:
The primary objective of this study is to evaluate the therapeutic effects of the combined alcoholic extracts from two botanical sources, Rosmarinus officinalis and Melissa officinalis. Additionally, this research aims to compare the individual therapeutic effects of each alcoholic extract separately within the study framework. Moreover, the investigation explores the impact of these extracts on renal tissue modifications resulting from inflammatory and apoptotic processes associated with the secondary consequences of spinal cord injury. Additionally, the study assesses the expression levels of genes related to apoptosis and inflammation. Male Wistar rat models were employed for this investigation, with spinal cord injuries induced through a compression technique. Previous studies have demonstrated that this method effectively establishes a spinal cord injury model characterized by gliosis, demyelination, connective tissue loosening, and the development of cavities within both the white and gray matter of the spinal cord. [
45,
47]. The findings of this study indicate that the presence of vacuoles in the samples, as illustrated in
Figure 2, signifies degeneration or loss of spinal axons. This observation is consistent with prior research and corroborates the effective induction of spinal cord injury in the rat models utilized. Furthermore, the investigation demonstrated that post-injury, the rats exhibited an inability to utilize their hind limbs for locomotion and displayed insufficient responses to sensory stimuli.
The current investigation revealed that subjects with induced spinal cord injury exhibited paralysis, which rendered them incapable of ambulation and unresponsive to sensory stimuli. Notably, the neuroprotective effects of alcoholic extracts from
Rosmarinus officinalis and
Melissa officinalis were highlighted in this study. The results indicated that treatment with these extracts, particularly when utilized in conjunction, led to marked improvements in both sensory and motor functions, as well as enhanced responsiveness to sensory stimuli, in contrast to the spinal cord injury control group. This functional enhancement may be attributed to the presence of anti-inflammatory and antioxidant compounds within the alcoholic extracts of these plant sources. Moreover, these extracts may exhibit acetylcholinesterase inhibitory properties, which could facilitate the reconnection of sensory transmitter cells with nicotinic receptors or modulate cholinergic receptors, thereby enhancing nerve signal transmission. Additionally, the neuroprotective effects of these extracts may mitigate neuronal cell loss, cavity formation, and astrogliosis in the ventral horn of the spinal cord, while also promoting increased myelination in the dorsal white matter. As a result, these effects contribute to the enhancement of sensory and motor functions. The observed improvement in functional recovery following spinal cord injuries can be attributed to the suppression of pro-inflammatory cytokines, inflammatory mediators, and apoptosis-related factors, including tumor necrosis factor-alpha, interleukins 1 and 6, Bax, and caspase 3. These factors are critical to the pathophysiology of spinal cord injuries, particularly regarding the secondary damage that occurs. Furthermore, spinal cord injuries can negatively impact the functionality of other organs and systems within the body, such as the kidneys. An increased expression of inflammatory mediators may exacerbate and perpetuate disease processes, while the upregulation of apoptosis-related genes can lead to cellular death and damage to nerve tissue. The current study demonstrates that the application of alcoholic extracts from
Rosmarinus officinalis and
Melissa officinalis, particularly in combination, can significantly modulate the expression of factors associated with inflammation and apoptosis. This finding indicates that these extracts may possess therapeutic potential in the treatment and management of spinal cord injuries (
p < 0.05), thereby improving disease management and resulting in substantial enhancements in both sensory and motor functions [
39,
48,
49,
50].
Empirical research has established that alcoholic extracts derived from
Rosmarinus officinalis and
Melissa officinalis exhibit neuroprotective effects through a variety of mechanisms, which can be linked to the presence of specific bioactive compounds within these extracts. A key compound identified in both extracts is rosmarinic acid, which serves as a potent antioxidant and anti-inflammatory agent. This compound alleviates oxidative stress and inflammation in neural tissues by inhibiting the generation of free radicals and the production of inflammatory cytokines. Furthermore, rosmarinic acid promotes the expression of antioxidant genes through the activation of the Nrf2/ARE signaling pathway and aids in the attenuation of inflammation by reducing the expression and activity of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), a pivotal regulator of inflammatory responses [
26,
51]. Another compound found in both alcoholic extracts shows notable antioxidant and anti-inflammatory effects. This compound reduces the production of inflammatory prostaglandins by blocking the activity of the enzymes cyclooxygenase-2 (COX-2) and lipoxygenase (LOX), which helps to lessen inflammation [
48]. Moreover, the alcoholic extract of
Rosmarinus officinalis is particularly rich in carnosic acid, a strong antioxidant and anti-inflammatory substance. Carnosic acid boosts the expression of antioxidant genes by activating the Nrf2/ARE signaling pathway, similar to the effects of rosmarinic acid. It has also been shown to enhance cognitive function and memory by inhibiting acetylcholinesterase, thus affecting the cholinergic pathway [
52,
53].
Linalool, an important element found in the alcoholic extract of
Melissa officinalis, has demonstrated a significant ability to lessen neural damage. This is due to its anti-inflammatory and antioxidant characteristics. In particular, linalool alleviates oxidative stress by lowering free radical production and protects against cell death and neural tissue injury by modulating Bcl-2 and Bax proteins, in addition to inhibiting caspase activity [
34,
54,
55]. Recent studies show that alcoholic extracts from
Rosmarinus officinalis and
Melissa officinalis can greatly reduce inflammation and tissue damage after spinal cord injuries. This is accomplished by lowering the levels of inflammatory mediators, including tumor necrosis factor-alpha (TNF-α), and apoptotic factors such as caspases and Bax. Additionally, these extracts are linked to enhancements in sensory and motor functions. Several studies back these results; for example, one study found that
Rosmarinus officinalis extract lessens motor impairments in mouse models of spinal cord injury [
56]. Furthermore, research conducted by Dr. Hosseini and colleagues showed that a combination of
Melissa officinalis extract and dexamethasone significantly enhances sensory and motor functions in rats with neural damage [
47]. According to the current study, extracts from
Melissa officinalis and
Rosmarinus officinalis are both useful for managing and treating secondary problems related to spinal cord injury. Moreover, by optimizing the availability of every active ingredient in each extract and therefore raising the overall efficacy of treatment, the combination of extracts from these two plants produces greater therapeutic outcomes. This enhancement arises from the possibility that every extract contains distinct active ingredients with particular medicinal advantages. Together, these extracts have the potential to enhance each other's beneficial effects and produce better therapeutic results. Furthermore, as was already said, distinct extracts may use different processes to treat wounds and inflammation, and this diversity can aid in improving overall functionality and minimizing harm.
The current investigation examines the effects of extracts derived from
Melissa officinalis and
Rosmarinus officinalis on pro-inflammatory cytokines, particularly tumor necrosis factor-alpha (TNF-α), as well as their influence on the expression of apoptosis-related genes, namely caspase-3, Bax, and Bcl-2. A considerable amount of research suggests that spinal cord injury (SCI) triggers significant systemic inflammation, which can detrimentally impact various organs and tissues, including the kidneys, thereby compromising their functionality [
33,
34]. Following SCI, there is an observed upregulation in the production and secretion of inflammatory mediators such as TNF-α, which contributes to both inflammation and secondary damage to the spinal cord. This inflammatory response may subsequently result in increased activity and expression of pro-apoptotic genes, such as Bax and caspase-3, while simultaneously decreasing the expression of the anti-apoptotic gene Bcl-2, potentially intensifying both apoptosis and inflammation [
57]. Moreover, the present study revealed a notable increase in TNF-α gene expression within the SCI cohort in comparison to the control group, highlighting the significance of TNF-α as a pivotal inflammatory mediator in the initiation and progression of spinal cord injury and its ramifications for other organs, including the kidneys.
Following a spinal cord injury (SCI), there is a notable increase in the expression of tumor necrosis factor-alpha (TNF-α), which reaches its peak within the initial hours post-injury. This heightened expression persists for several hours before gradually diminishing. TNF-α is synthesized by a variety of neural cell types, including glial cells, astrocytes, neurons, and compromised endothelial cells. Researchers posit that TNF-α is closely associated with the inflammatory response that ensues after the injury, suggesting that the inflammatory processes triggered by the injury may contribute to the elevated levels of this cytokine. Moreover, TNF-α is integral to the mechanisms underlying the secondary effects that occur following spinal cord injury and is linked to the acceleration of disease progression [
58]. A 2021 study demonstrated that microRNA-221 (miR-221) targets genes involved in the production of tumor necrosis factor-alpha (TNF-α) in individuals with spinal cord injuries. This targeting is associated with reduced oxidative stress and inflammation, leading to improved functional outcomes [
59]. Additionally, another investigation found that the level of TNF-α expression following spinal cord injury is correlated with the degree of post-injury inflammation. It was noted that decreasing the expression of this inflammatory factor can result in a reduction of inflammatory response markers and pain-related behaviors [
60]. Furthermore, a research team in a 2020 study reported that spinal cord injuries are associated with increased TNF-α expression in affected patients, a finding that aligns with the results of the current study [
61].
Recent investigations have clarified that apoptosis and programmed cell death are essential mechanisms that are activated in response to spinal cord injury. Evidence suggests that multiple factors influence these processes, with Bax and Bcl-2, two key proteins from the Bcl-2 protein family, being particularly noteworthy. Bax is recognized for its role in initiating and facilitating apoptosis, serving as the primary pro-apoptotic member of this protein family. Typically found in the cytosol of mammalian cells, Bax translocates to the mitochondrial membrane at the commencement of apoptosis. In contrast, Bcl-2 acts as a regulator of gene expression, possessing the ability to either promote or inhibit apoptosis, thus functioning as a critical modulator of cell death. Furthermore, additional cellular and molecular pathways are involved in the induction of apoptosis, encompassing both caspase-3-dependent and caspase-3-independent mechanisms. Caspase-3, a crucial protease within the apoptosis pathway, remains inactive until it is activated. This activation occurs when activated DNase cleaves the caspase activator, ultimately culminating in cell death. Caspase-3 plays a pivotal role in the terminal phases of apoptosis [
62,
63].
The results of this study demonstrate that the expression levels of the caspase-3 and Bax genes in the spinal cord injury cohort were significantly higher than those observed in the control group. Importantly, the expression of these genes in the spinal cord injury group surpassed that of the Bcl-2 gene, which encodes an anti-apoptotic protein. Additionally, a notable decrease in Bcl-2 gene expression was recorded in the spinal cord injury group, indicating the onset of inflammatory processes and the advancement of apoptosis. This phenomenon contributes to cellular and tissue death, ultimately leading to damage across various organs and systems within the body, which are secondary effects of spinal cord injury. These consequences were rapidly evident in rat models following the injury. The levels of inflammatory and apoptosis-related factors exhibited a swift increase post-injury, suggesting the potential for further progression and dissemination to other organs and tissues. A 2017 study highlighted that spinal cord injury can activate mitochondrial apoptosis, a critical process initiated by intrinsic biochemical alterations that culminate in cell death, regulated by the p53 protein. The findings indicated that this condition occurs when Bcl-2 expression diminishes while the expression of caspase-3 and Bax genes escalates [
64]. Recent investigations have underscored the role of Triad1 in modulating the neuronal apoptosis process mediated through the p53-caspase-3 pathway following spinal cord injury [
65]. In a 2023 study conducted by Dr. Akbari and colleagues, it was revealed that targeting the Bax/Bcl-2 pathway and modulating TNF-α/IL-10 using platelet-rich plasma-derived exosomes loaded with dexamethasone led to significant improvements in symptoms associated with spinal cord injury [
66]. Alterations in the activity of genes linked to cell death and immune response, particularly the increased expression of the TNF-α gene—a pivotal inflammatory factor—contribute to the onset and progression of spinal cord injury to other regions of the body, such as the kidneys.
This study examines the therapeutic efficacy of alcoholic extracts derived from
Rosmarinus officinalis and
Melissa officinalis in the context of spinal cord injuries and their related complications. These complications, which encompass inflammation and cellular apoptosis that may result in dysfunction of other organs, such as the kidneys, are partially linked to the bioactive compounds present in these plant extracts. The results revealed a significant elevation in the expression levels of the caspase-3, Bax, and Bcl-2 genes within the spinal cord injury cohort, while a marked reduction in the expression of the anti-apoptotic Bcl-2 gene was observed. Conversely, the treatment groups exhibited a substantial increase in gene expression levels. Notably, the group receiving a combination of alcoholic extracts from
Rosmarinus officinalis and
Melissa officinalis demonstrated superior therapeutic efficacy compared to the other treatment groups. Furthermore, the group treated solely with the alcoholic extract of
Rosmarinus officinalis exhibited a more significant rate of improvement in comparison to the group receiving the alcoholic extract of
Melissa officinalis. A research team has identified that the alcoholic extract of
Rosmarinus officinalis can reduce cell death by lowering the levels of pro-inflammatory cytokines, such as interleukin-7 and TNF-α, while also inhibiting the activation of caspase-3 [
67]. Additionally, another study has indicated that the alcoholic extract of
Melissa officinalis possesses anti-inflammatory properties, which contribute to the reduction of inflammation and cell death by downregulating the expression of inflammation-related genes, including interleukin-6 and TNF-α [
68]. Moreover, research has suggested that certain compounds may decrease caspase-3 activity in the spinal cords of injured rats, indicating potential therapeutic advantages [
57].
A multitude of studies has established that the alcoholic extract of
Rosmarinus officinalis encompasses a wide range of bioactive compounds that possess notable therapeutic properties. This botanical is recognized for its analgesic, anti-inflammatory, headache-relieving, and muscle-relaxing effects, as well as its effectiveness in addressing ailments such as arthritis and rheumatism. Moreover, constituents such as carnosic acid and rosmarinic acid present in rosemary extract demonstrate antidepressant and neuroprotective properties, thereby rendering it a promising candidate for the treatment and management of neurological disorders. Additionally, the alcoholic extract of
Rosmarinus officinalis exhibits significant antimicrobial activity, which can be attributed to compounds including pinene, eugenol, and carnosic acid. Other active components within this plant, such as methyl carnosate, myrcene, cimicifuga, genkwanin, and rosmarinic acid, further augment its antimicrobial efficacy. Conversely,
Melissa officinalis is celebrated for its antioxidant and anticancer properties, containing compounds such as camphor and alpha-pinene. The presence of rosmarinic acid in the alcoholic extract of lemon balm enhances its anti-inflammatory, anti-allergic, antibacterial, and antiviral effects, suggesting its potential utility in cancer prevention and treatment [
69]. Research has also indicated that the alcoholic extract of
Melissa officinalis possesses analgesic, anti-inflammatory, and antioxidant properties, which are beneficial in the management of neurological disorders. The neuroprotective effects are primarily attributed to phenolic compounds, including hydroxycinnamic acid, flavonoids, rosmarinic acid, and caffeic acid, as well as luteolin and sialonic acid. Furthermore, the extract exhibits antimicrobial, antispasmodic, antiseptic, anti-inflammatory, and therapeutic effects on the digestive system [
70,
71]. A research team conducted a study in 2024 that showed
Rosmarinus officinalis has neuroprotective qualities because of its antioxidant effects. These qualities could potentially help lessen and enhance memory deterioration linked to aging [
72]. Another investigation underscored the anti-inflammatory properties of
Rosmarinus officinalis, which are ascribed to its distinctive diterpenoid compounds [
73]. Additionally, a separate study found that the alcoholic extract of
Melissa officinalis exhibits neuromodulating properties that may enhance emotional well-being and alleviate associated conditions [
74]. The alcoholic extracts of these plants have strong neuroprotective qualities and a variety of chemicals, which make them potentially useful for managing and treating spinal cord injuries and their associated secondary problems.
The examination of stained samples employing the Hematoxylin and Eosin (H&E) staining method across the studied groups indicated that spinal cord injury leads to modifications in the structural integrity and tissue composition of the kidneys. Notable disparities were identified in the sizes of renal corpuscles, glomeruli, and urinary spaces, in addition to variations in the height of distal and proximal epithelial cells, the diameters of distal and proximal tubules, and the lumen diameters of both distal and proximal tubules.
The current investigation revealed a significant reduction in the diameter of the renal corpuscle and glomerulus in the cohort of rats subjected to spinal cord injury. In relation to this finding, a study conducted by researchers in 2023 suggested that Rosemary oil extract may exert a positive influence on the structural alterations in kidney tissue linked to diabetes, leading to an increase in the diameter of the renal corpuscle [
75]. Additionally, in a previous investigation I undertook, the alcoholic extract of
Melissa officinalis was shown to influence the tissue and structural changes resulting from spinal cord injury, thereby affecting the architecture of the glomeruli, the urinary space, and the urinary tubules, which culminated in a marked improvement [
76]. The current study further elucidated that the combined administration of the alcoholic extracts of
Melissa officinalis and
Rosmarinus officinalis significantly augmented the sizes of renal corpuscles, glomeruli, and urinary spaces in rats with spinal cord injury.
In the spinal cord injury group, there was a notable decrease in the height of epithelial cells in both the proximal and distal convoluted tubules when compared to the control group. This decrease was especially significant in the proximal tubule cells near the glomerulus, likely due to their closer location to the glomerulus than the distal tubule cells. While the reduction in height of the distal tubule cells was less severe than that of the proximal cells, these changes in epithelial cell height—crucial for receiving and processing filtrate from the glomerulus and renal corpuscle—could have serious negative clinical consequences for the patient. Spinal cord injury is a complicated condition that can lead to the destruction, necrosis, and death of cells in the urinary system. The therapeutic effects of alcoholic extracts from Rosmarinus officinalis and Melissa officinalis on cells in the proximal area near the glomerulus were assessed in the studied groups. The findings showed that the group treated with the combined extract had significant improvements compared to both the spinal cord injury group and the other two treatment groups. Although all treatment groups showed better results than the spinal cord injury group, the group receiving the combined extract of Rosmarinus officinalis and Melissa officinalis had the most positive therapeutic outcomes. Histomorphometric analysis indicated that the size of epithelial cells in this group was nearly the same as that of the control group. Regarding the impact of the alcoholic extracts on the distal tubule cells, the most notable therapeutic effect was seen in the group treated with the alcoholic extract of Rosmarinus officinalis, where the height of these cells was nearly equivalent to that of the control group. Importantly, improved epithelial cell height was noted in all treated groups, but further research in this area is needed.
The kidney and spinal cord tissues in this investigation showed no signs of toxicity from the alcohol extracts. While some small structural changes were seen in kidney tissues, the effects on tissue function were not greatly affected by these modifications. For instance, the height of the epithelial cells was marginally higher in the group treated with the alcoholic extract of Melissa officinalis than in the control group. These modifications, however, were so little as to cause no functional disruptions and are therefore deemed insignificant.
A study conducted in 2019 revealed that the combination of alcoholic extracts from
Rosmarinus officinalis and two other plant sources can induce changes in the renal tubules and the epithelial cells that line them. This combination demonstrated significant therapeutic and protective effects against toxicities [
77]. Further research backs up these findings. For example, one study showed that dexmedetomidine can successfully reduce the structural alterations in the kidneys caused by spinal cord injury [
78]. The results of these studies align with a more recent investigation, which observed alterations in the diameter of both the distal and proximal tubular lumen following spinal cord injury. These changes were found to improve significantly in the treatment groups, particularly in the group receiving the combined alcoholic extract. Given the kidney's essential role in blood purification and waste elimination, any modifications in its tissue structure and size can negatively impact its functionality and pose a risk to patient health. Therefore, this study seeks to examine the therapeutic effects of the alcoholic extracts of
Melissa officinalis and
Rosmarinus officinalis, both individually and in combination, in murine models with spinal cord injuries. However, further research in this area is necessary. A 2018 study conducted by the European Union indicated that substances such as aspartame can affect the diameter of the renal corpuscle. The height of the epithelium in various kidney structures within the experimental groups significantly decreased compared to the control group, which is consistent with the findings of the current research. In a previous study I conducted, the alcoholic extract of
Melissa officinalis exhibited notable therapeutic effects on changes in kidney tissue and the expression of genes associated with inflammation and apoptotic processes following spinal cord injury in rat models [
76]. A 2012 study by Tae Wujang and colleagues investigated the combined effects of metformin,
Melissa officinalis, and dandelion on diabetic rats, revealing that this mixture could influence kidney histopathology and alter the vacuoles in urinary tubular cells [
79]. Additionally, a separate study conducted in 2023 by Dad and collaborators demonstrated that the natural antioxidants found in cinnamon and
Rosmarinus officinalis could offer protective benefits for the kidneys against the harmful effects of acrylamide. Consequently, these antioxidants may represent a promising therapeutic option for the enhancement of kidney health [
80].