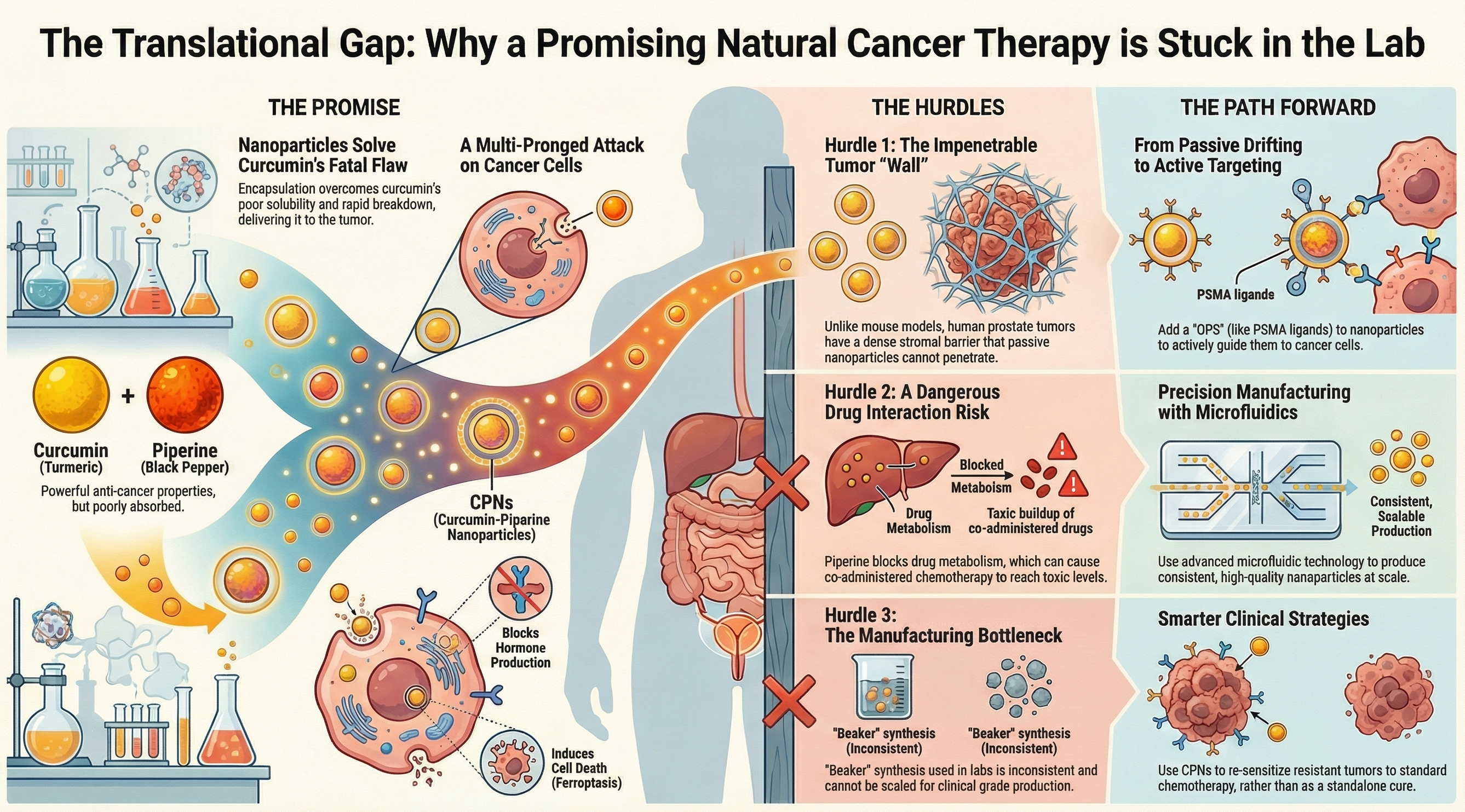
Introduction: Prostate cancer (PC) treatment is limited by resistance mechanisms and cumulative toxicities, necessitating novel therapeutic strategies. While curcumin and piperine exhibit potent anticancer properties, their clinical utility is severely compromised by poor bioavailability and rapid metabolism. Areas covered: This review critically analyzes the preclinical and clinical landscape of curcumin and piperine nanoformulations (CPN) for PC treatment. We utilized PubMed and Scopus (2000–2025) to evaluate molecular mechanisms, focusing on CYP17A1 inhibition, PI3K/Akt/mTOR signaling, and ferroptosis. The report examines the physicochemical attributes of nanocarriers, including PLGA and liposomes, and addresses translational barriers such as the heterogeneity of the Enhanced Permeability and Retention (EPR) effect, stromal density, and piperine-mediated drug–drug interaction risks. Expert opinion: While nano-encapsulation enhances the therapeutic index of curcumin, clinical translation remains stalled by a reliance on passive targeting and insufficient manufacturing scalability. Future success depends on shifting from "beaker" synthesis to microfluidic production (Quality by Design) and adopting active targeting (e.g., PSMA-directed delivery) to penetrate the prostate stroma. Without these strategic pivots and biomarker-driven trials, CPNs risk remaining an academic curiosity rather than evolving into a viable clinical intervention.