Submitted:
19 November 2025
Posted:
20 November 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Blood Collection and Fractionation
2.2. Isolation and Activation of Neutrophils to Produce Neutrophil Extracellular Traps (NETs)
2.3. Flow Cytometry of Isolated Neutrophils
2.4. (Immuno)Histochemical Examination of PMA-Activated Neutrophils and NETs
2.5. Scanning Electron Microscopy of Neutrophils and NETs
2.6. Blood Clot Contraction Assay
2.7. Thromboelastography (TEG)
2.8. Statistical Analysis
3. Results
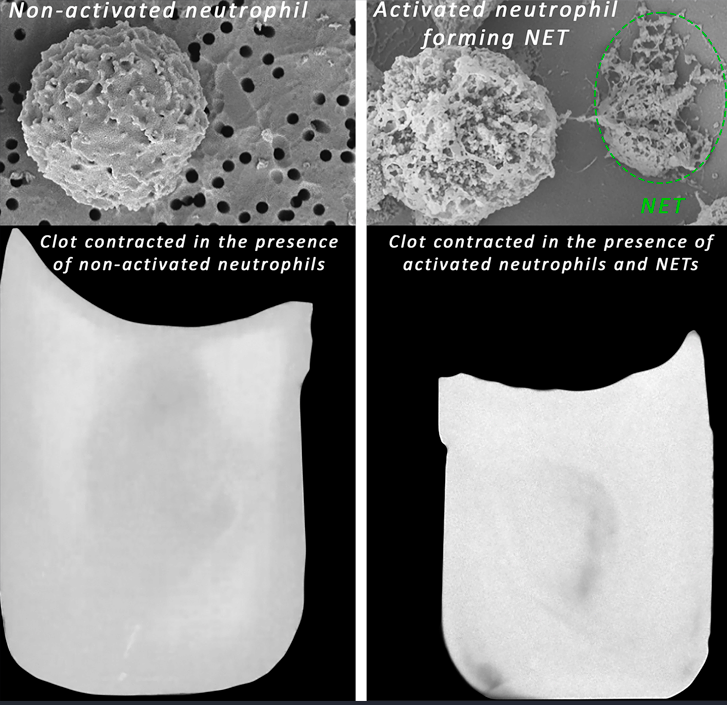
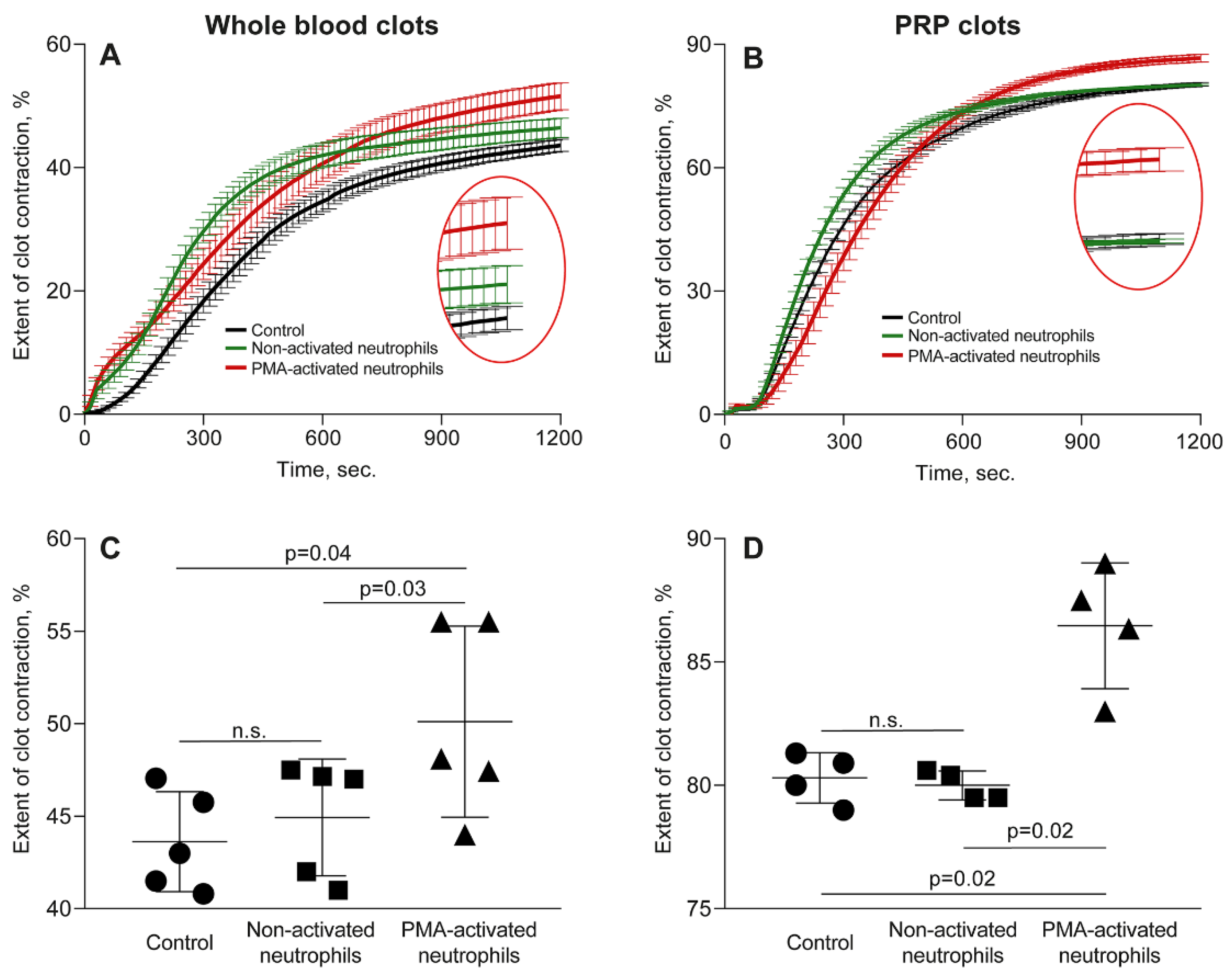
3.1. Effects of Activated Neutrophils on Clot Contraction
3.2. Kinetic Phase Analysis of Clot Contraction in the Presence of Activated Versus Non-Activated Neutrophils
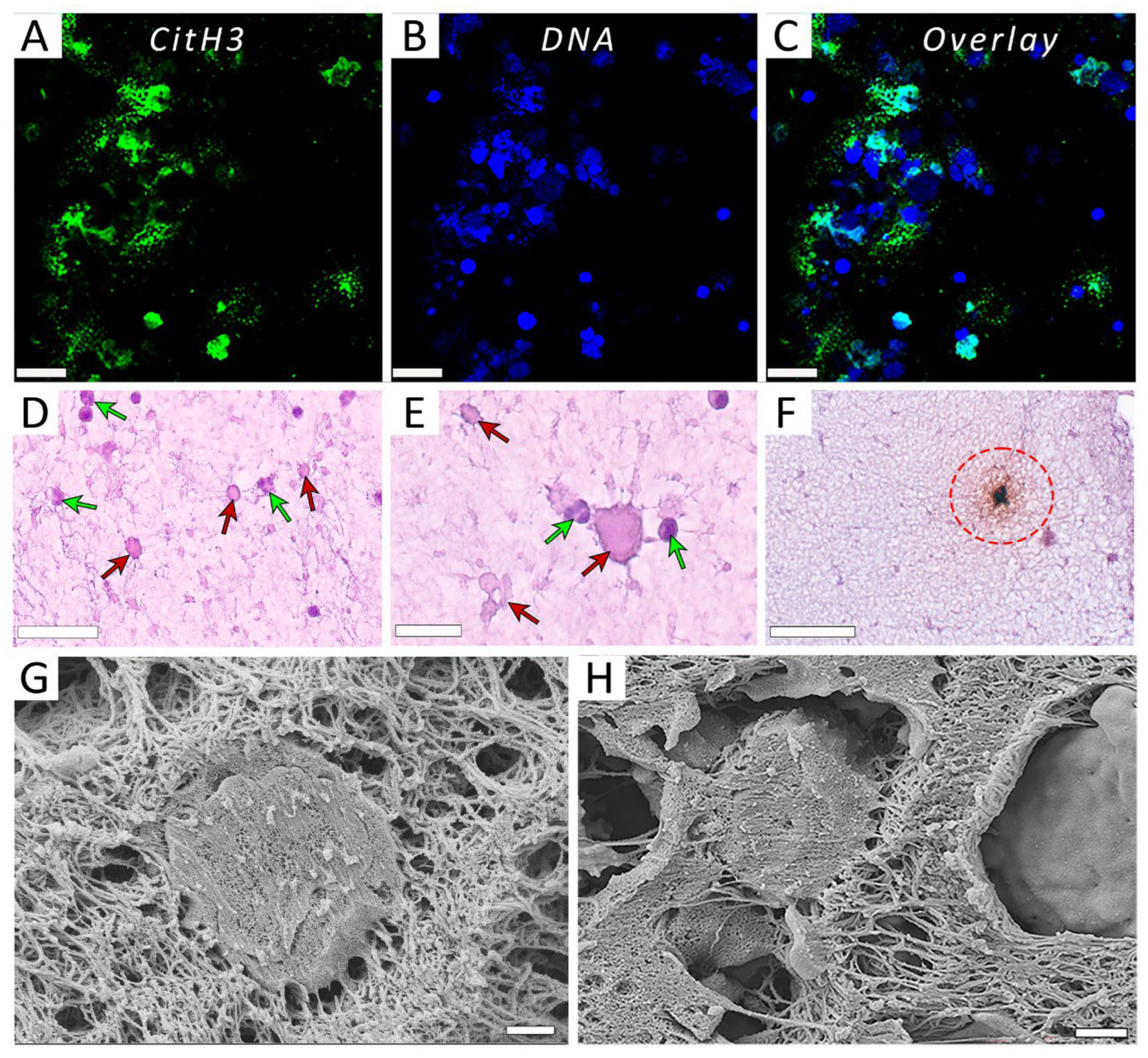
3.3. Visualization of NETs Produced by PMA-Activated Neutrophils
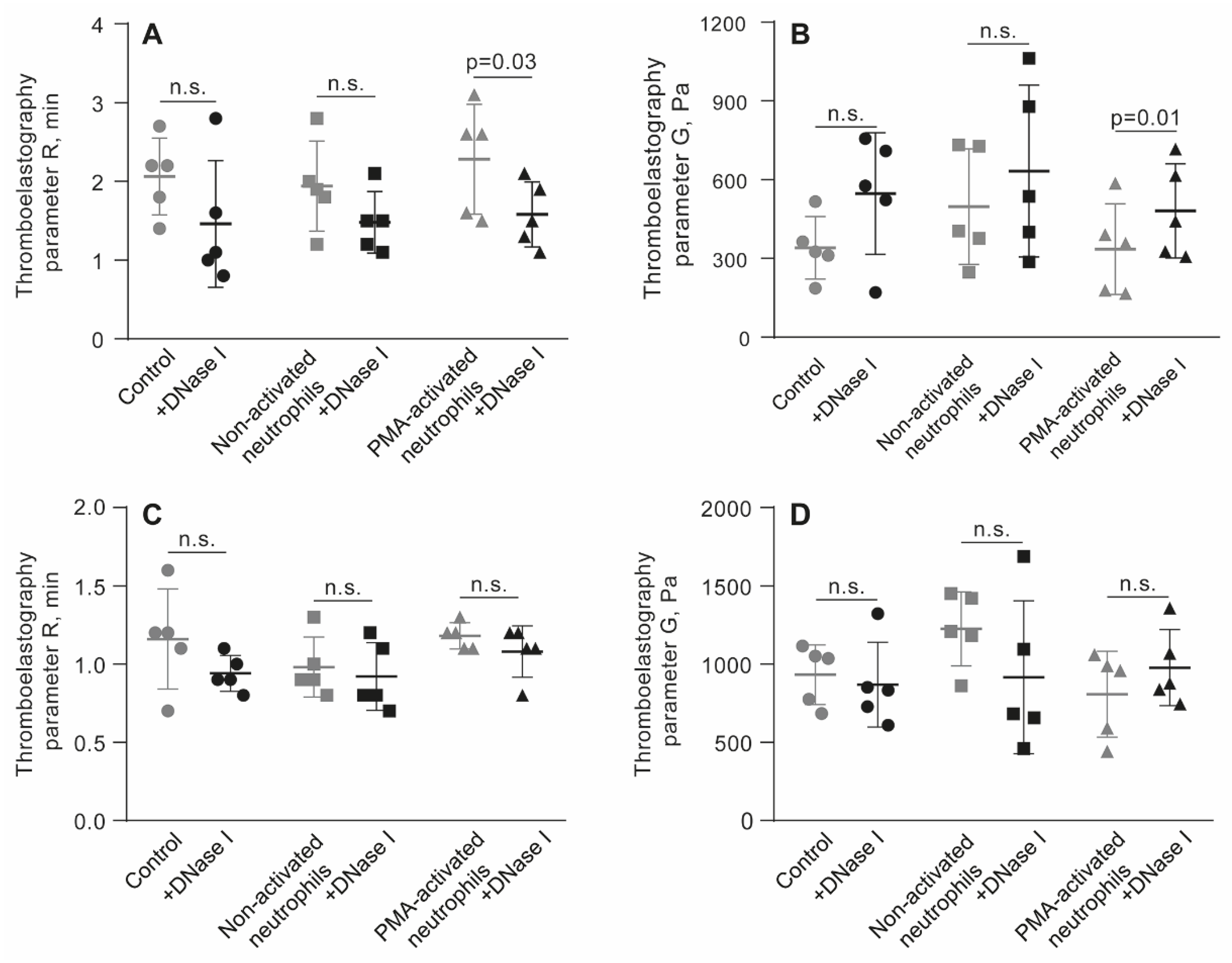
3.4. The Effects of Activated Neutrophils on Clot Contraction Are Associated with NETs Embedded Into a Clot
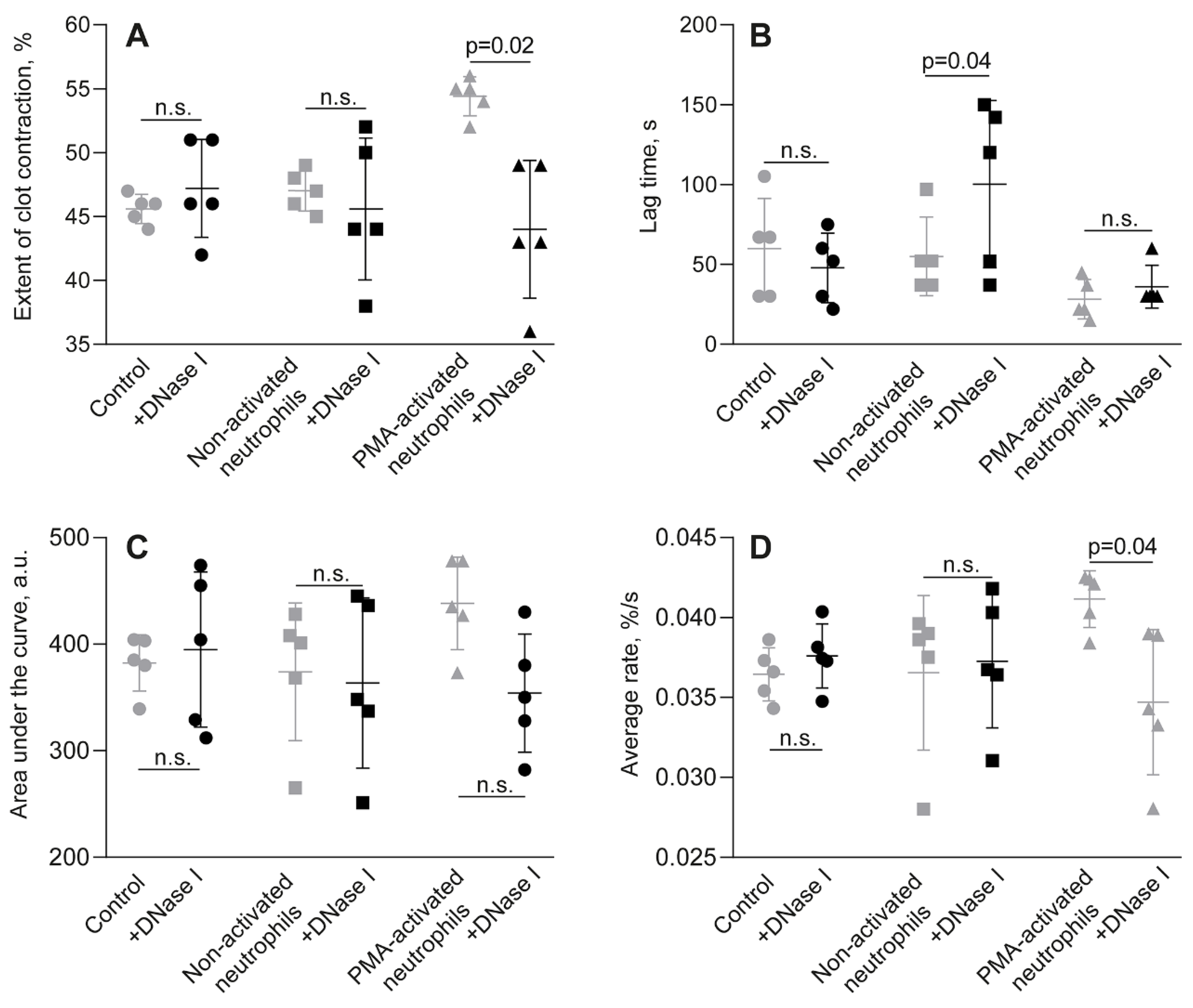
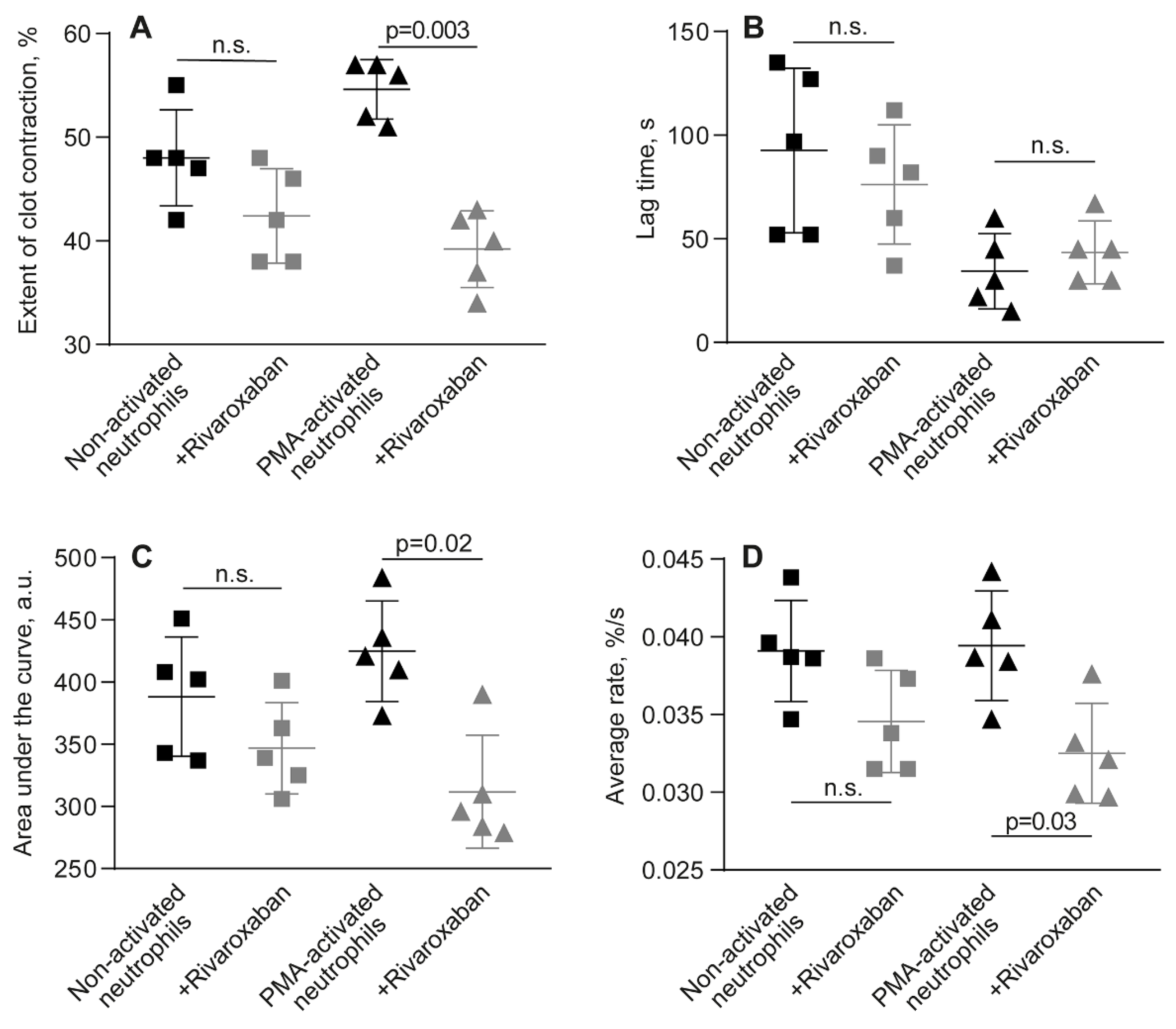
3.5. The Stimulating Effect of NETs on Clot Contraction Is Mediated by Enhanced Generation of Endogenous Thrombin
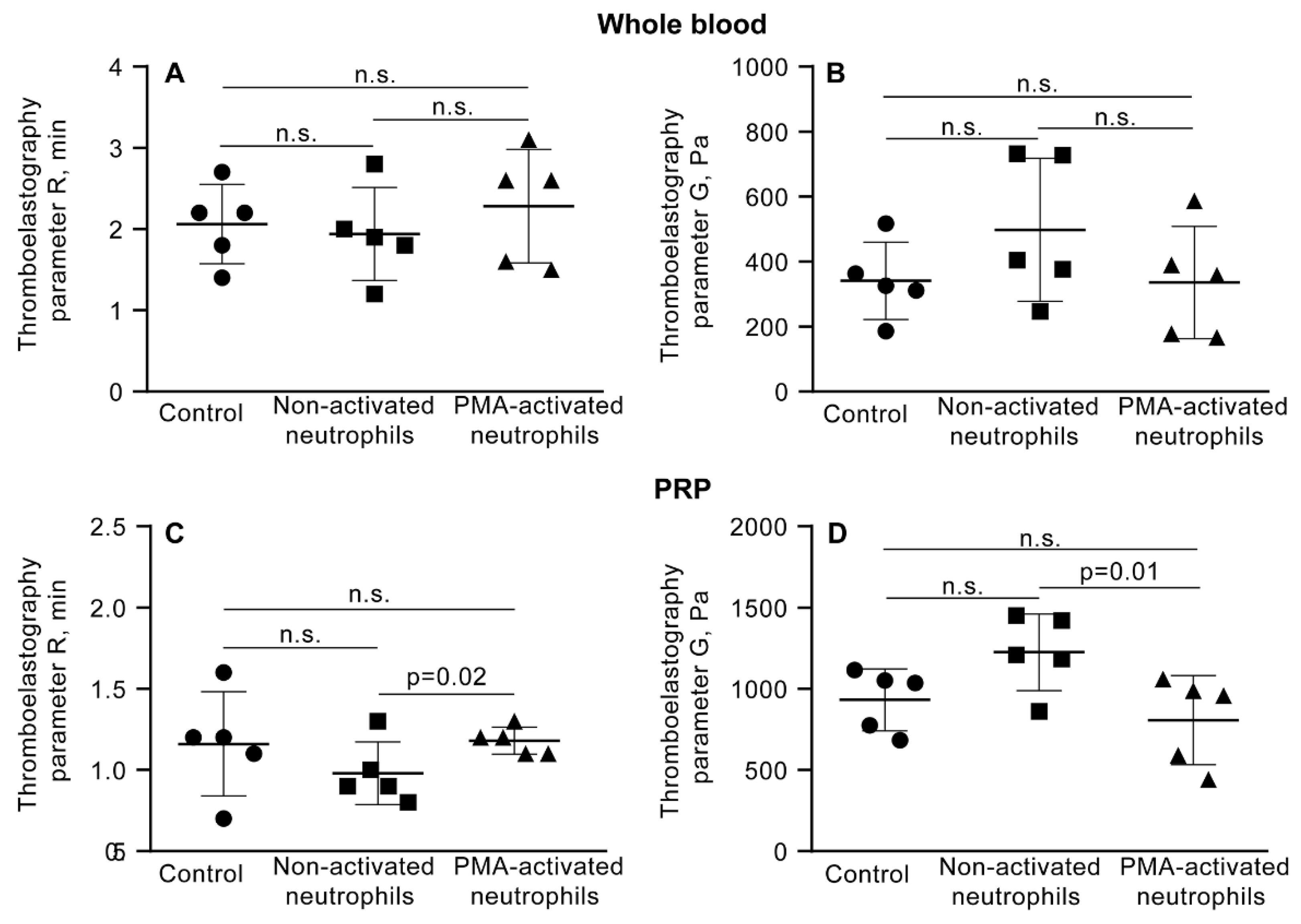
3.6. NETs Make the Fibrin Clot Softer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NETs | Neutrophil extracellular traps |
| PMA | Phorbol-12-myristate-13-acetate |
| PRP | Platelet-rich plasma |
| DNAse I | Deoxyribonuclease I |
| COVID-19 | Coronavirus disease 2019 |
| RBCs | Red blood cells |
| PPP | Platelet-poor plasma |
| PFP | Platelet-free plasma |
| DNA | Deoxyribonucleic acid |
| HBSS | Hanks’ balanced salt solution |
| DPBS | Dulbecco’s phosphate-buffered saline |
| TEG | Thromboelastography |
| ANOVA | Analysis of variance |
| R | Reaction time |
| G’ | Storage (elastic) modulus |
| MA | Maximal amplitude |
References
- Engelmann, B.; Massberg, S. Thrombosis as an Intravascular Effector of Innate Immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [CrossRef]
- Ma, L.; Willey, J. The Interplay Between Inflammation and Thrombosis in COVID-19: Mechanisms, Therapeutic Strategies, and Challenges. Thromb. Update 2022, 8, 100117. [CrossRef]
- Schrottmaier, W.C.; Assinger, A. The Concept of Thromboinflammation. Hamostaseologie 2024, 44, 21–30. [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, Regional, and National Sepsis Incidence and Mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [CrossRef]
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [CrossRef]
- Duarte-García, A.; Pham, M.M.; Crowson, C.S.; Amin, S.; Moder, K.G.; Pruthi, R.K.; Warrington, K.J.; Matteson, E.L.; et al. The Epidemiology of Antiphospholipid Syndrome: A Population-Based Study. Arthritis Rheumatol. 2019, 71, 1545–1552. [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; DE Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International Consensus Statement on an Update of the Classification Criteria for Definite Antiphospholipid Syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [CrossRef]
- Jorge, A.; Wallace, Z.S.; Lu, N.; Zhang, Y.; Choi, H.K. Renal Transplantation and Survival Among Patients With Lupus Nephritis: A Cohort Study. Ann. Intern. Med. 2019, 170, 240–247. [CrossRef]
- Holmqvist, M.E.; Neovius, M.; Eriksson, J.; Mantel, Ä.; Wållberg-Jonsson, S.; Jacobsson, L.T.; Askling, J. Risk of Venous Thromboembolism in Patients With Rheumatoid Arthritis and Association With Disease Duration and Hospitalization. JAMA 2012, 308, 1350–1356. [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA Traps Promote Thrombosis. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 15880–15885. [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [CrossRef]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled Up in NETs. Blood 2014, 123, 2768–2776. [CrossRef]
- Sun, Y.; Myers, D.R.; Nikolov, S.V.; Oshinowo, O.; Baek, J.; Bowie, S.M.; Lambert, T.P.; Woods, E.; Sakurai, Y.; Lam, W.A.; et al. Platelet Heterogeneity Enhances Blood Clot Volumetric Contraction: An Example of Asynchrono-Mechanical Amplification. Biomaterials 2021, 274, 120828. [CrossRef]
- Carr, M.E., Jr. Development of Platelet Contractile Force as a Research and Clinical Measure of Platelet Function. Cell Biochem. Biophys. 2003, 38, 55–78. [CrossRef]
- Myers, D.R.; Qiu, Y.; Fay, M.E.; Tennenbaum, M.; Chester, D.; Cuadrado, J.; Sakurai, Y.; Baek, J.; Tran, R.; Ciciliano, J.C.; et al. Single-Platelet Nanomechanics Measured by High-Throughput Cytometry. Nat. Mater. 2017, 16, 230–235. [CrossRef]
- Cines, D.B.; Lebedeva, T.; Nagaswami, C.; Hayes, V.; Massefski, W.; Litvinov, R.I.; Rauova, L.; Lowery, T.J.; Weisel, J.W. Clot Contraction: Compression of Erythrocytes Into Tightly Packed Polyhedra and Redistribution of Platelets and Fibrin. Blood 2014, 123, 1596–1603. [CrossRef]
- Khismatullin, R.R.; Abdullayeva, S.; Peshkova, A.D.; Sounbuli, K.; Evtugina, N.G.; Litvinov, R.I.; Weisel, J.W. Extent of Intravital Contraction of Arterial and Venous Thrombi and Pulmonary Emboli. Blood Adv. 2022, 6, 1708–1718. [CrossRef]
- Stalker, T.J.; Welsh, J.D.; Tomaiuolo, M.; Wu, J.; Colace, T.V.; Diamond, S.L.; Brass, L.F. A Systems Approach to Hemostasis: 3. Thrombus Consolidation Regulates Intrathrombus Solute Transport and Local Thrombin Activity. Blood 2014, 124, 1824–1831. [CrossRef]
- Litvinov, R.I.; Weisel, J.W. Blood Clot Contraction: Mechanisms, Pathophysiology, and Disease. Res. Pract. Thromb. Haemost. 2022, 7, 100023. [CrossRef]
- Tutwiler, V.; Litvinov, R.I.; Lozhkin, A.P.; Peshkova, A.D.; Lebedeva, T.; Ataullakhanov, F.I.; Spiller, K.L.; Cines, D.B.; Weisel, J.W. Kinetics and Mechanics of Clot Contraction Are Governed by the Molecular and Cellular Composition of the Blood. Blood 2016, 127, 149–159. [CrossRef]
- Peshkova, A.D.; Rednikova, E.K.; Khismatullin, R.R.; Kim, O.V.; Muzykantov, V.R.; Purohit, P.K.; Litvinov, R.I.; Weisel, J.W. Red Blood Cell Aggregation Within a Blood Clot Causes Platelet-Independent Clot Shrinkage. Blood Adv. 2025, 9, 3418–3428. [CrossRef]
- Barnes, B.J.; Adrover, J.M.; Baxter-Stoltzfus, A.; Borczuk, A.; Cools-Lartigue, J.; Crawford, J.M.; Daßler-Plenker, J.; Guerci, P.; Huynh, C.; Knight, J.S.; et al. Targeting Potential Drivers of COVID-19: Neutrophil Extracellular Traps. J. Exp. Med. 2020, 217, e20200652. [CrossRef]
- Mitchell, J.L.; Dunster, J.L.; Kriek, N.; Unsworth, A.J.; Sage, T.; Mohammed, Y.M.M.; De Simone, I.; Taylor, K.A.; Bye, A.P.; Ólafsson, G.; et al. The Rate of Platelet Activation Determines Thrombus Size and Structure at Arterial Shear. J. Thromb. Haemost. 2023, 21, 2248–2259. [CrossRef]
- Fuchs, T.A.; Abed, U.; Goosmann, C.; Hurwitz, R.; Schulze, I.; Wahn, V.; Weinrauch, Y.; Brinkmann, V.; Zychlinsky, A. Novel Cell Death Program Leads to Neutrophil Extracellular Traps. J. Cell Biol. 2007, 176, 231–241. [CrossRef]
- Muraro, S.P.; De Souza, G.F.; Gallo, S.W.; Da Silva, B.K.; De Oliveira, S.D.; Vinolo, M.A.R.; Saraiva, E.M.; Porto, B.N. Respiratory Syncytial Virus Induces the Classical ROS-Dependent NETosis Through PAD-4 and Necroptosis Pathways Activation. Sci. Rep. 2018, 8, 14166. [CrossRef]
- Onouchi, T.; Shiogama, K.; Matsui, T.; Mizutani, Y.; Sakurai, K.; Inada, K.; Tsutsumi, Y. Visualization of Neutrophil Extracellular Traps and Fibrin Meshwork in Human Fibrinopurulent Inflammatory Lesions: II. Ultrastructural Study. Acta Histochem. Cytochem. 2016, 49, 117–123. [CrossRef]
- Stark, K.; Massberg, S. Interplay Between Inflammation and Thrombosis in Cardiovascular Pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [CrossRef]
- Noubouossie, D.F.; Reeves, B.N.; Strahl, B.D.; Key, N.S. Neutrophils: Back in the Thrombosis Spotlight. Blood 2019, 133, 2186–2197. [CrossRef]
- Thålin, C.; Hisada, Y.; Lundström, S.; Mackman, N.; Wallén, H. Neutrophil Extracellular Traps: Villains and Targets in Arterial, Venous, and Cancer-Associated Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1724–1738. [CrossRef]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil Extracellular Traps in COVID-19. JCI Insight 2020, 5, e138999. [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Exploring the Thrombus Niche: Lessons Learned and Potential Therapeutic Opportunities. Blood 2025, 146, 1389–1399. [CrossRef]
- Ducroux, C.; Di Meglio, L.; Loyau, S.; Delbosc, S.; Boisseau, W.; Deschildre, C.; Ben Maacha, M.; Blanc, R.; Redjem, H.; Ciccio, G.; et al. Thrombus Neutrophil Extracellular Traps Content Impair tPA-Induced Thrombolysis in Acute Ischemic Stroke. Stroke 2018, 49, 754–757. [CrossRef]
- Savchenko, A.S.; Martinod, K.; Seidman, M.A.; Wong, S.L.; Borissoff, J.I.; Piazza, G.; Libby, P.; Goldhaber, S.Z.; Mitchell, R.N.; Wagner, D.D. Neutrophil Extracellular Traps Form Predominantly During the Organizing Stage of Human Venous Thromboembolism Development. J. Thromb. Haemost. 2014, 12, 860–870. [CrossRef]
- Laridan, E.; Denorme, F.; Desender, L.; François, O.; Andersson, T.; Deckmyn, H.; Vanhoorelbeke, K.; De Meyer, S.F. Neutrophil Extracellular Traps in Ischemic Stroke Thrombi. Ann. Neurol. 2017, 82, 223–232. [CrossRef]
- Mangold, A.; Alias, S.; Scherz, T.; Hofbauer, M.; Jakowitsch, J.; Panzenböck, A.; Simon, D.; Laimer, D.; Bangert, C.; Kammerlander, A.; et al. Coronary Neutrophil Extracellular Trap Burden and Deoxyribonuclease Activity in ST-Elevation Acute Coronary Syndrome Are Predictors of ST-Segment Resolution and Infarct Size. Circ. Res. 2015, 116, 1182–1192. [CrossRef]
- Gould, T.J.; Vu, T.T.; Swystun, L.L.; Dwivedi, D.J.; Mai, S.H.; Weitz, J.I.; Liaw, P.C. Neutrophil Extracellular Traps Promote Thrombin Generation Through Platelet-Dependent and Platelet-Independent Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1977–1984. [CrossRef]
- von Brühl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, Neutrophils, and Platelets Cooperate to Initiate and Propagate Venous Thrombosis in Mice In Vivo. J. Exp. Med. 2012, 209, 819–835. [CrossRef]
- Carr, M.E., Jr.; Martin, E.J.; Carr, S.L. Delayed, Reduced or Inhibited Thrombin Production Reduces Platelet Contractile Force and Results in Weaker Clot Formation. Blood Coagul. Fibrinolysis 2002, 13, 193–197. [CrossRef]
- Peshkova, A.D.; Le Minh, G.; Tutwiler, V.; Andrianova, I.A.; Weisel, J.W.; Litvinov, R.I. Activated Monocytes Enhance Platelet-Driven Contraction of Blood Clots via Tissue Factor Expression. Sci. Rep. 2017, 7, 5149. [CrossRef]
- Semeraro, F.; Ammollo, C.T.; Morrissey, J.H.; Dale, G.L.; Friese, P.; Esmon, N.L.; Esmon, C.T. Extracellular Histones Promote Thrombin Generation Through Platelet-Dependent Mechanisms: Involvement of Platelet TLR2 and TLR4. Blood 2011, 118, 1952–1961. [CrossRef]
- Massberg, S.; Grahl, L.; von Bruehl, M.L.; Manukyan, D.; Pfeiler, S.; Goosmann, C.; Brinkmann, V.; Lorenz, M.; Bidzhekov, K.; Khandagale, A.B.; et al. Reciprocal Coupling of Coagulation and Innate Immunity via Neutrophil Serine Proteases. Nat. Med. 2010, 16, 887–896. [CrossRef]
- Ruf, W.; Ruggeri, Z.M. Neutrophils Release Brakes of Coagulation. Nat. Med. 2010, 16, 851–852. [CrossRef]
- Komorowicz, E.; Farkas, V.J.; Szabó, L.; Cherrington, S.; Thelwell, C.; Kolev, K. DNA and Histones Impair the Mechanical Stability and Lytic Susceptibility of Fibrin Formed by Staphylocoagulase. Front. Immunol. 2023, 14, 1233128. [CrossRef]
- Longstaff, C.; Varjú, I.; Sótonyi, P.; Szabó, L.; Krumrey, M.; Hoell, A.; Bóta, A.; Varga, Z.; Komorowicz, E.; Kolev, K. Mechanical Stability and Fibrinolytic Resistance of Clots Containing Fibrin, DNA, and Histones. J. Biol. Chem. 2013, 288, 6946–6956. [CrossRef]
- Litvinov, R.I.; Weisel, J.W. Fibrin Mechanical Properties and Their Structural Origins. Matrix Biol. 2017, 60-61, 110–123. [CrossRef]
- Wakefield, T.W.; Myers, D.D.; Henke, P.K. Mechanisms of Venous Thrombosis and Resolution. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 387–391. [CrossRef]
- Tutwiler, V.; Peshkova, A.D.; Le Minh, G.; Zaitsev, S.; Litvinov, R.I.; Cines, D.B.; Weisel, J.W. Blood Clot Contraction Differentially Modulates Internal and External Fibrinolysis. J. Thromb. Haemost. 2019, 17, 361–370. [CrossRef]
- Henke, P.K.; Wakefield, T. Thrombus Resolution and Vein Wall Injury: Dependence on Chemokines and Leukocytes. Thromb. Res. 2009, 123, S72–S78. [CrossRef]
- Saha, P.; Humphries, J.; Modarai, B.; Mattock, K.; Waltham, M.; Evans, C.E.; Ahmad, A.; Patel, A.S.; Premaratne, S.; Lyons, O.T.; et al. Leukocytes and the Natural History of Deep Vein Thrombosis: Current Concepts and Future Directions. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 506–512. [CrossRef]
- Kolev, K.; Machovich, R. Molecular and Cellular Modulation of Fibrinolysis. Thromb. Haemost. 2003, 89, 610–621. [CrossRef]
- Jiménez-Alcázar, M.; Rangaswamy, C.; Panda, R.; Bitterling, J.; Simsek, Y.J.; Long, A.T.; Bilyy, R.; Krenn, V.; Renné, C.; Renné, T.; et al. Host DNases Prevent Vascular Occlusion by Neutrophil Extracellular Traps. Science 2017, 358, 1202–1206. [CrossRef]
- Peshkova, A.D.; Malyasyov, D.V.; Bredikhin R.A.; Le Minh, G.; Andrianova, I.A.; Tutwiler, V.; Nagaswami, C.; Weisel, J.W.; Litvinov, R.I. Reduced Contraction of Blood Clots in Venous Thromboembolism Is a Potential Thrombogenic and Embologenic Mechanism. TH Open 2018, 2, e104–e115. [CrossRef]
- Varjú, I.; Longstaff, C.; Szabó, L.; Farkas, Á.Z.; Varga-Szabó, V.J.; Tanka-Salamon, A.; Machovich, R.; Kolev, K. DNA, Histones and Neutrophil Extracellular Traps Exert Anti-Fibrinolytic Effects in a Plasma Environment. Thromb. Haemost. 2015, 113, 1289–1298. [CrossRef]
- Tan, Q.; Guo, P.; Zhou, J.; Zhang, J.; Zhang, B.; Lan, C.; Xian, J.; Ge, M.; Feng, H.; Chen, Z. Targeting Neutrophil Extracellular Traps Enhanced tPA Fibrinolysis for Experimental Intracerebral Hemorrhage. Transl. Res. 2019, 211, 139–146. [CrossRef]
- Kumar, R.; Patil, G.; Dayal, S. NLRP3-Induced NETosis: A Potential Therapeutic Target for Ischemic Thrombotic Diseases?. Cells 2023, 12, 2709. [CrossRef]
- Litvinov, R.I.; Weisel, J.W. What Is the Biological and Clinical Relevance of Fibrin?. Semin. Thromb. Hemost. 2016, 42, 333–343. [CrossRef]
- Collet, J.P.; Allali, Y.; Lesty, C.; Tanguy, M.L.; Silvain, J.; Ankri, A.; Blanchet, B.; Dumaine, R.; Gianetti, J.; Payot, L.; et al. Altered Fibrin Architecture Is Associated With Hypofibrinolysis and Premature Coronary Atherothrombosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2567–2573. [CrossRef]
- Wufsus, A.R.; Macera, N.E.; Neeves, K.B. The Hydraulic Permeability of Blood Clots as a Function of Fibrin and Platelet Density. Biophys. J. 2013, 104, 1812–1823. [CrossRef]
- Borissoff, J.I.; Joosen, I.A.; Versteylen, M.O.; Brill, A.; Fuchs, T.A.; Savchenko, A.S.; Gallant, M.; Martinod, K.; Ten Cate, H.; Hofstra, L.; et al. Elevated Levels of Circulating DNA and Chromatin Are Independently Associated With Severe Coronary Atherosclerosis and a Prothrombotic State. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2032–2040. [CrossRef]
- Knight, J.S.; Luo, W.; O’Dell, A.A.; Yalavarthi, S.; Zhao, W.; Subramanian, V.; Guo, C.; Grenn, R.C.; Thompson, P.R.; Eitzman, D.T.; et al. Peptidylarginine Deiminase Inhibition Reduces Vascular Damage and Modulates Innate Immune Responses in Murine Models of Atherosclerosis. Circ. Res. 2014, 114, 947–956. [CrossRef]
- Laridan, E.; Martinod, K.; De Meyer, S.F. Neutrophil Extracellular Traps in Arterial and Venous Thrombosis. Semin. Thromb. Hemost. 2019, 45, 86–93. [CrossRef]
- de Maat, M.P.; van Schie, M.; Kluft, C.; Leebeek, F.W.; Meijer, P. Biological Variation of Hemostasis Variables in Thrombosis and Bleeding: Consequences for Performance Specifications. Clin. Chem. 2016, 62, 1639–1646. [CrossRef]
- Sabnis, R.W. Novel Peptidylarginine Deiminase Type 4 (PAD4) Inhibitors. ACS Med. Chem. Lett. 2022, 13, 1537–1538. [CrossRef]
- Aiken, S.G.; Grimes, T.; Munro, S.; Zarganes-Tzitzikas, T.; La Thangue, N.B.; Brennan, P.E. A Patent Review of Peptidylarginine Deiminase 4 (PAD4) Inhibitors (2014–Present). Expert Opin. Ther. Pat. 2025, 35, 611–621. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




