Submitted:
10 November 2025
Posted:
11 November 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Pathophysiology of Acute CNS Damage: Common Cascades and Points of Divergence
2.1. Common Temporal Sequence Across Etiologies
2.2. Cardiovascular Etiologies: Focal Arterial Occlusion, Parenchymal Bleeding, and Whole-Brain Ischemia
2.2.1. Ischemic Stroke
2.2.2. Intracerebral Hemorrhage (ICH)
2.2.3. Subarachnoid Hemorrhage (SAH) and Global Ischemia After Cardiac Arrest
2.3. Traumatic CNS Injury: Mechanical Initiation, Biochemical Propagation
2.3.1. Traumatic Brain Injury (TBI)
2.3.2. Spinal Cord Injury (SCI)
2.4. Systemic and Metabolic Causes of Acute CNS Dysfunction
2.5. Cross-Cutting Mechanisms and Therapeutic Objectives
2.6. Therapeutic Objectives Across Time: What Current Care Tries to Achieve
3. The Orexin/Hypocretin System in Brief
3.1. Cellular Mechanisms and Receptor Signaling
3.2. State Control and Homeostatic Integration
3.3. From Drive to Movement: Orexins and the Neural Implementation of Action
3.4. System-Wide Neuromodulatory Integration
4. Strategies for Enhancing Orexinergic Tone
4.1. Synthetic Small-Molecule Agonists
4.2. Peptide-Based Orexin Replacement
4.3. Physiological and Behavioral Modulation
4.4. Neuromodulation and Electrical Stimulation
4.5. Cell and Gene Therapy
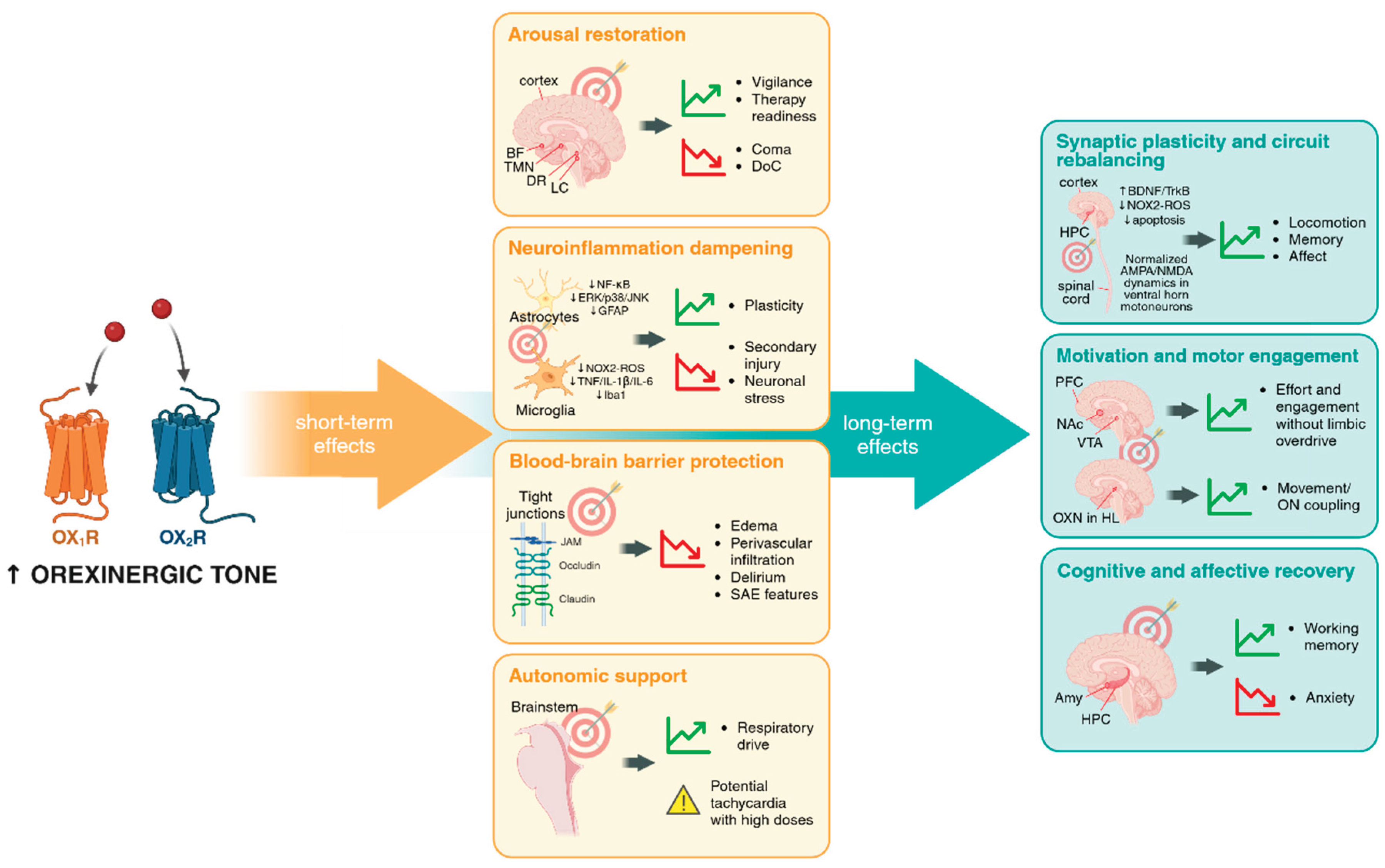
5. Experimental and Clinical Evidence of Orexinergic Modulation in Acute CNS Damage
5.1. Cerebrovascular and Global Ischemic Injuries
5.1.1. Orexin Alterations After Ischemic or Hemorrhagic Events
5.1.2. Experimental Modulation of Orexin Signaling
5.2. Traumatic Brain and Spinal Cord Injury
5.2.1. Orexin Alterations After Mechanical Trauma
5.2.2. Experimental Modulation of Orexin Signaling
5.3. Systemic Biological and Toxic Insults
5.3.1. Orexin Alterations During Sepsis and Systemic Metabolic Failure
5.3.2. Experimental Modulation of Orexin Signaling
5.4. Mechanistic Convergence Across Injury Types
5.4.1. Acute Phase: Metabolic Crisis, Excitotoxicity, and Orexin Silencing
5.4.2. Subacute Phase: Neurovascular Stabilization, Inflammation Tuning, and Circuit re-engagement
5.4.3. Chronic Phase: Arousal, Plasticity, and Long-Term Maladaptations
5.4.4. Mechanistic Convergence Across Injury Types
6. Translational Opportunities, Challenges and Perspectives
6.1. Temporal Precision: From Injury Phase to Circadian Alignment
6.2. Delivery Routes and Formulation Strategies
6.3. Receptor Selectivity and Subtype Balance
6.4. Combination and Multimodal Approaches
6.5. Safety Considerations and Potential Risks
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akbari: Y., Maybhate, A., Chen, C., Greenwald, E., Liang, L., Buitrago-Blanco, M., Jia, X., Thakor, N., & Geocadin, R. (2012). Abstract 2: Orexin-A Improves Arousal from Post--Cardiac Arrest Coma in a Rodent Model. Circulation, 126(suppl_21), A2-A2. [CrossRef]
- Ang, B. T., Tan, W. L., Lim, J., & Ng, I. (2005). Cerebrospinal fluid orexin in aneurysmal subarachnoid haemorrhage – a pilot study. Journal of Clinical Neuroscience, 12(7), 758-762. [CrossRef]
- Azadian, M., Tian, G., Bazrafkan, A., Maki, N., Rafi, M., Chetty, N., Desai, M., Otarola, I., Aguirre, F., Zaher, S. M., Khan, A., Suri, Y., Wang, M., Lopour, B. A., Steward, O., & Akbari, Y. (2021). Overnight Caloric Restriction Prior to Cardiac Arrest and Resuscitation Leads to Improved Survival and Neurological Outcome in a Rodent Model. Frontiers in Neuroscience, 14. [CrossRef]
- Baier, P. C., Hallschmid, M., Seeck-Hirschner, M., Weinhold, S. L., Burkert, S., Diessner, N., Göder, R., Aldenhoff, J. B., & Hinze-Selch, D. (2011). Effects of intranasal hypocretin-1 (orexin A) on sleep in narcolepsy with cataplexy. Sleep Medicine, 12(10), 941-946. [CrossRef]
- Barichello, T., Generoso, J. S., Singer, M., & Dal-Pizzol, F. (2022). Biomarkers for sepsis: More than just fever and leukocytosis-a narrative review. Critical Care (London, England), 26(1), 14. [CrossRef]
- Baumann, C. R., Bassetti, C. L., Valko, P. O., Haybaeck, J., Keller, M., Clark, E., Stocker, R., Tolnay, M., & Scammell, T. E. (2009). Loss of hypocretin (orexin) neurons with traumatic brain injury. Annals of Neurology, 66(4), 555-559. [CrossRef]
- Baumann, C. R., Stocker, R., Imhof, H.-G., Trentz, O., Hersberger, M., Mignot, E., & Bassetti, C. L. (2005). Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury. Neurology, 65(1), 147-149. [CrossRef]
- Baumann, C. R., Werth, E., Stocker, R., Ludwig, S., & Bassetti, C. L. (2007). Sleep–wake disturbances 6 months after traumatic brain injury: A prospective study. Brain, 130(7), 1873-1883. [CrossRef]
- Bémeur, C., & Butterworth, R. F. (2013). Liver-brain proinflammatory signalling in acute liver failure: Role in the pathogenesis of hepatic encephalopathy and brain edema. Metabolic Brain Disease, 28(2), 145-150. [CrossRef]
- Bigford, G. E., Bracchi-Ricard, V. C., Keane, R. W., Nash, M. S., & Bethea, J. R. (2013). Neuroendocrine and cardiac metabolic dysfunction and NLRP3 inflammasome activation in adipose tissue and pancreas following chronic spinal cord injury in the mouse. ASN Neuro, 5(4), 243-255. [CrossRef]
- Bigford, G. E., Bracchi-Ricard, V. C., Nash, M. S., & Bethea, J. R. (2012). Alterations in mouse hypothalamic adipokine gene expression and leptin signaling following chronic spinal cord injury and with advanced age. PloS One, 7(7), e41073. [CrossRef]
- Biswabharati, S., Jean-Xavier, C., Eaton, S. E. A., Lognon, A. P., Brett, R., Hardjasa, L., & Whelan, P. J. (2018). Orexinergic Modulation of Spinal Motor Activity in the Neonatal Mouse Spinal Cord. eNeuro, 5(5), ENEURO.0226-18.2018. [CrossRef]
- Blanco-Centurion, C., Liu, M., Konadhode, R., Pelluru, D., & Shiromani, P. J. (2013). Effects of orexin gene transfer in the dorsolateral pons in orexin knockout mice. Sleep, 36(1), 31-40. [CrossRef]
- Bonizzato, M., James, N. D., Pidpruzhnykova, G., Pavlova, N., Shkorbatova, P., Baud, L., Martinez-Gonzalez, C., Squair, J. W., DiGiovanna, J., Barraud, Q., Micera, S., & Courtine, G. (2021). Multi-pronged neuromodulation intervention engages the residual motor circuitry to facilitate walking in a rat model of spinal cord injury. Nature Communications, 12(1), 1925. [CrossRef]
- Born, J., Lange, T., Kern, W., McGregor, G. P., Bickel, U., & Fehm, H. L. (2002). Sniffing neuropeptides: A transnasal approach to the human brain. Nature Neuroscience, 5(6), 514-516. [CrossRef]
- Burdakov, D. (2019). Reactive and predictive homeostasis: Roles of orexin/hypocretin neurons. Neuropharmacology, 154, 61-67. [CrossRef]
- Burdakov, D. (2020). How orexin signals bias action: Hypothalamic and accumbal circuits. Brain Research, 1731, 145943. [CrossRef]
- Burdakov, D. (2021). Subsecond Ensemble Dynamics of Orexin Neurons Link Sensation and Action. Frontiers of Neurology and Neuroscience, 45, 52-60. [CrossRef]
- Burdakov, D., Jensen, L. T., Alexopoulos, H., Williams, R. H., Fearon, I. M., O’Kelly, I., Gerasimenko, O., Fugger, L., & Verkhratsky, A. (2006). Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron, 50(5), 711-722. [CrossRef]
- Campbell, B. C. V., & Khatri, P. (2020). Stroke. Lancet (London, England), 396(10244), 129-142. [CrossRef]
- Cangiano-Heath, A., Wirkowski, E., Gu, Y., Adachi, J., Ashcroft, B., Fiore, S., Mofakham, S., & Wright, B. (2020). Pilot Study of Orexin as an Indicator of Coma Recovery (820). Neurology, 94(15_supplement), 820. [CrossRef]
- Chen, Y., Lu, X., & Hu, L. (2023). Transcutaneous Auricular Vagus Nerve Stimulation Facilitates Cortical Arousal and Alertness. International Journal of Environmental Research and Public Health, 20(2), 1402. [CrossRef]
- Chen, Y.-H., Lee, H.-J., Lee, M. T., Wu, Y.-T., Lee, Y.-H., Hwang, L.-L., Hung, M.-S., Zimmer, A., Mackie, K., & Chiou, L.-C. (2018). Median nerve stimulation induces analgesia via orexin-initiated endocannabinoid disinhibition in the periaqueductal gray. Proceedings of the National Academy of Sciences of the United States of America, 115(45), E10720-E10729. [CrossRef]
- Cheng, Jocelyn. (2025, septiembre 8). E2086, a Selective Orexin Receptor-2 Agonist, Study for Promoting Wakefulness in Patients With Narcolepsy Type-1. World Sleep Congress, Singapore. https://ws2025.abstractserver.com/program/#/details/presentations/1510.
- Chieffi, S., Messina, G., Villano, I., Messina, A., Esposito, M., Monda, V., Valenzano, A., Moscatelli, F., Esposito, T., Carotenuto, M., Viggiano, A., Cibelli, G., & Monda, M. (2017). Exercise Influence on Hippocampal Function: Possible Involvement of Orexin-A. Frontiers in Physiology, 8, 85. [CrossRef]
- Cho, N., Squair, J. W., Aureli, V., James, N. D., Bole-Feysot, L., Dewany, I., Hankov, N., Baud, L., Leonhartsberger, A., Sveistyte, K., Skinnider, M. A., Gautier, M., Laskaratos, A., Galan, K., Goubran, M., Ravier, J., Merlos, F., Batti, L., Pages, S., … Courtine, G. (2024). Hypothalamic deep brain stimulation augments walking after spinal cord injury. Nature Medicine, 30(12), 3676-3686. [CrossRef]
- Christensen, J., MacPherson, N., Li, C., Yamakawa, G. R., & Mychasiuk, R. (2023). Repeat mild traumatic brain injuries (RmTBI) modify nociception and disrupt orexinergic connectivity within the descending pain pathway. The Journal of Headache and Pain, 24(1), 72. [CrossRef]
- Christensen, J., Vlassopoulos, E., Barlow, C. K., Schittenhelm, R. B., Li, C. N., Sgro, M., Warren, S., Semple, B. D., Yamakawa, G. R., Shultz, S. R., & Mychasiuk, R. (2024). The beneficial effects of modafinil administration on repeat mild traumatic brain injury (RmTBI) pathology in adolescent male rats are not dependent upon the orexinergic system. Experimental Neurology, 382, 114969. [CrossRef]
- Christiansen, J. R., & Kirkeby, A. (2024). Clinical translation of pluripotent stem cell-based therapies: Successes and challenges. Development (Cambridge, England), 151(7), dev202067. [CrossRef]
- Courtine, G., & Sofroniew, M. V. (2019). Spinal cord repair: Advances in biology and technology. Nature Medicine, 25(6), 898-908. [CrossRef]
- Craig, J., Carey, S., Gibbons, A., Kaplan, G., & Zielinski, M. (2024). 0049 Sleep and Electroencephalogram Effects from a Dual Orexin Receptor Antagonist in Mice with Traumatic Brain Injury. Sleep, 47(Supplement_1), A22. [CrossRef]
- Dauvilliers, Y. (2025, septiembre 8). The Radiant Light: Efficacy and Safety of Oveporexton (TAK-861), an Oral Orexin Receptor 2 Agonist for the Treatment of Narcolepsy Type 1. World Sleep Congress, Singapore. https://ws2025.abstractserver.com/program/#/details/presentations/3429.
- Dauvilliers, Y., Plazzi, G., Mignot, E., Lammers, G. J., Del Río Villegas, R., Khatami, R., Taniguchi, M., Abraham, A., Hang, Y., Kadali, H., Lamberton, M., Sheikh, S., Stukalin, E., Neuwirth, R., Swick, T. J., Tanaka, S., von Hehn, C., von Rosenstiel, P., Wang, H., … Olsson, T. (2025). Oveporexton, an Oral Orexin Receptor 2-Selective Agonist, in Narcolepsy Type 1. The New England Journal of Medicine, 392(19), 1905-1916. [CrossRef]
- de Lecea, L., Kilduff, T. S., Peyron, C., Gao, X., Foye, P. E., Danielson, P. E., Fukuhara, C., Battenberg, E. L., Gautvik, V. T., Bartlett, F. S., Frankel, W. N., van den Pol, A. N., Bloom, F. E., Gautvik, K. M., & Sutcliffe, J. G. (1998). The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America, 95(1), 322-327. [CrossRef]
- Deutschman, C. S., Raj, N. R., McGuire, E. O., & Kelz, M. B. (2013). Orexinergic Activity Modulates Altered Vital Signs and Pituitary Hormone Secretion in Experimental Sepsis. Critical Care Medicine, 41(11), e368. [CrossRef]
- Dhuria, S. V., Hanson, L. R., & Frey, W. H. (2010). Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. Journal of Pharmaceutical Sciences, 99(4), 1654-1673. [CrossRef]
- Dirnagl, U., Iadecola, C., & Moskowitz, M. A. (1999). Pathobiology of ischaemic stroke: An integrated view. Trends in Neurosciences, 22(9), 391-397. [CrossRef]
- Djupesland, P. G. (2013). Nasal drug delivery devices: Characteristics and performance in a clinical perspective-a review. Drug Delivery and Translational Research, 3(1), 42-62. [CrossRef]
- Dohi, K., Nishino, S., Nakamachi, T., Ohtaki, H., Morikawa, K., Takeda, T., Shioda, S., & Aruga, T. (2006). CSF orexin A concentrations and expressions of the orexin-1 receptor in rat hippocampus after cardiac arrest. Neuropeptides, 40(4), 245-250. [CrossRef]
- Dohi, K., Ripley, B., Fujiki, N., Ohtaki, H., Shioda, S., Aruga, T., & Nishino, S. (2005). CSF hypocretin-1/orexin-A concentrations in patients with subarachnoid hemorrhage (SAH). Peptides, 26(11), 2339-2343. [CrossRef]
- Dohi, K., Ripley, B., Fujiki, N., Ohtaki, H., Yamamoto, T., Goto, Y., Nakamachi, T., Shioda, S., Aruga, T., & Nishino, S. (2008). CSF orexin-A/hypocretin-1 concentrations in patients with intracerebral hemorrhage (ICH). Regulatory Peptides, 145(1), 60-64. [CrossRef]
- Dong, X., Papa, E. V., Liu, H., Feng, Z., Huang, F., & Liao, C. (2018). Vagus nerve stimulation causes wake-promotion by affecting neurotransmitters via orexins pathway in traumatic brain injury induced comatose rats.
- Dong, X., Ye, W., Tang, Y., Wang, J., Zhong, L., Xiong, J., Liu, H., Lu, G., & Feng, Z. (2021). Wakefulness-Promoting Effects of Lateral Hypothalamic Area–Deep Brain Stimulation in Traumatic Brain Injury-Induced Comatose Rats: Upregulation of α1-Adrenoceptor Subtypes and Downregulation of Gamma-Aminobutyric Acid β Receptor Expression Via the Orexins Pathway. World Neurosurgery, 152, e321-e331. [CrossRef]
- Dong, X.-Y., & Feng, Z. (2018). Wake-promoting effects of vagus nerve stimulation after traumatic brain injury: Upregulation of orexin-A and orexin receptor type 1 expression in the prefrontal cortex. Neural Regeneration Research, 13(2), 244-251. [CrossRef]
- Dorrier, C. E., Jones, H. E., Pintarić, L., Siegenthaler, J. A., & Daneman, R. (2022). Emerging roles for CNS fibroblasts in health, injury and disease. Nature Reviews. Neuroscience, 23(1), 23-34. [CrossRef]
- Du, Q., Huang, L., Tang, Y., Kang, J., Ye, W., & Feng, Z. (2022). Median Nerve Stimulation Attenuates Traumatic Brain Injury–Induced Comatose State by Regulating the Orexin-A/RasGRF1 Signaling Pathway. World Neurosurgery, 168, e19-e27. [CrossRef]
- Elliott, J. E., De Luche, S. E., Churchill, M. J., Moore, C., Cohen, A. S., Meshul, C. K., & Lim, M. M. (2018). Dietary therapy restores glutamatergic input to orexin/hypocretin neurons after traumatic brain injury in mice. Sleep, 41(3), zsx212. [CrossRef]
- Equihua-Benítez, A. C., Equihua-Benítez, J. A., Guzmán-Vásquez, K., Prospero-García, O., & Drucker-Colín, R. (2020). Orexin cell transplant reduces behavioral arrest severity in narcoleptic mice. Brain Research, 1745, 146951. [CrossRef]
- Eriksson, K. S., Sergeeva, O., Brown, R. E., & Haas, H. L. (2001). Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 21(23), 9273-9279. [CrossRef]
- España, R. A., Baldo, B. A., Kelley, A. E., & Berridge, C. W. (2001). Wake-promoting and sleep-suppressing actions of hypocretin (orexin): Basal forebrain sites of action. Neuroscience, 106(4), 699-715. [CrossRef]
- Evans, R., Kimura, H., Alexander, R., Davies, C. H., Faessel, H., Hartman, D. S., Ishikawa, T., Ratti, E., Shimizu, K., Suzuki, M., Tanaka, S., Yukitake, H., Dauvilliers, Y., & Mignot, E. (2022). Orexin 2 receptor-selective agonist danavorexton improves narcolepsy phenotype in a mouse model and in human patients. Proceedings of the National Academy of Sciences of the United States of America, 119(35), e2207531119. [CrossRef]
- Fu, T., Zhang, W., Guo, R., He, S., Yu, S., Wang, H., Zhang, Y., & Wu, Y. (2025). Inclusion of hypocretin-1 improved performance of poor sleep quality prediction for elderly patients with acute ischemic stroke: A prospective cohort study. Frontiers in Aging Neuroscience, 16. [CrossRef]
- Gao, X.-B., & Horvath, T. (2014). Function and dysfunction of hypocretin/orexin: An energetics point of view. Annual Review of Neuroscience, 37, 101-116. [CrossRef]
- Gaykema, R. P. A., & Goehler, L. E. (2009). Lipopolysaccharide challenge-induced suppression of Fos in hypothalamic orexin neurons: Their potential role in sickness behavior. Brain, Behavior, and Immunity, 23(7), 926-930. [CrossRef]
- Gouveia, F. V., Warsi, N. M., Suresh, H., Matin, R., & Ibrahim, G. M. (2024). Neurostimulation treatments for epilepsy: Deep brain stimulation, responsive neurostimulation and vagus nerve stimulation. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics, 21(3), e00308. [CrossRef]
- Guo, J., Kong, Z., Yang, S., Da, J., Chu, L., Han, G., Liu, J., Tan, Y., & Zhang, J. (2024). Therapeutic effects of orexin-A in sepsis-associated encephalopathy in mice. Journal of Neuroinflammation, 21(1), 131. [CrossRef]
- Guo, Y., Gharibani, P., Agarwal, P., Cho, S.-M., Thakor, N. V., & Geocadin, R. G. (2023). Hyperacute autonomic and cortical function recovery following cardiac arrest resuscitation in a rodent model. Annals of Clinical and Translational Neurology, 10(12), 2223-2237. [CrossRef]
- Guo, Y., Gharibani, P., Agarwal, P., Modi, H., Cho, S.-M., Thakor, N. V., & Geocadin, R. G. (2024). Endogenous orexin and hyperacute autonomic responses after resuscitation in a preclinical model of cardiac arrest. Frontiers in Neuroscience, 18. [CrossRef]
- Hamasaki, M. Y., Barbeiro, H. V., Barbeiro, D. F., Cunha, D. M. G., Koike, M. K., Machado, M. C. C., & Silva, F. P. da. (2016a). “Neuropeptides in the brain defense against distant organ damage”. Journal of Neuroimmunology, 290, 33-35. [CrossRef]
- Hamasaki, M. Y., Barbeiro, H. V., Barbeiro, D. F., Cunha, D. M. G., Koike, M. K., Machado, M. C. C., & Silva, F. P. da. (2016b). “Neuropeptides in the brain defense against distant organ damage”. Journal of Neuroimmunology, 290, 33-35. [CrossRef]
- He, G., Chu, W., Li, Y., Sheng, X., Luo, H., Xu, A., Bian, M., Zhang, H., Wang, M., & Zheng, C. (2025). [Orexin-A promotes motor function recovery of rats with spinal cord injury by regulating ionotropic glutamate receptors]. Nan Fang Yi Ke Da Xue Xue Bao = Journal of Southern Medical University, 45(5), 1023-1030. [CrossRef]
- Herring, W. J., Connor, K. M., Snyder, E., Snavely, D. B., Morin, C. M., Lines, C., & Michelson, D. (2019). Effects of suvorexant on the Insomnia Severity Index in patients with insomnia: Analysis of pooled phase 3 data. Sleep Medicine, 56, 219-223. [CrossRef]
- Hilkens, N. A., Casolla, B., Leung, T. W., & de Leeuw, F.-E. (2024). Stroke. Lancet (London, England), 403(10446), 2820-2836. [CrossRef]
- Hirota, K., Takekawa, D., Kushikata, T., & Kudo, M. (2018). Orexinergic tone affects survival in sepsis in rat. British Journal of Anaesthesia, 120(5), e19-e20. [CrossRef]
- Horvath, T. L., Peyron, C., Diano, S., Ivanov, A., Aston-Jones, G., Kilduff, T. S., & van Den Pol, A. N. (1999). Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. The Journal of Comparative Neurology, 415(2), 145-159.
- Hu, S., Ren, L., Wang, Y., Lei, Z., Cai, J., & Pan, S. (2023). The association between serum orexin A and short-term neurological improvement in patients with mild to moderate acute ischemic stroke. Brain and Behavior, 13(1), e2845. [CrossRef]
- Huang, L., Kang, J., Chen, G., Ye, W., Meng, X., Du, Q., & Feng, Z. (2022). Low-intensity focused ultrasound attenuates early traumatic brain injury by OX-A/NF-κB/NLRP3 signaling pathway. Aging, 14(18), 7455-7469. [CrossRef]
- Igarashi, S., Nozu, T., Ishioh, M., Kumei, S., Saito, T., Toki, Y., Hatayama, M., Yamamoto, M., Shindo, M., Tanabe, H., & Okumura, T. (2020). Centrally administered orexin prevents lipopolysaccharide and colchicine induced lethality via the vagal cholinergic pathway in a sepsis model in rats. Biochemical Pharmacology, 182, 114262. [CrossRef]
- Illum, L. (2000). Transport of drugs from the nasal cavity to the central nervous system. European Journal of Pharmaceutical Sciences: Official Journal of the European Federation for Pharmaceutical Sciences, 11(1), 1-18. [CrossRef]
- Irving, E. A., Harrison, D. C., Babbs, A. J., Mayes, A. C., Campbell, C. A., Hunter, A. J., Upton, N., & Parsons, A. A. (2002). Increased cortical expression of the orexin-1 receptor following permanent middle cerebral artery occlusion in the rat. Neuroscience Letters, 324(1), 53-56. [CrossRef]
- Islam, M. T., Rumpf, F., Tsuno, Y., Kodani, S., Sakurai, T., Matsui, A., Maejima, T., & Mieda, M. (2022). Vasopressin neurons in the paraventricular hypothalamus promote wakefulness via lateral hypothalamic orexin neurons. Current Biology: CB, 32(18), 3871-3885.e4. [CrossRef]
- Jia, X., Yan, J., Xia, J., Xiong, J., Wang, T., Chen, Y., Qi, A., Yang, N., Fan, S., Ye, J., & Hu, Z. (2012). Arousal effects of orexin A on acute alcohol intoxication-induced coma in rats. Neuropharmacology, 62(2), 775-783. [CrossRef]
- Kang, J., Ren, B., Huang, L., Dong, X., Xiong, Q., & Feng, Z. (2024). Orexin-A alleviates ferroptosis by activating the Nrf2/HO-1 signaling pathway in traumatic brain injury. Aging, 16(4), 3404-3419. [CrossRef]
- Kang, J., Ren, B., Wang, J., Tang, Y., & Dong, X. (2025). Vagus nerve stimulation: A promising strategy to combat pyroptosis and inflammation in traumatic brain injury through the OX-A/NLRP3/caspase-1/GSDMD signaling pathway. European Journal of Medical Research, 30(1), 586. [CrossRef]
- Kang, J., Zhou, Y., Xiong, Q., & Dong, X. (2024). Trigeminal nerve electrical stimulation attenuates early traumatic brain injury through the TLR4/NF-κB/NLRP3 signaling pathway mediated by orexin-A/OX1R system. Aging, 16(9), 7946-7960. [CrossRef]
- Kang, Y.-J., Tian, G., Bazrafkan, A., Farahabadi, M. H., Azadian, M., Abbasi, H., Shamaoun, B. E., Steward, O., & Akbari, Y. (2017). Recovery from Coma Post-Cardiac Arrest Is Dependent on the Orexin Pathway. Journal of Neurotrauma, 34(19), 2823-2832. [CrossRef]
- Karnani, M. M., Schöne, C., Bracey, E. F., González, J. A., Viskaitis, P., Li, H.-T., Adamantidis, A., & Burdakov, D. (2020). Role of spontaneous and sensory orexin network dynamics in rapid locomotion initiation. Progress in Neurobiology, 187, 101771. [CrossRef]
- Kitamura, E., Hamada, J., Kanazawa, N., Yonekura, J., Masuda, R., Sakai, F., & Mochizuki, H. (2010). The effect of orexin-A on the pathological mechanism in the rat focal cerebral ischemia. Neuroscience Research, 68(2), 154-157. [CrossRef]
- Koenig, M. A., Jia, X., Kang, X., Velasquez, A., Thakor, N. V., & Geocadin, R. G. (2009). Intraventricular orexin-A improves arousal and early EEG entropy in rats after cardiac arrest. Brain Research, 1255, 153-161. [CrossRef]
- Konduru, S. R., Isaacson, J. R., Lasky, D. J., Zhou, Z., Rao, R. K., Vattem, S. S., Rewey, S. J., Jones, M. V., & Maganti, R. K. (2022). Dual orexin antagonist normalized sleep homeostatic drive, enhanced GABAergic inhibition, and suppressed seizures after traumatic brain injury. Sleep, 45(12), zsac238. [CrossRef]
- Korotkova, T. M., Brown, R. E., Sergeeva, O. A., Ponomarenko, A. A., & Haas, H. L. (2006). Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. The European Journal of Neuroscience, 23(10), 2677-2685. [CrossRef]
- Kotan, D., Deniz, O., Aygul, R., & Yıldırım, A. (2013). Acute cerebral ischaemia: Relationship between serum and cerebrospinal fluid orexin-A concentration and infarct volume. Journal of International Medical Research, 41(2), 404-409. [CrossRef]
- Kukkonen, J. P., Jacobson, L. H., Hoyer, D., Rinne, M. K., & Borgland, S. L. (2024). International Union of Basic and Clinical Pharmacology CXIV: Orexin Receptor Function, Nomenclature and Pharmacology. Pharmacological Reviews, 76(5), 625-688. [CrossRef]
- Kuwaki, T. (2011). Orexin links emotional stress to autonomic functions. Autonomic Neuroscience: Basic & Clinical, 161(1-2), 20-27. [CrossRef]
- Lai, F., Cucca, F., Frau, R., Corrias, F., Schlich, M., Caboni, P., Fadda, A. M., & Bassareo, V. (2018). Systemic Administration of Orexin a Loaded Liposomes Potentiates Nucleus Accumbens Shell Dopamine Release by Sucrose Feeding. Frontiers in Psychiatry, 9, 640. [CrossRef]
- Li, F.-T., Yao, C.-H., Yao, L., Huo, Z.-F., & Liu, J. (2018). Study on the effects of parecoxib on hypothalamus orexin neuron of cerebral infarction rats. European Review for Medical and Pharmacological Sciences, 22(5), 1499-1505. [CrossRef]
- Li, H.-T., Viskaitis, P., Bracey, E., Peleg-Raibstein, D., & Burdakov, D. (2024). Transient targeting of hypothalamic orexin neurons alleviates seizures in a mouse model of epilepsy. Nature Communications, 15(1), 1249. [CrossRef]
- Li, J., Hu, Z., & de Lecea, L. (2014). The hypocretins/orexins: Integrators of multiple physiological functions. British Journal of Pharmacology, 171(2), 332-350. [CrossRef]
- Li, T., Xu, W., Ouyang, J., Lu, X., Sherchan, P., Lenahan, C., Irio, G., Zhang, J. H., Zhao, J., Zhang, Y., & Tang, J. (2020). Orexin A alleviates neuroinflammation via OXR2/CaMKKβ/AMPK signaling pathway after ICH in mice. Journal of Neuroinflammation, 17(1), 187. [CrossRef]
- Lim, M. M., Elkind, J., Xiong, G., Galante, R., Zhu, J., Zhang, L., Lian, J., Rodin, J., Kuzma, N. N., Pack, A. I., & Cohen, A. S. (2013). Dietary Therapy Mitigates Persistent Wake Deficits Caused by Mild Traumatic Brain Injury. Science Translational Medicine, 5(215), 215ra173-215ra173. [CrossRef]
- Liu, M., Blanco-Centurion, C., Konadhode, R. R., Luan, L., & Shiromani, P. J. (2016). Orexin gene transfer into the amygdala suppresses both spontaneous and emotion-induced cataplexy in orexin-knockout mice. The European Journal of Neuroscience, 43(5), 681-688. [CrossRef]
- Liu, R.-J., van den Pol, A. N., & Aghajanian, G. K. (2002). Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 22(21), 9453-9464. [CrossRef]
- Lo, E. H., Dalkara, T., & Moskowitz, M. A. (2003). Mechanisms, challenges and opportunities in stroke. Nature Reviews. Neuroscience, 4(5), 399-415. [CrossRef]
- Lochhead, J. J., & Thorne, R. G. (2012). Intranasal delivery of biologics to the central nervous system. Advanced Drug Delivery Reviews, 64(7), 614-628. [CrossRef]
- Lozano, A. M., Lipsman, N., Bergman, H., Brown, P., Chabardes, S., Chang, J. W., Matthews, K., McIntyre, C. C., Schlaepfer, T. E., Schulder, M., Temel, Y., Volkmann, J., & Krauss, J. K. (2019). Deep brain stimulation: Current challenges and future directions. Nature Reviews. Neurology, 15(3), 148-160. [CrossRef]
- Maas, A. I. R., Menon, D. K., Manley, G. T., Abrams, M., Åkerlund, C., Andelic, N., Aries, M., Bashford, T., Bell, M. J., Bodien, Y. G., Brett, B. L., Büki, A., Chesnut, R. M., Citerio, G., Clark, D., Clasby, B., Cooper, D. J., Czeiter, E., Czosnyka, M., … InTBIR Participants and Investigators. (2022). Traumatic brain injury: Progress and challenges in prevention, clinical care, and research. The Lancet. Neurology, 21(11), 1004-1060. [CrossRef]
- Magid-Bernstein, J., Girard, R., Polster, S., Srinath, A., Romanos, S., Awad, I. A., & Sansing, L. H. (2022). Cerebral Hemorrhage: Pathophysiology, Treatment, and Future Directions. Circulation Research, 130(8), 1204-1229. [CrossRef]
- Martins, L., Seoane-Collazo, P., Contreras, C., González-García, I., Martínez-Sánchez, N., González, F., Zalvide, J., Gallego, R., Diéguez, C., Nogueiras, R., Tena-Sempere, M., & López, M. (2016). A Functional Link between AMPK and Orexin Mediates the Effect of BMP8B on Energy Balance. Cell Reports, 16(8), 2231-2242. [CrossRef]
- Matsuura, W., Nakamoto, K., & Tokuyama, S. (2020). Involvement of descending pain control system regulated by orexin receptor signaling in the induction of central post-stroke pain in mice. European Journal of Pharmacology, 874, 173029. [CrossRef]
- Messina, G., Dalia, C., Tafuri, D., Monda, V., Palmieri, F., Dato, A., Russo, A., De Blasio, S., Messina, A., De Luca, V., Chieffi, S., & Monda, M. (2014). Orexin-A controls sympathetic activity and eating behavior. Frontiers in Psychology, 5, 997. [CrossRef]
- Meusel, M., Voß, J., Krapalis, A., Machleidt, F., Vonthein, R., Hallschmid, M., & Sayk, F. (2022). Intranasal orexin A modulates sympathetic vascular tone: A pilot study in healthy male humans. Journal of Neurophysiology, 127(2), 548-558. [CrossRef]
- Mihara, Y., Dohi, K., Yofu, S., Nakamachi, T., Ohtaki, H., Shioda, S., & Aruga, T. (2011). Expression and Localization of the Orexin-1 Receptor (OX1R) After Traumatic Brain Injury in Mice. Journal of Molecular Neuroscience, 43(2), 162-168. [CrossRef]
- Milbank, E., & López, M. (2019). Orexins/Hypocretins: Key Regulators of Energy Homeostasis. Frontiers in Endocrinology, 10, 830. [CrossRef]
- Mileykovskiy, B. Y., Kiyashchenko, L. I., & Siegel, J. M. (2005). Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron, 46(5), 787-798. [CrossRef]
- Modi, H. R., Wang, Q., Gd, S., Sherman, D., Greenwald, E., Savonenko, A. V., Geocadin, R. G., & Thakor, N. V. (2017). Intranasal post-cardiac arrest treatment with orexin-A facilitates arousal from coma and ameliorates neuroinflammation. PLOS ONE, 12(9), e0182707. [CrossRef]
- Mofatteh, M., Mohamed, A., Mashayekhi, M. S., Skandalakis, G. P., Neudorfer, C., Arfaie, S., MohanaSundaram, A., Sabahi, M., Anand, A., Aboulhosn, R., Liao, X., Horn, A., & Ashkan, K. (2025). Deep brain stimulation of the hypothalamic region: A systematic review. Acta Neurochirurgica, 167(1), 33. [CrossRef]
- Nakamachi, T., Endo, S., Ohtaki, H., Yin, L., Kenji, D., Kudo, Y., Funahashi, H., Matsuda, K., & Shioda, S. (2005). Orexin-1 receptor expression after global ischemia in mice. Regulatory Peptides, 126(1), 49-54. [CrossRef]
- Nedeljkovic-Kurepa, A., Abraham, M. N., Fernandes, T. D., Yaipen, O., Brewer, M. R., Taylor, M. D., Pavlov, V. A., & Deutschman, C. S. (2025). Loss of M1 Acetylcholine Receptor-mediated Orexinergic Activity Contributes to Immune Dysfunction in Experimental Sepsis. Research Square. [CrossRef]
- Ogawa, Y., Ezaki, S., Shimojo, N., & Kawano, S. (2021). Case Report: Reduced CSF Orexin Levels in a Patient With Sepsis. Frontiers in Neuroscience, 15. [CrossRef]
- Ogawa, Y., Irukayama-Tomobe, Y., Murakoshi, N., Kiyama, M., Ishikawa, Y., Hosokawa, N., Tominaga, H., Uchida, S., Kimura, S., Kanuka, M., Morita, M., Hamada, M., Takahashi, S., Hayashi, Y., & Yanagisawa, M. (2016). Peripherally administered orexin improves survival of mice with endotoxin shock. eLife, 5, e21055. [CrossRef]
- Ogawa, Y., Shimojo, N., Ishii, A., Tamaoka, A., Kawano, S., & Inoue, Y. (2022). Reduced CSF orexin levels in rats and patients with systemic inflammation: A preliminary study. BMC Research Notes, 15(1), 221. [CrossRef]
- O’Shea, T. M., Ao, Y., Wang, S., Ren, Y., Cheng, A. L., Kawaguchi, R., Shi, Z., Swarup, V., & Sofroniew, M. V. (2024). Derivation and transcriptional reprogramming of border-forming wound repair astrocytes after spinal cord injury or stroke in mice. Nature Neuroscience, 27(8), 1505-1521. [CrossRef]
- Pace, M., Adamantidis, A., Facchin, L., & Bassetti, C. (2017). Role of REM Sleep, Melanin Concentrating Hormone and Orexin/Hypocretin Systems in the Sleep Deprivation Pre-Ischemia. PLOS ONE, 12(1), e0168430. [CrossRef]
- Pace, M., Baracchi, F., Gao, B., & Bassetti, C. (2015). Identification of Sleep-Modulated Pathways Involved in Neuroprotection from Stroke. Sleep, 38(11), 1707-1718. [CrossRef]
- Palomba, L., Motta, A., Imperatore, R., Piscitelli, F., Capasso, R., Mastroiacovo, F., Battaglia, G., Bruno, V., Cristino, L., & Di Marzo, V. (2020). Role of 2-Arachidonoyl-Glycerol and CB1 Receptors in Orexin-A-Mediated Prevention of Oxygen–Glucose Deprivation-Induced Neuronal Injury. Cells, 9(6), 1507. [CrossRef]
- Peleg-Raibstein, D., Viskaitis, P., & Burdakov, D. (2023). Eat, seek, rest? An orexin/hypocretin perspective. Journal of Neuroendocrinology, 35(9), e13259. [CrossRef]
- Perekrest, S. V., Abramova, T. V., & Novikova, N. S. (2009). [Comparative analysis of orexin-containing neurons reactions in the rat hypothalamus after lipopolysaccharide injection in different doses]. Rossiiskii Fiziologicheskii Zhurnal Imeni I.M. Sechenova, 95(12), 1336-1345.
- Perekrest, S. V., Shainidze, K. Z., Loskutov, I. V., Abramova, T. V., Novikova, N. S., & Korneva, E. A. (2011). [Immunoreactivity of hypothalamic orexin neurons and expression level of preproorexin gene in them after lipopolysaccharide injection]. Rossiiskii fiziologicheskii zhurnal imeni I.M Sechenova, 97(6), 573-579.
- Perekrest, S. V., Shainidze, K. Z., Loskutov, Yu. V., Abramova, T. V., Novikova, N. S., & Korneva, E. A. (2013). Immunoreactivity of Orexin-Containing Business in the Hypothalamus and the Level of Expression of the Preproorexin Gene in These Cells after Administration of Lipopolysaccharide. Neuroscience and Behavioral Physiology, 43(2), 256-260. [CrossRef]
- Perkins, G. D., Neumar, R., Hsu, C. H., Hirsch, K. G., Aneman, A., Becker, L. B., Couper, K., Callaway, C. W., Hoedemaekers, C. W. E., Lim, S. L., Meurer, W., Olasveengen, T., Sekhon, M. S., Skrifvars, M., Soar, J., Tsai, M.-S., Vengamma, B., Nolan, J. P., & International Liaison Committee on Resuscitation. (2024). Improving Outcomes After Post-Cardiac Arrest Brain Injury: A Scientific Statement From the International Liaison Committee on Resuscitation. Resuscitation, 201, 110196. [CrossRef]
- Peyron, C., Tighe, D. K., van den Pol, A. N., de Lecea, L., Heller, H. C., Sutcliffe, J. G., & Kilduff, T. S. (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 18(23), 9996-10015. [CrossRef]
- Pintwala, S. K., Fraigne, J. J., Belsham, D. D., & Peever, J. H. (2023). Immortal orexin cell transplants restore motor-arousal synchrony during cataplexy. Current Biology: CB, 33(8), 1550-1564.e5. [CrossRef]
- Piper, D. C., Upton, N., Smith, M. I., & Hunter, A. J. (2000). The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. The European Journal of Neuroscience, 12(2), 726-730. [CrossRef]
- Plazzi, Giuseppe. (2025, septiembre 8). Vibrance-1: A Randomized Phase 2 Study Evaluating Safety and Efficacy of the Orexin 2 Receptor Agonist Alixorexton (ALKS 2680) in Patients with Narcolepsy Type 1. World Sleep Congress, Singapore. https://ws2025.abstractserver.com/program/#/details/presentations/1499.
- Quintana, D. S., Lischke, A., Grace, S., Scheele, D., Ma, Y., & Becker, B. (2021). Advances in the field of intranasal oxytocin research: Lessons learned and future directions for clinical research. Molecular Psychiatry, 26(1), 80-91. [CrossRef]
- Rahimi Darehbagh, R., Seyedoshohadaei, S. A., Ramezani, R., & Rezaei, N. (2024). Stem cell therapies for neurological disorders: Current progress, challenges, and future perspectives. European Journal of Medical Research, 29(1), 386. [CrossRef]
- Rejdak, K., Petzold, A., Lin, L., Smith, M., Kitchen, N., & Thompson, E. J. (2005). Decreased CSF hypocretin-1 (orexin-A) after acute haemorrhagic brain injury. Journal of Neurology, Neurosurgery & Psychiatry, 76(4), 597-598. [CrossRef]
- Ren, B., Kang, J., Wang, Y., Meng, X., Huang, Y., Bai, Y., & Feng, Z. (2024). Transcranial direct current stimulation promotes angiogenesis and improves neurological function via the OXA-TF-AKT/ERK signaling pathway in traumatic brain injury. Aging, 16(7), 6566-6587. [CrossRef]
- Saitoh, T., & Sakurai, T. (2023). The present and future of synthetic orexin receptor agonists. Peptides, 167, 171051. [CrossRef]
- Sakurai, T. (2007). The neural circuit of orexin (hypocretin): Maintaining sleep and wakefulness. Nature Reviews. Neuroscience, 8(3), 171-181. [CrossRef]
- Sakurai, T. (2014). The role of orexin in motivated behaviours. Nature Reviews. Neuroscience, 15(11), 719-731. [CrossRef]
- Sakurai, T., Amemiya, A., Ishii, M., Matsuzaki, I., Chemelli, R. M., Tanaka, H., Williams, S. C., Richardson, J. A., Kozlowski, G. P., Wilson, S., Arch, J. R., Buckingham, R. E., Haynes, A. C., Carr, S. A., Annan, R. S., McNulty, D. E., Liu, W. S., Terrett, J. A., Elshourbagy, N. A., … Yanagisawa, M. (1998). Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell, 92(4), 573-585. [CrossRef]
- Scammell, T. E., Nishino, S., Mignot, E., & Saper, C. B. (2001). Narcolepsy and low CSF orexin (hypocretin) concentration after a diencephalic stroke. Neurology, 56(12), 1751-1753. [CrossRef]
- Seigneur, E., & de Lecea, L. (2020). Hypocretin (Orexin) Replacement Therapies. Medicine in Drug Discovery, 8, 100070. [CrossRef]
- Sherman, D. L., Williams, A., Gd, S., Modi, H. R., Wang, Q., Thakor, N. V., & Geocadin, R. G. (2021). Intranasal Orexin After Cardiac Arrest Leads to Increased Electroencephalographic Gamma Activity and Enhanced Neurologic Recovery in Rats. Critical Care Explorations, 3(2), e0349. [CrossRef]
- Skopin, M. D., Kabadi, S. V., Viechweg, S. S., Mong, J. A., & Faden, A. I. (2015). Chronic Decrease in Wakefulness and Disruption of Sleep-Wake Behavior after Experimental Traumatic Brain Injury. Journal of Neurotrauma, 32(5), 289-296. [CrossRef]
- Smolensky, M. H., Hermida, R. C., Reinberg, A., Sackett-Lundeen, L., & Portaluppi, F. (2016). Circadian disruption: New clinical perspective of disease pathology and basis for chronotherapeutic intervention. Chronobiology International, 33(8), 1101-1119. [CrossRef]
- Sofroniew, M. V. (2015). Astrocyte barriers to neurotoxic inflammation. Nature Reviews. Neuroscience, 16(5), 249-263. [CrossRef]
- Sokołowska, P., Urbańska, A., Biegańska, K., Wagner, W., Ciszewski, W., Namiecińska, M., & Zawilska, J. B. (2014). Orexins Protect Neuronal Cell Cultures Against Hypoxic Stress: An Involvement of Akt Signaling. Journal of Molecular Neuroscience, 52(1), 48-55. [CrossRef]
- Somach, R. T., Jean, I. D., Farrugia, A. M., & Cohen, A. S. (2023). Mild Traumatic Brain Injury Affects Orexin/Hypocretin Physiology Differently in Male and Female Mice. Journal of Neurotrauma, 40(19-20), 2146-2163. [CrossRef]
- Sonneville, R., Verdonk, F., Rauturier, C., Klein, I. F., Wolff, M., Annane, D., Chretien, F., & Sharshar, T. (2013). Understanding brain dysfunction in sepsis. Annals of Intensive Care, 3(1), 15. [CrossRef]
- Sun, Y., Li, Q., Guo, H., & He, Q. (2022). Ferroptosis and Iron Metabolism after Intracerebral Hemorrhage. Cells, 12(1), 90. [CrossRef]
- Takekawa, D., Kushikata, T., Akaishi, M., Nikaido, Y., & Hirota, K. (2018). Influence of Orexinergic System on Survival in Septic Rats. Neuropsychobiology, 77(1), 45-48. [CrossRef]
- Tanaka, S., Takizawa, N., Honda, Y., Koike, T., Oe, S., Toyoda, H., Kodama, T., & Yamada, H. (2016). Hypocretin/orexin loss changes the hypothalamic immune response. Brain, Behavior, and Immunity, 57, 58-67. [CrossRef]
- Tanaka, S., Toyoda, H., Honda, Y., Seki, Y., Sakurai, T., Honda, K., & Kodama, T. (2015). Hypocretin/orexin prevents recovery from sickness. Biomedical Reports, 3(5), 648-650. [CrossRef]
- Ten-Blanco, M., Flores, Á., Cristino, L., Pereda-Pérez, I., & Berrendero, F. (2023). Targeting the orexin/hypocretin system for the treatment of neuropsychiatric and neurodegenerative diseases: From animal to clinical studies. Frontiers in Neuroendocrinology, 69, 101066. [CrossRef]
- Tesmer, A. L., Li, X., Bracey, E., Schmandt, C., Polania, R., Peleg-Raibstein, D., & Burdakov, D. (2024). Orexin neurons mediate temptation-resistant voluntary exercise. Nature Neuroscience, 27(9), 1774-1782. [CrossRef]
- Tesmer, A. L., Viskaitis, P., Donegan, D., Bracey, E. F., Grujic, N., Patriarchi, T., Peleg-Raibstein, D., & Burdakov, D. (2025). Orexin population activity precisely reflects net body movement across behavioral and metabolic states. eLife, 14, RP103738. [CrossRef]
- Thannickal, T. C., Moore, R. Y., Nienhuis, R., Ramanathan, L., Gulyani, S., Aldrich, M., Cornford, M., & Siegel, J. M. (2000). Reduced number of hypocretin neurons in human narcolepsy. Neuron, 27(3), 469-474. [CrossRef]
- Theus, M. H. (2024). Neuroinflammation and acquired traumatic CNS injury: A mini review. Frontiers in Neurology, 15, 1334847. [CrossRef]
- Thomasy, H. E., Febinger, H. Y., Ringgold, K. M., Gemma, C., & Opp, M. R. (2017). Hypocretinergic and cholinergic contributions to sleep-wake disturbances in a mouse model of traumatic brain injury. Neurobiology of Sleep and Circadian Rhythms, 2, 71-84. [CrossRef]
- Thomasy, H. E., & Opp, M. R. (2019). Hypocretin Mediates Sleep and Wake Disturbances in a Mouse Model of Traumatic Brain Injury. Journal of Neurotrauma, 36(5), 802-814. [CrossRef]
- Tsuneki, H., Wada, T., & Sasaoka, T. (2018). Chronopathophysiological implications of orexin in sleep disturbances and lifestyle-related disorders. Pharmacology & Therapeutics, 186, 25-44. [CrossRef]
- van den Pol, A. N. (1999). Hypothalamic hypocretin (orexin): Robust innervation of the spinal cord. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 19(8), 3171-3182. [CrossRef]
- Wang, C.-M., Pan, Y.-Y., Liu, M.-H., Cheng, B.-H., Bai, B., & Chen, J. (2017). RNA-seq expression profiling of rat MCAO model following reperfusion Orexin-A. Oncotarget, 8(68), 113066-113081. [CrossRef]
- Wang, T., Li, Y., Guo, M., Dong, X., Liao, M., Du, M., Wang, X., Yin, H., & Yan, H. (2021). Exosome-Mediated Delivery of the Neuroprotective Peptide PACAP38 Promotes Retinal Ganglion Cell Survival and Axon Regeneration in Rats With Traumatic Optic Neuropathy. Frontiers in Cell and Developmental Biology, 9, 659783. [CrossRef]
- Whiting, D. M., Tomycz, N. D., Bailes, J., de Jonge, L., Lecoultre, V., Wilent, B., Alcindor, D., Prostko, E. R., Cheng, B. C., Angle, C., Cantella, D., Whiting, B. B., Mizes, J. S., Finnis, K. W., Ravussin, E., & Oh, M. Y. (2013). Lateral hypothalamic area deep brain stimulation for refractory obesity: A pilot study with preliminary data on safety, body weight, and energy metabolism. Journal of Neurosurgery, 119(1), 56-63. [CrossRef]
- Willie, J. T., Lim, M. M., Bennett, R. E., Azarion, A. A., Schwetye, K. E., & Brody, D. L. (2012). Controlled Cortical Impact Traumatic Brain Injury Acutely Disrupts Wakefulness and Extracellular Orexin Dynamics as Determined by Intracerebral Microdialysis in Mice. Journal of Neurotrauma, 29(10), 1908-1921. [CrossRef]
- Wong, J. C., Shapiro, L., Thelin, J. T., Heaton, E. C., Zaman, R. U., D’Souza, M. J., Murnane, K. S., & Escayg, A. (2021). Nanoparticle encapsulated oxytocin increases resistance to induced seizures and restores social behavior in Scn1a-derived epilepsy. Neurobiology of Disease, 147, 105147. [CrossRef]
- Wu, G., Zhang, X., Li, S., Zhou, D., Bai, J., Wang, H., & Shu, Q. (2022). Overexpression of ORX or MCH Protects Neurological Function Against Ischemic Stroke. Neurotoxicity Research, 40(1), 44-55. [CrossRef]
- Xiong, X., White, R. E., Xu, L., Yang, L., Sun, X., Zou, B., Pascual, C., Sakurai, T., Giffard, R. G., & Xie, X. (Simon). (2013). Mitigation of Murine Focal Cerebral Ischemia by the Hypocretin/Orexin System is Associated With Reduced Inflammation. Stroke, 44(3), 764-770. [CrossRef]
- Xu, D., Kong, T., Cheng, B., Zhang, R., Yang, C., Chen, J., & Wang, C. (2021). Orexin-A alleviates cerebral ischemia-reperfusion injury by inhibiting endoplasmatic reticulum stress-mediated apoptosis. Molecular Medicine Reports, 23(4), 266. [CrossRef]
- Xu, D., Kong, T., Shao, Z., Liu, M., Zhang, R., Zhang, S., Kong, Q., Chen, J., Cheng, B., & Wang, C. (2021). Orexin-A alleviates astrocytic apoptosis and inflammation via inhibiting OX1R-mediated NF-κB and MAPK signaling pathways in cerebral ischemia/reperfusion injury. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1867(11), 166230. [CrossRef]
- Xu, D., Kong, T., Zhang, S., Cheng, B., Chen, J., & Wang, C. (2021). Orexin-A protects against cerebral ischemia-reperfusion injury by inhibiting excessive autophagy through OX1R-mediated MAPK/ERK/mTOR pathway. Cellular Signalling, 79, 109839. [CrossRef]
- Xu Y., Miao J., Jia Y., Dong W., & Xie P. (2011). Expression of the orexinergic system in ischemic cerebral injury and the modulation of the cerebellar fastigial nucleus through electrical stimulation. Chinese Journal of Physical Medicine and Rehabilitation, 100-105.
- Yamanaka, A., Beuckmann, C. T., Willie, J. T., Hara, J., Tsujino, N., Mieda, M., Tominaga, M., Yagami, K. ichi, Sugiyama, F., Goto, K., Yanagisawa, M., & Sakurai, T. (2003). Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron, 38(5), 701-713. [CrossRef]
- Yao, P., Zhou, Q., Ren, B., Yang, L., Bai, Y., & Feng, Z. (2025). Transcranial pulsed current stimulation alleviates neuronal pyroptosis and neurological dysfunction following traumatic brain injury via the orexin-A/NLRP3 pathway. Neuropeptides, 110, 102501. [CrossRef]
- Yenari, M. A., & Han, H. S. (2012). Neuroprotective mechanisms of hypothermia in brain ischaemia. Nature Reviews. Neuroscience, 13(4), 267-278. [CrossRef]
- Yoshida, Y., Fujiki, N., Nakajima, T., Ripley, B., Matsumura, H., Yoneda, H., Mignot, E., & Nishino, S. (2001). Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. The European Journal of Neuroscience, 14(7), 1075-1081. [CrossRef]
- Yuan, L., Dong, H., Zhang, H.-P., Zhao, R., Gong, G., Chen, X., Zhang, L., & Xiong, L. (2011). Neuroprotective Effect of Orexin-A Is Mediated by an Increase of Hypoxia-inducible Factor-1 Activity in Rat. Anesthesiology, 114(2), 340. [CrossRef]
- Zawilska, J. (2015). A new face of orexins action—Neuroprotection. SpringerPlus, 4(1), L59. [CrossRef]
- Zeitzer, J. M., Buckmaster, C. L., Parker, K. J., Hauck, C. M., Lyons, D. M., & Mignot, E. (2003). Circadian and homeostatic regulation of hypocretin in a primate model: Implications for the consolidation of wakefulness. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 23(8), 3555-3560. [CrossRef]
- Zhang, D., Cui, Y., Zhao, M., Zheng, X., Li, C., Wei, J., Wang, K., & Cui, J. (2022). Orexin-A exerts neuroprotective effect in experimental intracerebral hemorrhage by suppressing autophagy via OXR1-mediated ERK/mTOR signaling pathway. Frontiers in Cellular Neuroscience, 16. [CrossRef]
- Zhang, D., Perrey, D. A., Decker, A. M., Langston, T. L., Mavanji, V., Harris, D. L., Kotz, C. M., & Zhang, Y. (2021). Discovery of Arylsulfonamides as Dual Orexin Receptor Agonists. Journal of Medicinal Chemistry, 64(12), 8806-8825. [CrossRef]
- Zhang, Y., Song, D., Liu, R., Gong, H., Wang, C., Zhao, M.-G., & Zhang, K. (2025). Transcutaneous auricular vagus nerve stimulation inhibits food intake and body weight gain through the orexin dependent pathway in high fat diet mice. Scientific Reports, 15(1), 19286. [CrossRef]
- Zhao, D., Zeng, Z., Mo, H., Hu, W., Tian, S., Hu, D., Gong, L., & Hu, K. (2021). Orexin-A inhibits cerebral ischaemic inflammatory injury mediated by the nuclear factor-κB signalling pathway and alleviates stroke-induced immunodepression in mice. Brain Research Bulletin, 174, 296-304. [CrossRef]
- Zhong, Y.-J., Feng, Z., Wang, L., & Wei, T.-Q. (2015). Wake-promoting actions of median nerve stimulation in TBI-induced coma: An investigation of orexin-A and orexin receptor 1 in the hypothalamic region. Molecular Medicine Reports, 12(3), 4441-4447. [CrossRef]
- Zhu, M., Li, X., Guo, J., Zhang, Z., Guo, X., Li, Z., Lin, J., Li, P., Jiang, Z., & Zhu, Y. (2024). Orexin A protects against cerebral ischemia-reperfusion injury by enhancing reperfusion in ischemic cortex via HIF-1α-ET-1/eNOS pathway. Brain Research Bulletin, 218, 111105. [CrossRef]
| Strategy | Main features | Development stage | Advantages | Limitations | Potential in acute CNS injury |
|---|---|---|---|---|---|
| Synthetic small-molecule agonists (OX2R selective) | Oral/IV, BBB-penetrant; phase 2–3 efficacy in narcolepsy | Late-stage clinical | Drug-like; scalable; robust wake-promoting effects; clear PK; feasible in rodents & humans. | Only OX2R (no OX1R agonists yet); risk of insomnia, CV load; timing critical. | IV: arousal rescue in coma/ICU. Oral: time-locked to rehab to sustain vigilance and engagement. |
| Peptide replacement (orexin-A/B) | Non-invasive, tested in humans (pilot narcolepsy studies, healthy volunteers); robust preclinical benefit in injury models | Early translational | Engages both receptors; strong biological effect; non-invasive (IN). | Short half-life; repeated dosing; variable nasal deposition. | Acute rescue of arousal; peri-rehab adjunct to enhance plasticity, cognition, and BBB protection. |
| Advanced delivery systems (nano, liposomes, exosomes, BBB-shuttles) | Liposomes, nanoparticles, exosomes tested with other peptides (oxytocin, TRH, PACAP) | Preclinical (conceptual for orexin) | Stabilise peptides; prolong CNS action; targeting possible. | No orexin-specific primaries (post-2018); regulatory and manufacturing barriers. | Could enable sustained intranasal OXA or regional spinal depots in SCI. Proof-of-concept only. |
| Endogenous modulation (dietary/behavioral) | Exercise, diet (BCAA), metabolic cues | Preclinical/observational | Accessible, low-cost, scalable; aligns with rehab (mobilisation, exercise, diet). | Nonspecific; state-dependent; effects variable. | Exercise/diet as orexin primers; potential adjunct to pharmacology in rehab protocols. |
| Neuromodulation (MNS, VNS, DBS) | MNS/VNS tested in animals & humans (non-orexin fields), orexin mechanism shown in models | Experimental/Clinical (for other indications) | Provides causal proof; circuit-specific; inspires hybrid strategies. | Invasive (DBS/VNS); protocols not standardised; mostly animal models. | Proof-of-principle; could combine with low-dose agonists or IN-OXA to maximise benefit. |
| Cell/gene therapy replacement | Orexin grafts restore cataplexy in narcoleptic mice; AAV orexin reduces cataplexy | Preclinical/Translational (for narcolepsy) | Potential durable restoration of orexin tone. | Invasive; integration/tumorigenesis risks; distant from clinical use. | Unlikely near-term for CNS injury; future use may involve enhancing residual orexin neurons via vectors. |
| Disease context | Setting | Model / Condition | Intervention | Assessment phase | Primary outcomes | References |
|---|---|---|---|---|---|---|
| Ischemic stroke | Clinical | Acute ischemic stroke | N/A | Acute phase (1 - 30 dpi) |
• ↓ CSF and serum Orexin-A, correlated with poor sleep quality and neurological deficit. Excess of Orexin-A associated with post-stroke insomnia | (Fu et al., 2025; Hu et al., 2023; Kotan et al., 2013; Scammell et al., 2001) |
| Preclinical | CCAO MCAO |
N/A | Acute phase (1- 7 dpi) |
• ↑ cell death • ↑ OX1R expressed in neurons, astroglia and oligodendroglia within lesion core |
Irving et al., 2002; Nakamachi et al., 2005 | |
| BCAO MCAO 4-Vo |
icv Orexin-A pre- or post-injury | Acute phase (1 - 9 dpi) |
Orexin-A ameliorates stroke consequences: • ↓ infarct volume, ↓ neurological deficit, ↓ pain, ↑ blood pressure, flow and heart rate • Neuroprotection: ↓ apoptosis, ↓ autophagy in vitro and in vivo (via ↓ p-ERK1/2 and ↑ p-mTOR), ↑ OXR1, ↑ HIF-1α, eNOS, NO and BDNF expression, ↓ ET-1 expression • ↓ neuroinflammation: ↓ excessive activation of astrocytes, ↓ pro-inflammatory cytokines, NF-κB p65 pathway and MAPK pathway |
Kitamura et al., 2010; Matsuura et al., 2020; C.-M. Wang et al., 2017; Xiong et al., 2013; D. Xu, Kong, Cheng, et al., 2021; D. Xu, Kong, Shao, et al., 2021; D. Xu, Kong, Zhang, et al., 2021; Yuan et al., 2011; Zhao et al., 2021; Zhu et al., 2024 | ||
| MCAO CCAO |
Sleep deprivation pre-injury | Acute phase (3 - 7 dpi) |
Sleep deprivation ameliorates stroke consequences: • ↓ infarct volume, ↑ REM • ↓ upregulation of genes related to immune response and cell division |
Pace et al., 2015, 2017 | ||
| MCAO | FNS | Acute phase (1 - 24 hpi) |
FNS preconditioning ameliorates stroke consequences: • ↑ prepro-orexin Orexin-A in hypothalamus, ↓ OX1R |
(Xu Y. et al., 2011) | ||
| MCAO | Orexin overexpression plasmid 3 days before MCAO | Acute/Subacute phase (1 - 10 dpi) | Plasmid ameliorates stroke consequences: • ↓ infarct volume, ↑ sleep structure and neurological function (via Glu uptake and GABA levels) • Neuroprotection: ↓ neuronal apoptosis |
Wu et al., 2022 | ||
| MCAO | iv parecoxib (COX2 inhibitor) | Acute phase (72 hpi) |
Parecoxib ameliorates stroke consequences: • ↑ orexin positive cells and orexin levels in hypothalamus |
(F.-T. Li et al., 2018) | ||
| Hemorrhagic stroke | Clinical | ICH SAH |
N/A | Acute/Subacute phase (1 - 36 dpi) | • ↓ CSF orexin-A, correlated to ↓ consciousness and ↑ neurological deficit | (Ang et al., 2005; Dohi et al., 2005, 2008; Rejdak et al., 2005) |
| Preclinical | Injection of autologous blood into basal ganglion (ICH) | Intranasal or icv Orexin-A | Acute/Subacute/ Chronic phase (1 - 28 dpi) |
Orexin-A ameliorates ICH consequences: • ↓ neurological deficit, ↓ brain water content • Neuroprotection: ↓ neuronal damage, ↓ autophagy (via ERK/mTOR pathway), ↑ p-CaMKKβ, p-AMPK, and anti-inflammatory cytokines, ↓ p-NF-κB and pro-inflammatory cytokines |
(T. Li et al., 2020; D. Zhang et al., 2022) | |
| Cardiac arrest (CA) | Preclinical | ACA Major cardiac vessels compression (transient global ischaemia) |
N/A | Acute phase (0 - 7 dpi) |
• ↑ cell death. Better neurological function correlated with ↑ HR post-resuscitation, ↑ LF/HF ratio and ↑ gamma band power • ↑ CFS Orexin-A at 24 hpi but ↓ CSF Orexin-A at 2 and 4 dpi |
(Dohi et al., 2006; Y. Guo et al., 2023) |
| ACA | icv or intranasal Orexin-A 20-30 min after resucitation | Acute (0 - 72 hpi) and Subacute phase (12 dpi) | Orexin-A meliorates CA consequences: • ↓ neurological deficit, ↑ EEG entropy (IQ values), ↑ arousal (EEG gamma fraction) • ↑ OX1R expression • ↓ neuroinflammation: ↓ TNF-a, iNOS, CD11b |
Akbari et al., 2012; Koenig et al., 2009; Modi et al., 2017; Sherman et al., 2021 | ||
| ACA | ip suvorexant (OX1/OX2 antagonist) | Acute phase (0 - 72 hpi) |
Suvorexant has detrimental effects: • No neurological recovery, ↓ HR, ↓ LF/HF ratio |
(Y. Guo et al., 2024; Y.-J. Kang et al., 2017 | ||
| ACA | Caloric restriction O/N pre-injury | Acute phase (0 - 72 hpi) |
Caloric restriction ameliorates CA consequences: • ↓ neurological deficit • ↓ stress-induced hyperglycemia, ↑ blood ketone levels, no change in SIRT-1 • Neuroprotection: ↓ neurodegeneration, no change in BDNF |
(Azadian et al., 2021) | ||
| Hypoxia-Ischemia | Preclinical | Transient focal ischaemia in vivo | Orexin-A | Acute phase | Orexin-A ameliorates hypoxia-ischaemia consequences: • ↓ infarct volume • Neuroprotection: ↓ ROS accumulation and neuronal death, ↑ PI3K/Akt survival pathway |
(Palomba et al., 2020) |
| Cobalt chloride on primary cortical neuronal cell culture | Orexin-A/B | Acute phase (24 - 48 hpi) |
Orexin-A/B ameliorate hypoxia-ischaemia consequences: • Neuroprotection: ↑ neuronal viability (via Akt activation) |
(Sokołowska et al., 2014) | ||
| Chemical hypoxia | Orexin-A/B | Acute phase | Orexin-A/B ameliorate hypoxia-ischaemia consequences: • Neuroprotection: ↑ neuronal viability, ↓ oxidative stress (via MEKK and Akt activation) |
(Zawilska, 2015) | ||
| TBI | Clinical | Moderate to severe TBI | N/A | Acute (0 - 14 dpi), chronic (6 mpi) or post-mortem phase | • ↓ CSF orexin-A, correlated to ↑ daytime sleepiness and predictive of poor outcome | (Baumann et al., 2005, 2007, 2009; Cangiano-Heath et al., 2020) |
| Preclinical | CCI Fluid perfusion injury |
N/A | Acute/Subacute phase (1 - 30 dpi) |
• ↑ cognitive deficit, ↓ wakefulness, ↑ REM and NREM sleep, ↑ depressive-like symptoms, ↓ motor activity • ↓ Orexin-A, orexinergic neurons and orexinergic activity, • ↑ astrogliosis |
(Mihara et al., 2011; Skopin et al., 2015; Somach et al., 2023; Thomasy et al., 2017; Thomasy & Opp, 2019; Willie et al., 2012) | |
| Modified Feeney's method | icv orexin-A post-injury | Acute phase (12 - 72 hpi) |
Orexin-A ameliorates TBI consequences: • ↓ neurological deficit, ↓ lesion volume • Neuroprotection: ↓ neuronal damage, ↓ ferroptosis (via Nrf2/HO-1 pathway) • ↓ pro-inflammatory citokines |
(J. Kang, Ren, et al., 2024) | ||
| CCI | DORA (dual orexinergic antagonist) | Acute/Subacute/ Chronic (1 week - 3 mpi) |
Orexinergic inhibition contributed to TBI consequences: • ↑ sleep fragmentation, ↑ sleepines |
(Craig et al., 2024) | ||
| CCI | tPCS post-injury | Acute phase (1 - 7 dpi) |
tPCS ameliorates TBI consequences: • ↓ neurological, motor and cognitive deficit • ↑ orexin-A • Neuroprotection: ↓ tissue damage All effects are OX1R-dependent |
(Yao et al., 2025) | ||
| Free fall drop / Modified Feeney's method |
VNS post-injury MNS post-injury TNS post-injury |
Acute phase (1 - 24 hpi) |
VNS, MNS and TNS ameliorate TBI consequences: • ↑ consciousness (via ↑ RasGRF1 pathway), ↓ neurological deficit, ↓ brain edema • ↑ orexin-A and OX1R expression, • Neuroprotection: ↓ brain cellular and tissue damage, ↓ neuronal pyroptosis (via ↓ NLRP3/Caspase-1/GSDMD) • ↓ pro-inflammatory cytokines, ↓ TLR4/NF-κB/NLRP3 inflammasome All effects are OX1R-dependent |
(X. Dong et al., 2018; X.-Y. Dong & Feng, 2018; Du et al., 2022; J. Kang, Zhou, et al., 2024; J. Kang et al., 2025; Zhong et al., 2015) | ||
| Free fall drop | LH-DBS post-injury | Acute phase (1 - 12 hpi) |
LH-DBS ameliorates TBI consequences: • ↑ consciousness • ↑ orexin-A/OX1R expression All effects are OX1-dependent |
(X. Dong et al., 2021) | ||
| Free fall drop | LIFUS post-injury | Acute phase (3 dpi) |
LIFUS ameliorates TBI consequences: • ↓ neurological deficits, ↓ brain edema • ↑ Orexin-A and OX1R expression • Neuroprotection: ↓ tissue damage, ↓ necrotic neuronal degeneration • ↓ pro-inflammatory cytokines, ↓ NF-κB/NLRP3 inflammasome |
(Huang et al., 2022) | ||
| Fluid percussion injury | BCAA dietary supplementation | Acute/Subacute/ Chronic (4 - 30 dpi) |
BCAA ameliorates TBI consequences: • ↑ wakefulness, ↓ fragmented wake bouts ↑ Orexin neurons activation (via ↑ glutamatergic presynaptic density) |
(Elliott et al., 2018; Lim et al., 2013) | ||
| Repeated mild TBI | Chronic ICV orexin-A (daily, 5–33 dpi) | Chronic phase (2–4 wpi) | • ↓ orexin+ neurons, ↓ orexinergic projections to PAG • ↑ nociceptive sensitivity, ↓ anxiety-like behavior Orexin-A worsened outcomes: • ↑ anxiety and motor deficits • ↑ CSF metabolomic disruption |
(Christensen et al., 2023, 2024) | ||
| SCI | Preclinical | Complete spinal cord transection at T9 | intrathecal orexin-A post-Injury | Acute phase (1 - 7 dpi) |
Orexin-A ameliorates SCI consequences: • ↑ motor recovery • ↓ overactive/dysregulated Glu dynamics |
(He et al., 2025) |
| Systemic biological and toxic insults | Clinical | Meningitis, Encephalitis |
N/A | Acute phase (1 - 20 dpi) |
• ↓ CSF orexin-A levels • ↓ BBB integrity (Orexin-A leaked into the blood) |
(Ogawa et al., 2021, 2022) |
| Preclinical | ip LPS in WT and/or orexin-ablated (OX/AT3 TG) mice | N/A | Acute phase (1 - 3 dpi) |
• ↓ gait speed and exploration, ↑ nREM sleep, ↓ wakefulness • ↓ CSF orexin-A levels, ↓ orexin neurons, ↓ prepro-orexin expression, ↓ Fos expression in orexin neurons • ↑ cytokines In orexin-ablated mice: • ↓ survival |
(Gaykema & Goehler, 2009; Hirota et al., 2018; Ogawa et al., 2022; Perekrest et al., 2009, 2011, 2013; Takekawa et al., 2018; Tanaka et al., 2015, 2016) | |
| Acute pancreatitis (non-infectious) | N/A | Acute phase (12-24 hpi) |
• ↑ brain levels of β-endorphin, orexin, and oxytocin Neuropeptide upregulation occurred before local cytokine increase or microglial activation |
(Hamasaki et al., 2016b) | ||
| Cecal ligation & puncture | Icv or intranasal Orexin-A post-injury | Acute phase (1 - 7 dpi) |
Orexin-A ameliorates sepsis consequences: • ↑ survival, ↓ cognitive and emotional deficit, ↓ brain edema, ↑ responsiveness, ↑ pituitary function • ↓ tissue damage, ↓ BBB disruption, • ↓ neuroinflammation: ↓ pro-inflammatory citokines, ↓ microglia activation Effects mediated via OX2R and RAS/MAPK pathway |
(Deutschman et al., 2013; J. Guo et al., 2024) | ||
| Cecal ligation & puncture | ip xanomeline (mAChR agonist) post-injury | Acute phase (48 hpi) |
Xanomeline ameliorates sepsis consequences: • ↑ orexinergic activity, ↑ temp, HR and RR • ↑ pituitary function • ↓ pro-inflammatory cytokines Effects reversed by OX1/2R antagonist (almorexant) |
(Nedeljkovic-Kurepa et al., 2025) | ||
| LPS-induced endotoxemia | ict, ip or sc orexin-A post-injury | Acute phase (1-3 dpi) |
Central, peripheral orexin-A (crosses BBB in endotoxemia): • ↑ survival • restores body temperature, cardiovascular tone • ↓ cytokine levels • ↑ catecholamines, ↑ corticosterone Effects blocked by OX1R antagonist, vagotomy, or atropine |
(Igarashi et al., 2020; Ogawa et al., 2016) | ||
| Alcohol-induced coma | icv orexin-A or orexin-B | Acute phase (hpi) | Orexin-A/B ameliorates intoxication consequences: • ↓ coma duration, restores righting reflex • ↓ delta power in EEG, ↑ prefrontal cortex activity |
(Jia et al., 2012) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





