Introduction
Onychomycosis Overview
Onychomycosis is a common, chronic fungal infection of the nail presenting as white or yellow discolouration with thickening and brittleness of the nail plate. [
1] Toenail onychomycosis is predominantly caused by dermatophytes, with
Trichophyton mentagrophytes and
Trichophyton rubrum being the most common agents, however non-dermatophyte moulds and yeasts also play a role. [
2] Prevalence of onychomycosis in the general population is approximately 4% but varies greatly due to underlying factors. Significantly higher rates are found in at-risk populations, as well as in those spending time in warm humid environments that encourage fungal growth such as public swimming pools. [
3] Risk factors for developing onychomycosis include advancing age, obesity, diabetes mellitus, tinea pedis and vascular diseases. [
4] In addition to physical symptoms, psychological quality of life is impacted by onychomycosis, with an association with depression, anxiety, and self-esteem. [
5]
Toenail onychomycosis is not self-limiting [
3] and due to slow toenail growth rate, treatment of toenail onychomycosis may take 12-18 months. Treatment strategies for onychomycosis include oral or topical antifungal medications, laser treatment and combination treatments. [
6] Due to drug interactions, potential hepatotoxicity and long-term treatment required, oral treatments are limited in use. [
2] Topical antifungal treatments offer safer options however have lower efficacy rates and a difficult application process for those with mobility restrictions. [
2] As such, there is an unmet clinical need for safe, convenient and efficacious treatments that are appropriate for long term use, and potentially continued use to manage this chronic condition.
Manuka Oil
Manuka,
Leptospermum scoparium, is a small bush or tree in the
Myrtaeae plant family found throughout New Zealand and Australia with a history of traditional uses by Māori people. [
7] The volatile essential oil from the Manuka plant is extracted through steam distillation, mainly from the leaves and stems. [
7] The primary chemical constituents of Manuka oil include triketones, monoterpenes and sesquiterpenes, with varying levels depending geographic location of the plant. [
8] Manuka oil with high levels of β-triketones (>20%) is of particular interest due to antimicrobial properties and found only in the oil of Manuka plants from the small area of East Cape, New Zealand. [
8] The β-triketones in Manuka oil are made up of leptospermone, grandiflorone, flavesone and isoleptospermone. Manuka oil has been widely studied for its antimicrobial and anti-inflammatory effects. [
7,
9] Antifungal activity of manuka oil has been shown in vitro with multiple dilutions and preparations. [
10] While the exact mechanism is unknown, the antifungal properties of Manuka oil are suggested to be attributed to β-triketone content [
9] which may disrupt the fungal cell wall. [
7]
FunghiClear®
FunghiClear® is a plant-based toenail spray classified as a Class I Medical Device in the European Union (Medical Device Regulation 2007), United Kingdom and Australia, with a claim to combat and prevent toenail onychomycosis and pending registrations in South Africa, Taiwan and China. The formulation comprises a blend of essential oils, with the key ingredient of high-triketone organic Leptospermum scoparium (Manuka) oil sourced from the East Cape region of New Zealand, supported with Ocimum basilicum (basil), Mentha piperita (peppermint), and Lavandula (lavender) oils.
In vitro research demonstrated that Manuka oil with high β -triketones successfully inhibits >90% of the dermatophyte growth of
T. interdigitale, a variant of
T. mentagrophytes, with minimum inhibitory concentration (MIC) of 0.39%. [
11] Time-kill tests demonstrated fungal eradication at 48 hours at low concentrations. [
11]
This study was initiated by Norman Health Products & Services B.V, the manufacturer of FunghiClear® as part of an ongoing assessment of safety and efficacy, as required by medical device regulations.
The primary objectives of this study were to investigate the safety and efficacy of FunghiClear® for combatting onychomycosis and the secondary objective was to investigate user convenience. FunghiClear® has been available in Europe since 2012, with distribution in over 20 countries. It is quick-drying spray with a pleasant scent and dispensed in a spray bottle that can be used right side up or upside-down, making it easy to reach to the affected toenails. Directions are to be sprayed twice per day to the affected toenail(s) and continued until a full healthy nail has regrown.
Methods
Study Design
A 12-week, single arm clinical user study in adults aged 18 – 65 with onychomycosis was undertaken. One hundred ninety-two (192) participants completed the investigation as per study protocol in four sites: The Netherlands and Belgium, United Kingdom, Taiwan, and Scandinavia (Norway and Sweden).
Participants
Participants who were recommended to use FunghiClear® by their footcare professional were enrolled through their footcare professional between June 2021 and March 2025 and followed for 12 weeks. Reflecting the variations in cultural and healthcare frameworks across countries, participants were recruited through medical pedicurists in the Netherlands and Belgium, podiatrists and chiropodists in the UK, dermatologists in Taiwan and podiatrists in Scandinavia.
Inclusion criteria for participants were human volunteers between ages 18 and 65, confirmed toenail onychomycosis and no onychomycosis treatment for the past three months. In the Netherlands and Belgium, onychomycosis was confirmed through PCR testing of toenail clippings positive for fungal presence. In the UK and Scandinavia, rapid, in-office immunochromatography test confirming dermatophyte presence from a toenail clipping was used, specifically the test PreventID® Dermatophyte by Preventis. This test provided a quicker, more cost-effective method to confirm onychomycosis, which was not previously available during recruitment in the Netherlands and Belgium. In Taiwan, in-office rapid testing was not available and careful diagnosis of onychomycosis was made by skilled dermatologists.
Exclusion criteria included current use of prednisone, corticosteroid use on the feet, dermatologically sensitive skin, neurological conditions, undergoing oncology treatment, untreated HIV infection, poor wound healing as a result of diabetes mellitus, and conditions which severely compromise the immune system.
Participants were informed of the purpose and protocol of the study and able to ask their footcare professional any questions they had. All participants were informed that they can withdraw from the study at any time without reason and provided written informed consent prior to participation.
Study Protocol
The study took place over 12 weeks, with participant visits to the footcare professional at baseline (week 0), week 6 and week 12.
Enrollment was initiated at baseline after informed consent was obtained and dermatophyte testing was completed. During this visit, the footcare professional provided standard hygiene and treatment including cleaning the toenail, filing the nail to reduce if thickened, cutting the toenail and filing the corners, then cleaning again. Practitioners then sprayed the affected toenail with FunghiClear® from a distance of 2-3 cm, waiting one minute to dry. A photo of the affected toenail was taken, written questionnaires conducted and assessments performed.
Participants were provided FunghiClear® to take home and instructed by their footcare professional to spray the affected toenail(s) and surrounding skin twice per day, after washing and drying feet. Appointments were made for the participant’s return at weeks 6 and 12.
Upon return, standard hygiene and treatment was provided by the footcare professional at weeks 6 and 12. Additional bottles of FunghiClear® were sent home with the participant when needed. A photo was taken, questionnaires conducted and assessments were performed at each timepoint.
Assessments
Efficacy was measured through two distinct assessments, as well as user and footcare professional perceived efficacy. First, an Onychomycosis Severity Index (OSI) was taken at baseline and week 12. The OSI was developed by Carney et al in 2011 and is an established, objective and reproducible methodology to grade the severity of onychomycosis [
12] and has high levels of reliability between professionals, regardless of clinical experience. [
13,
14] The score is calculated using measurements of area of nail involvement, proximity to the matrix, and presence or absence of a dermatophytoma or subungual hyperkeratosis. Total score ranges from 0 to 35, with a higher score indicating a more severe condition. [
12] The participating footcare professionals in the study were trained on how to perform OSI calculations.
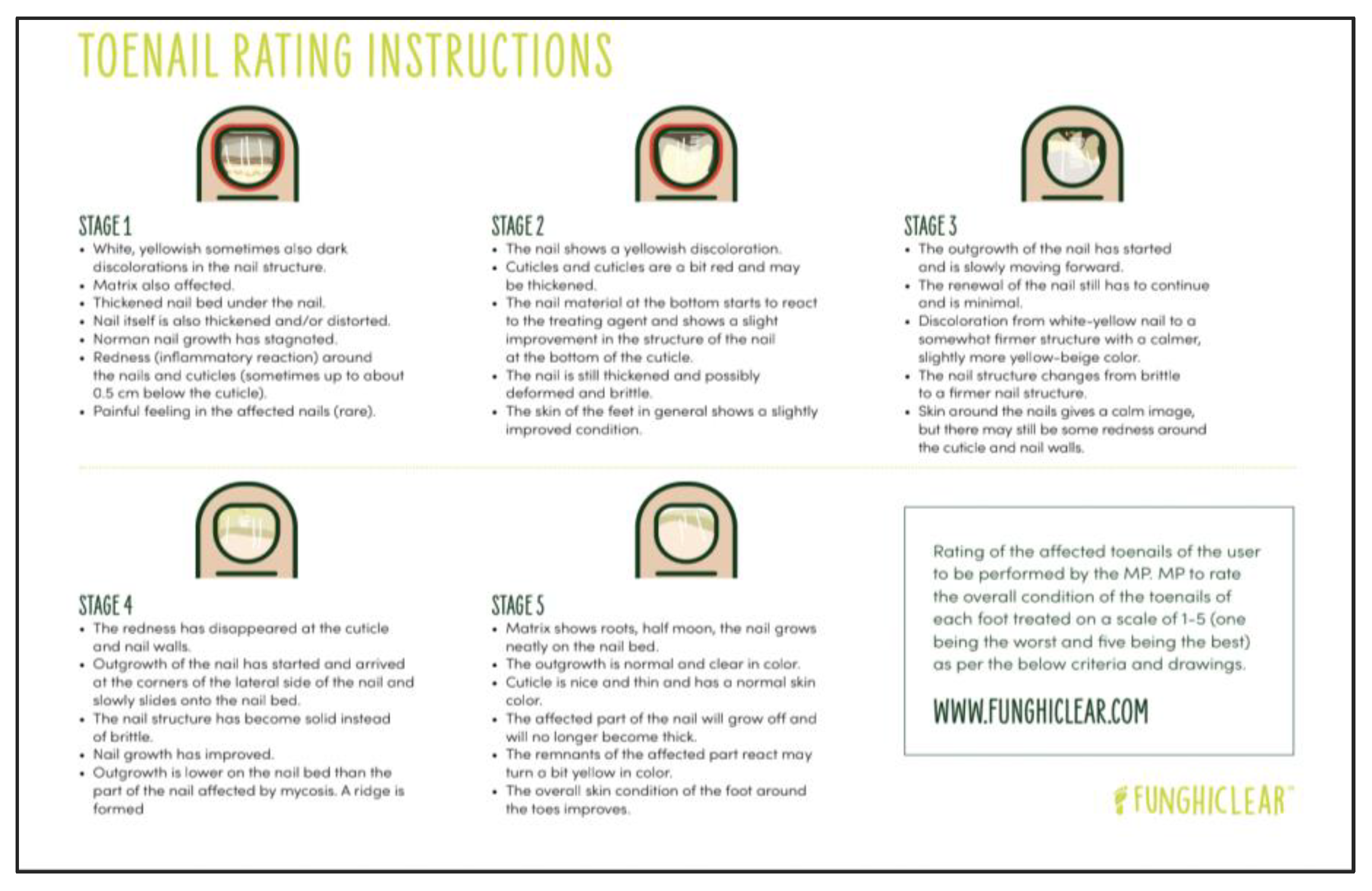
A second rating was made by the footcare professional on a five-stage Toenail Rating scale at baseline, week 6 and week 12. Four criteria were used to assign a stage, including the presence of signs of inflammation, structure of the nail, colour of the nail, and presence of regrowth of a healthy nail. A lower stage rating indicates a more severe condition. The infographic in
Figure 1 was provided to footcare professionals to inform the rating.
Subjective measures of efficacy were assessed through perceived change by the footcare professional as well as participant and were scored at week 6 and week 12. They were asked to provide an overall assessment of the affected toenail compared to the first visit, as much worse, worse, similar, better, or much better.
Safety was assessed at 6 and 12 weeks through a questionnaire. Participants were asked questions regarding pain or discomfort at the feet, inflammation or redness around the nail or other problems regarding feet or affected toenails. Convenience of the upside-down spray bottle, ability to comply with twice daily protocol, and overall user experience by participants was assessed at 6 and 12 weeks. Participants scored these factors on a scale from 1 to 5, with 1 being worst and 5 being best.
All analyses were performed on the per protocol set. Statistical analyses were performed using MS Excel and SPSS.
Results
In total, 192 participants completed the full protocol and were included in final analyses: 81 from The Netherlands, 61 from the United Kingdom (UK), 25 from Taiwan and 22 from Scandinavia (Norway and Sweden). The study was initiated with 265 participants, of whom 73 discontinued participation or did not return for follow up as per protocol. Losses to follow up were mainly due to COVID restrictions, practitioner burnout (Netherlands), and negative test for dermatophyte.
The mean age of participants was 49 years of age (SD 11.8), with the highest proportion of participants (39%) in the 55-65 age category. With regards to length of time that the participant has been suffering from suspected onychomycosis, 20% reported they have had it for over ten years, 27% for five to ten years, 44% for one to five years, and 9% for less than one year. The affected toenails evaluated were 85% the first digit of the right or left foot. Prior treatment for onychomycosis was reported by 97 participants (51%), with the highest proportions previously using cosmetic treatments (18%) or over-the-counter medication (16%). Previous use of prescription topical or prescription oral treatment was reported by 27 participants (14%).
After 12 weeks of using FunghiClear®, 129 participants (67%) had an improved OSI score (≥1) compared to baseline, while 35 participants (18%) had no change in OSI and 28 (15%) had worsened OSI scores. Efficacy results by country using OSI and toenail rating are found in
Table 1.
The OSI improved by 5.16 points between baseline and week 12, was statistically significant (p < .01, 95% CI [3.99, 6.33]) and corresponds to 28% improvement. Toenail Rating improved by 0.96 stages on the five-stage rating scale, also statistically significant (p < .01, 95% CI [0.77, 1.15]) and corresponds to 38% improvement.
Follow up analyses of the 129 responders, defined as an improved OSI score by at least one point after 12 weeks of using FunghiClear®, found a mean improvement in OSI of 8.82 points (SD 7.25), with a baseline mean score of 20.22 (SD 10.64) and week 12 mean score of 11.40 (SD 8.53). This corresponds to 43.6% improvement in mean OSI score in responders. Mean Toenail Rating improved by 1.09 (SD 1.35) stages on the five-stage rating scale, with mean rating of 2.50 (SD 1.15) at baseline and 3.59 (SD 0.91) at week 12, corresponding to 43.6% improvement in toenail rating.
There was a shared perception among footcare professionals and users that the affected toenail improved within 12 weeks of FunghiClear® use, with 143 participants (74%) reporting that their condition was better or much better than baseline, and footcare professionals of 141 participants (73%) reported improvement in toenail of the participant.
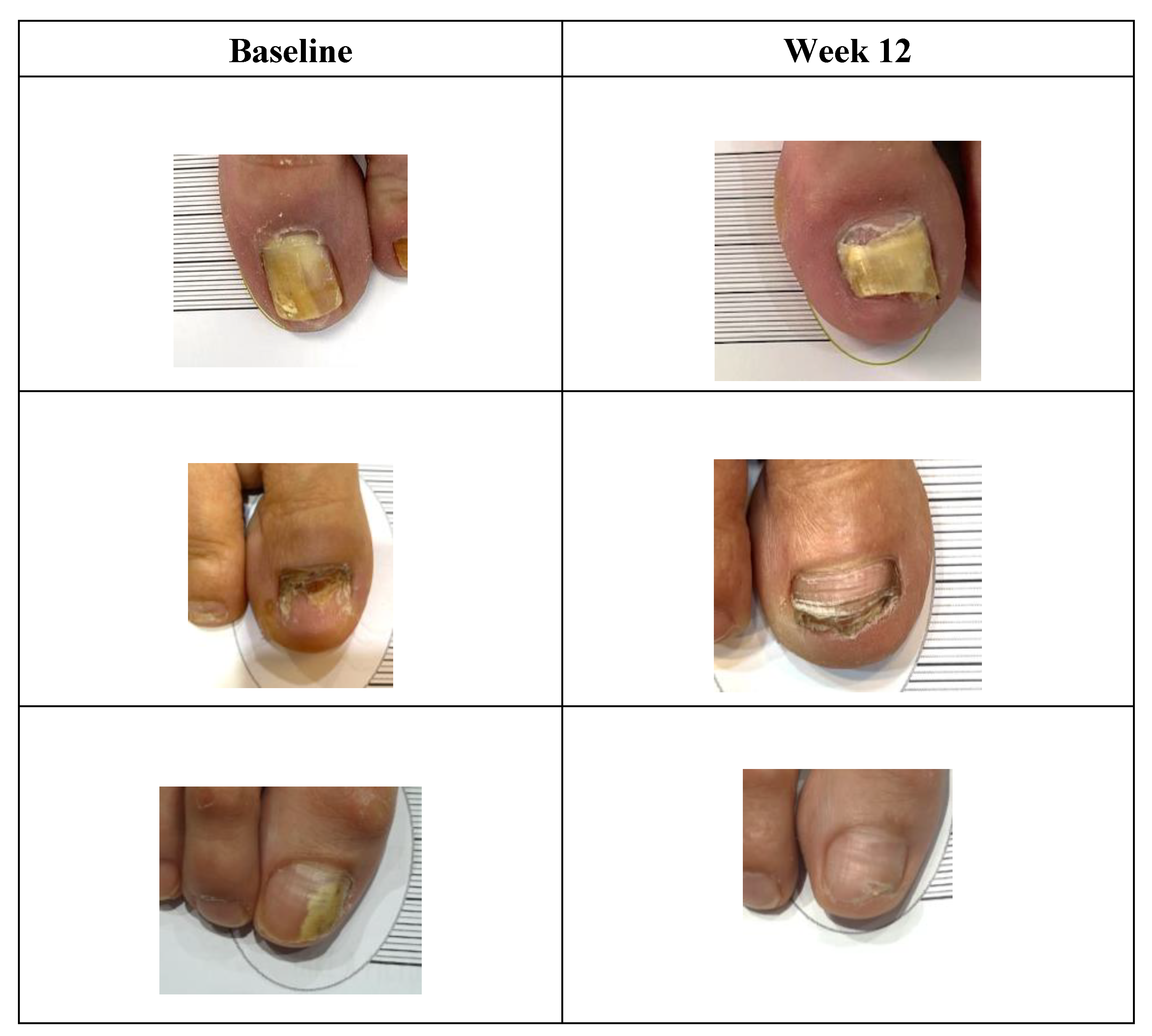
Figure 2.
Sample participant progress photos at baseline and week 12 after using FunghiClear®.
Figure 2.
Sample participant progress photos at baseline and week 12 after using FunghiClear®.
With respect to convenience at week 12, participants rated the mean convenience of the upside-down spray bottle at 4.4/5, the ability to comply with twice daily application at 4.5/5, and overall user experience at 4.5/5.
In response to the questions regarding safety and side effects at week 12, 191 participants (99.5%) reported no pain or discomfort at the site of application. The one participant who answered yes further elaborated that they noticed slight tingling when the product was applied. Two participants (1%) reported signs of inflammation or redness; on further review, one of these participants identified the site of inflammation on a different toe than the product was being used, and one participant did not provide further information. One participant in the Netherlands stopped using FunghiClear® during the study due to a blister that developed on the toe. Upon further investigation by the footcare professional, it was most likely a friction blister due to the absence of redness and inflammation, and not related to the product. To our knowledge, no other participants stopped using the product due to side effects.
Discussion
Dermatophyte onychomycosis non-self-limiting, often chronic condition and long-term treatment of multiple months is often required. [
15] Even still, reinfection and recurrence rates are high indicating that complete eradication of fungal elements is extremely difficult. [
15] Many conventional treatments focus on fungal infection elimination, however full healthy nail regrowth is slow and recurrence or reinfection may occur during this time or later. [
15] Long-term safe and effective management which focuses on healthy nail regrowth may be an alternative approach.
Clinical guidelines suggest that topical treatment should be recommended for 48 weeks [
6] and extended treatment even after resolution may be helpful to prevent reinfection or recurrence. [
15] As such, this 12-week study did not aim to elicit full resolution to a healthy toenail, but rather assess any improvement within the relatively short period of using FunghiClear®, which was found in 67% of participants, with the understanding that ongoing treatment is required for full healthy nail regrowth. Additionally, due to the progressive nature of onychomycosis, prevention of condition advancement may also be considered a positive outcome and was found in an additional 18% of participants.
Due to the length of treatment required for onychomycosis, convenience is essential for long-term adherence. Studies have shown that adherence to topical treatments for onychomycosis is low and suggest that length and frequency of application, as well as additional steps such as some lacquers requiring to be periodically peeled from the nails, may contribute to low convenience and adherence. [
6] FunghiClear® offers a unique mode of application through a pleasant smelling and quick-drying spray, with additional ease of use from the upside-down spray bottle, particularly for users with mobility challenges who may have difficulty reaching their toenails. These characteristics may contribute to the high overall rating results of 4.4/5 for convenience of FunghiClear® use.
Adverse effects frequently limit the use of certain treatments; for example, oral antifungals are associated with drug interactions and hepatotoxicity. [
2] Conventional topical treatments typically have minimal side effects due to lack of systemic absorption. [
6] Side effects observed in this study were not common and not serious.
Limitations of the study include lack of control group and blinding, relatively short length of study, participant drop out due to COVID restrictions. Selection bias is also a limitation, as all participants sought care from a footcare professional and were likely motivated. This is an ongoing study of safety and effectiveness of FunghiClear® toenail spray, and the sponsor plans to continue expand the study and extend the findings. Further research on long-term efficacy, in specific populations and advanced studies in using randomized, blinded participants, is warranted.
Conclusion
Prioritizing healthy nail regrowth and ongoing management in dermatophyte onychomycosis care may provide an innovative framework for patient care. There is currently a lack of safe, effective, and convenient treatment options to support the long-term regrowth of a healthy toenail. This study suggests that FunghiClear® is a safe and convenient option to manage dermatophyte toenail onychomycosis, which is critical for long term treatment adherence. Furthermore, based on objective and subjective improvements, FunghiClear® along with standard hygiene by a footcare professional, is effective to manage dermatophyte onychomycosis and to support healthy nail growth.
Disclosures
This study was financially supported by Norman Health Products & Services B.V., the manufacturer of FunghiClear®. Jan-Willem Eleveld is the owner of Norman Health Products & Services B.V. Ashley Weber and Annie Salsberg work for Norman Health Products & Services B.V.
Acknowledgments
M Tijssen MD, R Koevoets MD, Ellen van der Heijden.
References
- Falotico JM, Lipner SR. Updated Perspectives on the Diagnosis and Management of Onychomycosis. Clin Cosmet Investig Dermatol. 2022;15:1933-57. [CrossRef]
- Gupta AK, Stec N, Summerbell RC, Shear NH, Piguet V, Tosti A, et al. Onychomycosis: a review. J Eur Acad Dermatol Venereol. 2020, 34, 1972–1990. [CrossRef]
- Gupta AK, Wang T, Polla Ravi S, Mann A, Bamimore MA. Global prevalence of onychomycosis in general and special populations: An updated perspective. Mycoses. 2024, 67, e13725. [CrossRef]
- Albucker SJ, Falotico JM, Choo ZN, Matushansky JT, Lipner SR. Risk Factors and Treatment Trends for Onychomycosis: A Case-Control Study of Onychomycosis Patients in the All of Us Research Program. J Fungi (Basel). 2023;9(7). [CrossRef]
- Gupta AK, Mays RR. The Impact of Onychomycosis on Quality of Life: A Systematic Review of the Available Literature. Skin Appendage Disord. 2018, 4, 208–216. [CrossRef]
- Yousefian F, Smythe C, Han H, Elewski BE, Nestor M. Treatment Options for Onychomycosis: Efficacy, Side Effects, Adherence, Financial Considerations, and Ethics. J Clin Aesthet Dermatol. 2024, 17, 24–33.
- Mathew C, Tesfaye W, Rasmussen P, Peterson GM, Bartholomaeus A, Sharma M, et al. Manuka Oil-A Review of Antimicrobial and Other Medicinal Properties. Pharmaceuticals (Basel). 2020, 13. [CrossRef]
- Douglas MH, van Klink JW, Smallfield BM, Perry NB, Anderson RE, Johnstone P, et al. Essential oils from New Zealand manuka: triketone and other chemotypes of Leptospermum scoparium. Phytochemistry. 2004, 65, 1255–1264. [CrossRef]
- Christoph F, Kaulfers PM, Stahl-Biskup E. A comparative study of the in vitro antimicrobial activity of tea tree oils s.l. with special reference to the activity of beta-triketones. Planta Med. 2000, 66, 556–560. [CrossRef]
- Miastkowska M, Michalczyk A, Figacz K, Sikora E. Nanoformulations as a modern form of biofungicide. J Environ Health Sci Eng. 2020, 18, 119–128. [CrossRef]
- ten Haaf N, Buil, JB, Eleveld, JW. Potent Antifungal Activity of Manuka Oil Against Dermatophytes Microbiome & Probiotics R&D and Business Collaboration Forum April 2025; The Hauge, The Netherlands2025.
- Carney C, Tosti A, Daniel R, Scher R, Rich P, DeCoster J, et al. A new classification system for grading the severity of onychomycosis: Onychomycosis Severity Index. Arch Dermatol. 2011, 147, 1277–1282.
- Tirico M, Torezan L. Validation of the Onychomycosis Severity Index in a Brazilian population. Eur J Dermatol. 2024, 34, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Pérez D, García-Oreja S, Tardáguila-García A, León-Herce D, Álvaro-Afonso FJ, Lázaro-Martínez JL. Inter-observer reliability of the Onychomycosis Severity Index depending on clinical experience: A review of 50 cases. Mycoses. 2024, 67, e13694. [CrossRef]
- Tosti A, Elewski BE. Onychomycosis: Practical Approaches to Minimize Relapse and Recurrence. Skin Appendage Disord. 2016, 2, 83–87. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).