Introduction
Chronic pain occurring in vascular disorders of the lower limb (such as chronic venous leg ulcers [VLUs], chronic venous insufficiency, post-thrombotic syndrome, peripheral arterial disease [PAD] and chronic limb-threatening ischemia [CLTI]) imposes a considerable burden in terms of suffering, mobility limitation, quality of life and healthcare resource use [
1,
2]. Historically, this pain has been viewed primarily through a nociceptive lens; tissue injury (ulceration, ischemia) activates nociceptors, inflammation drives sensitization, and pain follows from a “source” of damage [
3,
4,
5]. Yet, recent studies challenge this simplistic view. For example, patients with VLUs frequently report burning, shooting or dysesthetic sensations, supportive of neuropathic pain involvement (e.g., 56% in one series) [
6], and electrophysiological data have revealed distal sensory abnormalities in chronic venous disease [
7]. Moreover, features of nociplastic pain, such as widespread hyperalgesia, temporal summation or impaired conditioned pain modulation, have been documented in analogous pain syndromes and increasingly recognized in vascular contexts, although the evidence remains scattered [
8,
9,
10]. From a conceptual standpoint, vascular-origin pain may therefore represent a prototypical mixed pain condition, with overlapping nociceptive (tissue/vascular injury), neuropathic (nerve injury from ischemia/venous hypertension) and nociplastic (central sensitization) mechanisms [
11]. This raises three important clinical and research challenges: (i) to quantify how often neuropathic features occur in vascular pain; (ii) to collate mechanistic evidence of nociplasticity; and (iii) to compare treatment outcomes across different mechanistic approaches (e.g., vascular correction vs neuropathic pharmacotherapy vs neuromodulation) and determine whether a mechanism-based stratification improves outcomes. Despite growing recognition of this complexity, these questions across the full spectrum of vascular pain conditions. To address these gaps, we present this protocol for a systematic review and meta-analysis. By prospectively registering this protocol (PROSPERO: CRD420251132877) and detailing our methods prior to data extraction and analysis, we aim to enhance transparency, minimize bias, and provide a rigorous foundation for evidence-based, mechanism-oriented management of vascular pain. The detailed methodology is outlined in the following sections.
Methods
1. Eligibility Criteria
This systematic review will adhere to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) guidelines. Eligibility criteria are structured according to the PICOS framework (Population, Intervention, Comparator, Outcome, Study design).
The target population comprises adults (≥18 years) with chronic pain (≥3 months duration) associated with lower limb vascular disorders. Eligible vascular conditions include: PAD or CLTI with rest pain or ischemic ulcer pain; chronic venous leg ulcers; chronic venous insufficiency with pain; post-thrombotic syndrome with pain; or mixed arterial-venous ulcers. Studies will be excluded if they focus exclusively on neuropathic diabetic foot ulcers without documented vascular compromise, vasculitic ulcers, ulcers due to primary inflammatory or rheumatological conditions, or other non-vascular neuropathic pain conditions, unless the vascular contribution to pain is explicitly demonstrated and quantified.
Primary outcomes will be aim-specific:
Aim 1: Prevalence of neuropathic pain features, defined as the proportion of patients screening positive on validated neuropathic pain screening tools (e.g., DN4 ≥4, painDETECT ≥19, LANSS ≥12, or other validated instruments). Secondary outcomes include prevalence stratified by specific neuropathic pain descriptors (burning, shooting, tingling, allodynia).
Aim 2: Physiological or psychophysical markers of nociplastic pain mechanisms, including: central sensitization indices; remote or widespread hyperalgesia (reduced pain thresholds in non-affected areas); temporal summation or wind-up; impaired conditioned pain modulation (CPM) or diffuse noxious inhibitory control (DNIC); altered brain neuroimaging findings (functional MRI, PET, or EEG signatures).
Aim 3: Treatment outcomes including: pain intensity measured on validated scales (Visual Analogue Scale [VAS], Numerical Rating Scale [NRS], Brief Pain Inventory); responder rates (≥30% or ≥50% pain reduction, or patient-reported meaningful improvement); opioid consumption (dose reduction or cessation); quality of life measures; and vascular-specific outcomes (ulcer healing rates, time to healing, recurrence rates, amputation-free survival).
Interventions and comparators: For Aim 3, eligible interventions encompass any therapeutic strategy with reported quantitative pain outcomes, including: (i) vascular-causal interventions (revascularization procedures, compression therapy, venous ablation); (ii) wound management strategies (dressings, debridement, topical agents); (iii) systemic pharmacotherapy (analgesics, neuropathic pain medications, anti-inflammatory agents); and (iv) neuromodulation or interventional pain techniques (spinal cord stimulation, peripheral nerve stimulation, nerve blocks). Comparators may include usual care, placebo, sham interventions, no treatment, or alternative active treatments. For Aims 1 and 2, interventions are not applicable as these are observational research questions.
3. Study Selection
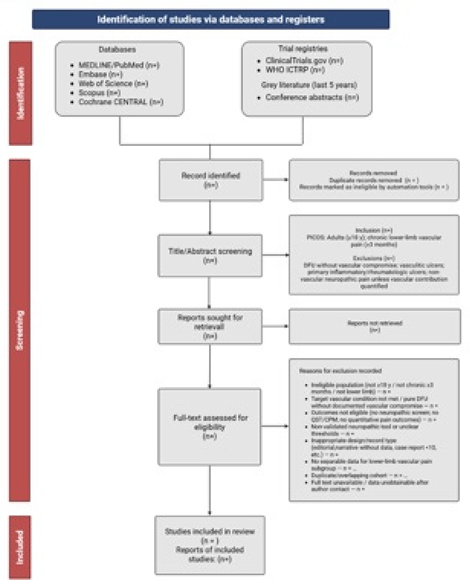
Two independent reviewers will screen titles and abstracts, followed by full-text evaluation. Disagreements will be resolved by consensus or a third reviewer. Exclusion reasons will be logged to generate a PRISMA flow diagram (Figure 1).
5. Risk of Bias Assessment
For prevalence studies we will use an adaptation of Hoy et al. [
12] tool assessing external validity (sampling frame, non-responders) and internal validity (measurement, case definition). For mechanism studies, we will apply JBI checklists for cross-sectional/cohort designs [
13]. RCTs will be assessed using Cochrane RoB 2; uncontrolled pre–post studies via ROBINS-I adapted domains (confounding, selection, missing data) [
14]. Risk of bias will inform sensitivity analyses and GRADE.
7. Certainty of Evidence
Using GRADE, we will assess certainty of evidence separately for each major domain: prevalence of neuropathic features, evidence for nociplastic mechanisms, treatment outcomes. Non-randomized evidence begins at low; may be upgraded for large effect, dose–response, consistency; downgraded for risk of bias, inconsistency, indirectness, imprecision, publication bias.
8. Ethics and Dissemination
As this is a review using published and anonymized data, no ethical approval is required. The protocol is registered on PROSPERO (registration number: CRD420251132877). Findings will be submitted for peer-reviewed publication. Probably they will also be presented at relevant vascular/wound/pain forums, and shared with patient advocacy groups.
Discussion
This protocol describes a comprehensive and methodologically rigorous systematic review and meta-analysis designed to elucidate the complex, mixed-pain mechanisms underlying vascular pain. By prospectively detailing our methods prior to study execution, we aim to enhance transparency, minimize bias, and provide a robust foundation for evidence synthesis. By systematically quantifying the prevalence of neuropathic features, charting the evidence for nociplastic mechanisms, and comparatively analyzing treatment outcomes in vascular-origin pain, this review seeks to clarify the role of vascular pain as a prototypical mixed-pain condition. In doing so, we intend to furnish both clinicians and health-policy makers with a robust evidence base to guide mechanism-based analgesic strategies. Should a high prevalence of neuropathic or nociplastic features emerge, it would substantiate the expansion of neuropathic-adjuvant therapies and support earlier therapeutic intervention in vascular management pathways. In turn, improved analgesia may enhance compression-therapy adherence, promote mobility, expedite wound healing, and potentially reduce opioid consumption.
The findings from this review are expected to have important implications for clinical practice and research. By systematically quantifying the prevalence of neuropathic features across different vascular pain populations, we will provide evidence to guide screening and assessment practices in vascular clinics and wound care centers. This would support the integration of mechanism-based assessment tools into routine clinical care and justify the expansion of neuropathic-adjuvant therapies in vascular management pathways. Understanding the mechanistic composition of vascular pain may also explain the substantial inter-individual variability in treatment response observed in clinical practice. Some patients achieve excellent pain relief following revascularization or compression therapy, while others experience persistent or refractory pain despite successful vascular intervention. If baseline neuropathic or nociplastic features predict treatment response, as our planned meta-regression analyses will explore, this could inform patient stratification and personalized treatment selection. For example, patients with prominent neuropathic features might benefit from earlier adjunctive therapy with gabapentinoids or serotonin-norepinephrine reuptake inhibitors, while those with evidence of central sensitization might be candidates for multimodal approaches including psychological interventions.
Our project is not without significant anticipated challenges. Heterogeneity in definitions of neuropathic features, owing to diverse screening tools (e.g., DN4, PainDETECT), varying threshold criteria and mixed patient populations, will complicate synthesis. For example, Eusen et al. [
15] found 58% neuropathic pain in chronic leg ulcers. The variable pathological substrate (venous vs arterial vs mixed ulcer types) further adds complexity [
16]. Experimental data on nociplastic mechanisms remain limited in vascular-pain cohorts, despite the broader pain-medicine literature defining nociplastic pain as “pain that arises from altered nociception” [
17]. Treatment-studies are frequently uncontrolled or small, reducing certainty of evidence. Nevertheless, the formal meta-analytic approach, with pre-specified subgroup analyses and meta-regression, will allow systematic exploration of effect moderators and gaps in knowledge.
Beyond individual patient care, this review has potential health-systems implications. Improved pain control through mechanism-based management may enhance adherence to compression therapy in venous disease, promote mobility and rehabilitation following revascularization, expedite wound healing, and potentially reduce long-term opioid consumption and its associated risks. These outcomes are particularly important given the global aging population and rising burden of chronic vascular disease.
Conclusion
This protocol outlines a comprehensive and methodologically rigorous systematic review designed to elucidate the complex, mixed-pain mechanisms underlying vascular pain. By integrating quantitative evidence on neuropathic and nociplastic features with analyses of treatment outcomes, it aims to advance a mechanistic understanding of pain in vascular disorders. Given the progressive ageing of the global population, the rising burden of chronic vascular disease, and the growing emphasis on mechanism-based and personalized analgesia, the findings of this review are expected to inform future clinical guidelines, optimize therapeutic strategies, and stimulate further translational research in vascular pain management.
Author Contributions
All authors have reviewed the final version to be published and agreed to be accountable for all aspects of the work. Concept and design: Giustino Varrassi, Giacomo Farì, Matteo Luigi Giuseppe Leoni. Drafting of the manuscript: Giustino Varrassi, Giacomo Farì, Tran Van Y, Phong Pham Van, Ameen A. Al Alwany, Annalisa Caruso, Matteo Luigi Giuseppe Leoni. Supervision: Giustino Varrassi.
Acknowledgments
The authors are thankful to the Paolo Procacci Foundation for its support in the editing process.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
- Nicolaides AN, Labropoulos N. Burden and Suffering in Chronic Venous Disease. Adv Ther. 2019 Mar;36(Suppl 1):1-4. [CrossRef]
- Haider R. Peripheral vascular disease. Cardiol Res Rep. 2023;5(4):01-13. [CrossRef]
- Leren L, Johansen E, Eide H, Falk RS, Juvet LK, Ljoså TM. Pain in persons with chronic venous leg ulcers: A systematic review and meta-analysis. Int Wound J. 2020 Apr;17(2):466-484. [CrossRef]
- Ma YC, Kang ZB, Shi YQ, Ji WY, Zhou WM, Nan W. The complexity of neuropathic pain and central sensitization: exploring mechanisms and therapeutic prospects. J Integr Neurosci. 2024 Apr 25;23(5):89. [CrossRef]
- Ntalouka MP, Chatzis A, Nana P, Spanos K, Bareka M, Matsagkas M, Arnaoutoglou E. Acute Pain Management in Peripheral Artery Disease: A Holistic, Beyond-Opioids, Individualized Multimodal Approach. Turk J Anaesthesiol Reanim. 2024 Dec 16;52(6):200-206. [CrossRef]
- Eastman DM, Dreyer MA. Neuropathic Ulcer. 2022 Nov 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan. PMID: 32644640.
- Leoni MLG, Farì G, Al-Alwany AA, Guillen MR, Varrassi G. Vascular pain: a narrative review on venous leg ulcers. Preprint 2025. [CrossRef]
- Kim J, Wilkie DJ, Weaver M, Lyon D, Kelly DL, Millan SB, Park J, Stechmiller J. Multidimensional Pain Characteristics in Older Adults with Chronic Venous Leg Ulcers. Adv Wound Care (New Rochelle). 2021 Oct;10(10):544-556. [CrossRef]
- Schmidt LJ, Parker CN, Parker TJ, Finlayson KJ. Clinical correlates of pain in adults with hard-to-heal leg ulcers: a cross-sectional study. J Wound Care. 2023 Jun 1;32(Sup6):S27-S35. [CrossRef]
- Dowling C, Chu L, Etkin Y, Oropallo A. Assessment and management of chronic venous, arterial, and diabetic wounds in older adults. Semin Vasc Surg. 2025 Sep;38(3):281-290. [CrossRef]
- Varrassi G, Farì G, Narvaez Tamayo MA, Gomez MP, Guerrero Liñeiro AM, Pereira CL, Samy Aziz E, Gharibo C, Kaye A, Garcia-Larrea L, Leoni ML. Mixed pain: clinical practice recommendations. Front Med. 2025 Oct 9;12:1659490. [CrossRef]
- Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012 Sep;65(9):934-9. [CrossRef]
- Munn Z, Aromataris E, Tufanaru C, Stern C, Porritt K, Farrow J, Lockwood C, Stephenson M, Moola S, Lizarondo L, McArthur A, Peters M, Pearson A, Jordan Z. The development of software to support multiple systematic review types: the Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI). Int J Evid Based Healthc. 2019 Mar;17(1):36-43. [CrossRef]
- Nejadghaderi SA, Balibegloo M, Rezaei N. The Cochrane risk of bias assessment tool 2 (RoB 2) versus the original RoB: A perspective on the pros and cons. Health Sci Rep. 2024 Jun 3;7(6):e2165. [CrossRef]
- Eusen M, Brenaut E, Schoenlaub P, Saliou P, Misery L. Neuropathic pain in patients with chronic leg ulcers. J Eur Acad Dermatol Venereol. 2016 Sep;30(9):1603-5. [CrossRef]
- Mayrovitz HN, Wong S, Mancuso C. Venous, Arterial, and Neuropathic Leg Ulcers With Emphasis on the Geriatric Population. Cureus. 2023 Apr 25;15(4):e38123. [CrossRef]
- Kosek E. The concept of nociplastic pain-where to from here? Pain. 2024 Nov 1;165(11S):S50-S57. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).