1. Introduction
It would be difficult to present all AD under a single umbrella due to the scope of these diseases and their deleterious effects. Systemic lupus Erythematosus (SLE) is a disease with the hallmark of autoantibodies, unpredictable degrees of inflammation, and often denies progenitive outcomes to females of child-bearing age. For many years the cause of SLE was thought to be a product of the adaptive immune system. However, in recent publications, it would appear that the InImS has a role in potentiation of this disease, especially through the action of neutrophils [
1]. It transpires that neutrophils play the major role in this disease by establish the neutrophil extracellular trap (NET) formation and thereby increasing reactive oxygen species and impairing trap clearance. Immune complexes are generated by the NLRP3 inflammasome, releasing inflammatory cytokines, and lupus low-density granulocytes acting to harm [
2] exquisitely adjusted function leading to physiological malfunction such as endothelial apoptosis by aberrant NET formation. In Type 1 diabetes, the loss of innate immunity to detect self-antigens leads to destruction of pancreatic β-islet cells and elsewhere in the gut [
3] through both innate and adaptive immune system activity. In other ADs we find that trained-innate immunity plays a role in multiple sclerosis, a condition where both innate immunity and environmental factors are involved which leads to neuropathology via a proinflammatory mechanism implicating macrophages and monocytes, that contribute to this fluctuating disease entity [
4]. There are also conditions with a blending of innate immunity with adoptive particularly invidious in rheumatoid arthritis and Still’s disease, involving cell populations of γδ lymphocytes and mucosal-associated invariant T cells [
5]. There does appear to be an interdigitating series of tasks undertaken by the innate immune system in various diseases such as IBD where, dendritic antigen presenting cells expose antigens to naïve T-cells. This part of the adaptive immune system has a potentially autoimmune function and, in concert with the innate immune system simultaneously, activates the reactive cytokine system mediating cell damage which is part and parcel of the autoimmune disorder [
6]. One of the most challenging ADs is Hidradenitis suppurativa, where innate and adoptive systems together with microbial dysbiosis blend to produce a progressive state of inflammation and morbidity [
7]. We will address this issue further in the interests of illuminating the immune issues involved. Sarcoidosis is an inflammatory disease involving major organs and this involvement has been linked to accumulation of type 1 innate lymphoid cells (ILC1s) in the blood and skin of human tissues which is not akin to other granulomatous disease [
8]. We believe that this disease straddles the “haves and have-nots” in terms of InImS activation. We will further probe the remaining entities in the results and discussion sections.
2. Materials and Methods
2.1. Database
The basis for the data collection was to enroll patients with increased risk for colorectal cancer (CRC) using a short questionnaire to determine who could qualify to undergo colonoscopy and did not have one in the 5 years preceding the procedure. The timing of future screening tests such as colonoscopy, barium enema and flexible sigmoidoscopy was left to the discretion of the primary care provider. Samples of bodily fluids were collected upon enrollment. Any newly diagnosed maladies were recorded to adjudicate fitness for remaining in the study. The study began in 1995 and closed in 2022 [
9] which caught the first couple of years of the COVID-19 pandemic which affected follow up by colonoscopy [
10].
2.2. Sample Collection
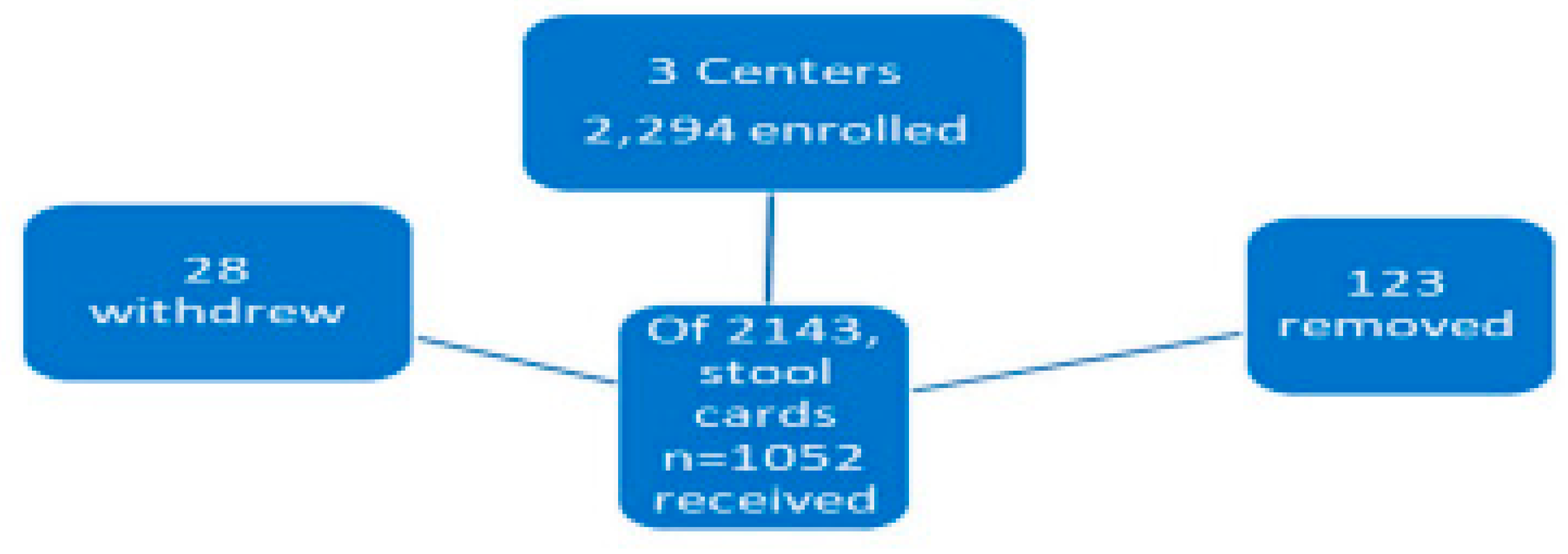
The samples collected fell into 2 categories, the first was the patients who sent 3 occult stool samples in the mail. And the second was the collection of unstimulated saliva, blood and urine just before colonoscopy and rectal fecal effluent aspirated through the endoscope during the procedure. There was a separate Phase 2 part of the section where patients volunteered to undergoing pinch biopsies during colonoscopy from 6 segments of the colon for immunohistochemistry and ELISA. The overall numbers are shown in
Figure 1 but the participants in this actual study were 1,228 in number, with 133 AD, 66 ID and 1,029 controls.
2.3. Exclusion Criteria
Patients who were unable to collect stool on the cards due to physical inability, or those unwilling to have a colonoscopy were excluded from the study. Any patient who lacked the capacity to understand the necessary steps of sample procurement were also excluded. Incidental serious diseases were also exclusionary. Inclusion criteria were the opposite of the exclusion criteria with patients showing a working understanding of the steps involved in the study.
2.4. Storage and Other Samples
The samples mentioned above were stored in Ultracold -70 degrees Celsius freezers. We ensured that results were reproducible after 10 years of storage by repeating the assay and we found that the results were identical [
11]. The general laboratory at the Detroit VAMC performed the urinary creatinine, ferritin and Vitamin D assays.
2.5. ELISA
3.0 A sandwich assay was used in which we incubated 5μg of protein per well as determined by the Bicinchoninic Acid (BCA) method available from Thermoscientific kit #AN53072 in a 96-well (Nunc microtiter plate Catalogue GS-120030 from Oldsmar, FL, USA now also available from Thermoscientific 243,656 to construct a standard curve for an Enzyme Linked Immunosorbent Assay (ELISA). Antibodies: The standard ELISA followed manufacturers’ instructions for the protein content determination of stool specimens diluted in phosphate-buffered saline (PBS). All subsequent washings between the primary and secondary antibodies were performed using PBS and 5% Tween-20 (Polyoxyethylenesorbitan monolaurate, TWEEN® 20) headquartered at Burlington, MA, USA) MilliporeSigma, Catalogue 93773, Burlington, MA, USA. The reagents were obtained from Vector Laboratories (Catalogue SA-5014-1, Newark CA, USA) for immunohistochemistry (IHC) by the ABC (Avidin,-Biotin Complex) amplifying method for the signal target antigen, following the manufacturer’s instructions ©Vector Laboratories, Inc. 2025 All Rights Reserved, The ELISA final step was developed by adding 40 μL of p-nitro-phenyl phosphate MilliporeSigma, Catalogue N7653 which develops a yellow color and read on a Thermo-Fisher cyto-spectrophotometer at 405 nm (Thermo Fisher Scientific 168 Third Avenue, Waltham, MA USA). The negative control antibody, designated UPC10 (MilliporeSigma, Catalogue M9144, Burlington, MA, USA), is an irrelevant antibody (anti-levan) with the same isotype (IgG2a) as the Adnab-9 monoclonal, and was used on half of the microtiter plate for background determination. The results were expressed as the optical density (OD) minus the background.
3. Results
The initial breakout of participants submitting FOBT cards and dropouts who were either withdrawn or removed are shown schematically in
Figure 1 below.
The demographical data of the participating patients are tabulated below in
Table 1.
The aim of the study was to compare the response of the InIms in ADs and IDs to each other and that of control patients, using the FERAD ratio. The enumerator was blood ferritin and the denominator was fecal p87. Most patients were at risk for CRC at the outset, however, on careful curation of patient follow-up, many were found to have been diagnosed with AD and IDs in the interim, which stood out from the CRC baseline. This unanticipated revelation allowed us to conduct a separate analysis.
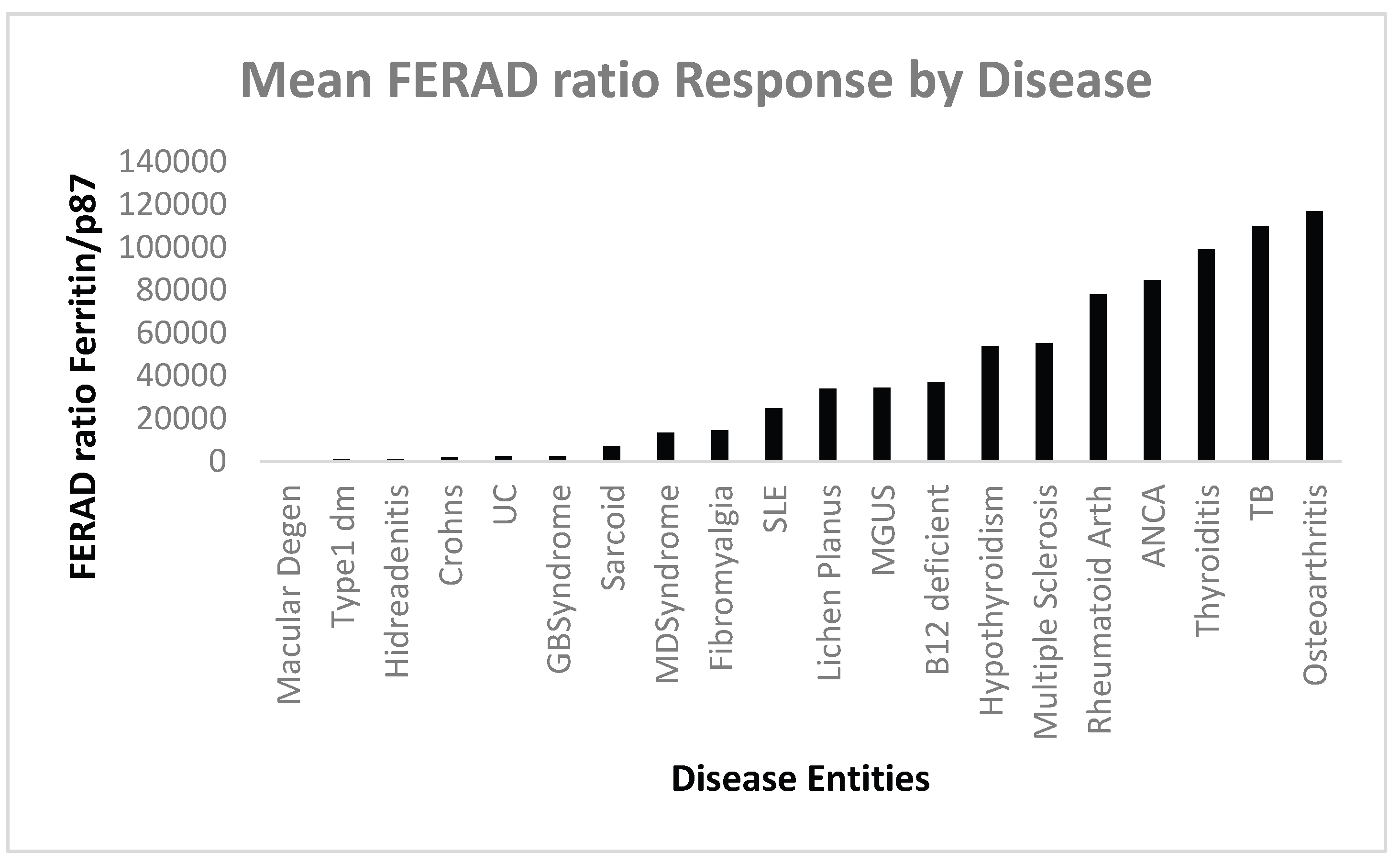
Figure 2.
A Bar Diagram of Mean FERAD Ratio versus Specific Disease Entities.
Figure 2.
A Bar Diagram of Mean FERAD Ratio versus Specific Disease Entities.
AM-macular degeneration to Lichen Planus; ID-from MGUS to Osetoarthritis.
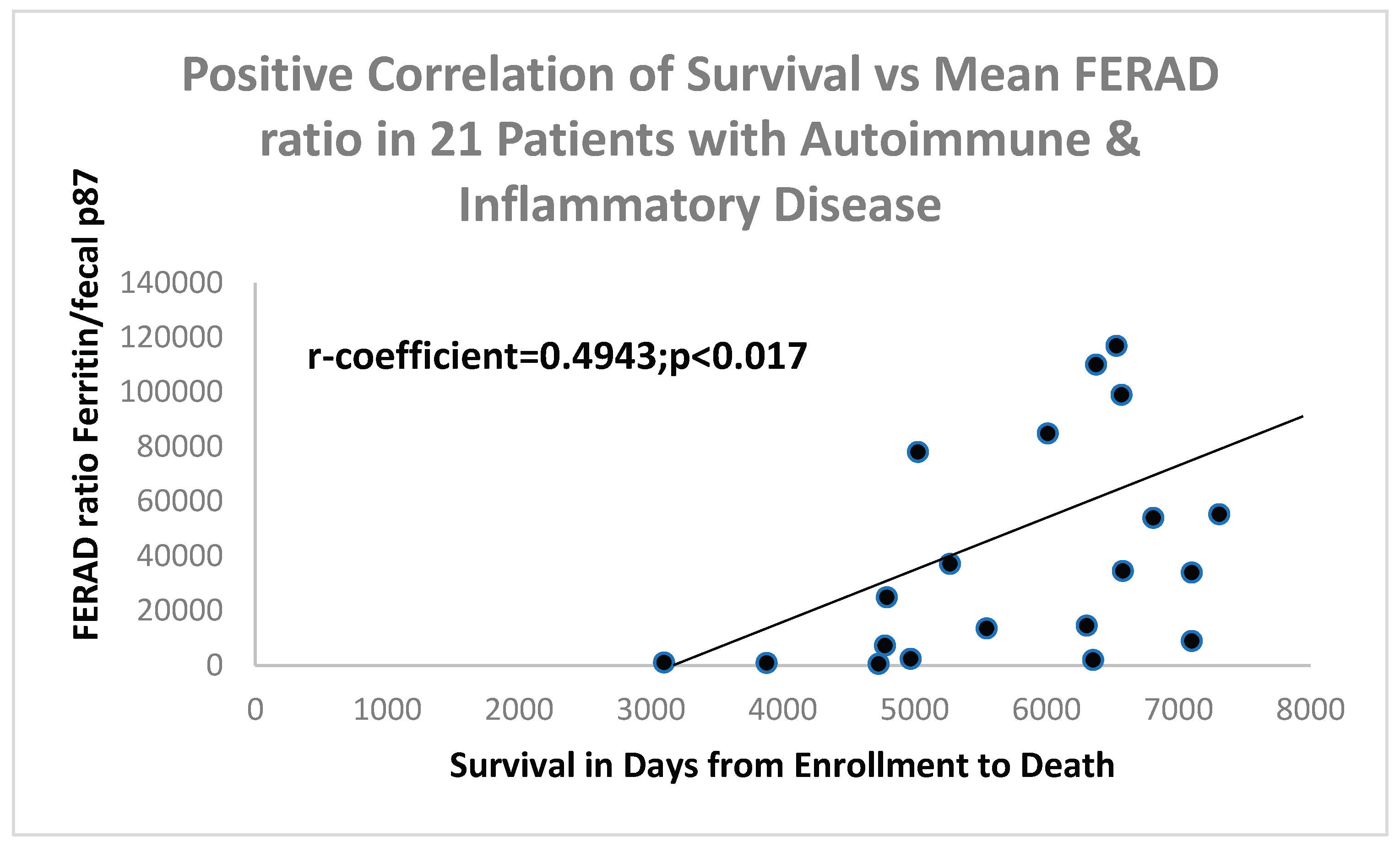
Figure 3.
A Scattergram with Positive Correlation between Survival and Mean FERAD ratios.
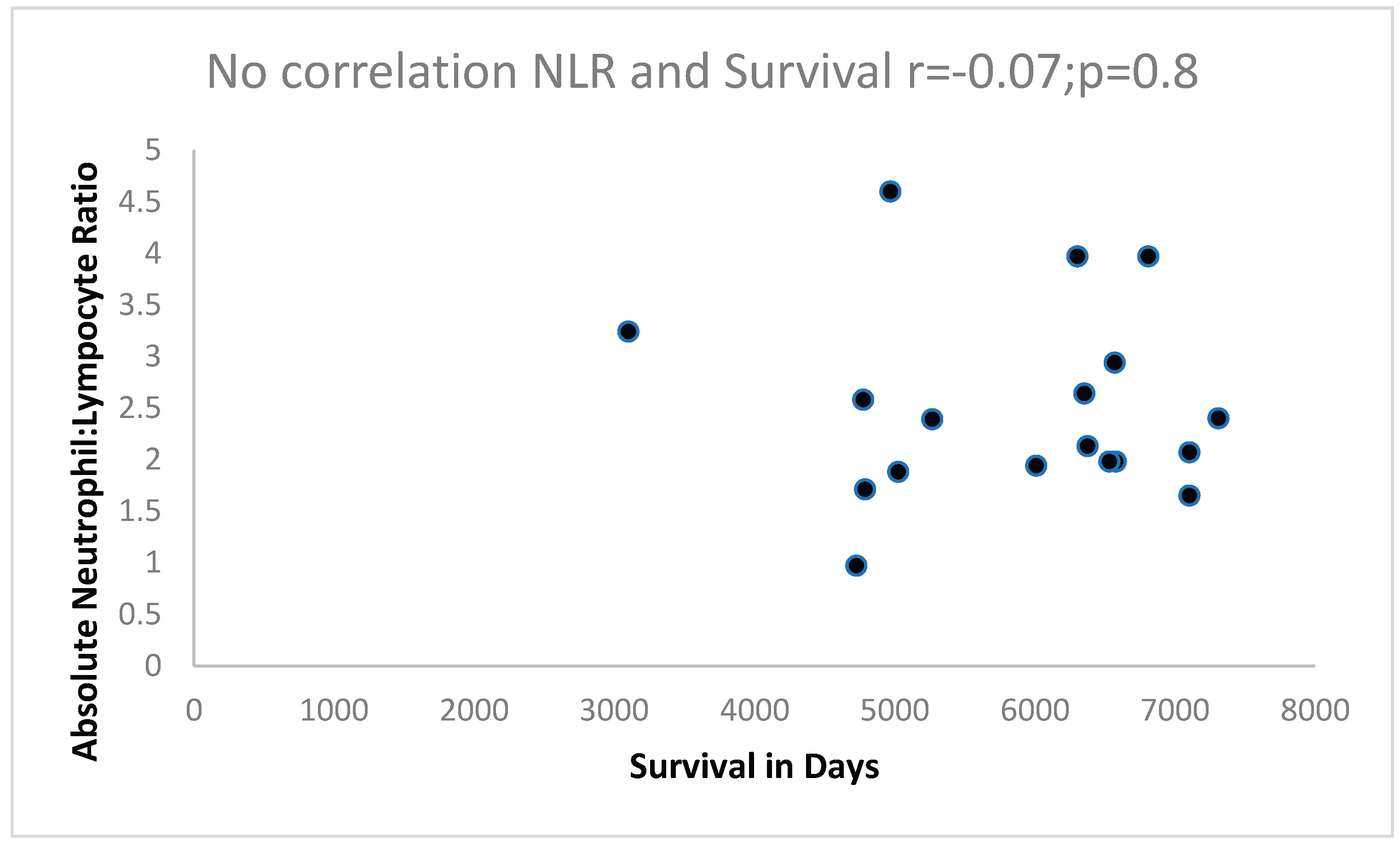
We wished to assess disease survival in the above groups and found that there was a significant correlation of FERAD and survival which did not apply when using a well-known prognostic biomarker, the absolute neutrophil:absolute lymphocyte ratio which was not significant.
Figure 4 below shows that the absolute neutrophil:lymphocyte ration (NLR) does not show a correlation with survival.
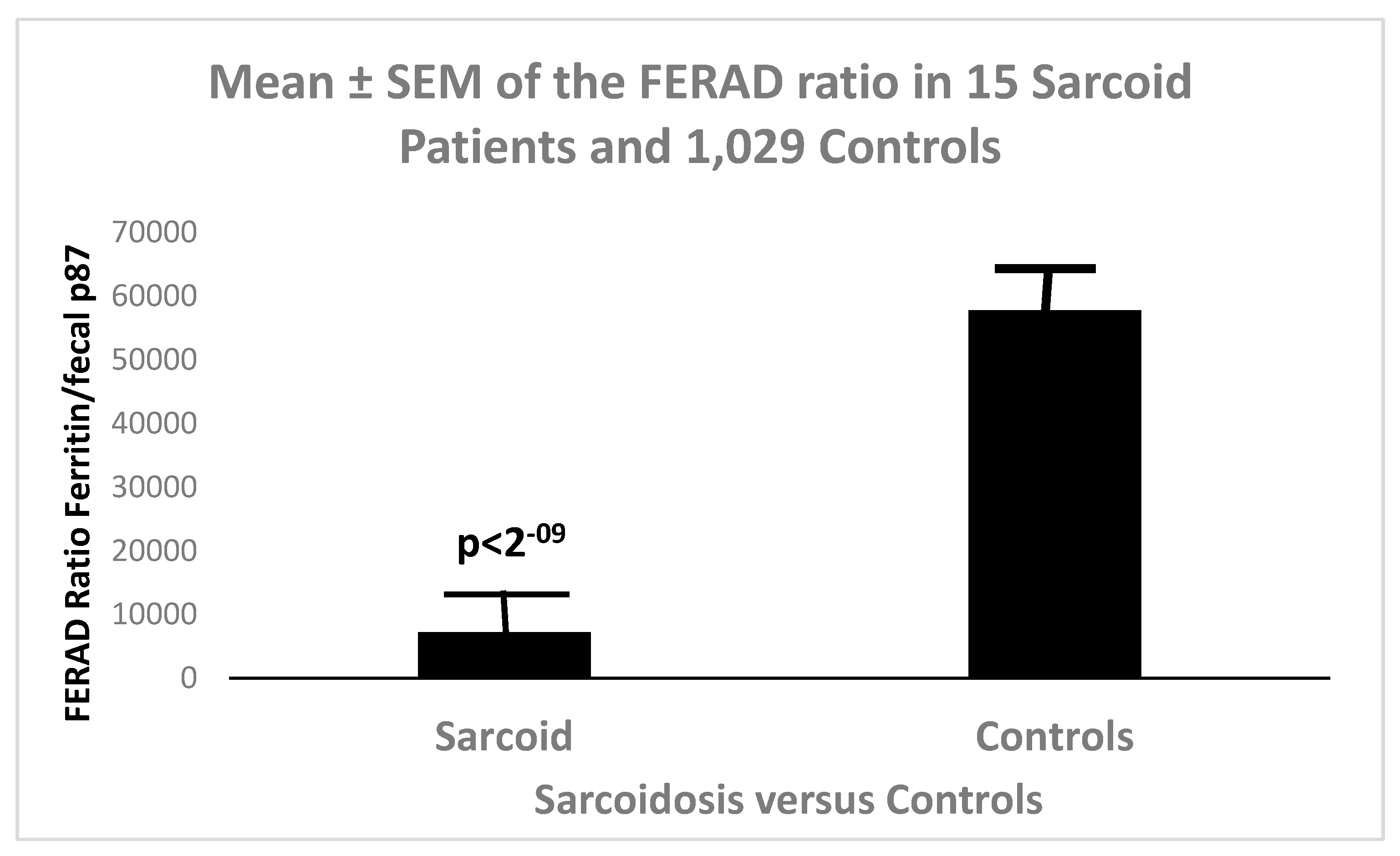
Figure 5 is a bar diagram showing the differences in FERAD between sarcoidosis and controls.
Figure 6.
Shows FEARD Ratios between Sarcoidosis and Controls with a Strong Difference.
Figure 6.
Shows FEARD Ratios between Sarcoidosis and Controls with a Strong Difference.
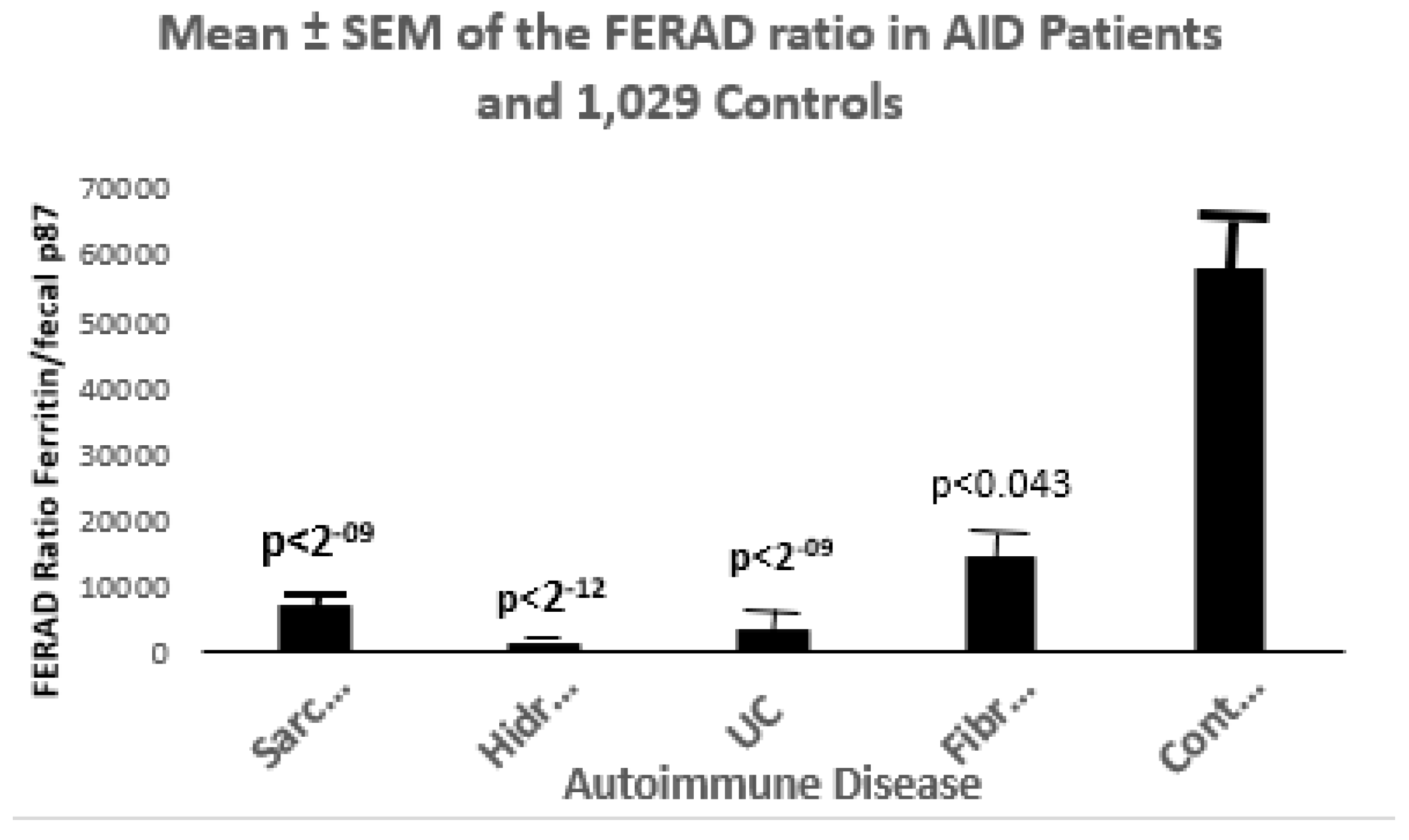
Figure 7.
A Bar Diagram Showing Differences of Autoimmune Diseases (AID) Compared to controls.
Figure 7.
A Bar Diagram Showing Differences of Autoimmune Diseases (AID) Compared to controls.
Sarc- Sarcoidosis n=15; Hidr- Hidradenitis Suppurativa n=4; UC-Ulcerative Colitis n=10; Fibro- Fibromyalgia n=7; Cont-Controls n=184.
An 4. ADs were implicated [
12]. A causal relationship was found in 4 ADs at risk for MDS: rheumatoid arthritis (p=0.02; multiple sclerosis p=0.04;myasthenia gravis (p= 0.042), and Hashimoto thyroiditis (p= 0.046). Of interest when comparing the FERAD ratio between 4 MDS patients (FERAD 13,491±10,211) and controls, the T-test means were significantly different at p<0.0005. Not surprisingly, the p87 fecal levels were significantly different (p<0.04). This will be further explored in the discussion section.
In general, patients with inflammatory disease had higher FERAD ratios than AD patients. Surprisingly, serum creatinine levels in myelodysplastic syndrome (MDS) patients were significantly less than those of controls, 0.75±0.07mg/dL versus 1.34±2.35mg/dL (p<0.00003) but BMI were not statistically significantly different at 24.32±2.31 in MDS patients versus 28.7±6.09 in controls.
Table 2.
below shows patient habits in AD, ID and Controls.
Table 2.
below shows patient habits in AD, ID and Controls.
| Habits/Condition |
Autoimmune % |
Inflammatory |
Controls |
p-Values |
| Smoking |
34+49- 41% |
19+34- 35.9% |
300+473- 38.8% |
NSS |
| Drinking |
15+53- 22.1% |
13+35- 27.1% |
127+341- 27.1% |
NSS |
| Diabetes |
37+74- 33.3% |
24+38- 38.7% |
359+647- 35.7% |
NSS |
| Flu Vaccination |
12+31- 27.9% |
16+18- 47.1% |
226+332- 40.5% |
0.083 |
| Mammography |
7+9- 43.8% |
5+3- 62.5% |
30+43- 41.1% |
NSS |
| PSA |
29+ 17- 63% |
27+9- 75% |
379+92- 80.5% |
P<0.009 ADvsC |
| Hepatitis C |
6+67- 8.2% |
9+ 33- 21.4% |
132+456- 22.5% |
P<0.006 ADvsC |
| Vitamin D Level |
13+31- 29.6% |
7+23- 23.3% |
50+194- 20.5% |
NSS |
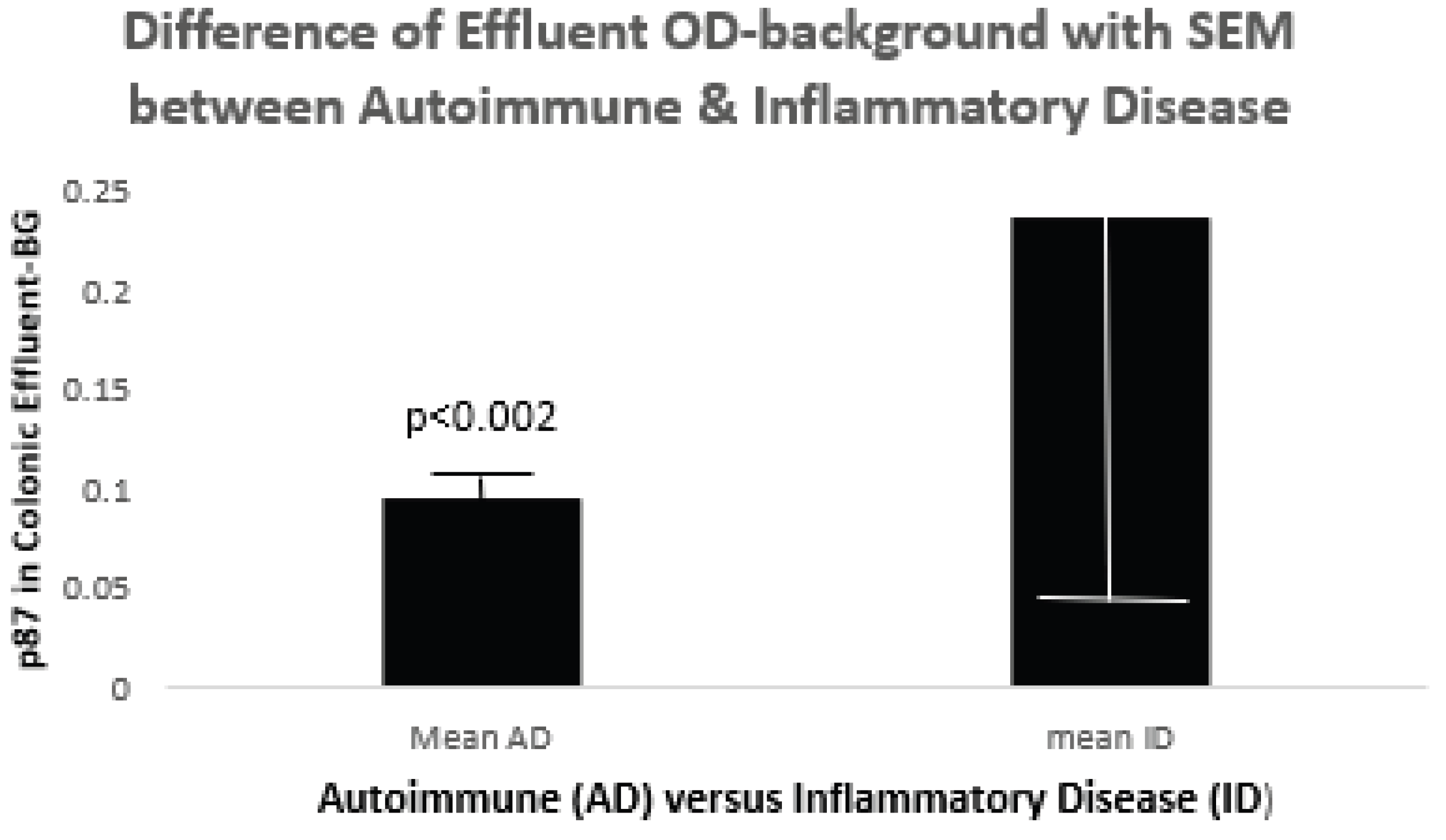
Since we were able to use secreted p87 colonic into effluent at the time of colonoscopy we could define significant difference as can be seen in
Figure 8 below.
4. Discussion
The study was embarked upon to assess the stance of the InImS in patients with AD and ID as these are few examples of such comparisons and the differences between these diseases may be obscured [
13], yet our FERAD biomarker singles out osteoarthritis (OA) as the ID with the highest FERAD ratio (
Figure 3), suggesting the most robust response of the innate immune system of all the diseases under consideration. Indeed, increasing attention has been directed at the role of the InImS in OA [
14]. Some have suggested that diseases like OA and erosive arthritis are indeed AD and not just inflammatory. They base their opinion on the release of cytokines and the fact that some antibiotics have anti-inflammatory properties (sulfamethoxazole/trimethoprim) and have been successfully used to the treatment of other established AD [
15]. Although it is a less common subset of OA, erosive OA appears to be a separate entity and shows erosion of articular cartilages. We did not have any well-defines cases for analysis and this more aggressive entity needs further investigation into cytokine cascades and inflammatory pathways [
16]. More exacting studies have been performed in another disease entity for AD consideration entitled aseptic particle-induced inflammation and osteolysis [
17]. This is postulated to occur in cyanoacrylate glued joint inserts which are prone to attrition and produce arthritic symptoms that respond to anti-IL10 treatment. Particulate deposition has been implicated along with some known inflammatory pathways.
Of interest is the abject lack of response of the InImS in macular degeneration. This condition is age-related and shows minimal inflammation and divided into wet (choroidal vascular changes seen) and dry forms and recently, inflammatory cytokines mainly circulating monocytes and monocyte-derived macrophages. CCL2 and CX2CL1 chemokines have been implicated [
18] with sub-retinal microglial changes and macrophage infiltration and danger of permanent blindness. Treatment has progressed with the finding of VEGF production and Flt1 pathways. The InImS has challenges in exerting a reaction in this disease as the blood-retina barrier excludes infiltration making this are an immune-privileged and protected area. This is probably the explanation of the extremely low FERAD ratio in Fig.3. It is thought that the innate immune cells have a protective role in maintaining the barrier. Type 1 diabetes is a close neighbor in terms of a mean FERAD ratio of only 817. The seat of this disorder is in the pancreas where the loss of self-tolerance plays the key pathogenetic role in this disease, and that of the activation of both the InImS inside the islets and adaptive immunity.
Hidradenitis suppurativa [
7] is another minimal InImS responder in terms of the FERAD ratio. The worldwide incidence of this disease is about 0.05-4.1%, yet it causes the afflicted individual long-term morbidity with painful nodules, skin tunnels, and tissue breakdown [
19]. Treatment depends on the 3 progressive stage with medical, medical and surgical, and finally surgical. Medical treatment relies on anti-TNF, IK-17 agents to counter the Innate Immune system action, supplanting the previously predominantly used antibiotics, Other co-morbidities require more specialized treatments are metabolic syndrome, inflammatory arthritis, and inflammatory bowel disease. This disease represents a paradox in that it is locally aggressive in integumental areas yet does excite only a barely measurable FERAD response.
Multiple sclerosis is a disease of the central nervous system wherein myelin is targeted and degeneration is induced but fortunately, immune cells can also mediate a remyelination process to repair the damage. The hallmark of the disease therefore is intervals of inflammation leading to loss of function but also periods of regeneration and period of close to normal functionality. The monocytes and macrophages show aspects of trained immunity which can participate in either one of these phases with CD16+-expressing macrophages driving the inflammatory process with additional involvement of epigenetic mechanisms [
4]. This review also introduces concept of Yin and Yang effects of Epstein Barr Virus (EBV) and cytomegalovirus (CMV) in disease causation and well as the effects of Bacille Calmette Guérin (BCG) in trained immunity, with a strong response having a beneficial effect on MS. The FERAD level of 55,250 represents a robust response of the InImS likely reflecting the lapses in disease activity. Another condition that is associated with Ads is myelodysplastic syndrome [
20,
21] which has a FERAD ratio of 13491, suggesting InImS activation.
Another disease that engenders trained-immunity which leads to turning the InImS against itself is systemic lupus erythematosus (SLE) which affects mainly females of child-bearing age [
22]. There are many heterogenous ways in which damage occurs such as autoantibodies, leading to excessive type 1 interferon production, interference with removal of apoptotic cell debris, poor construction of neutrophilic extracellular traps (NET), but also beneficial mitochondrial pathways use of nucleic caids that help regulate type 1 interferon production. The FERAD level of 24,835 hence portends a reasonable InImS response. Recently, the entry of the upregulation of the stimulator of interferon genes (cGAS-STING) pathway has been described in this disease as an intracellular DNA censor u[regulating deleterious responses in this disease [
23].
5. Conclusions
In general, the p87 moiety of the FERAD ratio does respond to dietary supplements, increasing with turmeric (dose response to be determined) and reduced with folic acid [
24]. Higher FERAD ratio may suggest better survival in patients compared to other cancers with lower FERAD ratios [
25]. We could theoretically manipulate the FERAD ratio accordingly, but additional studies will need to be undertaken to confirm these hypotheses.
Autoimmune disorders (ADs) are encountered in 10 to 20% of patients [
15] with myelodysplastic syndromes (MDS). Others have looked at larger numbers of patients and found up to a 30% associated and a 11% increase chance of death with this association [
16]. We believe that this paper represents a wider representation of ADs and IDs than commonly encountered and will generate both interest and additional research into the interesting interdigitation of this set of potentially devastating diseases.
Author Contributions
Conceptualization, M.T. ;Y.Y.T. methodology,; N.F.R.; validation, P.S.; formal analysis, M.L.; investigation, Y.Y..; data curation, M.L.; writing—original draft preparation, M.T.; writing—review and editing, M-P.M.; supervision, M.T.; project administration, M.L..; funding acquisition, B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
In this section, you should add the Institutional Review Board Statement and approval number, if relevant to your study. You might choose to exclude this statement if the study did not require ethical approval. Please note that the Editorial Office might ask you for further information. Please add “The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of NAME OF INSTITUTE (protocol code XXX and date of approval).” for studies involving humans. OR “The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of NAME OF INSTITUTE (protocol code XXX and date of approval).” for studies involving animals. OR “Ethical review and approval were waived for this study due to REASON (please provide a detailed justification).” OR “Not applicable” for studies not involving humans or animals.
Informed Consent Statement
Written consent was given by all the participants in the NIPCON study in accordance with the Detroit and Wayne State University School of Medicine Institutional Review Board. The H09-62-94 study was conducted in accordance with the Declaration of Helsinki and approved by the above Institutional Review Boards for studies involving humans. Informed consent was taken from all the subjects involved in this study. Online data for the PD-L1 data sources are given in the body of the manuscript or in the References Section and were obtained during the month of November 2023. The views expressed herein are purely those of the authors and do not necessarily reflect those of the United States Federal Government under whose auspices this study was conducted.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author under the Data Transfer Agreement and all the following conditions apply. An application must contain statements to the effect of the following: what data are being requested; what data may be used; how will the data be used; who will access the data; and how the data will be accessed, stored, and safeguarded. The applicant must also address how the data will be disposed of, after the completion of the data review. Suggested Data Availability Statements are available in the VHA directive 1200.12 of 3/9/2009. The VHA Handbook addresses both the use of data for research and the clinical and administrative data repositories for research. It also addresses the development and use of data research repositories.
Acknowledgments
The authors would like to thank our internal reviewers, and those who contributed as authors in the cited source documents. This paper is dedicated to the memory of Myrna Friedgood, Terea Harris, Alexis Shelokov, Donald Kuhn and Fanya Warhaftig.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dehdashtian, E.; Caricchio, R. From innate immunity to autoimmunity: Neutrophils in systemic lupus erythematosus pathogenesis. Lupus 2025, 34, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.K.; Kaplan, M.J. The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr Opin Rheumatol 2015, 27, 448–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bertrand, L.; Chervonsky, A.V.; Lehuen, A. Innate Immunity in Type 1 Diabetes. Cold Spring Harb Perspect Med 2025, 15, a041595. [Google Scholar] [CrossRef] [PubMed]
- Popa, E.; Cheval, H.; Zujovic, V. Cues of Trained Immunity in Multiple Sclerosis Macrophages. Cells 2025, 14, 1054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abacar, K.; Macleod, T.; Direskeneli, H.; McGonagle, D. How underappreciated autoinflammatory (innate immunity) mechanisms dominate disparate autoimmune disorders. Front Immunol. 2024, 15, 1439371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saferding, V.; Blüml, S. Innate immunity as the trigger of systemic autoimmune diseases. J Autoimmun. 2020, 110, 102382. [Google Scholar] [CrossRef] [PubMed]
- Burke, O.M.; Frerichs, V.R.; Garcia, D.F.; et al. Stone RC, Lev-Tov H, Czarnowicki T, Keane RW, Ojeh N, Marjanovic J, Pastar I, Tomic-Canic M, de Rivero Vaccari JP, Sawaya AP. The impact of innate immunity and epigenetics in the pathogenesis of hidradenitis suppurativa. Front Immunol 2025, 16, 1593253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, I.; Ji, A.L. Type 1 innate lymphoid cells: a biomarker and therapeutic candidate in sarcoidosis. J Clin Invest 2024, 134, e183708. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tobi, M.; Antaki, F.; Rambus, MA.; et al. The Non-Invasive Prediction of Colorectal Neoplasia (NIPCON) Study 1995-2022: A Comparison of Guaiac-Based Fecal Occult Blood Test (FOBT) and an Anti-Adenoma Antibody, Adnab-9. Int J Mol Sci. 2023, 24, 17257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cappell, M.S.; Tobi, M.; Friedel, D.M. The Impact of COVID-19 Infection on Miscellaneous Inflammatory Disorders of the Gastrointestinal Tract. Gastroenterol Clin North Am 2023, 52, 115–138. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tobi, M.; Khoury, N.; Al-Subee, O.; et al. Predicting Regression of Barrett's Esophagus-Can All the King's Men Put It Together Again? Biomolecules 2024, 14, 1182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miao, Z.; Zhu, W.; Zhou, Y.; Chen, H. Association between autoimmune diseases and myelodysplastic syndrome: a Mendelian randomization study. Hematology 2024, 29, 2433799. [Google Scholar] [CrossRef] [PubMed]
- Moulin, D,; Sellam, J. ; Berenbaum, F.; Guicheux, J.; Boutet, M.A. The role of the immune system in osteoarthritis: mechanisms, challenges and future directions. Nat Rev Rheumatol. 2025, 21, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Orlowsky, E.W.; Kraus, V.B. The role of innate immunity in osteoarthritis: when our first line of defense goes on the offensive. J Rheumatol. 2015, 42, 363–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rozin, A. Is osteoarthritis an infection-associated disease and a target for chemotherapy? Chemotherapy. 2007, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Punzi, L.; Ramonda, R.; Sfriso, P. Erosive osteoarthritis. Best Pract Res Clin Rheumatol. 2004, 18, 739–758. [Google Scholar] [CrossRef]
- Jiang, J.; Jia, T.; Gong, W.; Ning B, Wooley, P. H.; Yang, S.Y. Macrophage Polarization in IL-10 Treatment of Particle-Induced Inflammation and Osteolysis. Am J Pathol. 2016, 186, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Ascunce, K.; Dhodapkar, R.M.; Huang, D.; Hafler, B.P. Innate immune biology in age-related macular degeneration. Front Cell Dev Biol. 2023, 11, 1118524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sabat, R.; Alavi, A.; Wolk, K,; et al. Hidradenitis suppurativa. Lancet 2025, 405, 420–438. [Google Scholar] [CrossRef] [PubMed]
- Grignano, E.; Jachiet, V.; Fenaux, P.; Ades, L.; Fain, O.; Mekinian, A. Autoimmune manifestations associated with myelodysplastic syndromes. Ann Hematol 2018, 97, 2015–2023. [Google Scholar] [CrossRef] [PubMed]
- Adrianzen-Herrera, D.; Sparks, A.D.; Singh, R.; et al. Impact of preexisting autoimmune disease on myelodysplastic syndromes outcomes: a population analysis. Blood Adv 2023, 7, 6913–6922. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watanabe, H.; Matsumoto, Y.; Wada, J. Immunometabolic Regulation of Innate Immunity in Systemic Lupus Erythematosus. Acta Med Okayama. 2025, 79, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.A.; Blasini, A.M. Just Autoimmunity? The Role of the Innate Immune Response in Lupus. J Clin Rheumatol. 2025, 31, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Tobi, M.; Khoury, N.; Al-Subee, O.; et al. Predicting Regression of Barrett's Esophagus-Can All the King's Men Put It Together Again? Biomolecules 2024, 14, 1182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tobi, M.; Talwar, H.; Rossi,; N. F.; Lockette, W.; McVicker, B. A Practical Format to Organize Cancer Constellations Using Innate Immune System Biomarkers: Implications for Early Diagnosis and Prognostication. Int J Transl Med (Basel) 2024, 4, 726–739. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).