1. Introduction
Atrial fibrillation (AF) is the most common arrhythmia, with a prevalence of 1-2% in the general population that has tripled during the last five decades [
1,
2]. Stroke is a major complication of AF. Non-valvular AF confers a fivefold increased risk of stroke, with age further escalating this risk [
3,
4]. Historically, cardioembolic strokes (CS) due to AF have been estimated to account for 30% of ischemic events [
5], though more contemporary classifications suggest AF contributes to over 50% of such cases [
6].
AF can be classified according to its temporal pattern: paroxysmal (episodes <7 days with spontaneous reversion to sinus rhythm), persistent (episodes ≥7 days, aiming for cardioversion), or permanent (AF is an accepted rhythm, rhythm control is no longer pursued). Each type affects stroke risk differently due to variations in arrhythmia burden. Patients with paroxysmal AF exhibit a lower annual stroke risk (about 2% without anticoagulation) compared to those with persistent or permanent AF (around 3%) [
7], due to a markedly lower AF burden (5-11% vs. 70-100%) [
8]. Rhythm control strategies, including ablation, have shown potential to reduce this burden to 0.5 - 3% and, consequently, stroke rates below 1% annually [
9].
Nielsen et al. showed recently that female sex is not a risk factor for stroke per se among AF patients, but rather a risk modifier, since the excess risk for women only affected those with ≥2 non-sex-related stroke risk factors [
10], contributing to a change of the CHA
2DS
2-VASc risk score to the CHA
2DS
2-VA score in the latest European Society of Cardiology (ESC) AF guidelines [
11].
DOACs have revolutionized stroke prevention in AF patients by reducing thromboembolic events while offering superior safety over vitamin K antagonists like warfarin, including lower risks of severe bleeding and no need for routine monitoring [
12]. Good adherence to DOAC (defined as a proportion of days covered (PDC) >80%) is associated with a 31% reduction in ischemic stroke and 14% lower all-cause mortality [
13]. Moreover, strokes occurring on anticoagulation may be less severe, with reduced haemorrhagic transformation, potentially due to thrombin inhibition stabilizing the blood-brain barrier [
14]. A multicentre study by Meinel et al. demonstrated that intravenous thrombolysis in patients on DOAC therapy resulted in lower rates of intracranial bleeding compared to patients without anticoagulation [
15].
However, challenges persist in optimizing DOAC use. Observational data indicate that 30-38% of high-risk AF patients (CHA
2DS
2-VASc ≥2) remain untreated, underscoring gaps in guideline adherence [
16]. Recent data on Latvian AF patients showed that 55.8% were not taking DOAC medication regularly (their adherence was below 80% of the total intake time), while 30.6% of AF patients showed a ≥30-day gap in their DOAC usage [
17]. Rivaroxaban was the most popular DOAC. According to data from 2019, stroke incidence in Latvia is 183.6 per 100,000 inhabitants [
18].
Pauls Stradins Clinical University Hospital (PSKUS), and Riga East University Hospital (RAKUS) are located on either side of the Daugava River, which also divides their catchment areas in Riga and nearby suburbs, collectively serving around one million people, approximately 55% of the Latvian population [
19]. Coincidentally, these are the only two hospitals in Riga that treat stroke patients.
A noteworthy strength of the Latvian medical system, similar to those in Estonia and Lithuania, is its electronic interconnectivity. For example, to ensure that the diagnosis of a new-onset AF is correct, a treating doctor can access prior in- and outpatient discharge letters of the other university hospital, access a national archive of medical diagnostic data (i.e., previous ECGs), and see whether any anticoagulants have been prescribed to a patient previously, even whether the patient has obtained them in the pharmacy.
This retrospective study aimed, first, to analyse how recent DOAC adherence data translates into the real-world characteristics of stroke patients in both university hospitals in the years 2022 - 2024. In a second step, we evaluated the impact of DOACs on stroke severity in a cohort of CS patients with AF in PSKUS. Our primary hypothesis was that AF patients who were on DOAC therapy before their ischemic stroke would develop less severe stroke symptoms at arrival when compared to AF patients not using DOACs.
2. Materials and Methods
2.1. Patient Population
To assess the prevalence of DOAC usage, this retrospective study included AF patients with ischemic stroke of cardioembolic origin (including transient ischemic attack (TIA), which is subsequently omitted in text to improve readability) admitted to the Neurovascular Department of the RAKUS or the PSKUS, in accordance with the trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria [
20] - either with solely cardioembolic pathogenesis or of undetermined aetiology with a partly cardioembolic cause. AF was either known or newly diagnosed during the hospital stay.
Patients with AF admitted for CS were excluded if they had a cardioversion, cardiac intervention, or operation within the previous two weeks, or a non-stroke-related hospital stay within the previous week, as the cause of stroke was considered uncertain (58 patients excluded). A few stroke patients who were transferred to another university hospital for an immediate thrombectomy were attributed to the discharging hospital.
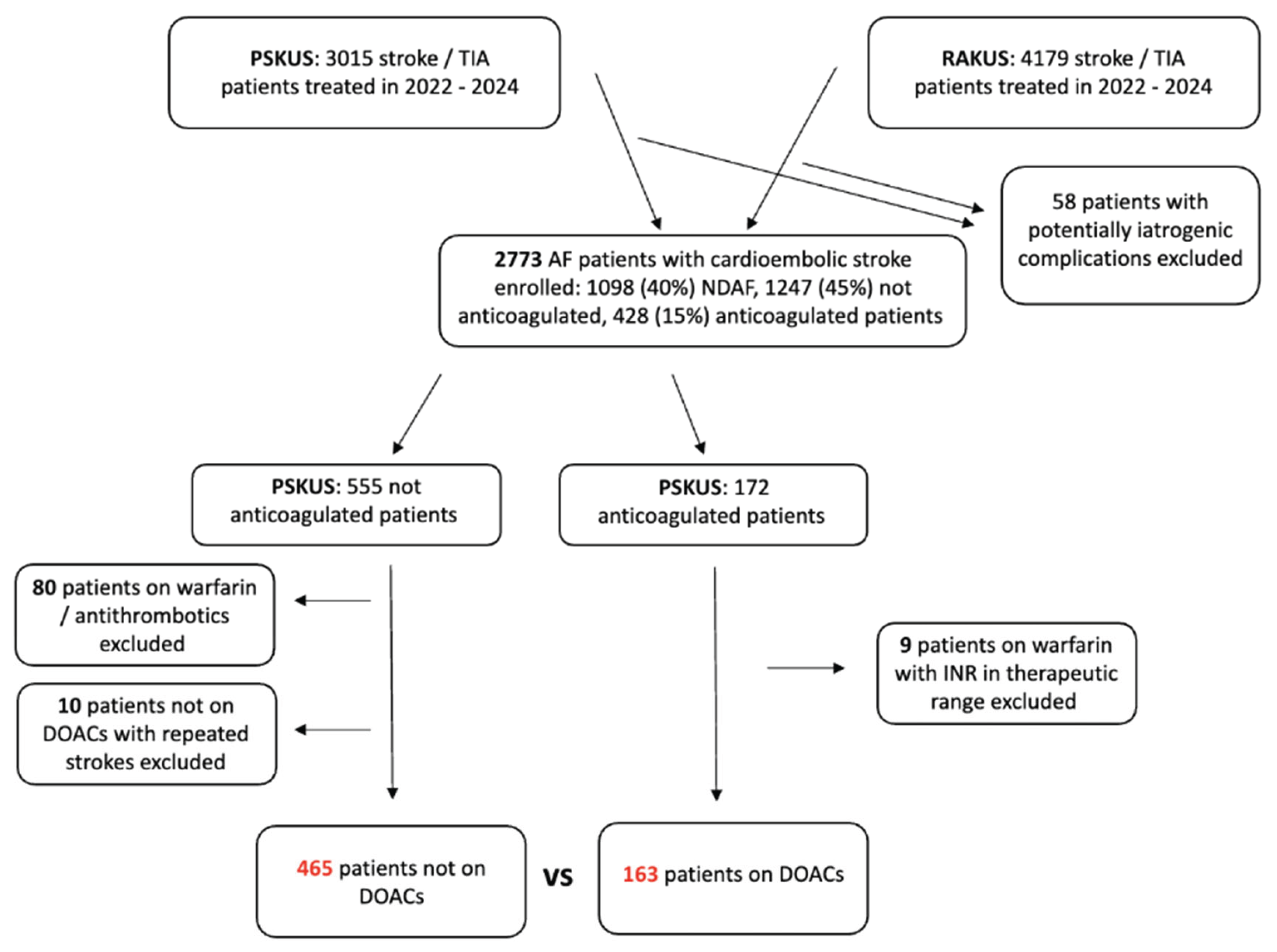
When analysing AF patients on DOAC therapy versus those who have not taken DOACs before the stroke, cases of repeated stroke were excluded, and the first stroke episode of that patient was included in the analysis; 10 cases in total. The study protocol is summarized in
Figure 1.
To evaluate the effect of DOAC in patients with known non-valvular AF, we analysed only the cohort admitted to PSKUS due to a more systematic measurement of factor Xa or factor IIa inhibitor concentrations to further improve diagnostic accuracy.
2.2. Co-Variates
Demographic factors: age at the time of admission and sex.
Clinical variables:CHA2DS2-VA score (isolated risk factors contributing to it, i.e. congestive heart failure, arterial hypertension, etc.) was retrospectively calculated according to the latest ESC AF guidelines11. The current stroke was not incorporated into the calculations. Only echo results performed during the hospital stay were considered when calculating the CHA2DS2-VA score.
The AF type was classified as paroxysmal and permanent, with the latter category also including persistent AF forms. A cardioversion of AF was not performed routinely during the hospital stay.
The severity of stroke in this cohort was assessed using a Latvian version of the National Institutes of Health Stroke Scale (
NIHSS) [
21].
The arrival time was defined as the time from the onset of stroke symptoms (or the last time a patient was seen healthy) to the first contact with a neurologist in the Emergency Department and is further categorized into ≤4.5h, 4.6 - 9h, and >9 h.
Medications prior to admission: Antiplatelet agents, direct oral anticoagulants and vitamin K antagonist - warfarin. Patients were classified as not being on appropriate anticoagulation therapy if they were using only antiplatelet agents, were on warfarin with an INR <1.9, or had not taken their DOAC within 48 hours before the stroke event. 48 hours were chosen as approximately one-third of stroke patients arrived late with stroke symptoms, including those with a so-called wake-up stroke.
Treatments: intravenous thrombolysis, mostly with alteplase, and mechanical thrombectomy.
Laboratory values: in cases when data from the National Health Service reimbursement prescription database suggested a DOAC purchase within the last one to three months (depending on the number of packages bought), but the doctor was not able to confirm the intake (from anamnesis), factor Xa inhibitor and factor IIa inhibitor concentrations were measured. The cut-off inhibitor concentrations to classify patients on rivaroxaban therapy or not were set at <20 µg/L and at <10 µg/L for other factor Xa inhibitors (since many patients are taking a reduced DOAC dose according to their age)17. For dabigatran the cut-off was also set at <20 µg/L. If the last intake of DOAC medicine was ≥24h, the decision to define the patient as on DOAC therapy was based solely on clinical data (i.e., anamnesis).
Echocardiographic values: We included the echocardiographic data up to 8 months post-stroke event. For this analysis, we included left ventricular ejection fraction (LVEF) and left atrial volume index (LAVI) [
22].
2.3. Statistical Analysis
Descriptive analysis was used to obtain the means and standard deviations. To compare between-group differences, the Mann-Whitney U test was used for continuous variables, and the chi-square test was used for categorical variables.
Due to the retrospective character of the study, we performed a propensity matching of the patient group who received no DOACs to patients taking DOACs based on age, sex, AF type, CHA2DS2-VA score, and arrival time.
In all the analyses, statistical significance was defined as p-value <0.05.
The ethical aspects of this retrospective study were approved by the Ethics Committee of Latvian University (nr. 13-22/90, May 2025) and both local university hospital ethical committees.
3. Results
3.1. General Characteristics, Assessment of Anticoagulation Among Stroke Patients with AF
Between January 2022 and December 2024, a total of 2773 patients were enrolled in this study. The median age was 81 years, and 34.0% of the patients were male.
The total number of patients with stroke / TIA treated in PSKUS was 887 / 122 in 2022 (433 patients with CS or TIA, or 42.9% were enrolled), 889 / 128 in 2023 (421 or 42.0% were enrolled), and 870 / 119 in 2024 (379 or 38.3% were enrolled). In RAKUS, out of 1257 stroke and 197 TIA patients, 525 patients (i.e., 36%) were enrolled. In 2023, out of 1396 stroke / 143 TIA patients, 542 patients (i.e., 39%) were enrolled. In 2024, out of 1329 stroke / 156 TIA patients, 473 patients (i.e., 36%) were enrolled.
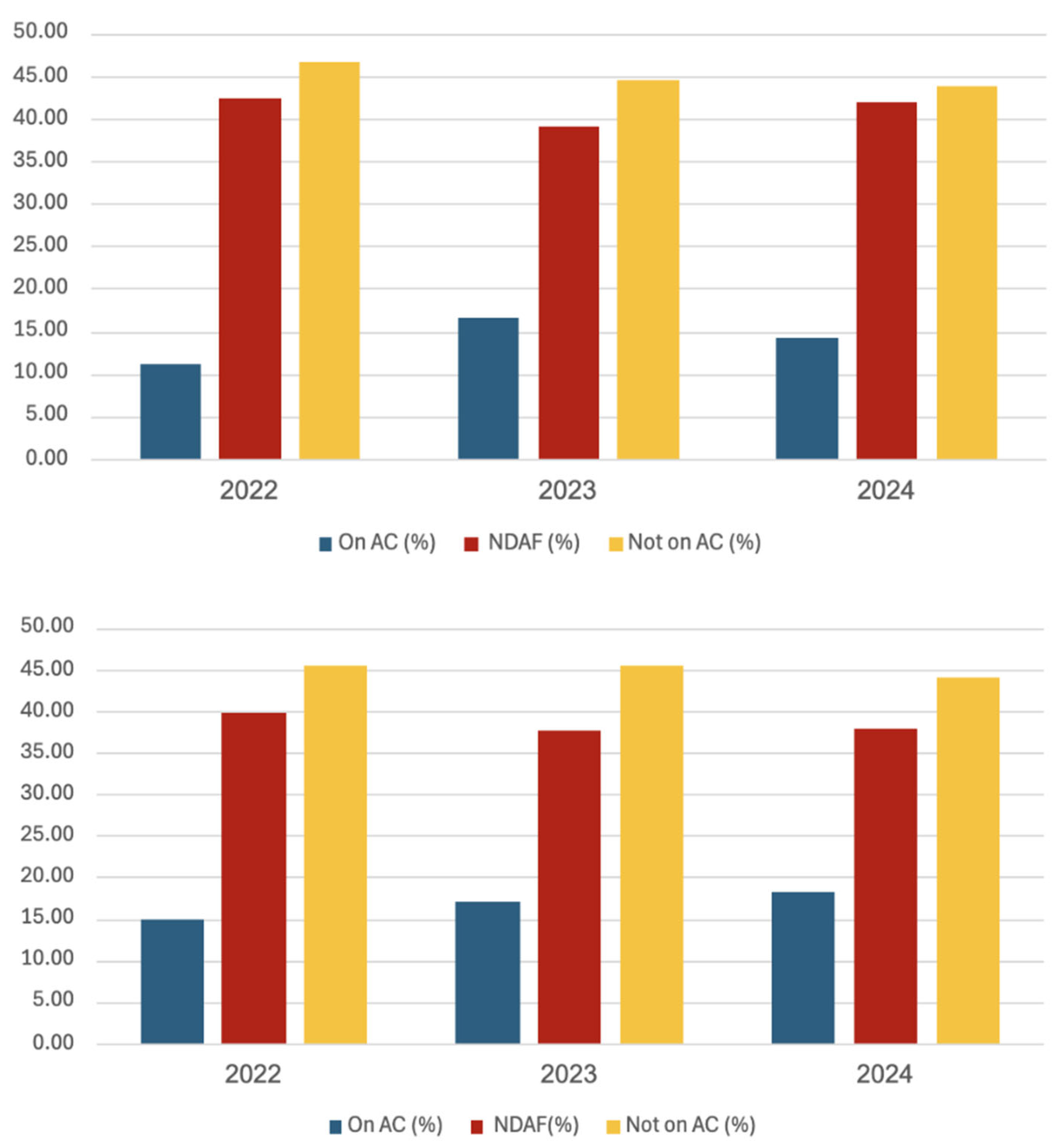
Stroke patients with known AF not on adequate anticoagulation treatment (DOAC or if on warfarin with INR <1.9) were consistently the largest patient group (>43%) throughout the years and in both hospitals, as shown in
Figure 2 and
Table 1. The proportion of stroke patients with newly diagnosed AF was around 40%. Regarding the patient numbers, no significant differences were found between RAKUS and PSKUS. When comparing the year 2024 with the year 2022, an absolute reduction of 106 CS cases (i.e., -11%) and a decrease of 66 cases (i.e., -15%) among patients not on adequate anticoagulation.
A further assessment of patients’ demographics showed that in the year 2022, the stroke patient group treated in RAKUS with newly diagnosed AF were significantly older compared to PSKUS patients (mean age (± standard deviation (SD)) 77.8 ± 9.7 vs. 80.3 ± 9.1 years, p=0.013). Also in 2024, those patients were older (77.8 ± 10.0 vs 80.1 ± 9.6 years, p=0.018), and there were significantly more females with stroke and new-onset AF (59.1% vs. 71.5%, p=0.023). In 2023, stroke patients not on adequate anticoagulation therapy admitted to RAKUS were significantly older (77.5 ± 10.0 vs. 79.8 ± 9.2, p=0.014). This baseline difference is important to acknowledge, as the subsequent analysis was performed exclusively on the PSKUS cohort for the reasons previously stated.
3.1.1. Characteristics of Stroke Patients with AF Not on Anticoagulation Therapy
When looking at PSKUS patients not on anticoagulation therapy prior to stroke, the median age of 555 patients in this category was 80 years, and 62% of patients were female and 38% were male. The largest group consisted of patients not taking DOACs for longer than 7 days (135 patients or 67% in 2022, 125 or 67% in 2023, and 139 or 83% in 2024) as shown in
Table 2. The proportion of these patients increased as other causes for not being on the DOAC therapy dwindled over time. AF patients solely on antithrombotics (such as aspirin or clopidogrel) decreased from 19 (or 9.4%) in 2022 to 8 (4.8%) in 2024. Similarly, patients on a subtherapeutic dose of warfarin (i.e., INR <1.9) were 20 (9.9%) in 2022 and 4 (2.4%) in 2024.
When comparing patients not using DOACs long-term (>7 days) versus short-term (≤7 days, including the inconsistent group), no significant differences in stroke severity (i.e., NIHSS score at admission) were observed, although the former group presented with more severe stroke (mean NIHSS score 12.11 ± 7.72 vs. 10.33 ± 6.96, p=0.08, median score 11 vs. 10).
3.1.2. Characteristics of Stroke Patients with AF on Anticoagulation Therapy
172 AF patients admitted with stroke to PSKUS were 69% female and 31% male, and 81 years was the median age. 9 (5.2%) were taking warfarin with their INR at admission ≥2. Among all four available DOACs, the most frequent was rivaroxaban (99 users out of 163, 60.7%), then edoxaban with 47 users (28.8%), 14 patients using dabigatran (8.6%), and 3 apixaban users (1.8%).
66 out of 163 (40.5%) patients were taking the reduced dose of DOACs, and in 36 patients (22.1%) it was inadequately reduced, likely based on the patient's age rather than established criteria. 16 patients suffered a fatal stroke; in 129 out of 147 patients (88%), another DOAC was recommended at discharge. In none of those patients was a left atrial appendage closure procedure recommended.
Out of 149 patients, 69 had symptom onset ≤4.5h from admission, for 41 patients, factor Xa inhibitor levels were available, and 25 had FXa inhibitor levels <100 µg/L, 12 had levels <50 µg/L. Two of them had thrombolysis, and 16 had mechanical thrombectomy (including the former two).
3.1.3. Comparison of Stroke Patients with Paroxysmal versus Permanent AF
To compare PSKUS patients with paroxysmal versus permanent (including persistent AF cases), we excluded those with newly diagnosed AF. The proportion of stroke patients with paroxysmal AF was 27.2% (68 out of 250) in 2022, 19.12% (48 out of 251) in 2023, and 22.54% (48 out of 213) in 2024. The median age of 164 paroxysmal AF patients was 78 years, 34% were males, while the median age of 550 patients with permanent AF was 80 years, and 37% were males. Significant differences were observed regarding age, CHA2DS2-VA score, LAVI, and LVEF, but not in the severity of stroke (NIHSS score at arrival permanent vs. paroxysmal AF, 11.23 ± 7.63 vs. 10.11 ± 7.41, p=0.09).
3.1.4. Comparison of Patients on DOAC Therapy Versus Those Who Are Not on DOAC Therapy
In a further step, we identified 163 patients on DOAC therapy with a median age of 81 years, and 31% were male, and compared them with 465 patients not on DOAC therapy with a median age of 79 years, 36% were male.
The analysis revealed that there was a significant difference regarding the CHA2DS2-VA score, with patients on DOAC therapy having a higher score than non-users (4.3 ± 1.5 vs. 4.7 ± 1.5, p=0.003) and significantly higher rates of prior stroke and/or thromboembolism events. There was a difference in arrival time between groups, with patients on DOAC therapy arriving significantly later. However, these DOAC users experienced significantly less severe strokes (NIHSS score at arrival 11.8 ± 7.6 vs. 9.8 ± 7.2, p=0.003).
After propensity matching based on the criteria described above, we repeated the comparison with the results shown in
Table 3.
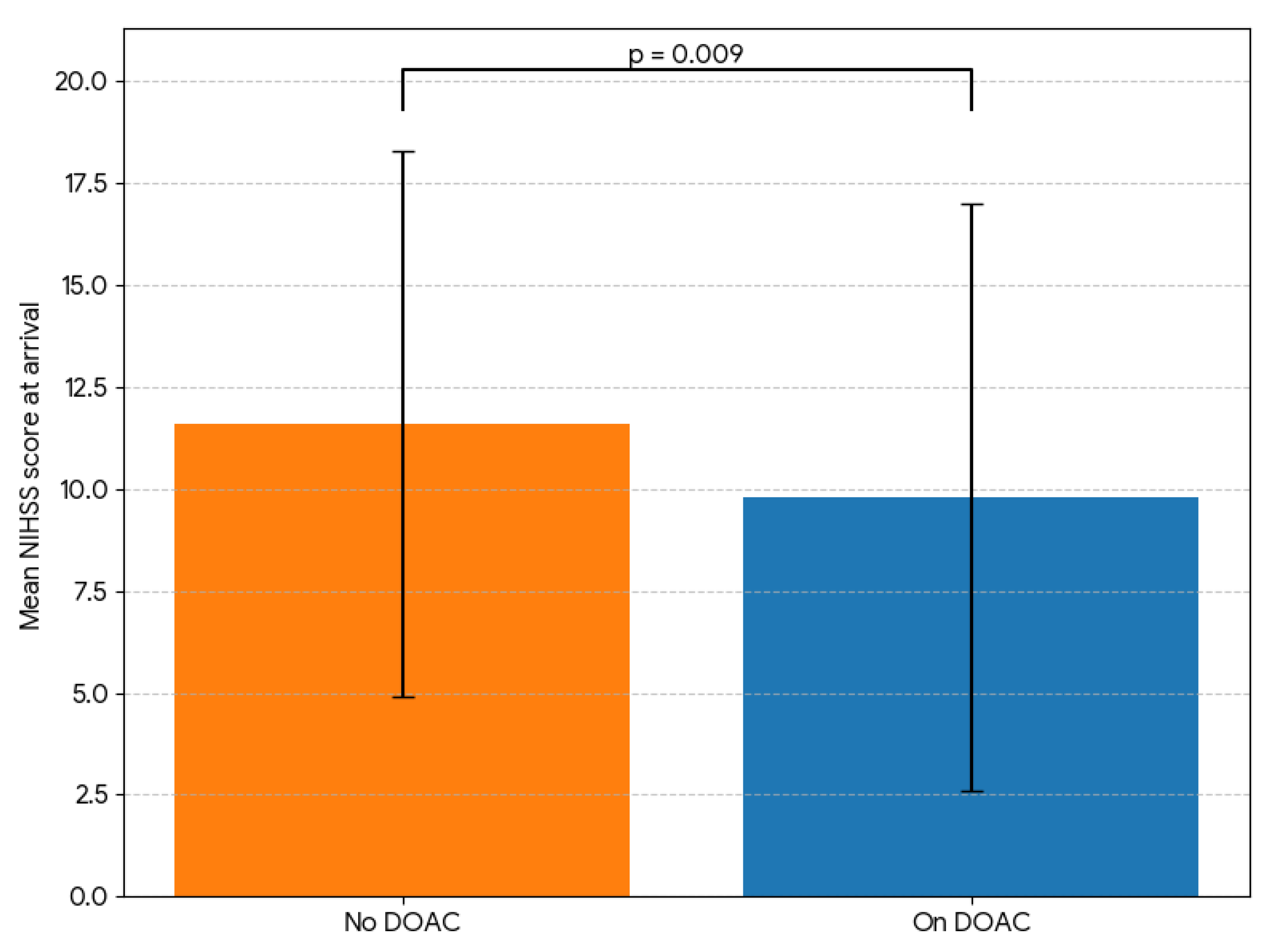
163 patients in each group differed significantly only regarding the stroke severity at arrival (NIHSS score 11.6 ± 6.7 vs. 9.8 ± 7.2 for non-users and DOAC users, respectively, p=0.009, see
Figure 3) and at discharge, and the frequency of thrombolysis (54 vs 4, p<0.001). There was no significant difference (p=0.132) in NIHSS improvement between groups (despite the thrombolysis therapy) only at first sight. When we split the non-DOAC users into two groups depending on the thrombolysis treatment, the thrombolysis treatment made a significant difference for 50 thrombolysed patients (6.56 ± 4.81 vs. 3.65 ± 5.05 for 141 (non-thrombolysed) DOAC users, p<0.001). No significant difference was observed between non-thrombolysed DOAC users and non-thrombolysed non-DOAC patients (3.65 ± 5.05 vs. 3.00 ± 4.46, p=0.296). After propensity matching based on age, sex, NIHSS score, arrival time, thrombolysis, and thrombectomy, we compared 81 patients in each group. We found no significant difference in stroke improvement between non-DOAC patients and DOAC users (2.17 ± 3.50 vs 2.62 ± 3.93, respectively, p=0.45).
A significant difference in LAVI remained between the propensity-matched non-DOAC and DOAC groups (48.0 ± 15.6 ml/m2 vs. 56.4 ± 20.7 ml/m2, respectively, p=0.004). There was no significant difference in time after the stroke event when the echo was performed, albeit a bit later in the “on DOACs” group (2.00 ± 1.75 months vs. 1.66 ± 1.5 months, p=0.255).
4. Discussion
AF has become the most significant cardiac rhythm disorder as its prevalence is increasing in the aging population [
1]. The age-adjusted mortality rate in Europe linearly increased from 12.3 in 2008 to 15.3 per 100,000 population in 2019, showing an average annual percentual change of +2.0% [
23], while some Eastern European countries showed higher mortality rate increases. In Latvia, particularly, the average annual percentual change of +37.6% (as the highest - from 0.4 per 100,00 inhabitants in 2008 to 5.9 in 2019) was observed during this period [
23], in stark contrast to decreases in mortality rates associated with coronary artery disease [
24]. The two largest Latvian university hospitals serve as the primary stroke centres for just over half of the Latvian population, and, therefore, data from these two hospitals may not be generalizable to the entire country. Furthermore, after the worldwide rollout of the COVID-19 vaccines in 2021 and a subsequent decrease in mortality, data on three full years are available, and a long-term prospect based on these is limited. However, by comparing the stroke numbers from 2022 with those from 2024, we noticed an 11% decrease in CS cases and a 15% decrease in AF patients who were not on adequate anticoagulation therapy before stroke. Accordingly, there was a 15% increase in the number of prescribed anticoagulants (from 451,323 in 2022 to 519,136 prescriptions in 2024) as per unpublished data from the National Health Service. Hopefully, it is the beginning of a long-term trend as we attributed about 40% of all stroke and TIA cases to cardioembolic pathogenesis (using the TOAST criteria), while this pathogenesis (AF, especially) has been responsible for up to 30% of all stroke and TIA patients in the 1990s [
5].
Anticoagulation is a cornerstone of (preventive) treatment in AF. It is important to emphasize recent data by Gavrilova et al. from the National Health Service, which shows that only 44.2% of AF patients achieve an adherence level >80% (i.e., regular DOAC intake) [
17]. Accordingly, we saw that CS patients with known AF, a mean CHA
2DS
2-VA score of 4.3 ± 1.5, and not on DOAC medication account for 45% of all AF patients with CS treated in PSKUS across the years 2022 – 2024. Similarly, around 40% of stroke patients presented with newly diagnosed AF, see
Table 1. This number exceeds the previously observed one from a meta-analysis of 50 studies from 2015, where the overall detection rate of (newly diagnosed) AF was 23.7% [
25].
Encouragingly, the number of AF patients with stroke taking antithrombotics or being on a subtherapeutic dose of warfarin has dwindled in this period, presumably due to an intensified education of all medical professionals, beyond cardiologists and neurologists. This is reflected by the unpublished data from the National Health Service, as there was an increase in DOAC prescriptions by 32.4%, while prescriptions of Vitamin K antagonists fell by 35.1% from 2022 to 2024.
An intensified patient education about the necessity to use DOACs (regularly) is a straightforward opportunity to dramatically improve AF-related mortality. It is also important to thoroughly evaluate pauses in DOAC usage before (minor) surgeries, as there are indications for “prothrombotic rebound” [
26].
A more expensive solution is to promote more electrophysiological pulmonary vein ablations to reduce the AF burden. An argument in favor of this costly procedure is data showing that the total cost of stroke amounts to 0.53% of Latvia's GDP, i.e., to €213 million in 2024 [
27]. Our data support the recently introduced “AF burden” paradigm as only about 25% of stroke patients with known AF had the paroxysmal form [
28]. Once the stroke occurred, no significant difference in stroke severity between AF forms was observed.
In accordance with the recent study by Grosse et al. [
14], we also saw a significant difference in stroke severity in patients on adequate DOAC therapy (a 2-point lower NIHSS score, on average) versus those who are not. After matching both groups, based on age, sex, AF type, and arrival time after stroke onset, the difference in stroke severity remained significant – with DOAC users having a less severe stroke (a 1.8-point lower NIHSS score). In the study by German colleagues, the difference was –2.5 points in NIHSS score and –1.8 points between those patients who did not receive thrombolysis and mechanical thrombectomy [
14]. During the further hospital stay, there was no significant difference regarding the improvement of stroke symptoms, when comparing DOAC users and non-DOAC patients.
A few previous studies demonstrated enlarged left atria (LA) in anticoagulated stroke patients [
29,
30]. Ogata et al. showed that increasing LA diameter is predictive of recurrent strokes even for patients under anticoagulation [
31], thus suggesting that LA enlargement promotes further thrombogenicity. To our knowledge, this is the first study to provide a significant numerical difference in LA dilatation for anticoagulated stroke patients, measured using LAVI, which is currently an established echo parameter [
22]. Hopefully, our study encourages other researchers to refine a potential cut-off of LAVI that promotes the thrombogenicity, as left atria in both groups (non-DOAC users vs. DOAC users) showed severely enlarged left atria (48.0 ± 15.6 ml/m2 vs. 56.4 ± 20.7 ml/m2, respectively).
Although there was a strong female predominance (approximately 66%) throughout all patient groups, one must consider that a female-to-male ratio in the Latvian population above 65 is roughly 2:1, based on data from 2016 [
32].
The strength of this study is the ability to classify patients on adequate anticoagulation, not on adequate anticoagulation, and with newly diagnosed AF due to access to multiple healthcare databases available for doctors in the Latvian university hospitals. Our obtained data are consistent with the previously published stroke incidence rate in Latvia and non-adherence to DOACs [
17,
18]. The study's findings, comparing DOAC users and non-users, primarily serve to generate hypotheses, as a relatively small sample size is one of the study's weaknesses. In a group of “inconsistent non-DOAC users” the measurement of DOAC concentrations played an important role. Although this group contributes only about 10% to all non-DOAC users, they would significantly distort the data of the DOAC users. Therefore, the RAKUS stroke patients were not considered in this comparison. Although the data between the university hospitals generally were not significantly different, in 2023 non-DOAC users were significantly older in the RAKUS cohort. Both university hospitals in Riga serve only 55% of the Latvian population, and since no regional hospitals were involved in this study, caution is warranted when generalizing these data to the national level. Furthermore, the study had a retrospective observational character. Therefore, not all information was obtained during the patient’s hospitalization, especially in those with severe stroke. Additionally, a collaboration between neurologists and cardiologists should be further improved, as indicated in
Table 3, where echocardiography data were available for roughly half of all patients.
5. Conclusions
In summary, AF-associated mortality rates have increased in Europe in the past decade, especially in Eastern Europe. Although the non-adherence to regular DOAC therapy is a worldwide problem, it is very important in Latvia, where 30.6% do not use DOACs and 55.8% (including those 30.6%) do not use DOACs regularly and, thus, are the largest group of patients with CS. Fortunately, the use of DOACs not only reduces the risk of stroke but also seems to reduce its severity, further favouring its use. Although LA were severely dilated among CS patients with AF, those patients on DOAC therapy had significantly higher LAVI. Further studies are required to define a cut-off for LAVI associated with an increased CS risk despite being on an adequate DOAC therapy.
Author Contributions
Conceptualization, V.S and K.K.; methodology, V.S.; software, V.S.; validation, V.S., I.O. and K.J.; formal analysis, V.S.; investigation, V.S.; resources, V.S.; data curation, V.S.; writing—original draft preparation, V.S.; writing—review and editing, K.K., K.J., I.O., G.K., E.M. and A.Ē; visualization, V.S.; supervision, A.Ē.; project administration, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Latvian University (nr. 13-22/90, May 2025).
Informed Consent Statement
Patient consent was waived due to the retrospective character of this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AF |
atrial fibrillation |
| CHF |
congestive heart failure |
| CS |
cardioembolic stroke |
| DOAC |
direct oral anticoagulants |
| ESC |
European Society of Cardiology |
| LA |
left atrium |
| LAVI |
left atrial volume index |
| LVEF |
left ventricular ejection fraction |
| NIHSS |
National Institutes of Health Stroke Scale |
| PDC |
proportion of days covered |
| PSKUS |
Paula Stradiņa klīniskās universitātes slimnīca (i.e., Paul Stradins Clinical University Hospital) |
| RAKUS |
Rīgas Austrumu klīniskās universitātes slimnīca (i.e., Riga East University Hospital) |
| TIA |
transient ischemic attack |
| TOAST |
trial of ORG 10172 in Acute Stroke Treatment |
References
- Schnabel R.B., Yin X., Gona P., Larson M.G., Beiser A.S., McManus D.D., et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154–62. [CrossRef]
- Kornej J., Börschel C.S., Benjamin E.J., Schnabel R.B. Epidemiology of Atrial Fibrillation in the 21st Century. Circ Res. 2020;127(1):4–20. [CrossRef]
- Marini C., De Santis F., Sacco S., Russo T., Olivieri L., Totaro R., et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36(6):1115–9.
- Christiansen C.B., Gerds T.A., Olesen J.B., Kristensen S.L., Lamberts M., Lip G.Y.H., et al. Atrial fibrillation and risk of stroke: a nationwide cohort study. Europace. 2016;18(11):1689–97. [CrossRef]
- Kolominsky-Rabas P.L. Epidemiology of Ischemic Stroke Subtypes According to TOAST Criteria. Stroke. 2001;12(12):2735-40. [CrossRef]
- Bogiatzi C., Wannarong T., McLeod A.I., Heisel M., Hackam D., Spence J.D. SPARKLE (Subtypes of Ischaemic Stroke Classification System), Incorporating Measurement of Carotid Plaque Burden: A New Validated Tool for the Classification of Ischemic Stroke Subtypes. Neuroepidemiology. 2014;42(4):243–51. [CrossRef]
- Vanassche T., Lauw M.N., Eikelboom J.W., Healey J.S., Hart R.G., Alings M., et al. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J. 2015;36(5):281-8. [CrossRef]
- Charitos E.I., Pürerfellner H., Glotzer T.V., Ziegler P.D.. Clinical Classifications of Atrial Fibrillation Poorly Reflect Its Temporal Persistence: Insights From 1,195 Patients Continuously Monitored With Implantable Devices. J Am Coll Cardiol. 2014;63(25, Part A):2840–8. [CrossRef]
- Kirchhof P., Camm A.J., Goette A., Brandes A., Eckardt L., Elvan A., et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N Engl J Med. 2020;383(14):1305–16. [CrossRef]
- Nielsen P.B., Overvad T.F. Female Sex as a Risk Modifier for Stroke Risk in Atrial Fibrillation: Using CHA2DS2-VASc versus CHA2DS2-VA for Stroke Risk Stratification in Atrial Fibrillation: A Note of Caution. Thromb Haemost. 2020;120(6):894–8. [CrossRef]
- Van Gelder I.C., Rienstra M., Bunting K.V., Casado-Arroyo R., Caso V., Crijns H.J.G.M., et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2024;45(36):3314–414. [CrossRef]
- Carnicelli A.P., Hong H., Connolly S.J., Eikelboom J., Giugliano R.P., Morrow D.A., et al. Direct Oral Anticoagulants Versus Warfarin in Patients With Atrial Fibrillation: Patient-Level Network Meta-Analyses of Randomized Clinical Trials With Interaction Testing by Age and Sex. Circulation. 2022;145(4):242–55. [CrossRef]
- Hurtado-Navarro I., García-Sempere A., Rodríguez-Bernal C., Santa-Ana-Tellez Y., Peiró S., Sanfélix-Gimeno G. Estimating adherence based on prescription or dispensation information: impact on thresholds and outcomes. A real-world study with atrial fibrillation patients treated with oral anticoagulants in Spain. Front Pharmacol. 2018;9:1353. [CrossRef]
- Grosse G.M., Hüsing A., Stang A., Kuklik N., Brinkmann M., Grond M., et al. Prior Anticoagulation and Risk of Hemorrhagic Transformation in Acute Stroke: A Post Hoc Analysis of the PRODAST Study. J Am Heart Assoc. 2025;14(3):e037014. [CrossRef]
- Meinel T.R., Wilson D., Gensicke H., Scheitz J.F., Ringleb P., Goganau I., et al. Intravenous Thrombolysis in Patients With Ischemic Stroke and Recent Ingestion of Direct Oral Anticoagulants. JAMA Neurol. 2023;80(3):233–43. [CrossRef]
- Yao X., Abraham N.S., Caleb Alexander G., Crown W., Montori V.M., Sangaralingham L.R., et al. Effect of Adherence to Oral Anticoagulants on Risk of Stroke and Major Bleeding Among Patients With Atrial Fibrillation. J Am Heart Assoc. 2016;5(2):1–12. [CrossRef]
- Gavrilova A., Zolovs M., Šmits D., Ņikitina A., Latkovskis G., Urtāne I. Role of a National Health Service Electronic Prescriptions Database in the Detection of Prescribing and Dispensing Issues and Adherence Evaluation of Direct Oral Anticoagulants. Healthcare. 2024;12(10):975. [CrossRef]
- Prendes C.F., Rantner B., Hamwi T., Stana J., Feigin V.L., Stavroulakis K., et al. Burden of stroke in Europe: An analysis of the global burden of disease study findings from 2010 to 2019. Stroke. 2024;55(2):432-42. [CrossRef]
- Latvia Healthcare Facilities Master Plan 2016-2025. Available from: https://www.vmnvd.gov.lv/sites/vmnvd/files/data_content/58b57889c736b1.pdf (accessed 14 Okt 2025).
- Adams H.P., Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. [CrossRef]
- Jurjāns K., Noviks I., Volčeka D., Zandersone L., Meilerte K., Miglāne E., et al. The adaption and evaluation of a Latvian version of the National Institutes of Health Stroke Scale. J Int Med Res. 2017;45(6):1861–9. [CrossRef]
- Lang R.M., Badano L.P., Mor-Avi V., Afilalo J., Armstrong A., Ernande L., et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1-39.e14. [CrossRef]
- Zuin M., Malagù M., Vitali F., Balla C., De Raffele M., Ferrari R., et al. Trends in atrial fibrillation-related mortality in Europe, 2008–2019. Eur Heart J Qual Care Clin Outcomes. 2024;10(5):467–78. [CrossRef]
- Stolpe S., Kowall B., Stang A. Decline of coronary heart disease mortality is strongly effected by changing patterns of underlying causes of death: an analysis of mortality data from 27 countries of the WHO European region 2000 and 2013. Eur J of Epidemiol. 2021;36(1):57-68. [CrossRef]
- Sposato L.A., Cipriano L.E., Saposnik G., Ruíz Vargas E., Riccio P.M., Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015;14(4):377–87.
- Frydrych M., Janeczek M., Małyszek A., Nelke K., Dobrzyński M., Lukaszewski M. Prothrombotic Rebound After Discontinuation of Direct Oral Anticoagulants Therapy: A Systematic Review. J Clin Med. 2024;13(21):6606. [CrossRef]
- Luengo-Fernandez R., Violato M., Candio P., Leal J. Economic burden of stroke across Europe: A population-based cost analysis. Eur Stroke J. 2020;5(1):17–25. [CrossRef]
- Becher N., Metzner A., Toennis T., Kirchhof P., Schnabel R.B. Atrial fibrillation burden: a new outcome predictor and therapeutic target. Eur Heart J. 2024;45(31):2824–38. [CrossRef]
- Paciaroni M., Agnelli G., Caso V., Silvestrelli G., Seiffge D.J., Engelter S., et al. Causes and Risk Factors of Cerebral Ischemic Events in Patients With Atrial Fibrillation Treated With Non–Vitamin K Antagonist Oral Anticoagulants for Stroke Prevention. Stroke. 2019;50(8):2168–74. [CrossRef]
- Yaghi S., Henninger N., Giles J.A., Guerrero C.L., Mistry E., Liberman A.L., et al. Ischaemic stroke on anticoagulation therapy and early recurrence in acute cardioembolic stroke: the IAC study. J Neurol, Neurosurg Psychiatry. 2021;92(10):1062-7. [CrossRef]
- Ogata T, Matsuo R, Kiyuna F, Hata J, Ago T, Tsuboi Y, et al. Left Atrial Size and Long-Term Risk of Recurrent Stroke After Acute Ischemic Stroke in Patients With Nonvalvular Atrial Fibrillation. J Am Heart Assoc. 2017;6(8):e006402. [CrossRef]
- CEIC Data. Latvia Population: Male: Aged 65 and Above. Available from: https://www.ceicdata.com/en/latvia/population-and-urbanization-statistics/lv-population-as--of-total-male-aged-65-and-above (accessed 14 Oct 2025).
Figure 1.
A flowchart of study design. In the first step, we categorized 2773 enrolled cardioembolic stroke patients with atrial fibrillation (AF) based on their (adequate) anticoagulation therapy. We further categorized the reasons for insufficient anticoagulation therapy in 555 patients. Finally, we compared 465 patients not on direct oral anticoagulants (DOACs) prior to stroke with those taking DOACs. AF, atrial fibrillation, DOACs, direct oral anticoagulants, INR, International Normalized Ratio, NDAF, newly diagnosed atrial fibrillation, PSKUS, Paul Stradins Clinical University Hospital, RAKUS, Riga East University Hospital.
Figure 1.
A flowchart of study design. In the first step, we categorized 2773 enrolled cardioembolic stroke patients with atrial fibrillation (AF) based on their (adequate) anticoagulation therapy. We further categorized the reasons for insufficient anticoagulation therapy in 555 patients. Finally, we compared 465 patients not on direct oral anticoagulants (DOACs) prior to stroke with those taking DOACs. AF, atrial fibrillation, DOACs, direct oral anticoagulants, INR, International Normalized Ratio, NDAF, newly diagnosed atrial fibrillation, PSKUS, Paul Stradins Clinical University Hospital, RAKUS, Riga East University Hospital.
Figure 2.
Characteristics of cardioembolic stroke patients from 2022 – 2024 treated in PSKUS and RAKUS. The above graph shows that PSKUS patients not being on adequate anticoagulation (AC) constitute the largest group of cardioembolic stroke patients, being more than twice as much as patients on adequate AC. About 40% are patients with newly diagnosed atrial fibrillation. The graph below shows similar results from RAKUS. AC, anticoagulation, NDAF, newly diagnosed atrial fibrillation, PSKUS, Paul Stradins Clinical University Hospital, RAKUS, Riga East University Hospital.
Figure 2.
Characteristics of cardioembolic stroke patients from 2022 – 2024 treated in PSKUS and RAKUS. The above graph shows that PSKUS patients not being on adequate anticoagulation (AC) constitute the largest group of cardioembolic stroke patients, being more than twice as much as patients on adequate AC. About 40% are patients with newly diagnosed atrial fibrillation. The graph below shows similar results from RAKUS. AC, anticoagulation, NDAF, newly diagnosed atrial fibrillation, PSKUS, Paul Stradins Clinical University Hospital, RAKUS, Riga East University Hospital.
Figure 3.
Comparison of stroke severity at arrival between AF patients taking no DOAC and those on DOAC therapy prior to the stroke event. After matching both groups based on age, sex, atrial fibrillation (AF) type, arrival time period, and CHA2DS2-VA score, we compared the stroke severity (based on symptoms measured by NIHSS score) at arrival in the hospital between patients taking no direct oral anticoagulant (DOAC) therapy prior to stroke and those on DOAC therapy and show that the former group presented with a significantly pronounced stroke symptoms (11.6 ± 6.7 vs. 9.8 ± 7.2 NIHSS score points, p=0.009). .
Figure 3.
Comparison of stroke severity at arrival between AF patients taking no DOAC and those on DOAC therapy prior to the stroke event. After matching both groups based on age, sex, atrial fibrillation (AF) type, arrival time period, and CHA2DS2-VA score, we compared the stroke severity (based on symptoms measured by NIHSS score) at arrival in the hospital between patients taking no direct oral anticoagulant (DOAC) therapy prior to stroke and those on DOAC therapy and show that the former group presented with a significantly pronounced stroke symptoms (11.6 ± 6.7 vs. 9.8 ± 7.2 NIHSS score points, p=0.009). .
Table 1.
Categorization of cardioembolic stroke patients with atrial fibrillation (AF) based on their (adequate) anticoagulation in both involved hospitals. .
Table 1.
Categorization of cardioembolic stroke patients with atrial fibrillation (AF) based on their (adequate) anticoagulation in both involved hospitals. .
| RAKUS |
|
|
|
|
|
|
|
| |
NDAF |
NDAF(%) |
Not on AC |
Not on AC (%) |
On AC |
On AC (%) |
Total |
| 2022 |
209 |
39.81 |
238 |
45.33 |
78 |
14.86 |
525 |
| 2023 |
204 |
37.64 |
246 |
45.39 |
92 |
16.97 |
542 |
| 2024 |
179 |
37.84 |
208 |
43.97 |
86 |
18.18 |
473 |
| PSKUS |
|
|
|
|
|
|
|
| |
NDAF |
NDAF (%) |
Not on AC |
Not on AC (%) |
On AC |
On AC (%) |
Total |
| 2022 |
183 |
42.26 |
202 |
46.65 |
48 |
11.09 |
433 |
| 2023 |
164 |
38.95 |
187 |
44.42 |
70 |
16.63 |
421 |
| 2024 |
159 |
41.95 |
166 |
43.80 |
54 |
14.25 |
379 |
Table 2.
Reasons for inadequate anticoagulation among PSKUS stroke patients with known atrial fibrillation.
Table 2.
Reasons for inadequate anticoagulation among PSKUS stroke patients with known atrial fibrillation.
| |
Longer Pause |
Shorter Pause |
Inconsistent |
Antithrombotics |
Subtherapeutic INR |
Total |
| 2022 |
135 |
4 |
24 |
19 |
20 |
202 |
| 2023 |
125 |
11 |
22 |
10 |
19 |
187 |
| 2024 |
139 |
8 |
7 |
8 |
4 |
166 |
Table 3.
A propensity-matched comparison of patients taking no direct oral anticoagulants (DOACs) before stroke and those on DOACs. .
Table 3.
A propensity-matched comparison of patients taking no direct oral anticoagulants (DOACs) before stroke and those on DOACs. .
| |
Total (n) |
No DOAC (n = 163) |
On DOAC (n = 163) |
p-value |
| Age |
326 |
78.5 ± 9.1 |
78.5 ± 9.2 |
0.951 |
| Sex |
326 |
113 F / 50 M |
113 F / 50 M |
1 |
| AF type (parox. / perm.) |
326 |
33 / 130 |
30 / 133 |
0.779 |
| CHA2DS2-VA Score |
326 |
4.6 ± 1.5 |
4.7 ± 1.5 |
0.782 |
| Congestive heart failure |
326 |
106 |
99 |
0.492 |
| Art. hypertension |
326 |
149 |
151 |
0.192 |
| Age (0 / 1 / 2 points) |
326 |
13 / 38 / 112 |
17 / 37 / 109 |
0.746 |
| Diabetes mellitus |
326 |
38 |
35 |
0.329 |
| Prior stroke / TIA / TE |
326 |
0 |
0 |
0.221 |
| Vascular diseases |
326 |
61 |
61 |
1 |
| Arrival time period (1 / 2 / 3) |
326 |
67 / 30 / 65 |
69 / 30 / 64 |
0.983 |
| NIHSS at arrival |
325 |
11.6 ± 6.7 |
9.8 ± 7.2 |
0.009 |
| NIHSS at discharge |
295 |
6.8 ± 6.5 |
5.3 ± 5.8 |
0.013 |
| NIHSS difference |
295 |
4.2 ± 4.9 |
3.7 ± 5.0 |
0.132 |
| Thrombolysis |
326 |
54 |
4 |
< 0.001 |
| Thrombectomy |
326 |
25 |
29 |
0.637 |
| LAVI (ml/m2) |
145 |
48.0 ± 15.6 |
56.4 ± 20.7 |
0.004 |
| LVEF (%) |
145 |
49.4 ± 11.8 |
52.5 ± 10.9 |
0.064 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).