1. Introduction
Kawasaki disease (KD), first described by Dr. Tomisaku Kawasaki in 1967, is a systemic medium-vessel vasculitis of unclear etiology that predominantly affects children under five years of age [
1]. It remains the leading cause of acquired heart disease in children in developed countries, owing to its potential to cause coronary artery aneurysms if untreated. While the typical age of onset falls between 6 months and 5 years, KD in adolescents is increasingly being recognized as a small yet significant subgroup [
2,
3]. These cases, comprising approximately 5-10% of all KD diagnoses, often diverge from classical presentations and carry a greater risk of cardiac involvement and treatment resistance [
4].
The adolescent period, defined by the World Health Organization as the ages between 10 and 19 years, is marked by profound physical, cognitive, and psychosocial growth. During this developmental stage, health conditions that disrupt school attendance, limit physical activity, or delay social milestones can have long-lasting effects not just on physical growth but also on identity formation, emotional health, and autonomy. Adolescent KD is uniquely positioned at the intersection of a potentially life-threatening medical condition and a sensitive stage of personal development.
The current diagnostic and treatment guidelines provided by the American Heart Association (AHA) in 2024 are primarily designed with a focus on young children [
5]. Adolescents with KD often lack one or more of the traditional diagnostic criteria, such as rash, conjunctival injection, or mucocutaneous changes [
2,
6]. Instead, they may exhibit atypical symptoms like abdominal pain, arthralgia, or headache, which can mislead clinicians and delay appropriate treatment. This diagnostic delay is of particular concern because early administration of IVIG (within the first 10 days of illness) is critical in reducing the risk of coronary artery aneurysm formation [
1].
Kawasaki disease in adolescents poses multipronged challenges, as it remains under-recognized and under-studied, with scarce epidemiological data on prevalence and outcomes in this age group. Compared to younger children, limited studies have investigated the response of adolescents with KD to standard treatment regimens. Long-term follow-up studies are lacking. Post COVID-19 pandemic, Multisystem Inflammatory Syndrome in Children (MIS-C) has emerged as a new disease entity associated with COVID infection, mainly affecting older children and adolescents. It finds a significant overlap with KD in adolescents, adding to even more diagnostic uncertainty and potential for misdiagnosis [
7].
Given these challenges, this scoping review aims to identify current gaps and synthesize available evidence to aid clinicians in the timely diagnosis and effective management of Kawasaki disease in adolescents. Specifically, the review aims to comprehensively evaluate the literature, clarify the clinical, cardiologic, developmental, and psychological aspects of the disease, and highlight opportunities for improving long-term care.
1.1. Pathophysiology Overview
The precise etiology of Kawasaki disease (KD) remains unknown, but current evidence supports a multifactorial process involving infectious triggers, immune dysregulation, and genetic susceptibility. Aberrant activation of the innate immune system initiates neutrophil recruitment, cytokine release, and endothelial injury, leading to medium-vessel vasculitis and progressive vascular wall damage that predisposes to coronary artery aneurysm formation. Proposed mechanisms include activation of the interleukin-1 pathway, inflammasome signaling, pyroptosis, neutrophil extracellular trap formation (NETosis), and oxidative stress, which collectively amplify vascular inflammation [
8,
9]. Genetic studies have identified polymorphisms in immune-regulating genes such as
ITPKC and
CASP3, which may partly explain geographic and ethnic variations in disease incidence [
1]. While most insights are derived from younger children, it remains unclear whether adolescents with KD exhibit distinct immunologic or vascular responses that contribute to their higher rates of treatment resistance and coronary complications.
2. Methods
A targeted literature search was conducted using the PubMed database to identify studies examining KD in adolescents aged 10 to 19 years. This scoping review was conducted in accordance with the PRISMA–ScR guidelines. The search strategy included combinations of the terms “Kawasaki disease” OR “Kawasaki syndrome” AND “adolescent,” “teenager,” or “older children.” Additional keywords included “clinical characteristics,” “presentation,” “complications,” “psychosocial impact,” and “quality of life.” The exact PubMed search string was: (“Kawasaki disease”[Title/Abstract] OR “Kawasaki syndrome”[Title/Abstract]) AND (“10 years”[All Fields] OR “11 years”[All Fields] OR “12 years”[All Fields] OR “13 years”[All Fields] OR “14 years”[All Fields] OR “15 years”[All Fields] OR “16 years”[All Fields] OR “17 years”[All Fields] OR “18 years”[All Fields] OR “19 years”[All Fields]) AND (“clinical characteristics” OR “presentation” OR “complications” OR “coronary” OR “psychosocial impact” OR “quality of life”) AND (“2000/01/01”[Date - Publication] : “2024/12/31”[Date - Publication]); Filters: Clinical Study, Comparative Study, Observational Study, Review.
Search filters were applied to include clinical studies, cohort studies, comparative studies, and reviews published in English. Studies were selected based on their focus on clinical presentation, diagnostic patterns, coronary artery involvement, treatment response, and psychosocial outcomes in the adolescent age group. Articles were excluded if they focused solely on children under 10 years, lacked age-specific data, or presented duplicate findings. Two reviewers independently screened all titles and abstracts, followed by full-text review. Discrepancies were resolved by discussion until consensus was reached.
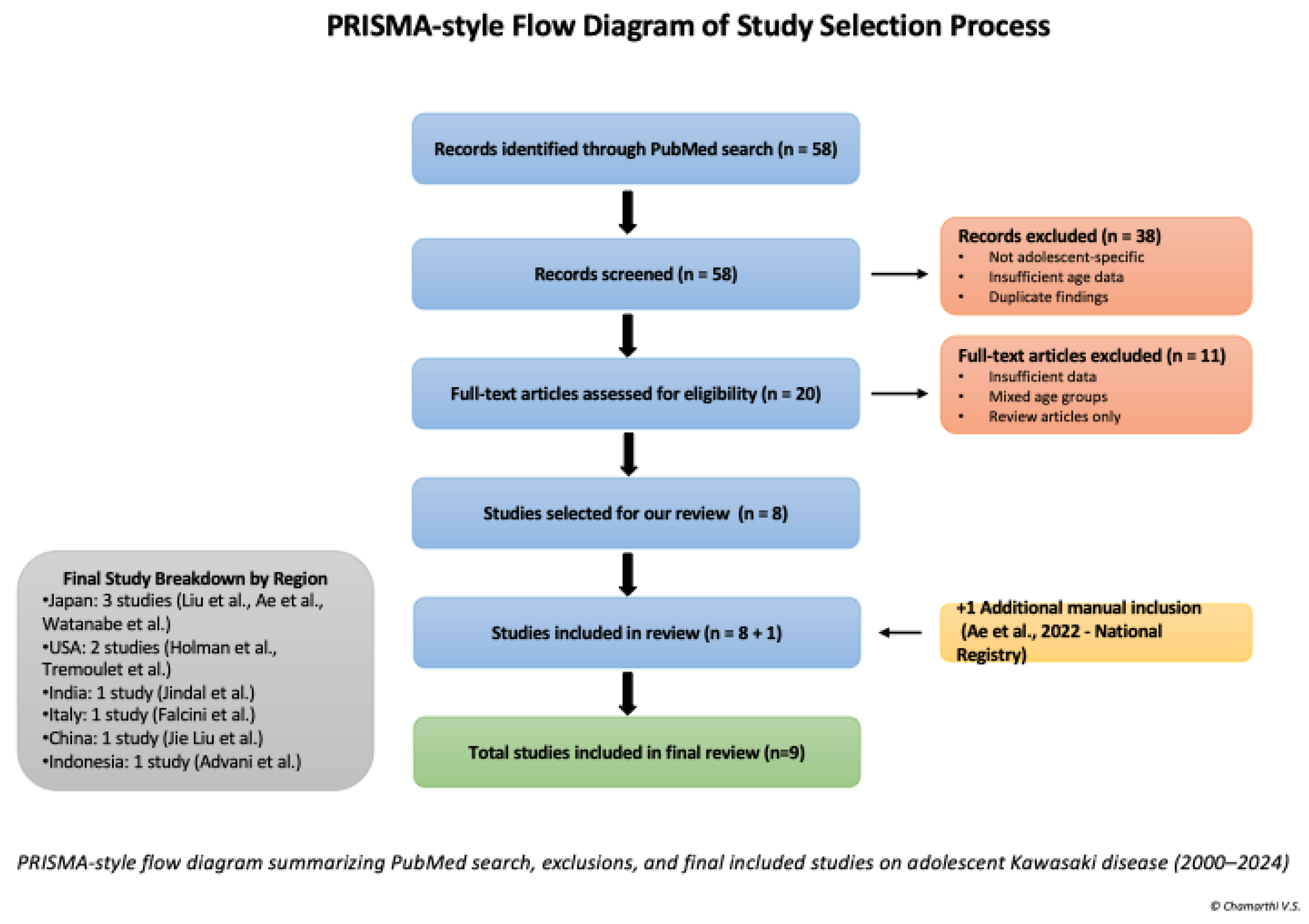
The search yielded 58 articles. Titles and abstracts were screened manually to identify studies reporting adolescent-specific data. After title and abstract screening, 20 full-text articles were reviewed, and nine studies were included in the final synthesis. An additional national surveillance study from Japan by Ae et al. was manually included due to its large cohort of adolescent KD cases and national significance [
6]. The study selection process is detailed in PRISMA Diagram of Study Selection Process [
Figure 1]. The characteristics and key findings of the included studies are summarized in
Table 1, which provides a thematic overview of study designs, sample sizes, demographics, and primary outcomes across the adolescent KD literature [
Table 1]. Data extraction was performed independently by two reviewers using a standardized charting form. Data items extracted included study design, year, country, sample size, demographics, clinical presentation, treatment response, and outcomes. No formal risk of bias or certainty assessment was performed, as is consistent with the scoping review methodology. To contextualize the unique features of adolescent presentations,
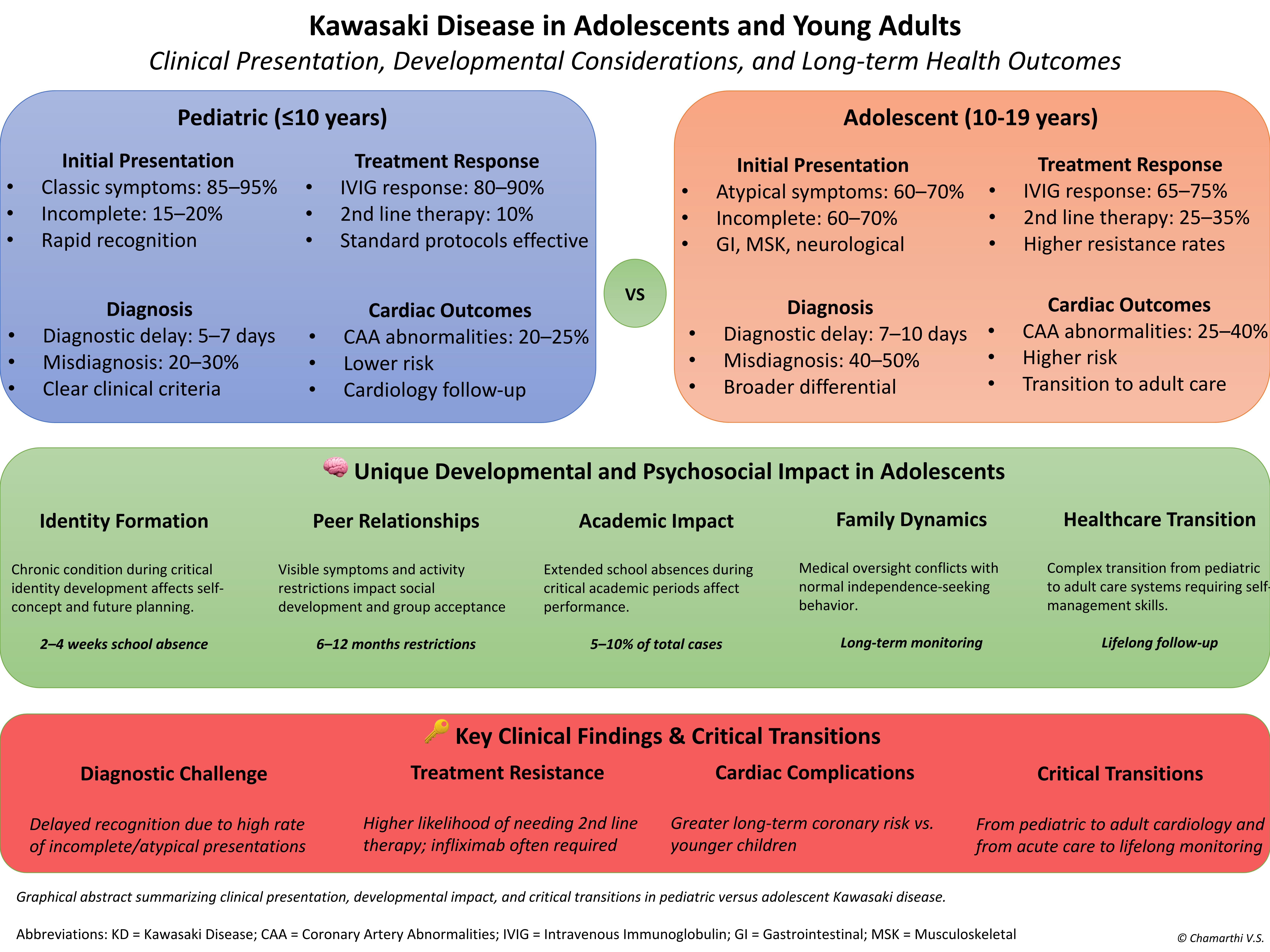
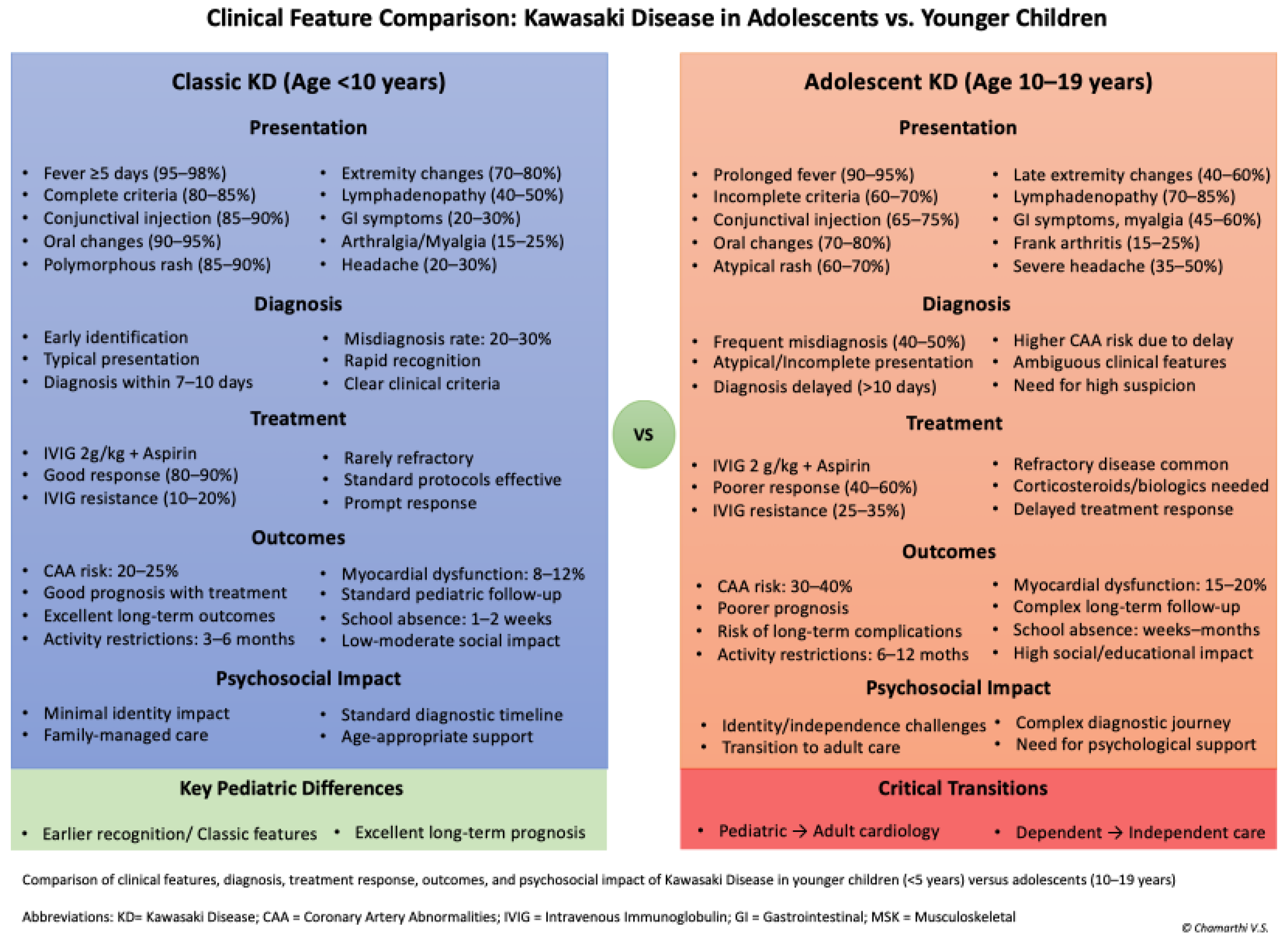
Figure 2 presents a comparative analysis of clinical features, treatment responses, and outcomes between adolescents (10-19 years) and younger children (<5 years) with Kawasaki disease [
Figure 2].
3. Epidemiology and Geographic Patterns
3.1. Global Incidence and Regional Variations
The global incidence of KD varies dramatically by geographic region and ethnicity, with adolescents representing a consistent minority across all populations studied. The highest rates are reported in Northeast Asian countries, with Japan reporting 371 cases per 100,000 children under 5 years in 2019 [
6]. Ae et al.'s national registry study of 1,234 adolescent cases demonstrated that patients aged 10-19 years constitute approximately 6.2% of all KD cases in Japan. South Korea demonstrated similarly elevated rates at 196.9 per 100,000 in 2020, while Taiwan reported 60.1 per 100,000 in 2011 [
13,
14].
In contrast, Western countries report substantially lower rates. England documented 8.9 per 100,000 in 2021, and North American rates typically 21.4 per 100,000 children under 5 years [
15,
16]. Holman et al.'s population-based study of 2,162 adolescent cases in the United States found that adolescents comprised 8% of all KD hospitalizations, with higher complication rates than younger children [
3].
3.2. Demographic Characteristics and Ethnic Disparities
The male-to-female ratio in adolescent KD mirrors that seen in younger children, with a predominance of approximately 1.2-1.5:1 across all studies reviewed [
2,
4,
6]. Ethnic disparities in KD incidence are particularly pronounced in multi-ethnic populations. In Hawaii, ethnically Japanese children demonstrate rates of 210.5 per 100,000, while Caucasian children show rates of only 13.7 per 100,000, representing a 20-fold difference [
17].
Seasonal patterns in adolescent KD generally follow those observed in younger children, with peaks typically occurring in winter and spring months in temperate climates. However, some studies suggest that seasonal variation may be less pronounced in adolescent cases compared to younger children, possibly reflecting different environmental exposures or immune responses in this age group [
18].
4. Clinical Presentation and Diagnostic Challenges
4.1. Incomplete Presentations and Atypical Features
Adolescents with KD frequently deviate from the classical pediatric presentation profile, posing significant diagnostic challenges. Studies consistently demonstrate that 60-73% of adolescent patients present with incomplete disease, compared to only 15-20% of children under 5 years [
2,
4,
6,
8].
Liu et al.'s cross-sectional study of 89 adolescent patients found that over two-thirds had incomplete disease, resulting in an average diagnostic delay of 2-4 days compared to younger children [
2]. This finding was corroborated by Jindal et al., who reported incomplete presentations in 73% of their 45 Indian adolescent patients, with delayed diagnosis being prevalent [
4]. Similarly, Falcini et al. found incomplete criteria in 63% of their 38 Italian adolescents, with 2-3-day diagnostic delays [
8]. Watanabe et al. documented incomplete presentations in 62% of their 34 Japanese adolescents, while Liu et al. reported 68% incomplete disease in their 52 Chinese patients [
10,
11]. Even Advani et al.'s smaller Indonesian cohort of 36 patients showed 60% incomplete presentations [
12].
Fever remains the most consistent presenting feature in adolescent KD, occurring in 90-95% of cases. However, classical mucocutaneous features show notable age-related differences. Conjunctival injection is present in only 65-75% of adolescent cases compared to 85-90% in younger children. Similarly, the characteristic polymorphous rash occurs in 60-70% of adolescent cases versus 85-90% in typical pediatric presentations [
2,
4].
The incomplete presentation algorithm developed by the American Heart Association has proven particularly valuable in adolescent populations. This algorithm includes elevated C-reactive protein (≥3.0 mg/dL) or erythrocyte sedimentation rate, plus three or more supplemental laboratory criteria, including anemia, hypoalbuminemia, elevated alanine aminotransferase, thrombocytosis after day 7, sterile pyuria, or echocardiographic evidence of coronary artery abnormalities [
1]. Studies examining the application of these algorithms in adolescent cohorts demonstrate improved diagnostic sensitivity while maintaining specificity for KD diagnosis.
4.2. Gastrointestinal Manifestations
Adolescents with KD demonstrate a higher prevalence of certain atypical features that can lead to diagnostic confusion. Gastrointestinal symptoms, including nausea, vomiting, and abdominal pain, occur in 45-60% of adolescent cases compared to 20-30% in younger children [
2,
6]. Jie Liu et al.'s retrospective cohort of 52 Chinese adolescents specifically highlighted the prevalence of gastrointestinal and neurological symptoms, which were often mistaken for appendicitis, gastroenteritis, or other acute abdominal conditions [
11]. Watanabe et al.'s single-center study of 34 Japanese adolescents also noted that abdominal and musculoskeletal symptoms were widespread and often led to initial misdiagnosis [
10].
The gastrointestinal manifestations in adolescent KD can be particularly misleading. Abdominal pain may be severe and localized, mimicking surgical conditions. This may have led to unnecessary appendectomies in some cases before the correct diagnosis was established [
19]. The pain may be associated with hepatomegaly and elevated liver enzymes, further complicating the diagnostic picture.
4.3. Musculoskeletal Manifestations
Musculoskeletal complaints represent another distinctive feature of adolescent KD. Arthralgia and myalgia occur in 45-60% of cases, significantly higher than the 15-25% reported in younger children [
2,
4]. Frank arthritis may develop in 15-25% of adolescent patients, typically affecting large joints including knees, ankles, and wrists. These symptoms can interfere with sports participation and physical education, creating additional psychosocial challenges during an age when peer activities are significant.
The arthritis in adolescent KD tends to be oligoarticular, affecting fewer than five joints, and is typically non-erosive. The temporal relationship between joint symptoms and other manifestations of KD can vary, with arthritis sometimes preceding or following the acute febrile phase. Joint effusions may be present, and in some cases, the arthritis may be the predominant presenting feature. [
1].
4.4. Neurological and Other Manifestations
Neurological manifestations appear more frequently in adolescent KD than in younger children. Headaches are reported in 35-50% of cases, often described as severe and refractory to standard analgesics [
6]. Headaches may be accompanied by photophobia and neck stiffness, raising concern for bacterial meningitis. Jie Liu et al. specifically noted neurological symptoms as a prominent feature in their Chinese adolescent cohort, often contributing to diagnostic confusion [
13].
In adolescents, mood changes and irritability may present more subtly and are often assumed to reflect the stress of illness, which can obscure their recognition as direct disease manifestations. Some adolescents may experience significant behavioral changes, including anxiety, depression, or cognitive difficulties, which can persist beyond the acute illness phase. Meningismus occurs in 15-25% of adolescent cases and may prompt evaluation for bacterial meningitis, particularly when fever and headache are prominent [
2].
Cervical lymphadenopathy shows an interesting pattern in adolescent KD, being present in 70-85% of cases compared to 40-50% in younger children [
2]. This higher prevalence may contribute to misdiagnosis as infectious mononucleosis or bacterial lymphadenitis, particularly when other classical features are absent or subtle. The lymphadenopathy in adolescent KD is typically unilateral, non-fluctuant, and measures at least 1.5 cm in diameter.
Other manifestations that may be more prominent in adolescents include respiratory symptoms such as cough and chest pain in 25-35% of cases. While these are generally mild, they can add to diagnostic confusion, particularly during respiratory illness seasons when viral infections are common. Some adolescents may develop pulmonary nodules or infiltrates visible on chest imaging, which can further complicate the diagnostic process [
2].
4.5. Laboratory and Imaging Findings
Laboratory findings in adolescent KD generally align with those seen in younger children, including elevated erythrocyte sedimentation rate and C-reactive protein, with thrombocytosis typically developing in the subacute phase. However, some studies suggest that the magnitude of inflammatory marker elevation may be less pronounced in adolescents, potentially contributing to diagnostic uncertainty [
6]. Anemia is common, occurring in approximately 60% of cases, while leukocytosis is present in about 60% of adolescent patients.
Hypoalbuminemia is frequently observed and may be more pronounced in adolescents with gastrointestinal symptoms. Elevated liver enzymes occur in 40-60% of cases and may be particularly prominent in adolescents presenting with abdominal pain. Sterile pyuria is found in approximately 30-40% of adolescent patients and can be a useful supportive finding when other criteria are incomplete. Echocardiographic findings at presentation may show early coronary artery changes in some adolescent patients. While frank aneurysms are typically not present at diagnosis, increased coronary artery brightness or slight dilation may be observed. The higher baseline coronary artery dimensions in adolescents compared to younger children require age-specific normative data for accurate interpretation of z-scores [
2].
The broader differential diagnosis in adolescent KD includes infectious mononucleosis, streptococcal infections, viral syndromes, systemic lupus erythematosus, juvenile idiopathic arthritis, inflammatory bowel disease, drug reactions, and other vasculitides. This expanded differential, combined with the lower clinical suspicion for KD in this age group, contributes to the consistently reported diagnostic delays averaging 2-4 days longer than in younger children [
2,
6].
5. Cardiovascular Complications and Treatment Outcomes
5.1. Coronary Artery Involvement
Adolescents with KD face increased risks for cardiovascular complications, particularly coronary artery abnormalities (CAA). Studies consistently report higher rates of coronary involvement in adolescents compared to younger children. Jindal et al. documented coronary abnormalities in 31% of their adolescent KD patients, while Liu et al. reported 28%, and Falcini et al. found rates as high as 42% in Italian adolescents [
2,
4,
10]. Ae et al.'s extensive national registry documented a 32% rate of coronary involvement among Japanese adolescents [
6]. Even in regions with historically lower rates, such as Indonesia, Advani et al. found coronary complications in 25% of their 36 adolescent patients [
14].
The pathophysiology of coronary complications in KD involves inflammatory damage to the arterial wall, leading to weakening and potential aneurysm formation. The risk is highest during the acute phase, particularly in the first 2-3 weeks of illness. Giant aneurysms (≥8mm diameter) represent the most severe complication and carry significant long-term morbidity and mortality risks. Age greater than six years is an independent risk factor for cardiovascular sequelae [
7]. In addition, adolescents are more likely to receive IVIG treatment after 10 days of illness, which independently increases the risk of coronary involvement.
5.2. Treatment Resistance and Advanced Therapies
Treatment protocols for adolescent KD follow established pediatric guidelines, with high-dose IVIG (2 g/kg) as the cornerstone of acute therapy, administered alongside aspirin [
1]. However, adolescents demonstrate higher rates of IVIG resistance, defined as persistent or recurrent fever 36 hours after initial IVIG administration. Tremoulet et al.’s clinical trial sub-analysis of 67 adolescents reported that 35% experienced IVIG resistance, requiring additional therapy beyond initial IVIG [
11].
Recent evidence from the KIDCARE trial has provided important insights into the management of IVIG-resistant KD. This randomized controlled trial demonstrated that infliximab (a TNF-α inhibitor) was superior to a second IVIG dose for treatment-resistant cases, resulting in faster fever resolution, shorter hospitalization, and fewer adverse effects, including hemolytic anemia [
22]. For adolescents with IVIG resistance, treatment options include a second IVIG dose, corticosteroids (methylprednisolone or prednisolone), or infliximab. The choice should consider patient factors, institutional experience, and recent evidence favoring infliximab in appropriately selected cases. Due to diagnostic ambiguity, adolescents often present later in the disease course, with prolonged inflammation—potentially leading to elevated inflammatory markers like ESR, CRP, pro-BNP, neutrophilia, hyponatremia, anemia, hypoalbuminemia, etc., all of which are risk factors for IVIG failure [
23].
6. Psychosocial and Developmental Impact
6.1. Academic and Social Disruptions
The diagnosis of KD during adolescence can have profound effects on psychosocial development, occurring during a critical period of identity formation and peer relationship development. Studies examining psychosocial outcomes report substantial disruptions in school attendance, with most patients requiring 2-4 weeks of absence during the acute phase [
6,
14]. Advani et al. specifically noted academic disruptions and peer withdrawal as significant issues in their Indonesian cohort of 36 adolescents [
14]. Academic performance may be affected not only by missed school days but also by fatigue, mood changes, and ongoing medical appointments that continue well beyond the acute illness. The cognitive effects of systemic inflammation during KD may persist for weeks to months after the acute phase, affecting concentration, memory, and learning capacity [
14].
As discussed in
Section 4.3, musculoskeletal manifestations such as persistent arthralgia or arthritis can further interfere with daily functioning, contributing to limitations in sports participation, difficulties with school activities, and chronic pain that exacerbates psychosocial stress.
Physical activity restrictions pose particular challenges for adolescents, especially those with coronary complications. These limitations can lead to social isolation from sports-based peer groups and may require significant modifications to physical education curricula. For adolescents whose identities are closely tied to athletic participation, such restrictions can have profound psychological impacts. The duration of activity restrictions varies depending on coronary status, ranging from no restrictions for those with normal coronary arteries to indefinite restrictions for those with giant aneurysms. Teachers and school counselors often require education about the condition to understand the student's needs and provide appropriate accommodations [
14].
The visible manifestations of KD can also affect adolescents' willingness to participate in social activities. During the acute phase, the rash, swollen hands and feet, and peeling skin can cause significant embarrassment. Even after resolution, some adolescents may develop anxiety about their appearance or fear of symptom recurrence that affects their social engagement [
1].
6.2. Personal Identity and Autonomy Challenges
Body image concerns may be reported in adolescents with KD due to visible manifestations such as rash, extremity changes, or desquamation. During an adolescent developmental stage when appearance and peer acceptance are particularly important, these changes could contribute to social withdrawal or reduced self-esteem [
24]. The characteristic "strawberry tongue" and cracked lips can be particularly distressing for adolescents concerned about their appearance [
1]. The need for ongoing medical surveillance and potential activity restrictions has the potential to conflict with the adolescent’s developmental drive toward independence and autonomy. Parental anxiety following diagnosis may contribute to overprotectiveness, occasionally creating tension within family dynamics as adolescents seek greater independence [
24].
Medication adherence may also be a challenge for adolescents with KD who require long-term antiplatelet or anticoagulant therapy. The transition from parent-managed to self-managed healthcare requires careful support, including attention to education, monitoring, and fostering self-advocacy skills. Some adolescents may resist taking daily medications, particularly when asymptomatic and the potential complications feel abstract or distant. Accepting the label of having a “heart condition” can be difficult for some, and this identity challenge may contribute to denial, non-adherence, or risk-taking behaviors in efforts to feel “normal” among peers [
24].
6.3. Family Dynamics and Support Systems
The diagnosis of KD during adolescence may influence family dynamics, including shifts in parental roles and sibling relationships. Parents may become highly attentive to the adolescent’s health, which can sometimes be perceived as limiting independence. Siblings may also feel overshadowed by the medical needs of the affected child. Financial stress related to medical care, time off work, and long-term surveillance may further contribute to strain within families. In a Canadian study, the total healthcare expenditures for KD patients were estimated at CAD 13.9 million within one year and CAD 54.8 million over the follow-up period [
25].
Psychosocial outcomes also warrant attention. A population-based study from Taiwan found that children with KD had a higher risk of developing psychiatric disorders, including anxiety and depression, later in life [
26]. These findings highlight the need to consider mental health and family support as part of long-term care. The American Heart Association (AHA) scientific statement similarly emphasizes that psychosocial support and transition planning are essential aspects of comprehensive KD management [
1]. Support beyond the family can assist adolescents in adapting to school and social life, with school-based resources such as nurses, counselors, and teachers playing an important role in facilitating return to academics and providing accommodations when needed.
7. Long-Term Outcomes and Prognosis
7.1. Cardiovascular Sequelae
The long-term prognosis for adolescents with KD depends largely on the presence and severity of coronary artery involvement. While many recover without sequelae, those with coronary abnormalities remain at increased cardiovascular risk that extends into adulthood [
2,
4,
6,
10]. Coronary artery aneurysms demonstrate different patterns of evolution: small and medium-sized aneurysms may regress to near-normal dimensions within 1–2 years, whereas giant aneurysms (≥8 mm) rarely normalize and carry significant risks for thrombosis, stenosis, and myocardial infarction [
27,
28]. Studies consistently show that adolescents have higher rates of coronary abnormalities compared with younger children, reflecting diagnostic delays and treatment resistance in this age group [
2,
4,
10].
7.2. Surveillance and Risk Factor Management
Ongoing surveillance strategies are guided by coronary artery status. Patients without coronary involvement or with only transient dilation typically require no long-term restrictions or therapy beyond the acute phase. Those with small persistent aneurysms may need low-dose aspirin and periodic cardiology follow-up, while patients with medium or giant aneurysms often require dual antiplatelet or anticoagulation therapy, activity modifications, and regular imaging or stress testing [
1,
22]. In addition, adolescents with a history of KD may be predisposed to traditional cardiovascular risk factors such as hypertension, dyslipidemia, and diabetes mellitus, further underscoring the importance of preventive care and structured follow-up [
1].
8. Transition to Adult Care
8.1. Challenges in Healthcare Transition
The transition from pediatric to adult healthcare represents a critical challenge for adolescents with KD, particularly those with ongoing coronary complications. Many adult cardiologists lack experience managing the sequelae of childhood-onset KD, creating potential gaps in care and surveillance [
29]. Successful transition requires careful planning beginning in mid-adolescence. Key components include patient education about their diagnosis and prognosis, development of self-advocacy skills, understanding of medication regimens and potential side effects, recognition of cardiac symptoms requiring urgent evaluation, and identification of appropriate adult cardiovascular specialists.
8.2. Structured Transition Programs
Transition readiness assessment should be ongoing throughout adolescence, with gradual transfer of responsibility from parents to patients. Structured transition programs have shown promise in improving patient knowledge and reducing gaps in care [
1,
29]. These programs typically involve joint visits with pediatric and adult providers, comprehensive medical summaries, and ongoing support during the transition period. The process should be individualized based on the patient’s coronary status, cognitive and emotional maturity, family dynamics, and available adult care resources. Patients with complex coronary abnormalities may require transition to specialized adult cardiology services rather than general practice settings [
1,
29].
9. Limitations
This review has several important limitations. This scoping review was not prospectively registered in PROSPERO or any other registry, which may limit reproducibility. The search strategy was limited to a single database (PubMed), due to restricted access to other databases, which may have resulted in the exclusion of relevant studies published in non-indexed or regional journals. This is particularly relevant given the geographic variation in KD incidence and the likelihood that important regional studies may not be captured. Restriction to English-language publications may also have excluded significant findings from non-English-speaking countries. Another limitation is that critical appraisal of individual sources was not performed, which is consistent with scoping review methodology but limits assessment of study quality.
The heterogeneity in study designs, sample sizes (ranging from 17 to 89 patients), and outcome measures limited opportunities for meta-analysis and precise pooled estimates. Most studies were retrospective, introducing potential selection bias and limiting data quality for psychosocial outcomes and long-term follow-up. In addition, the inclusion of studies across a broad time period (2000–2024) may have introduced temporal bias as diagnostic criteria and treatment protocols evolved. The lack of standardized psychosocial assessment tools further limited the ability to quantify the impact of adolescent KD on quality of life and development, highlighting the need for prospective, multicenter studies with harmonized methodologies.
10. Future Directions and Research Gaps
Despite growing recognition of KD in adolescents, significant research gaps remain. Extensive, prospective multicenter studies focusing specifically on this age group are needed to refine diagnostic criteria and management algorithms. Age-specific biomarkers and imaging strategies could help distinguish KD from mimics such as appendicitis or other acute abdominal conditions, which are particularly relevant in adolescents. More research is needed to address these diagnostic challenges and improve timely recognition and treatment.
The psychosocial dimensions of adolescent KD also remain poorly understood. Longitudinal studies examining quality of life, mental health, adherence, and social development are essential. Future priorities include the development of standardized care pathways for transition to adult care, validation of age-specific biomarkers, standardized psychosocial assessment tools, and long-term outcome studies extending into adulthood. Establishing international registries for adolescent KD would also facilitate large-scale data collection, support collaboration, and guide consensus-based care strategies.
11. Conclusions
Adolescent Kawasaki disease is a distinct entity requiring specialized recognition, management, and long-term care. Compared with younger children, adolescents more often present with incomplete or atypical features, face delays in diagnosis, demonstrate higher rates of treatment resistance, and carry an elevated risk of coronary complications. These factors highlight the importance of heightened clinical awareness and careful consideration of developmental context when evaluating older patients. Beyond the acute phase, the illness affects not only physical health but also school performance, peer relationships, family dynamics, and identity development, making a comprehensive, developmentally informed approach essential.
The challenges of adolescent KD call for coordinated, multidisciplinary care that integrates pediatric and adult cardiology, mental health, and primary care perspectives. Strengthening healthcare provider education, refining transition protocols, and addressing psychosocial needs are key to improving long-term outcomes. As understanding of this condition advances, prioritizing research that explores both the medical and developmental dimensions of adolescent KD will be critical to guiding future care and ensuring better health trajectories for this vulnerable population.
Author Contributions
Conceptualization, V.S.C.; Methodology, V.S.C., P.S., and K.S.; Formal Analysis, V.S.C., P.S., and K.S.; Investigation, V.S.C.; Data Curation, V.S.C., P.S., and K.S.; Writing – Original Draft Preparation, V.S.C., P.S., and K.S.; Writing – Review & Editing, V.S.C., P.S., and K.S.; Visualization, V.S.C.; Supervision, V.S.C. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CAA = Coronary artery abnormalities |
| GI = Gastrointestinal |
| IVIG = Intravenous immunoglobulin |
| KD = Kawasaki disease |
| M:F = Male-to-female ratio |
| MIS-C = Multisystem Inflammatory Syndrome in Children |
| MSK = Musculoskeletal |
| NR = Not reported |
References
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [CrossRef]
- Xiaoliang Liu; Liu, X.; Fang Wang; Wang, F.; Kaiyu Zhou; Zhou, K.; Hua, Y.; Yimin Hua; Hua, Y.; Mei Wu; et al. Clinical Characteristics of Kawasaki Disease in Adolescents. Journal of International Medical Research 2021, 49, 3000605211056839. [CrossRef]
- Holman, R.C.; Belay, E.D.; Christensen, K.Y.; Folkema, A.M.; Steiner, C.A.; Schonberger, L.B. Hospitalizations for Kawasaki Syndrome among Children in the United States, 1997-2007. Pediatr Infect Dis J 2010, 29, 483–488. [CrossRef]
- Jindal, A.K.; Pilania, R.K.; Guleria, S.; Vignesh, P.; Suri, D.; Gupta, A.; Singhal, M.; Rawat, A.; Singh, S. Kawasaki Disease in Children Older Than 10 Years: A Clinical Experience From Northwest India. Front Pediatr 2020, 8, 24. [CrossRef]
- Jone, P.-N.; Tremoulet, A.; Choueiter, N.; Dominguez, S.R.; Harahsheh, A.S.; Mitani, Y.; Zimmerman, M.; Lin, M.-T.; Friedman, K.G.; on behalf of the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Radiology and Intervention; and Council on Clinical Cardiology Update on Diagnosis and Management of Kawasaki Disease: A Scientific Statement From the American Heart Association. Circulation 2024, 150, e481–e500. [CrossRef]
- Ae, R.; Makino, N.; Kosami, K.; Kuwabara, M.; Matsubara, Y.; Nakamura, Y. Epidemiology, Treatments, and Cardiac Complications in Patients with Kawasaki Disease: The Nationwide Survey in Japan, 2017-2018. J Pediatr 2020, 225, 23-29.e2. [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med 2020, 383, 334–346. [CrossRef]
- Hara, T.; Yamamura, K.; Sakai, Y. The Up-to-Date Pathophysiology of Kawasaki Disease. Clinical & Translational Immunology 2021, 10, e1284. [CrossRef]
- Vaňková, L.; Bufka, J.; Křížková, V. Pathophysiological and Clinical Point of View on Kawasaki Disease and MIS-C. Pediatrics & Neonatology 2023, 64, 495–504. [CrossRef]
- Falcini, F.; Cimaz, R.; Calabri, G.B.; Picco, P.; Martini, G.; Marazzi, M.G.; Simonini, G.; Zulian, F. Kawasaki’s Disease in Northern Italy: A Multicenter Retrospective Study of 250 Patients. Clin Exp Rheumatol 2002, 20, 421–426.
- Tremoulet, A.H.; Best, B.M.; Song, S.; Wang, S.; Corinaldesi, E.; Eichenfield, J.R.; Martin, D.D.; Newburger, J.W.; Burns, J.C. Resistance to Intravenous Immunoglobulin in Children with Kawasaki Disease. The Journal of Pediatrics 2008, 153, 117-121.e3. [CrossRef]
- Watanabe, Y.; Ikeda, H.; Watanabe, T. Differences in the Clinical Characteristics of Kawasaki Disease Between Older and Younger Children (2015-2019): A Single-Center, Retrospective Study. The Journal of Pediatrics 2023, 253, 266–269. [CrossRef]
- Liu, J.; Huang, Y.; Chen, C.; Su, D.; Qin, S.; Pang, Y. Risk Factors for Resistance to Intravenous Immunoglobulin Treatment and Coronary Artery Abnormalities in a Chinese Pediatric Population With Kawasaki Disease: A Retrospective Cohort Study. Front Pediatr 2022, 10, 812644. [CrossRef]
- Najib Advani; Advani, N.; Lucyana Alim Santoso; Santoso, L.A.; Sudigdo Sastroasmoro; Sastroasmoro, S. Profile of Kawasaki Disease in Adolescents: Is It Different? Acta medica Indonesiana 2019, 51, 42–46.
- Huang, Y.-H.; Lin, K.-M.; Ho, S.-C.; Yan, J.-H.; Lo, M.-H.; Kuo, H.-C. Increased Incidence of Kawasaki Disease in Taiwan in Recent Years: A 15 Years Nationwide Population-Based Cohort Study. Front Pediatr 2019, 7, 121. [CrossRef]
- Kim, G.B.; Eun, L.Y.; Han, J.W.; Kim, S.H.; Yoon, K.L.; Han, M.Y.; Yu, J.J.; Choi, J.-W.; Rhim, J.W. Epidemiology of Kawasaki Disease in South Korea: A Nationwide Survey 2015-2017. Pediatr Infect Dis J 2020, 39, 1012–1016. [CrossRef]
- Odingo, M.; Rutter, M.; Bowley, J.; Peach, E.J.; Lanyon, P.C.; Grainge, M.J.; Stillwell, P.; McPhail, S.; Bythell, M.; Aston, J.; et al. The Incidence of Kawasaki Disease Using Hospital Admissions Data for England 2006–2021. Rheumatology (Oxford) 2023, 62, 3117–3125. [CrossRef]
- Taslakian, E.N.; Wi, C.-I.; Seol, H.Y.; Boyce, T.G.; Johnson, J.N.; Ryu, E.; King, K.S.; Juhn, Y.J.; Choi, B.S. Long-Term Incidence of Kawasaki Disease in a North American Community: A Population-Based Study. Pediatr Cardiol 2021, 42, 1033–1040. [CrossRef]
- Holman, R.C.; Christensen, K.Y.; Belay, E.D.; Steiner, C.A.; Effler, P.V.; Miyamura, J.; Forbes, S.; Schonberger, L.B.; Melish, M. Racial/Ethnic Differences in the Incidence of Kawasaki Syndrome among Children in Hawaii. Hawaii Med J 2010, 69, 194–197.
- MacGillivray, D.M.; Kollmann, T.R. The Role of Environmental Factors in Modulating Immune Responses in Early Life. Front Immunol 2014, 5, 434. [CrossRef]
- Sato, M.; Fujita, Y.; Naganuma, J.; Sekine, K.; Watanabe, S.; Ogino, K.; Suzuki, K.; Yoshihara, S. Kawasaki Disease Preceded by Acute Appendicitis Treated with Intravenous Immunoglobulin without Surgery. Rheumatol Advanc Pract 2025, 9, rkaf003. [CrossRef]
- Burns, J.C.; Roberts, S.C.; Tremoulet, A.H.; He, F.; Printz, B.F.; Ashouri, N.; Jain, S.S.; Michalik, D.E.; Sharma, K.; Truong, D.T.; et al. Infliximab versus Second Intravenous Immunoglobulin for Treatment of Resistant Kawasaki Disease in the USA (KIDCARE): A Randomised, Multicentre Comparative Effectiveness Trial. Lancet Child Adolesc Health 2021, 5, 852–861. [CrossRef]
- Kim, B.Y.; Kim, D.; Kim, Y.H.; Ryoo, E.; Sun, Y.H.; Jeon, I.-S.; Jung, M.-J.; Cho, H.K.; Tchah, H.; Choi, D.Y.; et al. Non-Responders to Intravenous Immunoglobulin and Coronary Artery Dilatation in Kawasaki Disease: Predictive Parameters in Korean Children. Korean Circ J 2016, 46, 542–549. [CrossRef]
- Chen, D.T.-L.; Chang, J.P.-C.; Cheng, S.-W.; Chang, H.-C.; Hsu, J.-H.; Chang, H.-H.; Chiu, W.-C.; Su, K.-P. Kawasaki Disease in Childhood and Psychiatric Disorders: A Population-Based Case-Control Prospective Study in Taiwan. Brain, Behavior, and Immunity 2022, 100, 105–111. [CrossRef]
- Robinson, C.; Chanchlani, R.; Gayowsky, A.; Darling, E.; Seow, H.; Batthish, M. Health Care Utilization and Costs Following Kawasaki Disease. Paediatr Child Health 2022, 27, 160–168. [CrossRef]
- Chen, D.T.-L.; Chang, J.P.-C.; Cheng, S.-W.; Chang, H.-C.; Hsu, J.-H.; Chang, H.-H.; Chiu, W.-C.; Su, K.-P. Kawasaki Disease in Childhood and Psychiatric Disorders: A Population-Based Case-Control Prospective Study in Taiwan. Brain, Behavior, and Immunity 2022, 100, 105–111. [CrossRef]
- Manlhiot, C.; Millar, K.; Golding, F.; McCrindle, B.W. Improved Classification of Coronary Artery Abnormalities Based Only on Coronary Artery Z-Scores after Kawasaki Disease. Pediatr Cardiol 2010, 31, 242–249. [CrossRef]
- Tsuda, E.; Abe, T.; Tamaki, W. Acute Coronary Syndrome in Adult Patients with Coronary Artery Lesions Caused by Kawasaki Disease: Review of Case Reports. Cardiol Young 2011, 21, 74–82. [CrossRef]
- Gorelik, M.; Chung, S.A.; Ardalan, K.; Binstadt, B.A.; Friedman, K.; Hayward, K.; Imundo, L.F.; Lapidus, S.K.; Kim, S.; Son, M.B.; et al. 2021 American College of Rheumatology/Vasculitis Foundation Guideline for the Management of Kawasaki Disease. Arthritis Care Res (Hoboken) 2022, 74, 538–548. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).