1. Introduction
Although the standard of care in the management of recurrent pleural effusion is the VATS talc poudrage [
1,
2], this option is not applicable in some complex cases of recurrent pleural effusion. These complex cases mainly consist of two groups of patients. The first is represented by pluri-comorbidities patients considered unfit for surgery, who are unable to withstand narcosis. The second group is represented by those patients with an associated trapped lung syndrome, a condition in which the lung is unable to fully expand, determining the partial or complete unapposition of the parietal and visceral pleura [
3], in which a chemical pleurodesis is not effective. In those patients, the aim should be to offer them long-term relief of symptoms and to reduce hospitalization through procedures with minimal complications [
4,
5]. Accordingly, the BTS guidelines [
6] and ERS/EACTS statement [
3] suggest the indwelling pleural catheter (IPC) to be the most adequate choice.
Nowadays, IPC is composed of a distal portion, usually connected to a unidirectional valve, that is left external to the body of the patient, and a proximal portion represented by a multi-fenestrated silicon catheter tunneled subcutaneously [
7]. This type of device, while having several advantages, presents various criticalities, mainly related to the external distal portion. The most common issues are infection of the site (~3%) [
8], accidental dislocation, the patient's difficulty in accepting it from a psychological point of view and the concern of many physicians to administer chemotherapy [
7].
In recent times, a fully implantable pleural system has been developed (Celsite® DRAINAPORT), aiming the reduction of the infections and the dislocation rate, the improvement of the self-image of the patients, the lowering of the costs due to the lack of the single-use vacuum bottle and a safer administration of chemotherapy in those patients who could benefit from it.
We report a retrospective series of patients who have undergone a pleural port implantation to describe the efficacy of Celsite® DRAINAPORT in the management of recurrent pleural effusion in unsuitable patients for pleurodesis.
2. Materials and Methods
2.1. Design of the Study
A single-center, observational, retrospective study was conducted on a series of patients who underwent the positioning of Celsite® DRAINAPORT from April 2018 to August 2024 for a recurrent pleural effusion.
The aim of the study was to verify the safety and efficacy of the Celsite® DRAINAPORT as an option for palliating recurrent pleural effusion in patients unsuitable for pleurodesis with sclerosing agents.
2.2. Device Specifications, Technical Properties and Surgical Technique
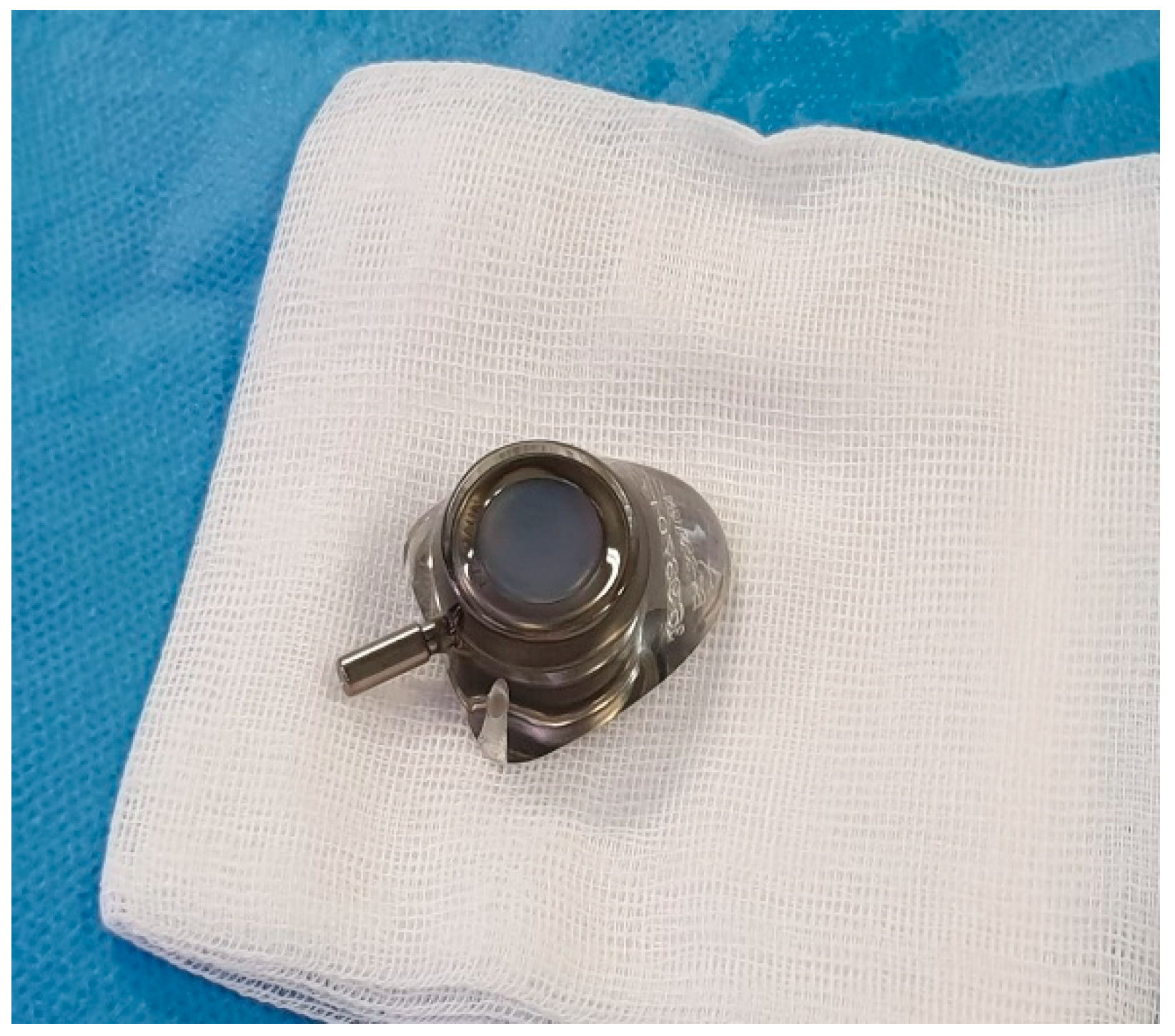
The Celsite® DRAINAPORT, manufactured by B. Braun (Carl-Braun-Straße 1, Melsungen, Hessen, Germany), is composed of a multiperforated 15F silicone catheter with 49 oval holes, a catheter cuff that promotes tissue in-growth and a reservoir, with a silicone puncture area that allows the drainage of pleural fluid. The main advantage of this device is that both the catheter and the reservoir are implanted completely subcutaneously.
This device is known mainly for its use for intra-peritoneal administration of chemotherapy, hydration, and drainage of malignant ascites, although in its technical sheet it is indicated also for the pleural effusion drainage [
9]. Data in literature about its use is scarce.
Furthermore, we conducted an experimental test to evaluate the full potential of the device and to detect the maximum suction capacity of air and liquid with various sizes of Huber needles. The materials used comprehended a Celsite® DRAINAPORT, a digital suction system, a container filled with saline solution and a set of Huber needles including the 19G and 20G ones. We tested the maximum suction capacity of the system with a 19G Huber needle, a 20G Huber needle, and then with a double gripper connected to the pleural port (including 19+19G, 20+20G, 19+20G). The suction pressure, given by digital suction system, was gradually increased in each step of 5 cmH2O starting from -5 cmH20 up to -100 cmH2O. The test was first conducted in the air, and then by submerging the drainage tube of the system in saline solution.
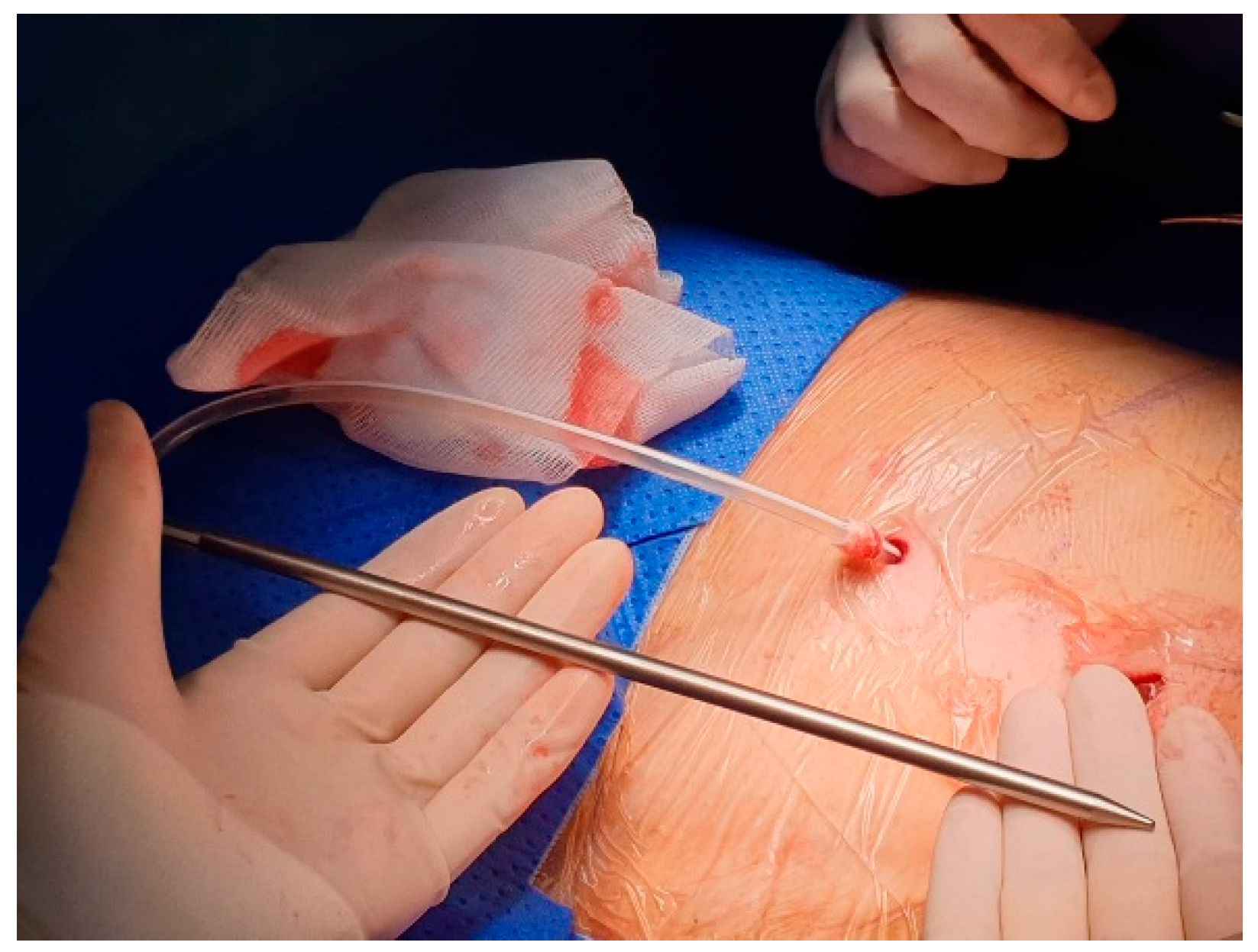
Typically, Celsite® DRAINAPORT is implanted using the modified Seldinger technique. The implantation kit is composed of an 18G Seldinger puncture needle, a J guidewire, 12F-14F dilator, 16F peelable introducer and a tunneling rod.
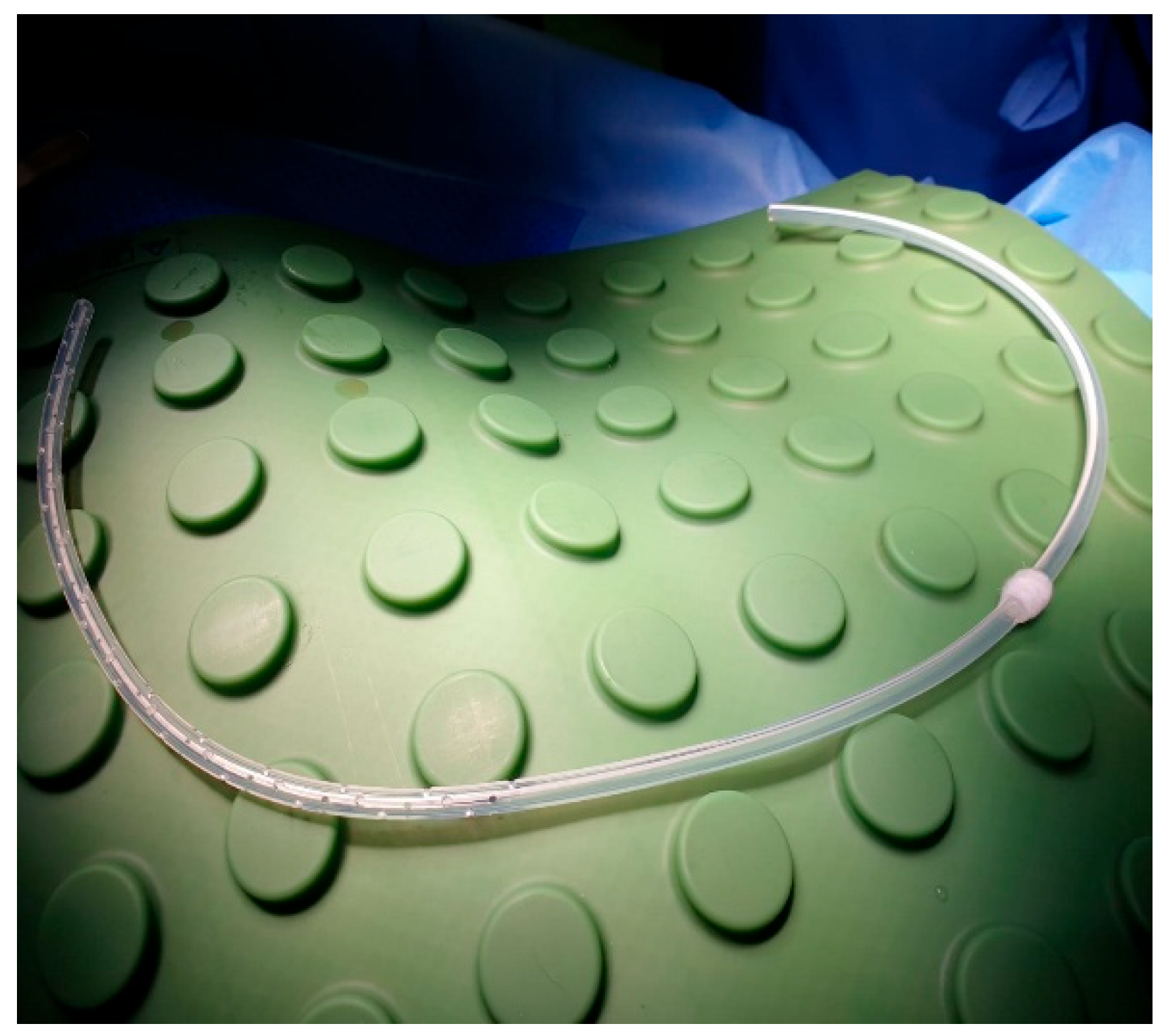
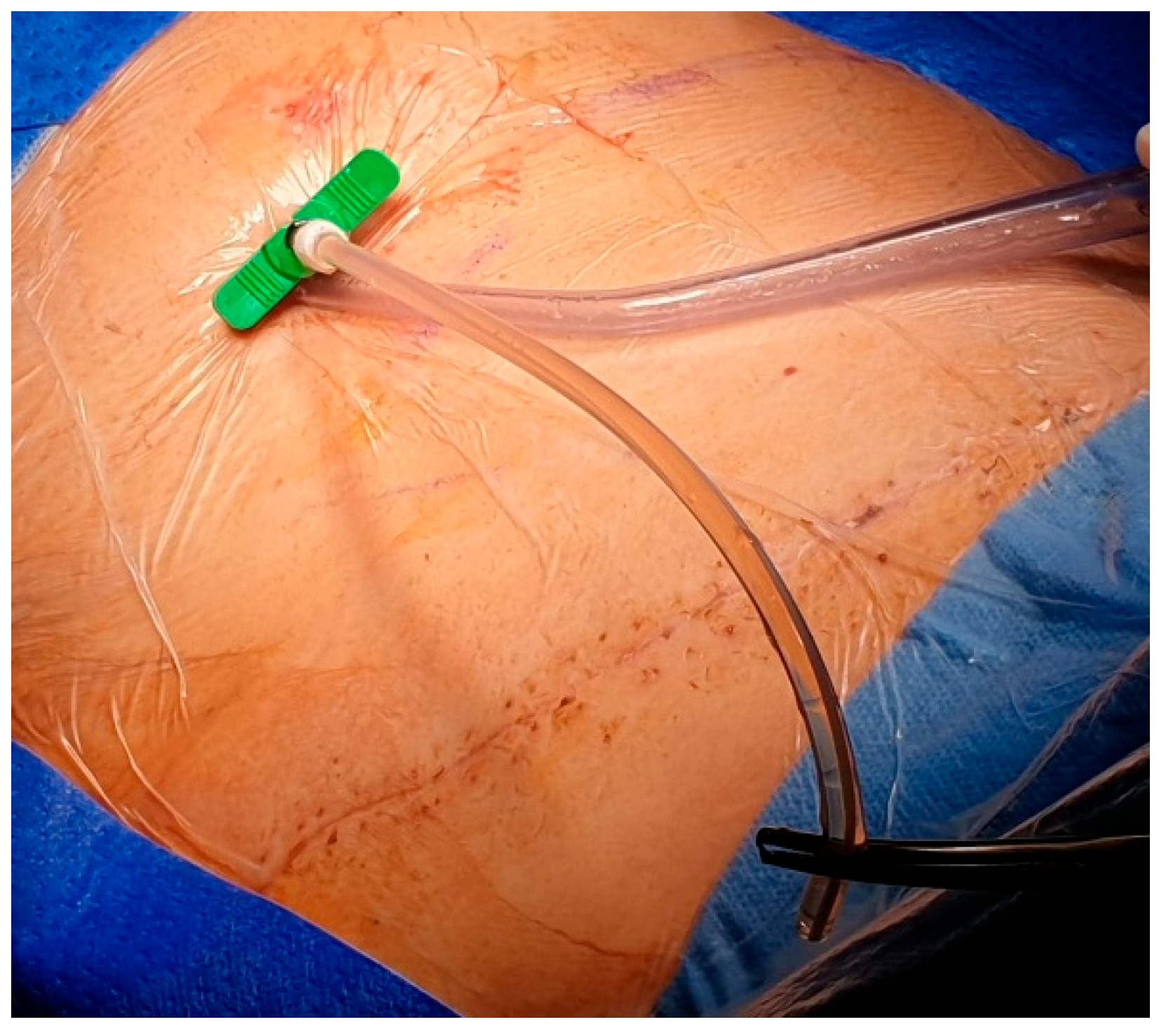
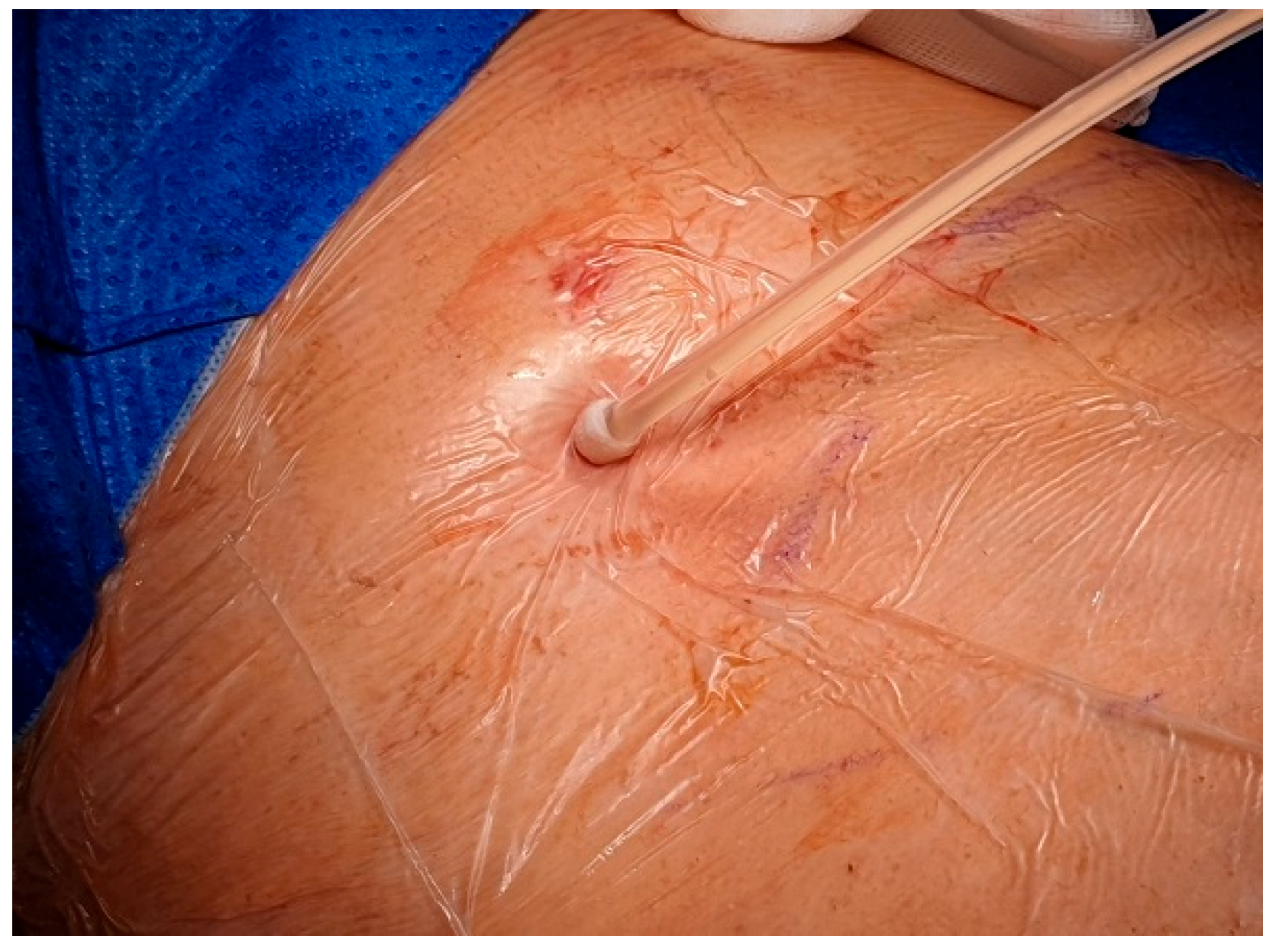
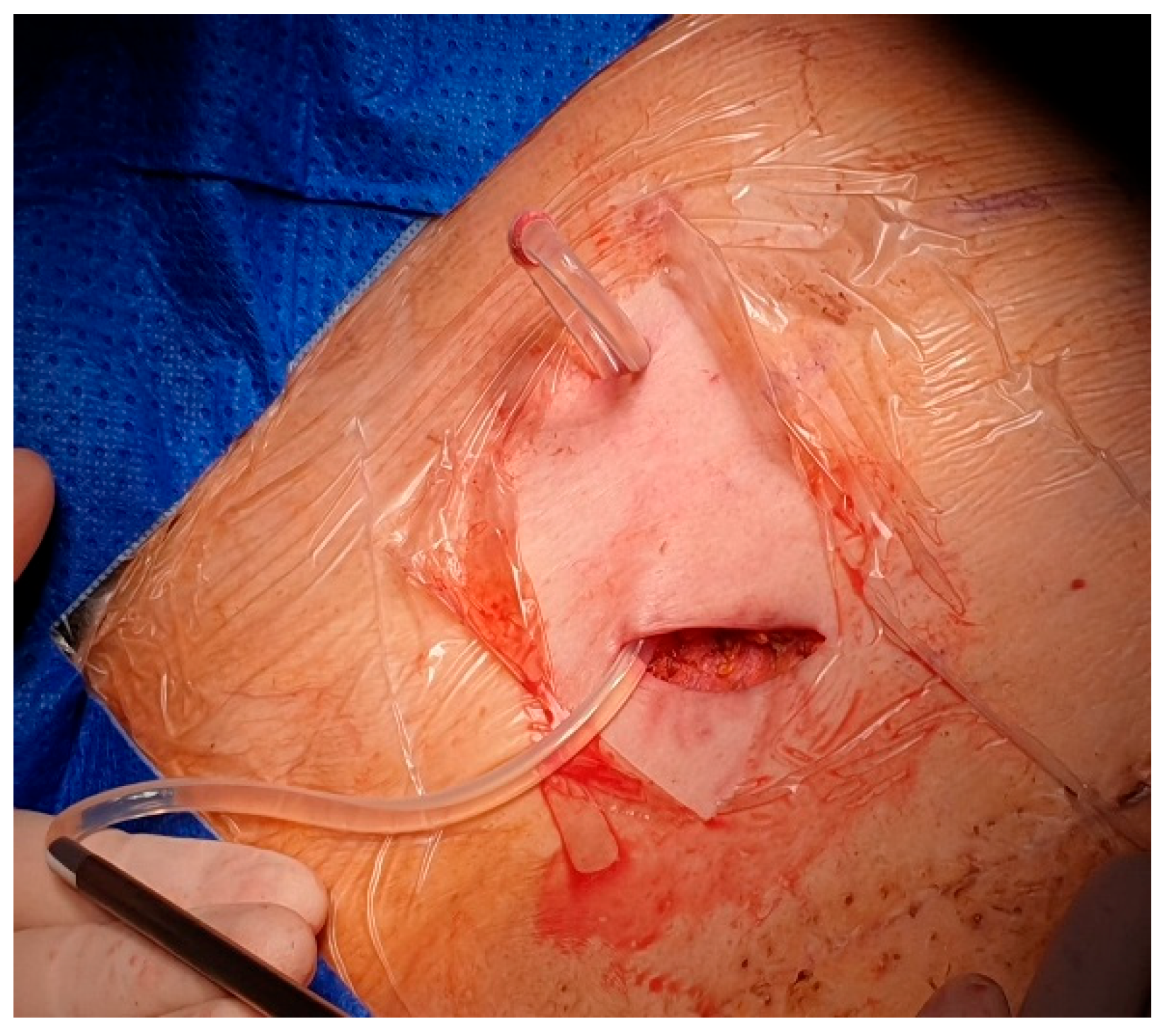
After local anesthesia, with the patient in lateral decubitus, the intercostal space (usually the VII) is identified, and then an explorative puncture is performed using the 18G Seldinger needle. Then the J guidewire is introduced and the Seldinger needle removed. The following steps are the insertion, via the guidewire, of the dilator and then the peelable introducer through which the multi-fenestrated drainage (
Figure 1 and
Figure 2) is introduced in the pleural cavity. Then, the peelable introducer is removed (
Figure 3), the free portion of the drainage tube is connected to the tunneling rod (
Figure 4 and
Figure 5) and is tunneled subcutaneously to be connected to the port reservoir (
Figure 6), which is accommodated in a subcutaneous pouch previously created, and held in position by sutures. The last step after the wound closure is the connection of a Huber needle to the system allowing drainage.
2.3. Clinical Data
The population of the study is composed of patients affected by recurrent pleural effusion associated with a trapped lung syndrome or with an excessively elevated risk for general anesthesia, that therefore are not eligible for chemical pleurodesis surgery.
The patients are classified, according to their pathology, in oncological ones and benign ones, represented by a small number of patients with cardiac failure or rheumatological disease associated to a pleural effusion unresponsive to medical treatment and impactful on the quality of life.
The device was implanted under local anesthesia in those patients considered unfit for surgery and after a thoracoscopy in those in which the re-expansion test gave evidence of a trapped lung.
The population of the study was also described in term of demographic and pre-operative variables, such as age, sex, main diagnosis and the eventual oncological treatment.
Additionally, data about the post operative period have been collected to evaluate the outcome of the positioning of the Celsite® DRAINAPORT. In particular, we have registered 1) Percentage of complication of the procedure (intraoperative, early: during the hospital stay, late: after discharge), 2) Homely or clinical outpatient follow up, 3) Number of patients that received oncological treatment after implantation, 4) Percentage of symptoms palliation, 5) Survival rate at six months (180 days) and cause of death.
2.4. Statistical Analyses
The overall survival at six months was calculated by the Kaplan-Meyer method. A log-rank test was performed to verify if there are any statistically significant differences in the survival of the group included in the study. Statistical analyses were conducted using Stata software version 18 (Stata—Corp, College Station, TX, USA).
3. Results
3.1. Device Properties
Based on our results we can assess that the maximum suction capacity of the 20G gripper was 1200 ml/min of air and 50 ml/min of fluid, while the 19G gripper’s maximum suction capacity was 700 ml/min and 35 ml/min, respectively.
With a double gripper connected to the device, the flow rate reached its maximum with the 19G+19G gripper combination, obtaining 2200 ml/min of maximum suction of air, and 80 ml/min of fluid.
The full results of the test are shown by
Table 1.
3.2. Clinical Results
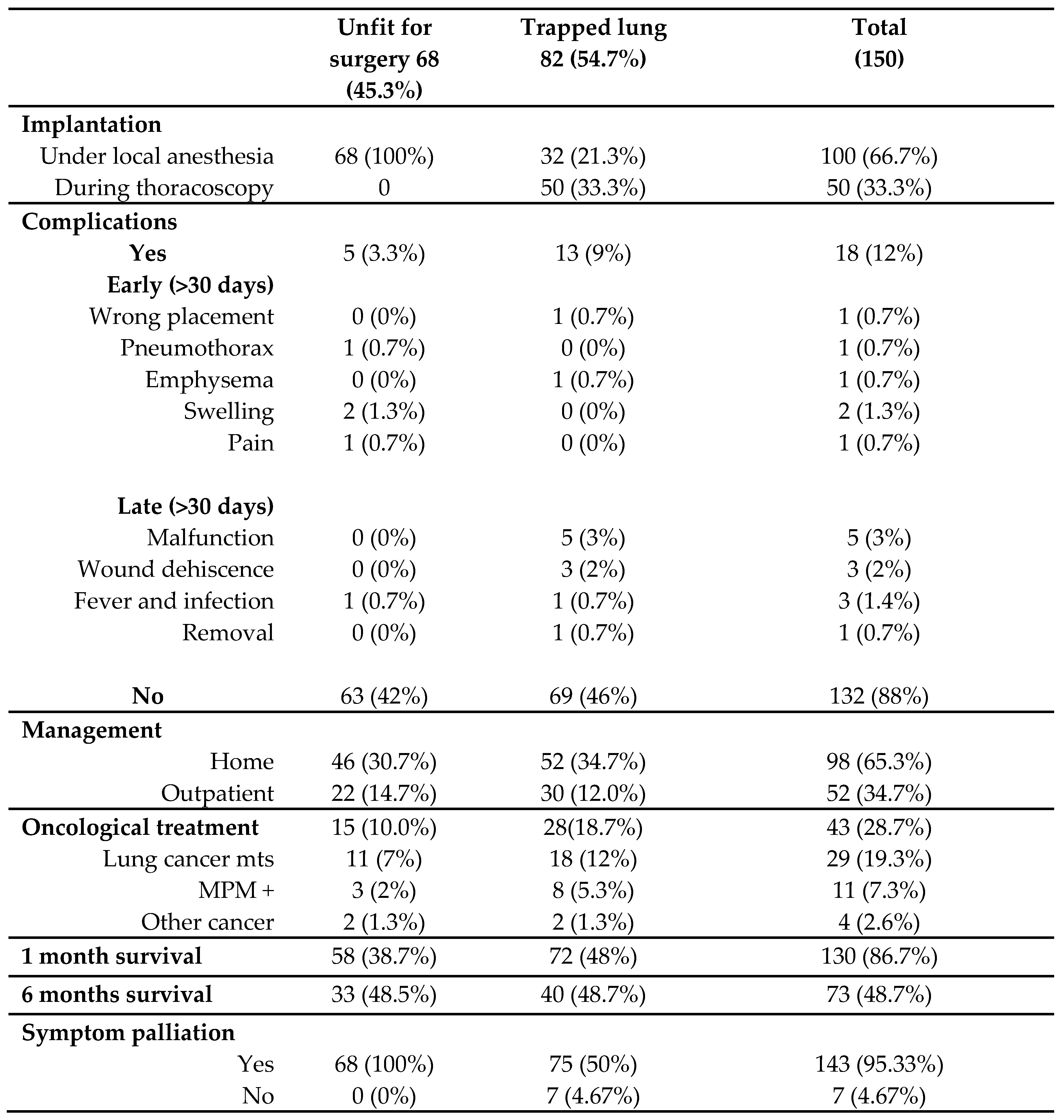
The population of study was composed by 150 patients, 99 (66%) male and 51 (34%) female, with a mean age of 74.8 years (SD 10.52). 82 patients (55%) suffered from trapped lung syndrome, while 68 (45%) suffered from recurrent pleural effusion in pluri-comorbidities conditions, and therefore considered unfit for surgery. 140 patients were oncological patients (93%), while 10 patients (7%) underwent device implantation for benign disease (mainly inflammatory and cardiac disease), as shown by
Table 2.
The totality of the unfit for surgery patients underwent Celsite® DRAINAPORT implantation under local anesthesia, while in the trapped lung syndrome sub-group, 32 patients (21%) underwent implantation under local anesthesia and 50 patients (33%) during a thoracoscopy with evidence of an unexpandable lung.
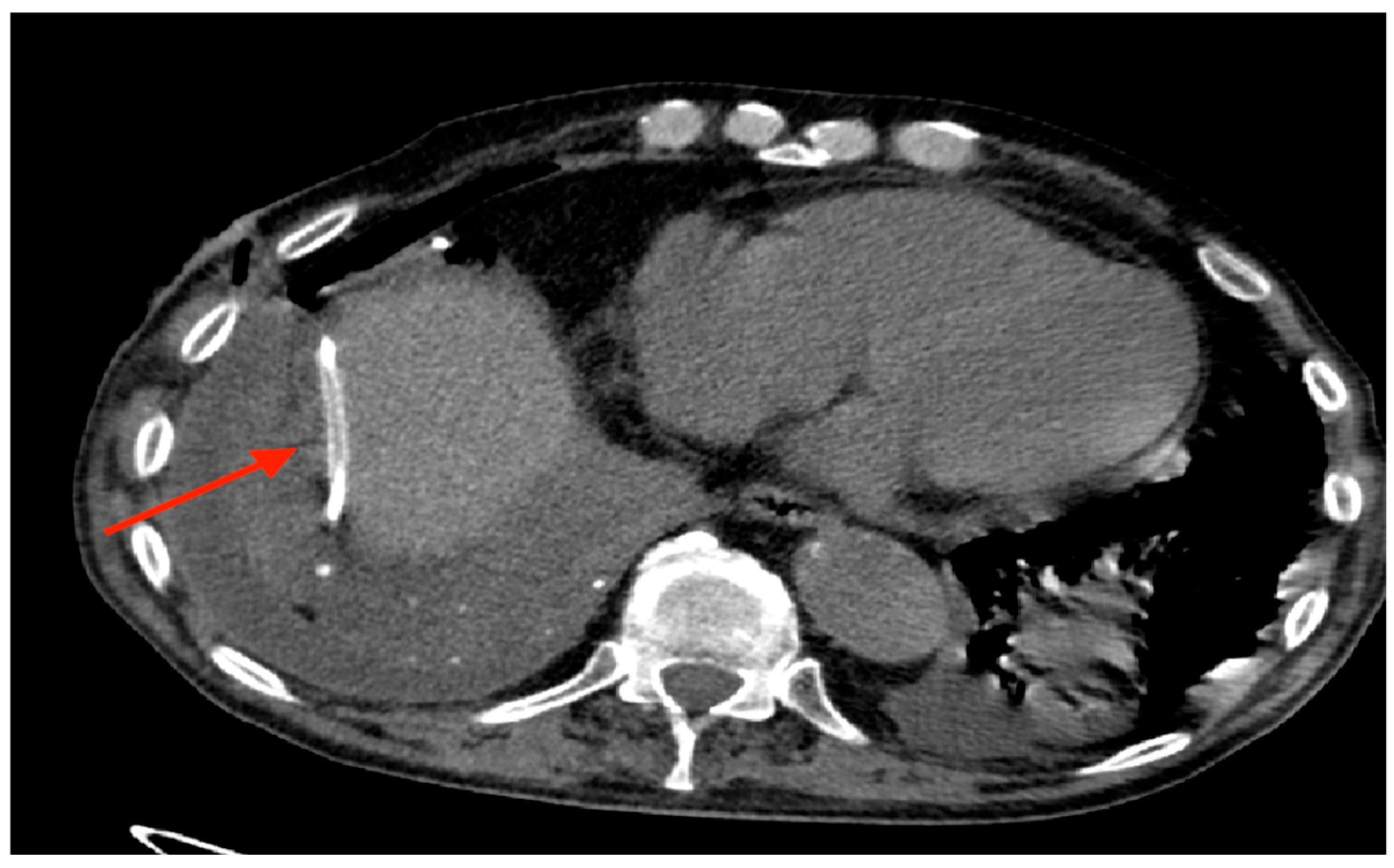
Out of 150 patients, there was a single case in which the drainage tube was implanted in the abdomen (
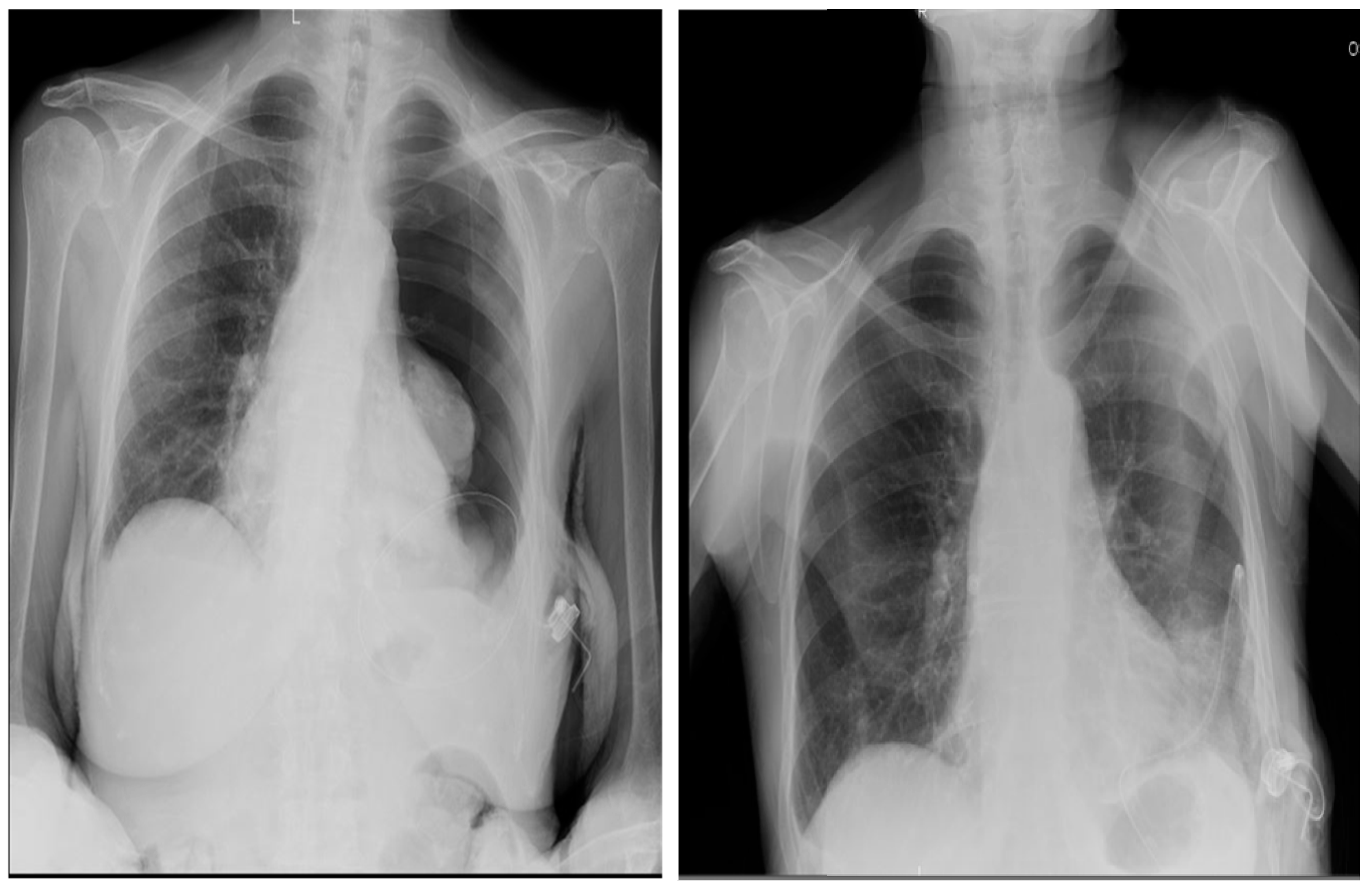
Figure 7) and only one case of total post-implantation pneumothorax, as shown by
Figure 8-A and 8-B, which however didn’t require an additional chest drainage tube and was resolved by connecting the port to a digital suction system. During follow up 18 (12%) patients developed complications (
Table 3), mainly in the trapped lung sub-group (9%), with malfunction (and thus failure) of the device and wound dehiscence as the most represented ones.
The home nursing services took correct care of 98 patients (65%), while the others required outpatient management.
A total of 43 patients (28.7%) received oncological treatment (such as chemotherapy) after the implantation of Celsite® DRAINAPORT, the majority of which was affected by lung cancer metastases.
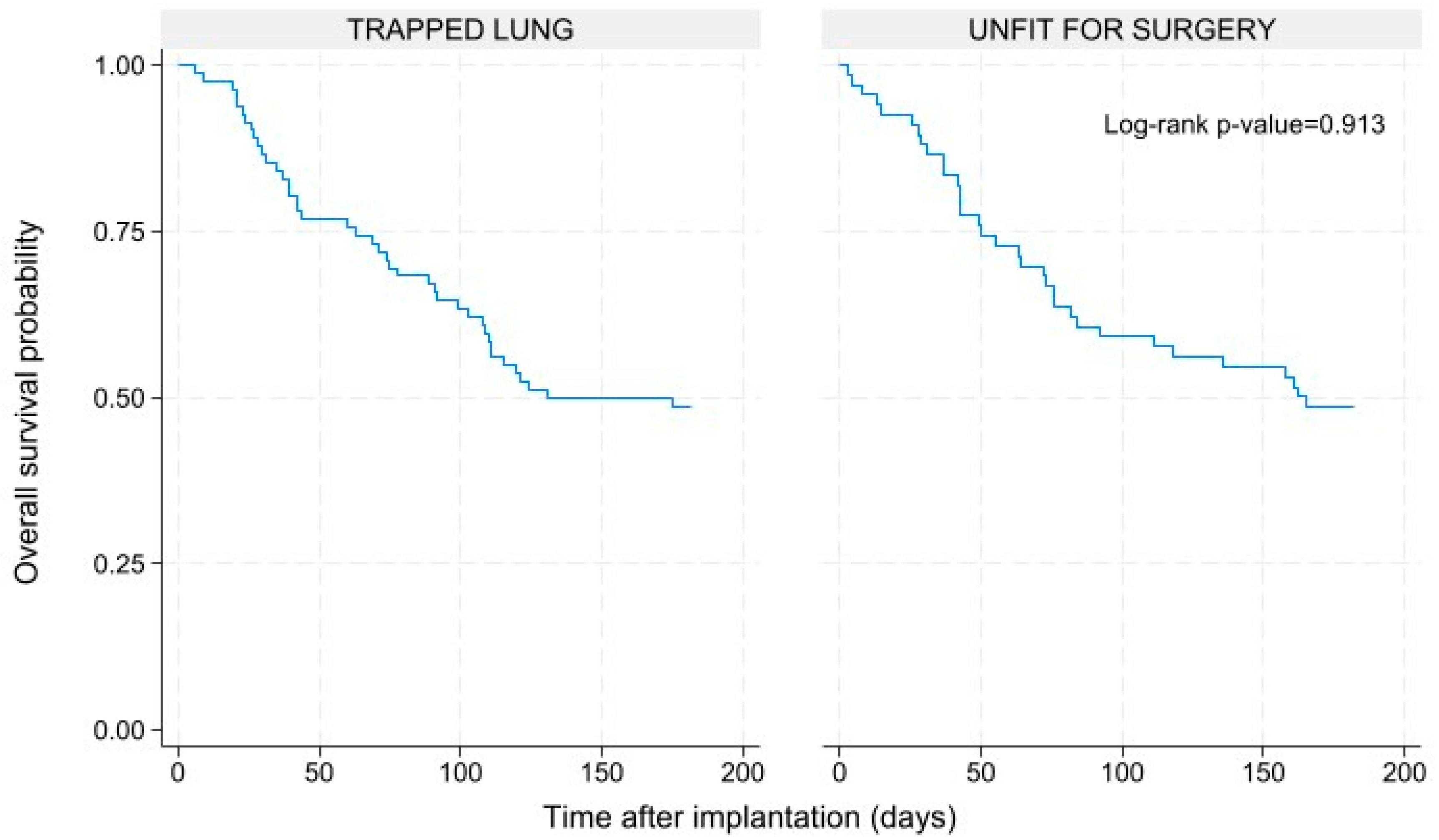
After six months (180 days), 73 patients (49%) were still alive, while the others died due to the oncological disease progression. The deaths were equally distributed in the unfit for surgery and the trapped lung sub-group, with no statistically significative difference between the two groups (Log-rank p-value=0.913). The Kaplan-Meyer survival curves are shown by
Figure 9.
The symptoms palliation rate (and thus the success of the device) was reported in 143 (95.33%) cases.
4. Discussion
The management of recurrent pleural effusion is a complex matter, both for the choice of the best treatment and for the patient governance.
Pleurodesis with a sclerosing agents, which remains the first choice of treatment in patients fit enough for surgery, is not effective in those patients with an un-expandable lung, in which the visceral and parietal pleura are unopposed, and is not a viable option in those patients who could not withstand a narcosis.
In those patients, other options are available, such as repeated thoracocentesis which, however, are painful, have a risk of bleeding and of iatrogenic pneumothorax and requires multiple access to the healthcare institute.
In these cases, when the life expectancy is adequate, the implantation of an indwelling pleural catheter may be the most adequate choice of treatment, as stated by international guidelines [
3,
6].
The most widely used device worldwide is the PleurX™ catheter, manufactured by CareFusion (McGaw Park, Ill., USA) [
7] and first approved for use in malignant pleural effusions by the Food and Drug Administration in 1997 [
8]. It is composed of a fenestrated 15.5-Fr-diameter silicone catheter, which is inserted in the pleural cavity, and a cuffed portion tunneled subcutaneously. The remaining portion, which comprehends a unidirectional valve, is external to the body and allows the attachment of the drainage kit. While this device holds many advantages, the fact that a portion of the catheter is left outside of the body leads to various complications and criticality, such as site infections, skin maceration, dislocation and the concern of many physicians to administer chemotherapy [
7].
In literature, there are some data describing the efficacy of indwelling catheters, in general, and of the PleurX™, in particular, showing their low rate of complications [
11,
12,
13].
Van Meter et al [
8] showed a very low rate of complications, less than 3% for major ones and less than 10% for minors. Additionally, they have described a 47% of spontaneous pleurodesis allowing the removal of the device.
In general, literature data report as main complication infectious events occurred in 3-12% of patients [
8,
14], dislocation of the catheter in 18% [
14] and malfunction and obstruction in 13% [
8], with a total complication rate of ~13% [
8]. Dilkaute et al. [
15] reported a complication rate of 25% in a series of 76 patients.
Many of these complications can be ascribed to the distal portion of the system, which is left outside of the body of the patient. Additionally, the outside-of-body portion, can have a significant impact on the patients' self-image, affecting their self-perception, psychological well-being and social interactions [
16]. The presence of the catheter can cause feelings of physical discomfort and limitations in daily movements. These changes can lead to a decrease in self-confidence and a negative perception of one's own body, and thus to a decrease in quality of life.
In our thoracic surgery division, the fully implantable Celsite
® DRAINAPORT was introduced as daily practice for the management of patients affected by a trapped lung syndrome or by recurrent pleural effusion and considered unfit for surgery. At the same time, we collected clinical data of those patients with the aim of verifying if the total subcutaneous implantation was related to less post operative complications and an improvement in the quality of life. As far as we know, this is the largest series of patients who have undergone this procedure with this type of device, although a similar study was conducted by Kriegel et al. in patients with symptomatic recurrent malignant pleurisy [
17].
In our study, the complication rate was 12%. The main complications were malfunction (3%), fever and infection (1.4%), and wound dehiscence (2%). The malfunction of the device, and hence its failure, mostly occurred in cases of multi-loculation of the effusion or obstruction of the drainage tube. The cases of fever and infection were mild and were all properly treated and resolved with adequate antibiotic therapy. The wound dehiscence, which mainly occurred in patients in active chemotherapy administration, required an average of two dressings per week to be properly resolved.
There was only one case of wrong placement of the device, with the drainage tube implanted in the abdomen, between the diaphragm and the liver. The device was readily repositioned in the correct location, without any consequences for the patient.
Thanks to the low complication rate and the simplicity of the implantation procedure, the mean hospital stay was one day for those patients that received the implantation under local anesthesia and three days for those who received implantation after a thoracoscopy. After discharge, patients were seen in the outpatient clinic for suture removal and surgical site control. Thereafter, most patients were properly cared for by home nursing services. The remaining cases that were managed on an outpatient basis were partly due to the absence of home nursing services in the patients' residence area, and partly due to those patients receiving active oncological treatments, in which the drainage procedure was carried out at the same time as the therapy administration sessions.
Almost all patients were satisfied with the procedure, and were able to lead normal lives without limitations, within the limits imposed by their underlying pathology.
This study has certain limitations. One of these is due to the fact that the data originates from a single center and may therefore not be representative of the general population. Another limitation is the fact that it is a retrospective study, in which a proportion of patients were followed up after the procedure by the oncologists, which may influence the completeness and the quality of the data collected. Subsequent research may confirm our findings and results and validate our clinical practice in the care management of this group of patients.
5. Conclusions
From the data we analyzed, we can infer that Celsite® DRAINAPORT is a valid, effective and safe option, not inferior to the other devices used worldwide, in the management of patients with recurrent pleural effusion associated or not with trapped lung, and is able to ensure an improvement in the quality of life of this group of patients, with a low rate of complications and a general level of satisfaction, both from the patient and the care-giver side.
Author Contributions
Conceptualization, M.M. and F.L; methodology, F.L. and M.M.; validation, F.L., M.M. and S.S.; formal analysis, S.S.; investigation, F.L, S.S., F.V. and L.E.; resources, S.S, F.V. and L.E.; data curation, M.M., F.M., S.S., ; writing—original draft preparation, M.M.; writing—review and editing, M.M., F.L., F.V. and S.S.; visualization, F.V., A.L., S.R. and C.G.; supervision, F.L.; project administration, F.L.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of A.O.U. San Luigi Gonzaga, Orbassano, Italy (protocol code TRS-2018-00000689)
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VATS |
Video-assisted thoracoscopic surgery |
| IPC |
Indwelling Pleural Catheter |
| BTS |
British Thoracic Society |
| ERS/EACTS |
European Respiratory Society/European Association for Cardio-Thoracic Surgery |
| MPM |
Malignant pleural mesothelioma |
References
- Sahn SA. Talc should be used for pleurodesis. Am J Respir Crit Care Med. 2000;162(6):2023-2024; discussion 2026. [CrossRef]
- Sioris T, Sihvo E, Salo J, Räsänen J, Knuuttila A. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35(5):546-551. [CrossRef]
- Bibby AC, Dorn P, Psallidas I, et al. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1):1800349. [CrossRef]
- Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129(2):362-368. [CrossRef]
- Musani AI, Haas AR, Seijo L, Wilby M, Sterman DH. Outpatient Management of Malignant Pleural Effusions with Small-Bore, Tunneled Pleural Catheters. Respiration. 2004;71(6):559-566. [CrossRef]
- Roberts ME, Neville E, Berrisford RG, Antunes G, Ali NJ, on behalf of the BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65(Suppl 2):ii32-ii40. [CrossRef]
- Bhatnagar R, Maskell NA. Indwelling Pleural Catheters. Respiration. 2014;88(1):74-85. [CrossRef]
- Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and Safety of Tunneled Pleural Catheters in Adults with Malignant Pleural Effusions: A Systematic Review. J Gen Intern Med. 2011;26(1):70-76. [CrossRef]
- Celsite® DRAINAPORT | B. Braun. Accessed October 19, 2024. https://catalogs.bbraun.com/en-01/p/PRID00004318/celsite-drainport-drainage-maligment-ascites?bomUsage=documents.
- Summary of Safety and Effectiveness Denver PleurX Pleural Catheter Kit, Denver PleurX Home Drainage Kit. Rockville, Food & Drug Administration, 1997. https://www.accessdata.fda.gov/cdrh_docs/pdf/K971753.pdf.
- Efthymiou CA, Masudi T, Charles Thorpe JA, Papagiannopoulos K. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg. 2009;9(6):961-964. [CrossRef]
- Pollak JS. Malignant pleural effusions: treatment with tunneled long-term drainage catheters: Curr Opin Pulm Med. 2002;8(4):302-307. [CrossRef]
- Chalhoub M, Saqib A, Castellano M. Indwelling pleural catheters: complications and management strategies. J Thorac Dis. 2018;10(7):4659-4666. [CrossRef]
- van den Toorn LM, Schaap E, Surmont VFM, Pouw EM, van der Rijt KCD, van Klaveren RJ. Management of recurrent malignant pleural effusions with a chronic indwelling pleural catheter. Lung Cancer Amst Neth. 2005;50(1):123-127. [CrossRef]
- Dilkaute M, Klapdor B, Scherff A, Ostendorf U, Ewig S. [PleurX drainage catheter for palliative treatment of malignant pleural effusion]. Pneumol Stuttg Ger. 2012;66(11):637-644. [CrossRef]
- Zhang J, Liang J, Kadwani O, et al. S138 Malignant pleural effusions: evaluating the psychosocial impact of indwelling pleural catheters on patients (MY-IPC) – an interim analysis. Thorax. 2023;78(Suppl 4):A100. [CrossRef]
- Kriegel I, Daniel C, Falcou MC, et al. Use of a Subcutaneous Implantable Pleural Port in the Management of Recurrent Malignant Pleurisy: Five-Year Experience Based on 168 Subcutaneous Implantable Pleural Ports. J Palliat Med. 2011;14(7):829-834. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).