Background
The World Health Organization (WHO) introduced the Expanded Program for Immunization (EPI) in 1974 to control vaccine-preventable diseases (VPDs). Vaccination helped a lot in terms of reducing child morbidity and mortality through wise use of resources. Immunization is one of the major cost-effective interventions through which globally more than 4.4 million children’s life is saved annually [
1,
2,
3,
4,
5]. The reduction in childhood mortalities due to immunization is also capitalized in the African region [
6].
Ethiopia introduced vaccines in 1980 and currently vaccines are provided in either static, outreach, or mobile modalities throughout healthcare facilities in the country [
7]. Vaccines are crucial as well as highly sensitive health commodities that are sensitive to light, heat, and freezing. These necessitate a robust supply chain system that operates from manufacturing to service delivery points. Around the millennium and earlier, vaccine supply chain management systems used to involve the administrative structure of the country. The supply chain followed the existing administrative structures, which constituted six levels: the central, regional, zonal, woreda (district,) health facility, and health post. Such extended levels increased the number of actors, which made it more difficult to achieve visibility and ensure the quality and accessibility of vaccination service. This also brought a lack of accountability as there are many people responsible for these activities because as hierarchy extends, responsibility becomes less elastic. Other issues such as lack of investment, low capacity for supply chain management, and trained staff attrition played a role in inefficient iSC management [
8]. For this reason, several transformation and transition plans took place. Firstly in 2005, when the Ethiopian Pharmaceuticals Fund and Supply Agency was established and the supply chain was transformed into last-mile delivery [
8]. The vaccine supply chain management transition plan of 2014, helped with the relocation of existing cold rooms to PFSA hubs, the design, and implementation of a logistic management information system (LMIS), the development of standard operating procedures (SOPs), and training and recruitment of staff; development of an overall Immunization Supply Chain Management Strategy, 2018-2023 [
9].
Currently, the health system transformation system of the country is built on core principles of improving supply chain and logistics management. Ethiopia has a three-tier healthcare delivery system. The procurement storage and distribution of medical-related products including vaccines are managed by the Ethiopian pharmaceutical supply service. The vaccine supply management should extend into districts and health facilities to ultimately protect vaccine reach at each level at the right quantity and at the right time. This system involves proper customs clearances, transferring vaccines from importation to central storage, maintaining appropriate temperature zones for storage and transportation, and effective stock management at all levels [
8,
9]. Apart from EPSS, the ministry and all hierarchical offices bear the responsibility of overseeing and guaranteeing the long-term availability of vaccines and supplies in all healthcare institutions [
10].
Effective Vaccine Management (EVM) is an initiative launched by WHO and UNICEF in 2009 to assess the iSC of countries through standard tools and materials. The initiative provides guidance and tools to help countries develop continuous improvement plans based on EVM assessment findings. EVM is used to assess the status of the immunization supply chain of a given country and prioritize anticipated improvements. This can be achieved through repeated assessments of iSC against established quality management standards. EVM has been used globally; so far, 70 countries and about 50 countries have conducted EVM2 assessments, with some having conducted more than one EVM assessment[
11]. Many countries have used EVM to assess iSC and benefitted in improving their iSC through its insights. India assessed its iSC in 2013 and improved the supply chain system and the change has been found in subsequent assessments of EVM in 2018 [
12]. Benin and Mozambique used an opportunity from EVM assessment to identify bottlenecks for the efficiency of iSC and redesign the supply chain management through system redesign. Surprisingly Benin found notable improvements from 40% to 100% between the baseline and endline assessments in 2012 and 2014. In Mozambique, the redesign brought a significant reduction in stockouts between the baseline and end-line time points [
13].
EVM assessment in Ethiopia was conducted in 2013, and 2019 this and subsequent assessment reports identified several areas that helped strengthen the envisaged transformation plan by then, which included replacing old cold chain equipment, training cold chain equipment technicians, ensuring implementation of regular temperature monitoring procedures, and putting in place effective stock management procedures. However, because timely assessments could not be done, progressive challenges encountered in supply chain management in Ethiopia were not identified, quantified and opportunities counted [
7,
8,
9].
This assessment is therefore designed to assess the status or performance of the immunization supply chain. Thus, this evaluation research will contribute to a better understanding of the country’s progress, identify areas for improvement, and inform evidence-based decision-making to enhance immunization programs.
Methods
Study Settings
This is a national assessment conducted on randomly selected facilities from all levels of the immunization supply chains nationwide. In 2023 the country had 11 administrative regions and 9 had been included in this evaluation while one was excluded due to security reasons at the time. The regions included in this assessment are Oromia, Amhara, Addis Ababa, Somali, Sidama, Gambella, Dire Dawa, Benshangul Gumuz, and the former SNNP region.
Study Design
A facility-based descriptive study design was used to evaluate the immunization supply chain of Ethiopia at all levels. As the study assessed the current iSC of the nation a descriptive study design allows to assess and describe the current status of the iSC of the country.
Sample Size and Data Collection
The study assessed 302 nationally representative health institutions, which were in randomly selected enumeration areas, Primary centers (EPSS centers), Sub-national (EPSS hubs), lower distributions, and service provision levels. The selection is illustrated in
Table 1 below. The health institutions were sampled from a large National EPI evaluation survey and the sampling procedures of the survey were presented in the evaluation report [
14].
Data Analysis and Presentation
EVM defines almost 900 requirements that an immunization supply chain should meet. These requirements are organized by category (input, output, and performance). Inputs are resources needed to carry out a function, Outputs are tangible outputs of a function while performance refers to the intended outcome of a function.
Criteria are the supply chain functions that a facility is required to carry out, divided into facility operations and facility management. Criteria and category scores, based on their aggregated requirement scores, are calculated at each supply chain level. A composite score is derived from the supply chain level scores by taking the geometric mean of each level’s score. Each level score, in turn, is calculated as the arithmetic mean of its corresponding criteria (E1-E9 and M1-M4).
The variables were analyzed internally by the EVM assessment standard tools and finally presented into facility operations (9 E’s) and facility management (4 M’s) requirements. The requirements for facility operations (E’s) are namely Vaccine arrival (E1), Temperature management (E2), Storage and transport capacity (E3), Facility infrastructure and equipment (E4), Maintenance and repair (E5), Stock management (E6), Distribution of vaccines and dry goods (E7), Vaccine management (E8) and waste management (E9). The facility management criteria (M’s) include Annual needs forecast (M1), Annual Work planning (M2), Supportive supervision (M3), and iSC performance monitoring (M4) [
15]. Each criteria score was based on inputs, processes, and performance categories. The inputs include infrastructure, equipment, information technology, human resources, policies and procedures, and financial resources. The weight of components depends on how much it is essential to the criterion and ranges from one to five in increasing order of importance, 1 nice to have and 5 essentials.
Finally, the full report containing heat maps at each level of the supply chain is generated by the EVM. The report contains a percentage of the performance of each requirement concerning each level of the supply chain.
Ethical Clearance
Ethical approval was obtained from the Hawassa University College of Health Science Institutional Review Board (Reference number: IRB/288/15). Support letter was written from the ministry to the regional health bureau and from the regional health bureau to respective districts and health facilities. Each institution’s management was informed about the purpose, risks benefits, and confidentiality of the study. Participants involved in each interview and assisted observation were also informed about the purpose, risks, benefits, confidentiality, and voluntary participation.
Results
Profile of Study Setting
The assessment covered one primary store (EPSS center) and 15 sub-national stores (EPSS hubs). The EPSS hubs were geographically distributed across various Ethiopian regions, including four in Oromia, three in Amhara, two in Addis Ababa, and one each in Somali, Sidama, Gambella, Dire Dawa, Benshangul Gumuz, and former SNNP. It also included 89 lower distribution levels (Woreda Health Offices) and 196 service provision facilities [101 (35.3%) health centers (HC), 83 (29%) health posts, and the remaining 12 (4.2%) hospitals].
Vaccine Arrival (E1)
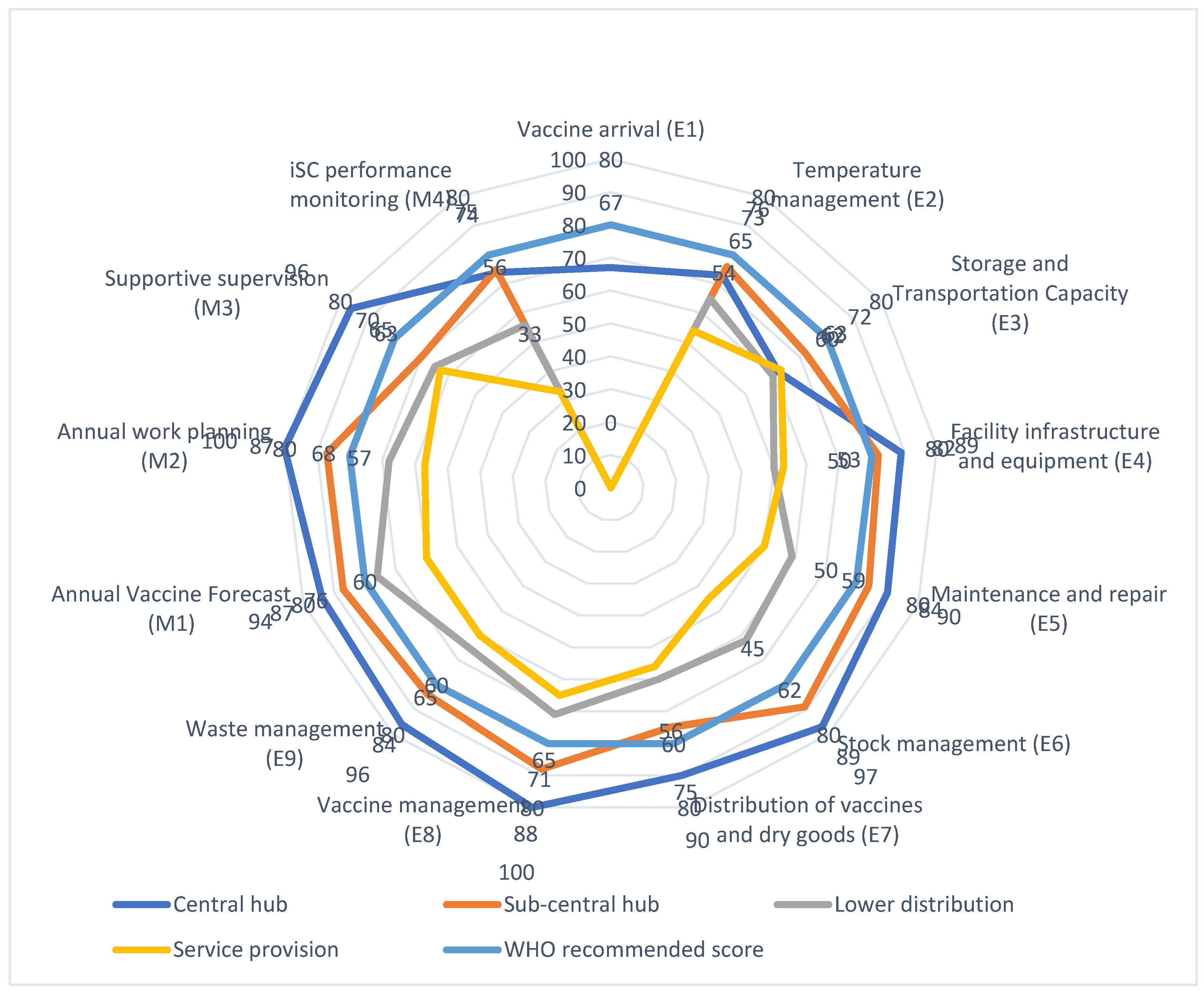
The Primary store (EPSS center) is responsible for inspecting vaccine shipment, customs clearance, and transporting the vaccine from the port/Airport to the storage facility. However, its performance in transporting vaccines from the port of entry to the primary store was notably poor. While the hub performed well in many aspects of vaccine arrival indications, it fell short in areas related to timely transportation (within 72 hours) and having a formal agreement (MoU) with port authorities. These shortcomings lowered the overall vaccine arrival score to only 67%.
Temperature Management (E2)
Vaccine temperature management was assessed based on requirements such as computerized temperature monitoring systems, backup equipment, refrigerated vehicles, staffing, training, and staff knowledge of temperature control procedures. Additionally, standard operating procedures (SOPs) for temperature mapping were considered. Based on these requirements, the overall performance of the assessed facilities on temperature management criteria was 64%. The primary store achieved a temperature management criterion score of 73%. The Sub-national centers scored 76% and lower distribution and service provision facilities scored 65% and 74% respectively, all below the standard.
Storage and Transport Capacity (E3)
Storage and transport capacity were estimated based on requirements related to the capacity and utilization of available resources. Among the E’s, all levels of iSCM consistently scored lowest in this requirement, the overall score on storage and transportation is 64% while the Primary store scored only 62%, much below the WHO’s recommended target of 80%. The Sub-national centers scored 72% and the lower distribution and service provision levels scored 60% and 63% respectively. These poor performances were primarily attributed to insufficient capacity to accommodate the expected maximum stock levels of dry goods, diluents, syringes, safety boxes, cold storage for vaccines, coolant pack storage, and vaccine vehicles.
Facility Infrastructure and Equipment (E4)
These requirements include electricity, communication (presence and functionality), water sanitation and hygiene, iSC office security, cold rooms, and cold storage equipment (appropriateness for meeting target requirements). It also investigated the presence of fire alarms and extinguishers, warm coats for working in cold rooms, computer and printer availability and functionality, and overall cleanliness and dryness of the facility and equipment. The overall score of this requirement was 67%, while the Primary store and Sub-national centers scored 89% and 82% respectively. At lower distribution and service provision levels, the facility infrastructure and equipment scored 50% and 53% respectively.
Maintenance and Repair (E5)
This included assignment and training of at least one maintenance staff, inventory, Standard Operating Procedures (SOPs) and guidelines for maintenance and budgeting, receipt of operational costs, preventive maintenance records, and standardized and up-to-date fault reporting. Based on these requirements, the overall facilities level score was 69% while the primary level/Primary store scored 90%. The Sub-national centers, lower distribution levels, and service provision levels scored 84%, 59%, and 50% respectively. This emanated from a lack of policies and procedures on maintenance care and repair and a lack of preventive maintenance records.
Stock Management (E6)
Stock management assessment included records with required fields, stock issuance, and computerized management systems. It also evaluated issues related to staffing, training, and staff knowledge of key principles and procedures, Standard Operating Procedures (SOPs) for vaccine stock transactions, and documentation related to receiving, releasing, and accounting for vaccine losses. Additionally, regular physical stock counts and accurate stock records were considered. The Primary store stock management score was 97%, and the Sub-national hubs 89% both scoring above the target WHO score. The lower distribution scored 63%, and service provision levels scored 45% in stock management. As a result, the overall score of stock management was 70%.
Distribution of Vaccines and Dry Goods (E7)
These requirements included for distribution of vaccines and dry goods is composed of the availability of transportation for scheduled vaccine distribution, collection, and outreach activities, as well as the staffing of individuals responsible for vaccine distribution tasks. Additionally, they evaluated issues related to Standard Operating Procedures (SOPs) and guidelines for vaccine distribution and transport emergency contingencies. Financial aspects such as the budget for fuel, transportation sufficiency of budgets, and documented vaccine distribution plans were also assessed. The requirements also encompassed the storage of insulated containers and coolant packs, recording of vaccine transportation trips, loading of refrigerated vehicles according to standards, and transportation as per the schedule. Based on these requirements, the overall score was 69% and the Primary store scored 90%. The Sub-national centers scored 75%, while lower distribution and service provision levels scored 60% and 56% respectively.
Vaccine Management (E8)
The assessment included staff knowledge of key principles and procedures for vaccine management, the presence of required Standard Operating Procedures (SOPs) and guidelines, shake tests performed in response to low-temperature alarms, correct diluent use for reconstituting freeze-dried vaccines, and the marking of opened multidose vials with the date of opening. Since vaccination does not occur on-site at the Primary store, Sub-national centers, and lower distribution levels, some requirements were not applicable at these levels. Based on the applicable requirements, the Primary store scored 100% in vaccine management. The Sub-national centers scored 88%, lower distribution 71%, and service provisions scored 65% whereas the overall score was 80%.
Waste Management (E9)
The evaluation also included the adequacy of facilities and equipment, such as safety boxes, waste burial facilities, incinerators, and needle pits, for safe waste handling, storage, and disposal. Staff training, knowledge, and understanding of key immunization waste management principles and the use of personal protective equipment were also assessed. Additionally, financial aspects, including budget allocation, receipt of funds, and budget sufficiency for immunization waste management, were considered. Performance-related requirements evaluated the use of recommended syringes, recording of needle-stick injuries, safe storage of filled safety boxes, cleanliness of immunization rooms, and the maintenance of a storage and disposal area free from used syringes, needles, vials, and ampoules. Based on applicable requirements, the Primary store scored 96% in waste management. Sub-national centers scored 84% in waste management while lower distribution levels and service provision levels scored 65% and 60% respectively, the overall score being 75%. As indicated in
Supplementary Table S5 at service provision the waste management falls in policies and procedures, knowledge of human resources, and financial resources for proper immunization waste management.
Annual Need Forecast (M1)
The annual need forecast was a weighted score that evaluated issues related to forecasting vaccines and dry goods, including staff preparedness and training. Performance-related requirements included the existence of vaccine and dry goods forecasts for the current year, adherence to standard forecasting methods, and the accuracy of the forecasts. Based on these requirements, the Primary store and Sub-national centers scored 94% and 87% respectively. The lower distribution scored 76% and service provision levels scored 60%, leaving the overall score of the annual need forecast at 78%.
Annual Work Planning (M2)
Annual work planning was also another important criterion for effective immunization supply chain management. Variables included under this requirement were budget and sufficiency of budgets for annual work activities and implementation status of work plan activities recording of incomes and expenditures. It also assessed the availability of trained staff who handle annual planning tasks, sufficient funds for staff salaries received and paid in full and on time, turnover of iSC staff kept low, and the presence of guidance materials for annual work planning. So based on these requirements, the Primary store scored 100% and Sub-national centers scored 87% in annual work planning, both levels hitting the target recommended score of WHO. However, lower distribution and service provision levels scored 68% and 57%, respectively dragging down the overall score to 76%.
Supportive Supervision (M3)
Supportive supervision was another criterion used to assess effective supply chain management. It involved the use of standardized supervision checklists covering key vaccine management areas and the training of staff in supportive supervision techniques. The availability of guidance materials for supervision was also evaluated. Financial aspects included funds budgeted for supportive supervision, reliable fuel supply for vehicles, and the availability of transportation for scheduled supervision visits. Facilities were assessed to determine if they received sufficient funds for supervision activities. Additionally, the adherence to a fixed supervision schedule, the completion of all scheduled visits, and the provision of staff feedback were evaluated. Overall, supportive supervision among the assessed supply chain actors was 72%, with the Primary store scoring 96% and the Sub-national centers 70%. Lower distribution and service provision facilities scored 65% and 57%, respectively.
Discussions
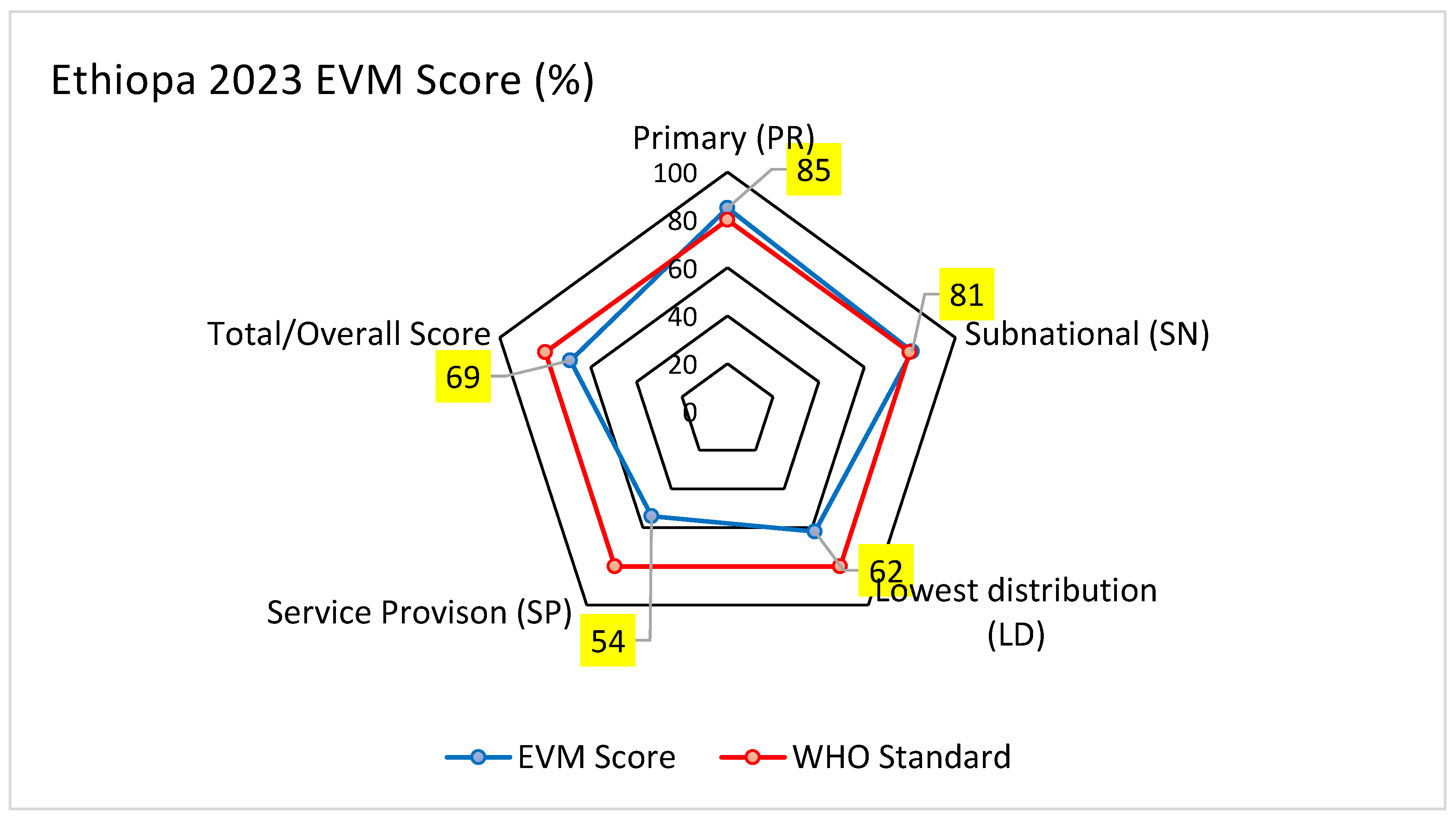
The national iSC management composite score was only 69% less than the 80% recommended target score. At primary (central) and sub-central levels the EVM score was around 80% hitting the target score. In each criterion score, the lowest distribution and service provision levels scored far less than the required target score, which led to the composite score being underestimated.
The EVM composite scores and many of the requirements in primary store and sub-national centers were promising as they are very close to 80%, these make the country among the few countries that had a better score in the African region [
11]. Though the country has comparable status with other African countries’ achievements overall performance is still behind, compared to global best-performing countries Thus there is a need to aspire for better performance and work towards achieving an overall effective immunization supply management performance giving special focus for SP levels of iSC [
13,
16]
Ethiopia achieved the target score of EVM in one of the vaccine management requirements. Better performance in the requirements was also observed in other developing countries such as Malawi, India, Benin, and Mozambique [
13,
16].
The service provision and lower distribution levels of iSC score are still below WHO targets in vaccine management. The criteria look into requirements that ensure operational and managerial aspects that pursue adequate and high quality vaccine availability at service points. Thus though the overall score is beyond the target, at lower levels where vaccine management should be highly stringent it falls behind. This might be related to a lack of resources or training of staff on vaccine management issues and unavailability of policies and procedures at service points. Thus if such interventions are done effectively at lower levels it can boost overall achievement to better scores.
At all levels temperature management, storage and transportation, and iSC performance monitoring criteria scores were consistently below the WHO target score levels. The temperature management requirement at PR is more stringent compared to SP’s and this is because at PR large volume of vaccines are kept. Effective supply chain management helps a lot in ensuring uninterrupted continuous supply and protecting vaccine efficacy and safety throughout its journey from manufacturer to end user [
8]. According to WHO data analysis of different countries that conducted EVM assessments in the year between 2013-2020, a similar finding was observed [
11]. The WHO global analysis also shows temperature management criteria were consistently below the targets for all iSC levels in the African region, southeast Asia, Eastern Mediterranean, and Pacific region being on target for only European and American regions [
11]. Temperature management is one of the critical indicators to maintain vaccine potency and efficacy there is a need to invest in functional cold chain equipment at all levels. The impact of lack of proper temperature management in immunization can be reduced vaccine potency, loss of efficacy, and increased wastage that hampers the ultimate goal of immunizations. Further ensuring proper temperature management is beyond equipment and it must include workforce training, energy stability, and real-time monitoring. Without these improvements, vaccine effectiveness is at risk, ultimately undermining immunization programs and public health outcomes [
5,
17].
A storage and transportation capacity (E3) criteria at all levels is also consistently below WHO target scores. The finding is much below compared to the global data analysis output where in most regions including the African region the criteria scores were above the WHO targets for all iSC levels [
11]. Storage and transportation capacity plays a pivotal role in iSC as it determines the maintenance of vaccine efficacy during storage, vaccine availability, and distribution at all levels. With incapacitated storage and transportation capacity, it will be very difficult to dream of an interrupted and safe immunization service. Haidari et al. argue investments are needed for both storage and transport capacity, as doubling storage capacity at service points or districts can only increase availability by 11% while doubling transportation capacity can increase availability by 26% [
18]. Thus the country’s health systems and other partners working on iSC should give due attention to strengthening storage and transport capacity for immunization.
Preventive maintenance (E5) and stock management (E6) criteria were on targets for PR and SN levels which was better than many African regions as shown in WHO global data analysis findings. But in this finding the indicators get far lower than WHO targets at SP and LD levels. This is consistently compared to the African region’s findings whereas it gets down to the lower distribution E5 gets a much lower score [
11]. The finding implies the stock management and preventive maintenance criteria are better functioning at higher levels which is encouraging, while at LD and SP where stock management is crucial, there are significant gaps to be considered. The absence of robust stock management systems at the service provision level may contribute to both vaccine unavailability during demand surges and avoidable wastage due to expiration or mismanagement.
At the Primary store, there were great achievements on some of the requirements scoring 90% and above including requirement scores of annual work planning, vaccine management, stock management, annual vaccine forecast, waste management, supportive supervision, and distribution of vaccines and dry goods. Sub-national centers also scored optimum in these criteria scoring 80-89%. But as we go down to the lower distribution and service provision levels the scores decline much below 80% in some cases even below 50%. This calls upon great attention from the state and other stakeholders working on immunization supply chain management.
Low waste management scores, particularly in service provision (60%) and lower distribution (65%), indicate significant gaps in the handling of immunization waste, which poses a serious health risk due to the infectious nature of the waste. Storage of waste in segregated areas was not common among institutions. There seems to be a lack of understanding or recklessness in to use of bins, color-coded bins, and proper disposal methods, and overfilling of waste poses risks of hazardous or infectious waste spills, endangering the health of the community.
Vaccine forecast, annual work planning, supportive supervision, and performance monitoring scores at service provision and lower distribution decline much below the targets. Ethiopia forecasts routine vaccine antigens every year and includes in GAVI vaccine renewal based on previous consumptions mainly for vaccines procured under co-financing like (PCV, IPV, Penta, Rota, Measles, and HPV) [
19]. Annual need forecast practice scored 60% among service provision health facilities and 76% among lower distribution facilities. There is a need to implement a policy requiring comprehensive work plans and forecasts at lower levels including SP and LD as it helps to have accurate estimates and ensures accountability for the performance of plans. Surprisingly at service provision immunization service performance monitoring practice score is only 33% this is an indication of gaps in proper design and implementation of supportive supervision and performance monitoring at lower levels. In order to address there is a need to have a coordinated approach involving stakeholders to enhance knowledge and understanding and create enabling environment and systems for the performance of supervision and monitoring.
The findings from this assessment were also compared to the 2013 and 2019 EVM assessment findings [
20,
21].
Table 3 compares the EVM assessment output in 2013, 2019, and 2023. Accordingly, on some variables, such as temperature management, the EVM assessment output of the three years shows a consistent value less than the target scores. Except for the SN level, temperature management greatly deteriorated over the years. However, there is an improvement between 2013 and 2023 for Central and Sub-national centers, from 40 to 73 for the former and 54 to 76 for the latter. The requirement showed a decrease in the score for LD and SP levels from 2013 to 2023. Temperature management is important during storage and transportation to prevent vaccine damage. So, the findings from the assessment tools call for immediate intervention to enhance proper temperature management, including enhancements to functional information technology systems and monitoring proper temperature management in storage and during transportation.
Vaccine arrival is one of the requirements where the country scored below the required target WHO score. Other developing countries such as Malawi had also scored similar scores [
12,
16]. The Primary store is involved in vaccine arrival activities and the comparison showed an increment in score from 2013 to 2023 but the score is lower compared to 2019. Overall, the scores in all three years are lower than the WHO-required target score.
The primary store demonstrated commendable improvement in stock management, vaccine management, maintenance and repair, and distribution of vaccines and dry goods between 2013 and 2023. Scores rose from well below the WHO target to around or above 90. This might be attributable to changes made based on the findings from previous EVM assessments of 2013 and 2019 including the cold chain.
The current assessment covered many facilities, with about 286 lower distribution and service provision facilities that are more than 4 folds included in either of 2013 or 2019 assessments [
20,
21]. Some criteria, surprisingly, are getting worse compared to the 2013 assessment; this includes storage and transportation capacity at all levels and facility infrastructure and equipment in lower distribution and service provision facilities. In almost all requirements the score for lower distribution and service provision facilities was much lower than the target WHO recommended score and mostly gets worse than in previous years. The decline observed in the requirements might be attributable to the population growth and introduction of new vaccines that require expansion of infrastructure and contrasting underinvestment in health systems. It can also be linked to internal conflicts in the country. Conflicts affect immunization services in various ways including the destruction of health facilities, staff turnovers, inaccessibility, challenges in transportation, and security concerns in transporting vaccines [
19,
22].