1. Introduction
The COVID-19 pandemic clearly demonstrated how politics, society, and science were compelled to modify long-standing and previously taken-for-granted principles to maintain functionality and resilience. This global health crisis required rapid adaptations across public and private life. Social interactions were minimized, physical distancing was instituted, and mask-wearing became the standard practice. These changes were implemented not only as temporary crisis measures but as part of a broader reconfiguration of societal norms, informed by evolving epidemiological evidence and public health guidelines. Subsequently, mRNA vaccination was established as one of the primary public health interventions, and health authorities asserted its safety and efficacy. This statement became part of the prevailing paradigm, framing mRNA vaccines as inherently safe, which in turn influenced what questions were deemed legitimate to investigate and how emerging safety signals were interpreted. The scientific community was subject to pressures, both explicit and implicit, to align itself with positions perceived as being on the “right side” of the consensus.
The newly established mRNA vaccines are estimated to have saved 14–20 million lives worldwide in the first year of rollout, according to the World Health Organization [
1]. However, a more recent analysis estimated that approximately 1 to 4 million lives were saved between 2020 and 2024, although the benefit was mostly limited to older individuals [
2]. Concerns about vaccine safety were raised following the rollout of mRNA vaccines, particularly given that published literature has suggested a possible link between excess mortality and the widespread deployment of these vaccines. Paradoxically, despite Omicron’s milder disease profile, countries with extensive vaccination coverage registered unanticipated increases in excess mortality during and after the Omicron waves [
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13]. The temporal association between widespread COVID-19 vaccination campaigns and concurrent changes in excess mortality patterns requires rigorous systematic investigation.
“Correlation does not imply causation” is a fundamental concept in epidemiology and statistics. It emphasizes the possibility that observed associations are due to confounding variables, bias, or random chance, rather than a direct causal relationship [
14,
15]. Although it is crucial for avoiding hasty conclusions, it has often been misused, leading to the premature decision not to initiate investigations into possible causal relationships [
14]. As Thomas S. Kuhn (1922-1996) observed, scientific communities generally resist anomalous findings that challenge the prevailing paradigm. This resistance can lead to the premature dismissal of emerging causal hypotheses if they are incompatible with the accepted theoretical framework [
16]. Kuhn questioned the widely held belief that science develops via the steady accumulation of empirical data in his seminal book,
The Structure of Scientific Revolutions (1962). Instead, he argued that scientific progress occurs within paradigms, shared frameworks of theory, method, and assumption that determine what questions scientists consider legitimate, what evidence they deem valid, and what explanations they find acceptable [
16]. A paradigm, according to Kuhn, defines not only the answers but also the very structure of permissible research. Scientists operating within a paradigm are engaged in what he termed “normal science”, a process of puzzle-solving within the boundaries of accepted theory. Findings that defy the conventional paradigm are referred to as “anomalies”, and they are frequently ignored or dismissed. Only when these anomalies accumulate to an inflection point does the prevailing paradigm enter a “crisis phase”, which can potentially lead to a paradigm shift and a radical restructuring of the conceptual framework [
16].
During the COVID-19 pandemic, an interesting pattern in public and academic discourse on vaccine safety emerged: Any perceived link between vaccination and severe adverse events (SAEs) was disregarded on the basis that a correlation did not prove causation. Although theoretically sound, this argument prevented further research by discouraging rigorous empirical inquiry. Reports of temporally associated SAEs, such as autoimmune reactions, neurological syndromes, cardiovascular events, or sudden death, were labelled as ’coincidental’ without thorough studies being performed. The principle that “correlation does not imply causation” subtly shifted from an invitation to deepen investigation into a reason to foreclose it.
Here, we examine how this shift has unfolded from historical, philosophical, and practical perspectives, and consider its implications for the integrity of science and public health. Furthermore, we analyze a case study that introduced an immunohistochemical (IHC) method that supposedly could demonstrate whether mRNA-based vaccines against SARS-CoV-2 contributed to or were the cause of death.
2. The Correlation-Causation Distinction: Origins and Utility
Researchers and physicians sometimes fail to distinguish between correlation and causation, a differentiation that is essential in medical research. Despite demonstrating a connection between two variables, correlation does not necessarily mean that changes in one variable result in changes in the other. This misunderstanding may lead to poor clinical judgments and public health policy [
14,
15,
17]. David Hume (1711-1766), a preeminent philosopher of the 18th century who fundamentally reshaped the philosophical understanding of causation through his rigorous analysis of causal inference. He suggested the human tendency to perceive causal connections is rooted not in logical necessity or direct observation, but rather in psychological habit and expectations [
18]. In the 20th century, statisticians such as Austin Bradford Hill (1897-1991) and Ronald A. Fisher (1890-1962) developed rigorous methodologies to evaluate whether correlations in observational data could indicate causal relationships, especially in epidemiology [
19]. From the landmark epidemiological association between smoking and lung cancer, the well-substantiated neurodevelopment impairments attributable to lead exposure, and the extensive mortality risk associated with air pollution, their groundbreaking contributions to public health have proven essential in a variety of important discussions. Notably, in each case, correlation served as the foundation for further investigation rather than a reason to dismiss it [
19].
3. Challenging the Dogma: Do Vaccines Cause Harm?
Vaccination has played a critical role in reducing infectious disease morbidity and mortality rates, helping to meet Sustainable Development Goal (SDG) 3, which aims to guarantee healthy lives and promote well-being at all ages [
20,
21,
22]. By reducing uneven access to healthcare and economic disparities among various socioeconomic groups, vaccines are largely regarded as one of the most effective strategies and as being crucial to advancing global health [
23]. As demonstrated in several reports, specific vaccines are highly effective in preventing child mortality from several diseases, including beneficial non-specific effects [
20,
24,
25,
26,
27,
28,
29,
30]. Health authorities must rigorously assess the benefits of vaccination against potential risks when addressing diseases with significant morbidity and mortality. While vaccines can cause AEs and induce theoretical unknown risks, the full safety profile only emerges after widespread population use. These potential harms can complicate vaccination decisions at both individual and policy levels, making comprehensive risk assessment challenging until extensive real-world deployment occurs [
31].
In the context of emerging vaccine safety signals, such as myocarditis, thrombotic events, anaphylaxis, myocardial infarction, pulmonary embolism, or death, the correlation-causation maxim has often been utilized anticipatorily to guide the interpretation of observational data studies. Public health authorities, regulatory agencies, and media outlets have stated that “there is no evidence of causality,” even though causality had not yet been investigated in the early stages of surveillance.
This rhetorical strategy serves two functions:
- (i)
It protects the vaccine program from reputational harm.
- (ii)
It delegitimizes anecdotal or post-marketing reports as “anti-vaccine” or “misinformation”.
As a result, a legitimate scientific principle becomes a defensive dogma. Both institutional constraints and cognitive biases can affect scientific inquiry. Research topics that pose a threat to established theoretical frameworks, financial or political interests, are usually marginalized or systematically excluded from mainstream scientific discourse and sometimes bullied by health authorities. If manifest outside the established surveillance timeframe or occur in forms unanticipated by trial designers, they may remain unrecognized. According to Kuhn, when anomalies arise, they are often initially overlooked or excluded because they do not fit within the established paradigm, which inherently limits what is observed and reported [
16]. This aligns with Carl Sagan’s maxim, “the absence of evidence is not evidence of absence”, which underscores that phenomena may exist despite not being detected within the constraints of the current paradigm [
32].
According to Kuhn, paradigms influence the questions scientists formulate, the approaches they adopt to address those issues, the standards for valid evidence, and the interpretation of findings. Scientists work within this framework, focusing on solving puzzles defined by the accepted paradigm [
16]. Unfortunately, the prevailing paradigm surrounding the vaccination of people against SARS-CoV-2, which asserts the efficacy of vaccines in preventing severe illness and death, has become a dogma that precludes consideration of the possibility that vaccines may also be associated with SAEs.
4. Case Report Analysis Under the Bradford Hill Criteria
In 1965, Austin Bradford Hill evaluated the conditions under which causation may be inferred from observed associations. To overcome this challenge, he presented a system of nine criteria for guiding causal inference in epidemiology: strength of association, consistency, specificity, temporality, biological gradient, plausibility, coherence, experimental evidence, and analogy [
33]. Despite recognized limitations, particularly in precision and subjectivity, and the emergence of newer alternatives, Hill’s criteria have stood the test of time and are still widely used as a conceptual guide, especially in public health [
34,
35].
In 2022, Dr. Michael Mörz published a case report titled:
“Multifocal Necrotizing Encephalitis and Myocarditis after BNT162b2 mRNA Vaccination against COVID-19”[
36]. Here, we will analyze the main findings from that work and evaluate them through the lens of the Bradford Hill criteria. Dr. Mörz reported the case of a 76-year-old male patient diagnosed with Parkinson’s disease (PD) who died 21 days after receiving a third COVID-19 vaccine. He was immunized with two doses of the BNT162b2 vaccine in July and December 2021, following the initial administration of the ChAdOx1 nCoV-19 vector vaccine in May 2021. Because the clinical symptoms were unclear before death, the family requested an autopsy. Post-mortem investigations confirmed PD. Additionally, there were clear signs of systemic arteriosclerosis and aspiration pneumonia.
However, histopathological examinations of the brain revealed previously uncovered abnormalities, such as multifocal necrotizing encephalitis of unknown cause, characterized by severe inflammation and a glial and lymphocytic response. There was also evidence of acute vasculitis, which was primarily lymphocytic, but no evidence of mild acute lymphohistiocytic myocarditis and vasculitis in the heart, alongside persistent cardiomyopathy.
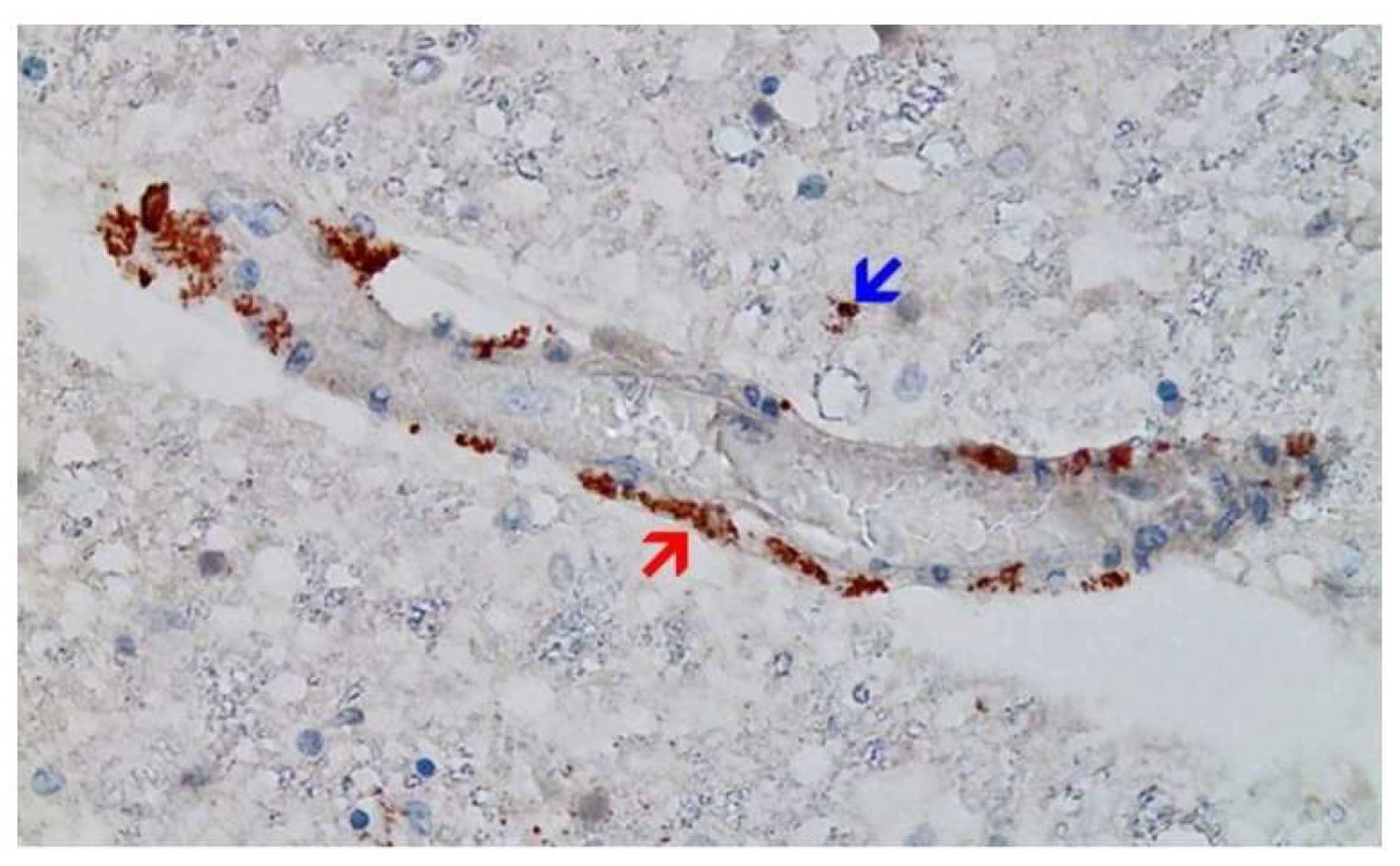
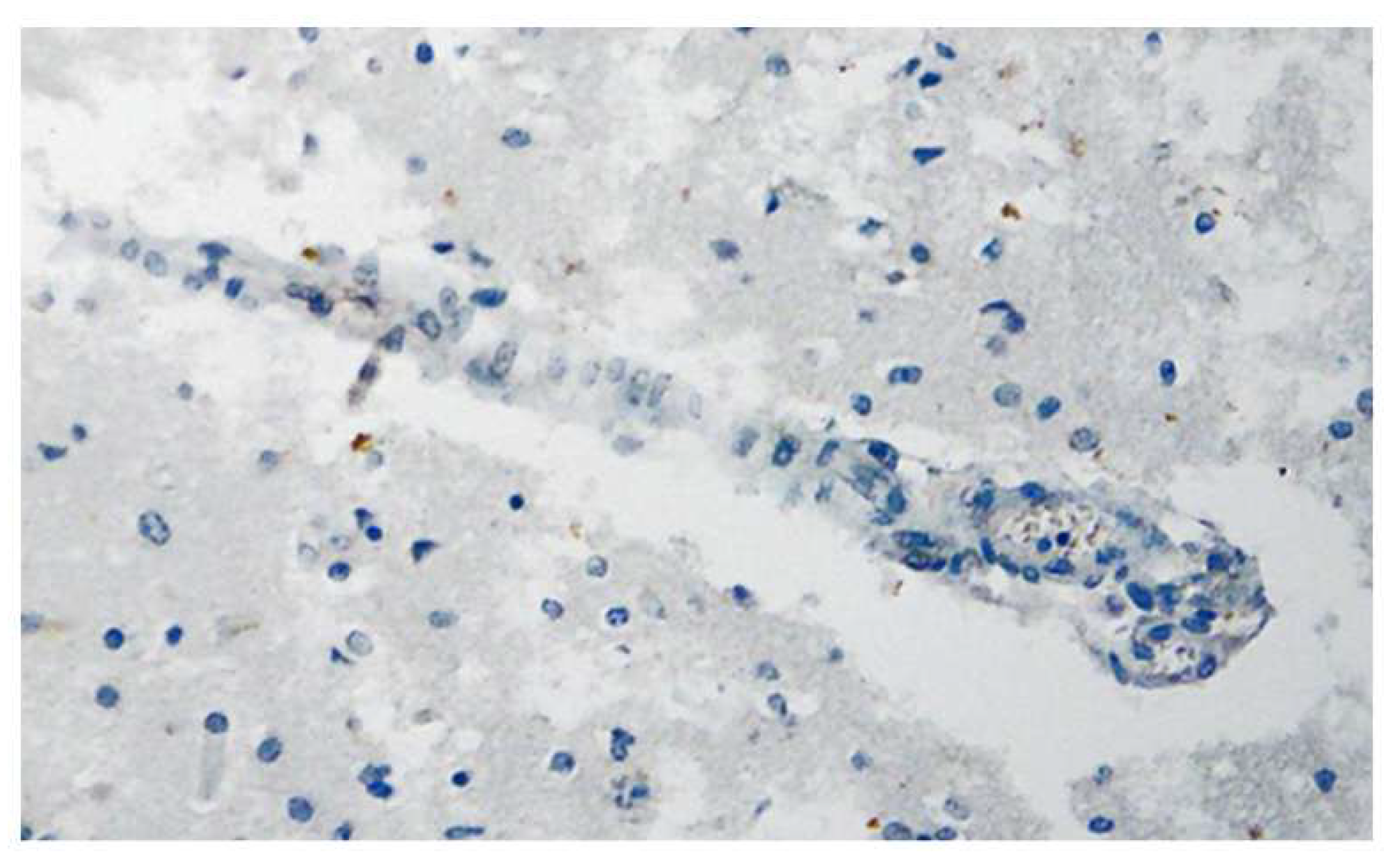
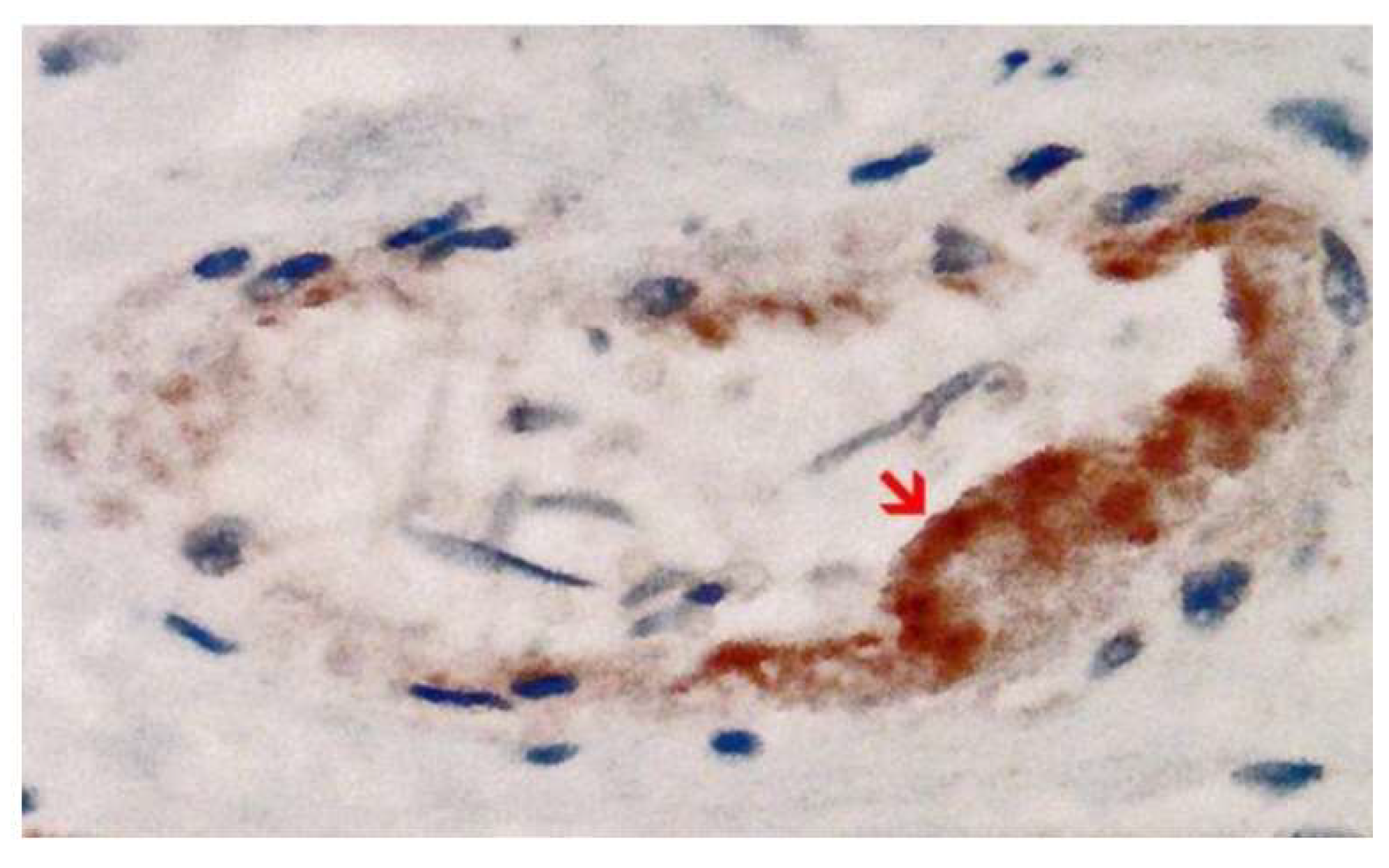
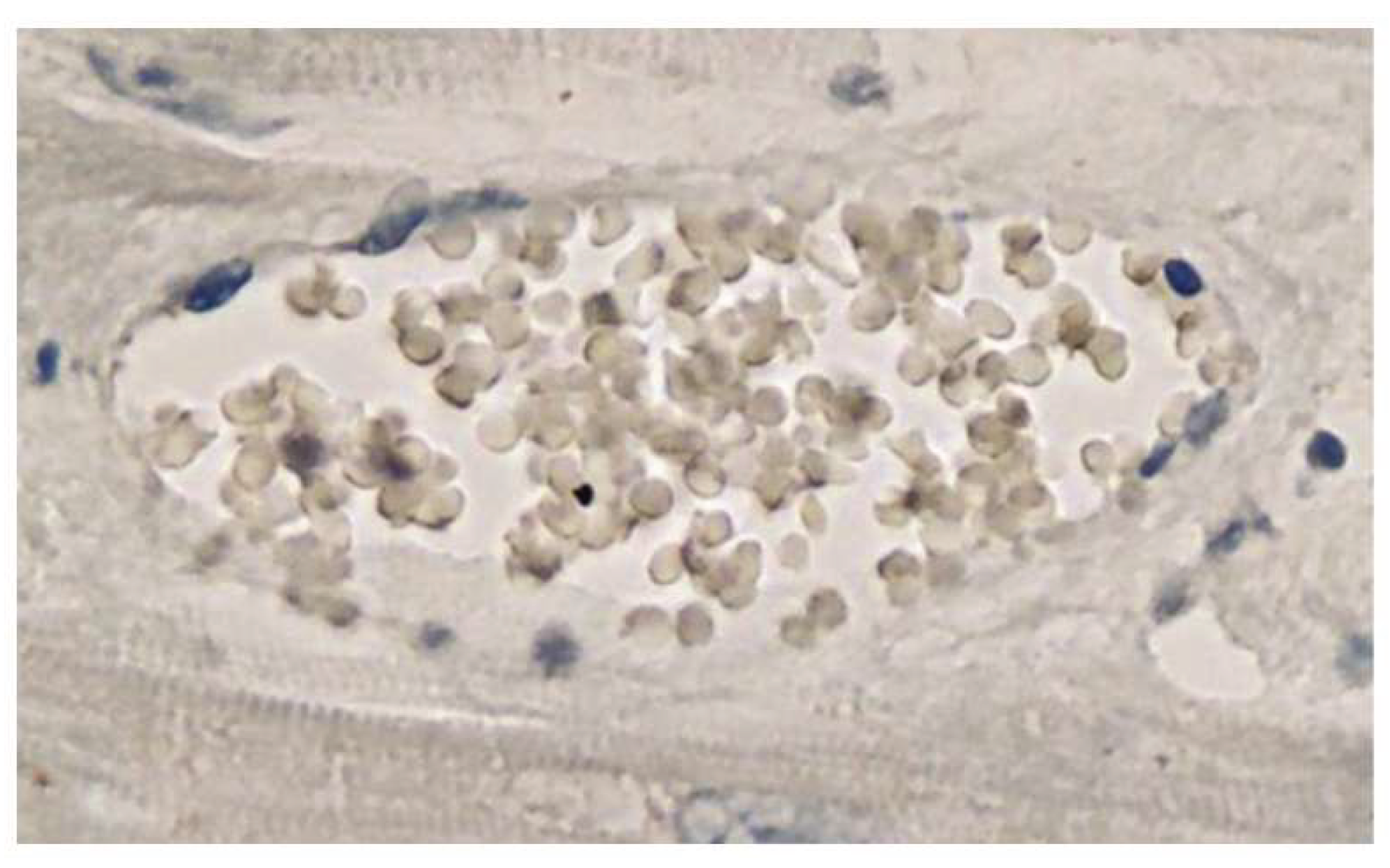
The man did not have a confirmed SARS-CoV-2 infection. IHC was performed to identify viral antigens, specifically the nucleocapsid (N) and spike proteins (S). Remarkably, that technique revealed the presence of S, but not the N protein, within inflammatory areas of the brain and heart, notably in the endothelial cells of small blood vessels. These two proteins are expressed during SARS-CoV-2 infection. On the other hand, mRNA-based vaccines only encode the genetic information for the S protein. Thus, the presence of the S protein and absence of the N protein in the brain (
Figure 1 and Figure 2) and heart (
Figure 3 and Figure 4), respectively
, was inferred to be a consequence of the vaccination rather than evidence of infection [
36].
The official cause of death was recorded as chronic aspiration-related pneumonia, a common complication in PD [
37,
38]. Additionally, it revealed necrotizing encephalitis and myocarditis. While the histopathological signs of myocarditis were relatively modest, the encephalitis had resulted in significant multifocal necrosis and may have contributed to the fatal consequence. Encephalitis frequently results in epileptic seizures, and the tongue bite observed during the autopsy indicated that this may have occurred in this instance [
36]. Although this is a single case report and therefore limited in its generalizability, it can be used to evaluate whether an observed association may be causal. Here, we will analyze this single case study to determine which of the nine criteria are fully, partially, or not fulfilled.
I. Temporality
SAEs such as neurological and cardiac events occurred following the administration of the second and third doses of the Pfizer-BioNTech (BNT162b2) vaccine. The last dose (BNT162b2) was administered three weeks before death, and the timeline of symptom progression has been documented.
Assessment: Fulfilled
II. Plausibility
The presence of the S protein without the N protein in brain and heart tissue (including endothelial cells, glial cells, and microglia) was proposed to be consistent with vaccine-derived S protein rather than natural infection [
36]. It has been demonstrated that the synthetic S protein persists for several weeks [
39], potentially triggering vascular inflammation and tissue damage, a mechanism that is both biologically plausible and supported by existing literature [
40,
41,
42]. A study by Schreiber
et al. investigated whether the SARS-CoV-2 S S1 protein by itself can affect the brain. In mice, intravenous, intranasal, and intracerebral administration of S1 triggered neuroinflammation and alterations in α-synuclein levels in regions linked to PD. The effects varied by exposure route and sex, with intravenous S1 causing the strongest α-synuclein accumulation and microgliosis. The findings suggest that circulating or tissue-retained S protein may contribute to long-term neurological changes, even in the absence of active viral infection [
43]. Failure to perform aspiration during the administration of mRNA vaccines in humans could potentially lead to the unintended injection of the vaccine into the bloodstream [
44].
Assessment: Fulfilled
III. Coherence
The S protein derived from vaccination or infection can induce, in some cases, endothelial dysfunction, immune cell infiltration, and tissue inflammation. The pathology is consistent with previously reported vaccine-related myocarditis and encephalitis [
45,
46,
47,
48,
49,
50].
Assessment: Fulfilled
IV. Specificity
Although it is one of the weaker criteria in modern epidemiology (as most exposures can have multiple effects, and they may stem from diverse causes), it still offers value when a cause leads to a narrowly defined effect. This criterion was not completely fulfilled since Parkinson’s disease was the patient’s underlying diagnosis. The potential contribution of this underlying illness to the encephalitis and myocarditis observed during post-mortem investigation warrants consideration. However, the temporal characteristics clearly distinguish these conditions: PD had been present as a chronic condition for years, whereas the encephalitis represented an acute inflammatory process. On the other hand, no case record of PD causing subsequent necrotizing encephalitis has been reported, nor is there a convincing mechanism. This suggests that the observed encephalitis was unlikely to be a direct consequence of PD [
36]. Although the N protein was absent, the patient may have had an asymptomatic infection. Following COVID-19 vaccination, cases of necrotizing encephalitis and encephalomyelitis have been reported [
45,
46,
51,
52,
53,
54,
55,
56,
57], and numerous studies have reported that SARS-CoV-2 infection can also trigger encephalitis [
58,
59,
60,
61,
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73,
74,
75,
76,
77,
78,
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89,
90].
It should be considered that most of those encephalitis cases occurred in 2020, before the initiation of COVID-19 mass vaccination campaigns. This suggests the causal agent was the virus. Of particular interest are five case reports, the first from 2020, on SARS-CoV-2-associated acute hemorrhagic, necrotizing encephalitis presenting with cognitive impairment in a 44-year-old woman without comorbidities [
91], and the second from 2021 reported the case of fatal acute hemorrhagic necrotizing encephalitis in a two-month-old boy [
92]. The remaining cases from 2021 reported fatal necrotizing encephalitis associated with COVID-19 [
93,
94].
Another important confounding factor is the fact that the official cause of death was aspiration pneumonia. Nevertheless, Mörz reported that the patient experienced a sudden collapse during dinner, notably without coughing or other signs of food aspiration, simply falling from his chair. This opens a debate of whether the sudden loss of consciousness was the result of aspiration pneumonia [
36].
Assessment: Partially fulfilled
V. Biological Gradient (Dose-Response Relationship)
The case report lacks statistical quantification. The observed clinical deterioration following each vaccine dose is consistent with a biological gradient. Furthermore, existing reports from peer-reviewed literature have noted: (i) An increased incidence of myocarditis, particularly in young males, after the second dose compared to the first dose of mRNA vaccines [
95,
96,
97], (ii) Recent research indicates that individuals with pancreatic cancer who received more COVID-19 booster shots had a worse overall survival rate. Interestingly, the study showed that a poor prognosis for these patients was associated with elevated IgG4 antibody levels brought on by vaccination [
98].
Assessment: Partially fulfilled
VI. Analogy
Similarities can be drawn with other reported cases of vaccine-induced myocarditis and encephalitis [
45,
46,
47,
48,
49,
50], as well as with the effects of natural infection, which also involve S protein expression and vascular inflammation [
40,
41,
42]. After Mörz’s work, Mikami et al. [
54] described a case of an 84-year-old man who died approximately 10 weeks after receiving his fourth COVID-19 vaccination, after being admitted to the hospital due to fever and impaired consciousness. Upon autopsy, the thalamus, pons, and cerebellum showed perivascular T-cell infiltration and acute ischemic change with microhemorrhage, which were thought to be connected to neurological symptoms. Ascites, pleural fluid accumulation, and right ventricular dilatation all pointed to right heart failure. Despite a negative COVID-19 polymerase chain reaction test, IHC analysis for S and N SARS-CoV-2 antigens was conducted to determine the cause of death. The thalamus, pons, pituitary, and adrenal glands were found to contain just the SARS-CoV-2 S protein, which was unexpected. Like Mörz [
36], Mikami et al. [
54] concluded that the presence of the S protein was likely attributable to vaccination rather than viral infection, as no N protein was detected. However, the absence of the N protein is insufficient to definitively confirm a mRNA vaccine-derived origin of the S protein.
Assessment: Partially fulfilled.
VII. Consistency
Although Mörz referenced comparable cases in the literature, there are only two cases reported in the literature [
36,
54]; therefore, the consistency of findings across studies and populations has not been demonstrated.
Assessment: Not fulfilled
VIII. Experimental Evidence
Mörz argued that the IHC findings in the report provided direct and compelling evidence that protein from the vaccine was present in the affected tissues and that this was spatially associated with the lesions. According to Mörz, his study was the first to provide evidence of vaccine-derived S protein within encephalitic lesions, suggesting that vaccination rather than SARS-CoV-2 infection was the cause of death [
36]. However, such affirmation is incorrect because the IHC technique employed does not possess the analytical specificity to distinguish between naturally occurring S protein and that of synthetic origin, and because the absence of the N protein does not rule out an asymptomatic infection [
99]. Besides, the case report included positive control stained anti-S1 of the S protein but lacked a negative control. Isotype control staining to determine unspecific binding was not performed.
The balance between production and degradation is reflected in the quantity of proteins [
100]. The stability of cellular proteins varies widely, ranging from a few minutes to several hours, and can be strictly controlled in response to a range of pathophysiological inputs, both internal and external [
101]. Li et al. [
102] employed immunoblot analysis to quantify the half-lives of SARS-CoV-2 proteins in lung epithelial cells, thus considering the dynamic nature of viral protein expression. 0.4 to 8 hours was the short half-life of eighteen of these unstable proteins. With half-lives of more than eight hours, the remaining seven proteins—NSP2, NSP5, NSP10, NSP15, S, N, and M—were comparatively stable. These proteins are degraded by the ubiquitin-proteasome system after the acute infection resolves [
102]. These findings strongly suggest that the N protein should remain detectable for 8 hours to a few days during acute symptomatic or asymptomatic infections. Therefore, its short half-life cannot be used to infer that its absence demonstrates a vaccine origin. In contrast, the half-life of the S protein is longer. Coordinated SARS-CoV-2-specific CD4
+ and CD8
+ T cell responses are associated with mild disease, and infected cells expressing S protein are cleared by cytotoxic T lymphocytes (CD8
+) cells within 1–2 weeks after infection [
103]. However, Rong et al. [
104] found that long after the virus was cleared, SARS-CoV-2 S protein continued to accumulate in the skull-meninges-brain axis of long COVID patients.
Therefore, the definitive method to confirm the presence of vaccine-derived S protein requires amino acid sequence analysis of the S protein itself to verify its unique molecular characteristics. A review of the published literature revealed that only the study by Brogna et al. [
39] has conclusively demonstrated the presence of the synthetic S protein in blood. These researchers revealed that although the mRNA from the BioNTech/Pfizer (BNT162b2) and Moderna (mRNA-1273) vaccines is different, both direct the synthesis of an identical recombinant S protein [
39]. This recombinant S differs from the wild-type S protein due to a double amino acid substitution at residues 986 and 987 (K986P and V987P), replacing lysine and valine with two proline residues (herein designated PP-spike). These alterations stabilize the S protein in an inactive prefusion conformation and eliminate a tryptic digestion site, thereby enabling differentiation of vaccine-derived synthetic S protein from naturally occurring S protein in biological fluids via tryptic digestion and mass spectrometry [
39].
Trypsin, a hydrolase enzyme, cleaves proteins into smaller polypeptides by targeting peptide bonds adjacent to arginine (R) and lysine (K) residues [
105]. This specificity enables differentiation between synthetic and natural S proteins based on their distinct tryptic digestion profiles:
- (i)
Trypsin digestion of the PP-S protein, encoded by vaccine mRNA, yields a characteristic LD
PPEAEVQIDR fragment (PP-spike marker) [
39].
- (ii)
In contrast, trypsin digestion of the viral S protein generates two smaller fragments: LDK and VEAEVQIDR [
39].
This methodological approach specifically identifies the source of S protein in tissues or fluids [
39,
105]. The absence of this mutation in over 6,600,000 sequenced SARS-CoV-2 genomes has shown that none, including the Omicron variant, harbor the K986P and V987P mutations [
106], further validating the technique’s reliability in ruling out natural infection as the source of detected S protein.
Ota
et al.[
99] conducted in situ hybridization (ISH) to determine if the S protein expression was caused by SARS-CoV-2 infection or mRNA vaccination, since some cases displayed positive staining for the S protein via IHC. Such a method was performed for cases of hemorrhagic stroke where the infection history was unclear, or when a considerable period had passed following vaccination. The vaccine and SARS-CoV-2 mRNA were both identified by ISH. This indicates that the vaccine cannot be solely responsible for the spike protein-positive staining seen in patients who have received SARS-CoV-2 vaccination but have no recorded history of viral infection. Notably, the N protein was consistently negative in all cases, confirming the high sensitivity of ISH. This capability allows for the detection of traces of mRNA, which may indicate undetected asymptomatic infections. These results emphasize the importance of being cautious in assuming that the S protein originates exclusively from mRNA vaccination [
99]. While Ota
et al. [
99] detected vaccine mRNA, suggesting possible spike protein synthesis, the simultaneous presence of viral mRNA prevents definitive attribution without protein-level sequencing. There is a possibility that the hemorrhagic stroke was caused by a past asymptomatic SARS-CoV-2 infection. Although the N protein was not detected (due to its short half-life), the presence of viral mRNA has been detected long after the acute infection had passed. For example, a study found that viral mRNA was identified in 16 (30%) of 53 solid tissue samples collected at 1 month, 38 (27%) of 141 samples collected at 2 months, and 7 (11%) of 66 samples collected at 4 months. 10 distinct solid tissue types (liver, kidney, stomach, intestine, brain, blood vessel, lung, breast, skin, and thyroid) were found to have viral mRNA. Furthermore, 26 (43%) of the 61 solid tissue samples that tested positive for viral RNA also tested positive for subgenomic RNA [
107].
Assessment: Partially fulfilled
IX. Strength of Association
This criterion addresses the statistical strength of the association between an exposure (vaccination) and an outcome (AE). It is often measured using metrics such as the relative risk (RR), the odds ratio (OR), and risk difference. A larger effect size (i.e., higher RR or OR) suggests a stronger association [
108]. This criterion is not met, as it relies on a single case report without clearly defined proof of vaccine-derived S protein presence in the examined tissues. Here, isotype, negative and positive controls must be added.
Assessment: Not fulfilled
5. Discussion
A comprehensive analysis of Mörz’s work [
36], as evaluated through the Bradford Hill criteria, revealed that three of the nine criteria presented strong evidence for causality, four were partially fulfilled, and two were not fulfilled. Overall, the report provides approximately 55% evidential support for a causal relationship. This is suggestive but not conclusive evidence for a causal relationship between the effects of the BNT162b2 vaccine and multifocal necrotizing encephalitis and myocarditis. The temporal association is strong; however, being a single-case report, potential confounding by comorbidities (PD and pneumonia), insufficient strength of association, and incomplete experimental data limit the capacity to establish definitive causality.
Importantly, the absence of the N protein cannot serve as definitive evidence for demonstrating a vaccine origin [
99]. Even though Mörz [
36], Mikami et al. [
54], and Ota
et al. [
99] could not specifically detect the presence of the S protein derived from the mRNA vaccine, the relevance of these studies lies in the fact that, from the Kuhnian perspective, they constitute “anomalies”. These investigations, alongside the research conducted by Brogna et al. [
39] have initiated what Kuhn termed the “crisis phase”, which emerges when a mismatch between empirical evidence and established paradigms becomes increasingly apparent. These anomalous phenomena encounter a strong resistance from adherents to the prevailing paradigm, even if they constitute an accumulating body of evidence that generates epistemological tension within the scientific community and progressively creates the conditions for revolutionary science and a paradigm shift [
16].
At present, the only method capable of distinguishing between the naturally occurring SARS-CoV-2 S protein and vaccine-derived S protein is the tryptic digestion and mass spectrometry analysis employed by Brogna et al. [
39]. This method is particularly valuable in post-mortem and clinical investigations of SAEs potentially associated with mRNA COVID-19 vaccines, enabling precise attribution of pathological findings to vaccination rather than viral infection. Additionally, the technique has the potential to enhance the range of methodologies employed in the post-marketing surveillance of mRNA vaccines. This would facilitate a thorough investigation into the molecular mechanisms underpinning rare SAEs, thereby contributing to the optimization of vaccine design and the establishment of robust safety monitoring protocols.
This technique should be employed in future studies, encompassing large-scale case series of healthy individuals who died unexpectedly following an mRNA vaccination, in addition to controlled clinical studies or detailed mechanistic investigations. The works by Mörz [
36], Brogna et al.[
39], Mikami et al.[
54], and Ota et al. [
99] exemplify the epistemological constraints that may limit pathological interpretation when empirical findings challenge established paradigmatic frameworks, potentially resulting in systematic underappreciation of novel causal mechanisms. The molecular differentiation [
39] between vaccine-induced and infection-induced S protein represents a paradigmatically disruptive innovation that provides empirical evidence for vaccine-related pathology, previously attributed to coincidental occurrence or alternative etiologies. As boosters continue to be promoted, it is urgent to implement the recommended strategies.
Kuhn’s work is particularly relevant to the COVID-19 pandemic, where the rapid development of novel vaccine technologies coincided with intense pressure to maintain public trust and policy coherence. In this context, any data that challenged the dominant paradigm that vaccines are inherently safe, effective, and largely free of SAEs was at risk of being treated not as anomalies warranting further study but as threats to be neutralized. Statements such as ‘the vaccine remains localized at the injection site’ and ‘the S protein is rapidly degraded’ served to reinforce the dominant narrative by providing reassurance. Yet emerging data refuted these claims. For instance, biodistribution studies have documented S protein expression both as a membrane-bound antigen on transfected cells and as a soluble protein detected in the blood circulation [
39,
109], indicating systemic antigen dissemination beyond the injection site. Several works have reported that, rather than remaining localized at the injection site, the mRNA vaccines (or their components) can travel to distant organs, including the liver [
109,
110], lungs [
109,
111], kidneys [
109,
111], lymph nodes [
112,
113,
114], spleen [
109,
111], heart [
109,
114], and brain [
109,
115].
A recent study revealed that half of the blood samples examined contained synthetic S protein, which is inherently difficult to degrade. The intervals between immunization and the subsequent detection of the vaccine-derived S protein were 69 and 187 days, respectively [
39]. Another study found S protein expression in cerebral arteries in patients with hemorrhagic stroke up to 17 months after vaccination, suggesting potential long-term tissue persistence [
99]. The biodistribution of mRNA vaccines and their long-term safety were seriously called into question by the discovery that 43.8% (7 out of 16) of vaccinated individuals had S protein expression [
99]. However, not all of these cases necessarily had only the S protein derived exclusively from the vaccine. ISH was conducted on three of these seven cases, and it detected both the vaccine-derived mRNA and SARS-CoV-2 viral mRNA, indicating a mixed origin of the S protein expression. The three cases (4, 5, and 15) represented 18.75% of the total 16 vaccinated individuals in the study. The authors acknowledged that asymptomatic SARS-CoV-2 infection could not be entirely excluded as a contributing factor [
99].
This finding further complicates the determination of causality, as the observed hemorrhagic stroke cases may result from the combined effects of both viral and synthetic spike proteins. Indeed, recent work has proposed that SAEs associated with the COVID-19 mRNA vaccines might be amplified by SARS-CoV-2 infection. The authors have coined the “Hybrid Harm Hypothesis”, which posits that “interactions between COVID-19 mRNA injections and later coronavirus infections may explain the manifestation and/or persistence of SAEs in previously vaccinated individuals, even after the emergence of milder Omicron variants. In many cases, the biological impact of COVID-19 mRNA vaccination may constitute a precursor event, predisposing the individual to develop the post-COVID-19 sequelae. Coronavirus infections may amplify the adverse effects of previous COVID-19 vaccinations over the course of years rather than months” [
116].
According to another study, the S1 subunit of the S protein, which is associated with inflammation and symptoms resembling those of post-acute COVID-19 sequelae, can remain in some monocytes for up to 245 days following vaccination [
117]. This suggests that some individuals may experience prolonged symptoms potentially attributable to the persistence of vaccine-induced S protein [
117]. Recent work found that two out of 40 individuals with post-vaccination syndrome (PVS) had measurable S protein levels for over 700 days after vaccination; however, this was not consistent for all PVS patients. The majority of those with detectable S protein levels had it in the lower range (26-300 days). Although additional research is required, this prolonged persistence may be linked to chronic symptoms [
118]. However, these last two studies were unable to differentiate between the SARS-CoV-2 S protein and the synthetic one, so they cannot be used to draw causal inferences in forensic pathology.
This work contends that hasty invocation of the principle “correlation does not imply causation” was not always intended to ensure methodological rigor, but rather to dismiss inconvenient evidence that threatened the established paradigm. By categorically dismissing reports of SAEs occurring after vaccination as “coincidental” without adequate investigation, the principle was transformed from a tool of caution into a shield against epistemic disruption. The history of science reminds us that progress does not come from the passive accumulation of data but from the willingness to question prevailing paradigms and critically evaluate correlations for causal meaning. As Kuhn cautioned, the entrenchment of dominant frameworks can blind the scientific community to anomalies and inhibit exploration of alternative explanations. Much of science advances precisely by uncovering correlations that are later shown to be either causative or, more often, spurious. But if causality is not actively investigated, science risks falling into fallacies that not only erode the integrity of research but also jeopardize the well-being of populations who rely on it. True scientific progress demands vigilance, openness to anomalous findings, and a continual readiness to reassess what we think we know. Thus, scientific anomalies should be met with enthusiastic curiosity, precisely because they hold the potential to shift prevailing paradigms rather than merely threaten the comfort of established narratives.
Author Contributions
Conceptualization, A.R.-C., V.N.U. and E.M.R.; formal analysis, V.N.U., A.R.-C. and E.M.R.; investigation, A.S.S., E.M.R., D.C., M.R., M.F., V.N.U., M.P., C.B., S.N.G., and A.R.-C.; data curation, E.M.R., D.C., V.N.U., C.B. and A.R.-C.; writing—original draft preparation, V.N.U., A.R.-C.; writing—review and editing, E.M.R., D.C., V.N.U., M.P., C.B., M.P., M.R., M.F., A.S.S., S.N.G., and A.R.-C.; visualization, A.R.-C. and V.N.U.; supervision, V.N.U. and A.R.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A.; Pezzullo, A.M.; Cristiano, A.; Boccia, S. Global Estimates of Lives and Life-Years Saved by COVID-19 Vaccination During 2020-2024. JAMA Health Forum 2025, 6, e252223. [Google Scholar] [CrossRef]
- Kuhbandner, C.; Reitzner, M. Estimation of excess mortality in Germany during 2020-2022. Cureus 2023, 15. [Google Scholar] [CrossRef]
- Forthun, I.; Madsen, C.; Emilsson, L.; Nilsson, A.; Kepp, K.P.; Björk, J.; Vollset, S.E.; Lallukka, T.; Skrindo Knudsen, A.K. Excess mortality in Denmark, Finland, Norway and Sweden during the COVID-19 pandemic 2020–2022. European Journal of Public Health 2024, 34, 737–743. [Google Scholar] [CrossRef]
- Jang, M.; Kim, S.; Choi, S.; Ryu, B.; Choi, S.Y.; Choi, S.; An, M.; Kim, S.-S. Estimating Excess Mortality During the COVID-19 Pandemic Between 2020–2022 in Korea. J Korean Med Sci 2024, 39, e267. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Jang, H.; Oh, J. Excess mortality during the Coronavirus disease pandemic in Korea. BMC Public Health 2023, 23, 1698. [Google Scholar] [CrossRef] [PubMed]
- Scherb, H.; Hayashi, K. Annual all-cause mortality rate in Germany and Japan (2005 to 2022) with focus on the COVID-19 pandemic: hypotheses and trend analyses. Med Clin Sci 2023, 5, 1–7. [Google Scholar] [CrossRef]
- Okoro, E.O.; Ikoba, N.A.; Okoro, B.E.; Akpila, A.S.; Salihu, M.O. Paradoxical increase in global COVID-19 deaths with vaccination coverage: World Health Organization estimates (2020–2023). International Journal of Risk & Safety in Medicine 2025, 09246479251336610. [Google Scholar] [CrossRef] [PubMed]
- Aarstad, J. Is There a Link between the 2021 COVID-19 Vaccination Uptake in Europe and 2022 Excess All-Cause Mortality? 2023.
- Aarstad, J. Deaths among young people in England increased significantly in 10 of 11 weeks after COVID-19 vaccination and doubled in three. EXCLI journal 2024, 23, 908. [Google Scholar]
- Mostert, S.; Hoogland, M.; Huibers, M.; Kaspers, G. Excess mortality across countries in the Western World since the COVID-19 pandemic:‘Our World in Data’estimates of January 2020 to December 2022. BMJ Public Health 2024, 2. [Google Scholar] [CrossRef]
- Alessandria, M.; Malatesta, G.M.; Berrino, F.; Donzelli, A. A critical analysis of all-cause deaths during COVID-19 vaccination in an Italian Province. Microorganisms 2024, 12, 1343. [Google Scholar] [CrossRef]
- Alessandria, M.; Malatesta, G.; Di Palmo, G.; Cosentino, M.; Donzelli, A. All-cause mortality according to COVID-19 vaccination status: An analysis of the UK office for National statistics public data. F1000Research 2025, 13, 886. [Google Scholar] [CrossRef]
- Barrowman, N. Correlation, causation, and confusion. The New Atlantis 2014, 23–44. [Google Scholar]
- Negri, F. Correlation is not causation, yet… matching and weighting for better counterfactuals. In Causality in Policy Studies: a Pluralist Toolbox; Springer International Publishing Cham, 2023; pp. 71–98. [Google Scholar]
- Kuhn, T.S.; Hacking, I. The structure of scientific revolutions; University of Chicago press Chicago, 1970; Volume 2. [Google Scholar]
- Karamitros, G.; Grant, M.P.; Lamaris, G.A. Associations in Medical Research Can Be Misleading: A Clinician’s Guide to Causal Inference. Journal of Surgical Research 2025, 310, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Hume, D. A treatise of human nature: Volume 1: Texts. 1739.
- Ward, A.C. The role of causal criteria in causal inferences: Bradford Hill’s” aspects of association”. Epidemiologic Perspectives & Innovations 2009, 6, 2. [Google Scholar]
- Li, X.; Mukandavire, C.; Cucunubá, Z.M.; Londono, S.E.; Abbas, K.; Clapham, H.E.; Jit, M.; Johnson, H.L.; Papadopoulos, T.; Vynnycky, E. Estimating the health impact of vaccination against ten pathogens in 98 low-income and middle-income countries from 2000 to 2030: a modelling study. The Lancet 2021, 397, 398–408. [Google Scholar] [CrossRef]
- Viliani, F.; Winkler, M.S. Ensure Healthy Lives and Promote Well-Being for All at All Ages. In Mining, Materials, and the Sustainable Development Goals (SDGs); CRC Press, 2020; pp. 15–28. [Google Scholar]
- Pan, J.; Wang, Y.; Cao, L.; Wang, Y.; Zhao, Q.; Tang, S.; Gong, W.; Guo, L.; Liu, Z.; Wen, Z. Impact of immunization programs on 11 childhood vaccine-preventable diseases in China: 1950–2018. The Innovation 2021, 2. [Google Scholar] [CrossRef]
- Ozawa, S.; Clark, S.; Portnoy, A.; Grewal, S.; Brenzel, L.; Walker, D.G. Return on investment from childhood immunization in low-and middle-income countries, 2011–20. Health Affairs 2016, 35, 199–207. [Google Scholar] [CrossRef]
- Morley, D. Saving children’s lives by vaccination. BMJ: British Medical Journal 1989, 299, 1544. [Google Scholar] [CrossRef]
- Rubio-Casillas, A.; Rodriguez-Quintero, C.M.; Redwan, E.M.; Gupta, M.N.; Uversky, V.N.; Raszek, M. Do vaccines increase or decrease susceptibility to diseases other than those they protect against? Vaccine, 42, 426-440. 2024.
- Aaby, P.; Benn, C.S. Developing the concept of beneficial non-specific effect of live vaccines with epidemiological studies. Clinical Microbiology and Infection 2019, 25, 1459–1467. [Google Scholar] [CrossRef]
- Benn, C.S.; Netea, M.G.; Selin, L.K.; Aaby, P. A small jab–a big effect: nonspecific immunomodulation by vaccines. Trends in immunology 2013, 34, 431–439. [Google Scholar] [CrossRef]
- Benn, C.S.; Fisker, A.B.; Whittle, H.C.; Aaby, P. Revaccination with live attenuated vaccines confer additional beneficial nonspecific effects on overall survival: a review. EBioMedicine 2016, 10, 312–317. [Google Scholar] [CrossRef]
- Benn, C.S.; Fisker, A.B.; Rieckmann, A.; Sørup, S.; Aaby, P. Vaccinology: time to change the paradigm? The Lancet infectious diseases 2020, 20, e274–e283. [Google Scholar] [CrossRef]
- Siebner, A.S.; Habib, M.; Osmani, V.; Adegnika, A.A.; Bogdan, C.; Breloer, M.; Elliott, A.; Fathi, A.; Hendrickx, G.; Nono, J.K. Interdisciplinary symposium on challenges and opportunities for vaccines: A comprehensive approach of current and future vaccine strategies to improve vaccine effectiveness in complex chronic infectious contexts. Vaccine: X 2025, 100615. [Google Scholar] [CrossRef]
- Poland, G.A.; Jacobson, R.M. Vaccine safety: injecting a dose of common sense. In Proceedings of the Mayo Clinic Proceedings; 2000; pp. 135–139. [Google Scholar]
- Feres, M.; Feres, M.F.N. Absence of evidence is not evidence of absence. 2023, 31, ed001.
- Hill, A.B. The environment and disease: association or causation? 1965.
- Imbens, G.W.; Rubin, D.B. Causal inference in statistics, social, and biomedical sciences; Cambridge university press: 2015.
- Phillips, C.V.; Goodman, K.J. The missed lessons of sir Austin Bradford Hill. Epidemiologic Perspectives & Innovations 2004, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Mörz, M. A case report: multifocal necrotizing encephalitis and myocarditis after BNT162b2 mRNA vaccination against COVID-19. Vaccines 2022, 10, 1651. [Google Scholar] [CrossRef]
- Won, J.H.; Byun, S.J.; Oh, B.-M.; Park, S.J.; Seo, H.G. Risk and mortality of aspiration pneumonia in Parkinson’s disease: a nationwide database study. Scientific reports 2021, 11, 6597. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sengoku, R.; Saito, Y.; Kakuta, Y.; Murayama, S.; Imafuku, I. Sudden death in Parkinson’s disease: a retrospective autopsy study. Journal of the Neurological Sciences 2014, 343, 149–152. [Google Scholar] [PubMed]
- Brogna, C.; Cristoni, S.; Marino, G.; Montano, L.; Viduto, V.; Fabrowski, M.; Lettieri, G.; Piscopo, M. Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: Possible molecular mechanisms. PROTEOMICS–Clinical Applications 2023, 2300048. [Google Scholar]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Critical care 2020, 24, 353. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circulation research 2021, 128, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Avolio, E.; Carrabba, M.; Milligan, R.; Kavanagh Williamson, M.; Beltrami, A.P.; Gupta, K.; Elvers, K.T.; Gamez, M.; Foster, R.R.; Gillespie, K. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease. Clinical Science 2021, 135, 2667–2689. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, C.S.; Navarro Ramil, L.; Bieligk, J.; Meineke, R.; Rimmelzwaan, G.; Käufer, C.; Richter, F. Intravenous SARS-CoV-2 Spike protein induces neuroinflammation and alpha-Synuclein accumulation in brain regions relevant to Parkinson’s disease. Brain Behav Immun 2025, 129, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Fal, A. To aspirate or not to aspirate? Considerations for the COVID-19 vaccines. Pharmacological Reports 2022, 74, 1223–1227. [Google Scholar] [CrossRef]
- Jarius, S.; Bieber, N.; Haas, J.; Wildemann, B. MOG encephalomyelitis after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): case report and comprehensive review of the literature. Journal of neurology 2022, 269, 5198–5212. [Google Scholar] [CrossRef]
- Ancau, M.; Liesche-Starnecker, F.; Niederschweiberer, J.; Krieg, S.M.; Zimmer, C.; Lingg, C.; Kumpfmüller, D.; Ikenberg, B.; Ploner, M.; Hemmer, B. Case series: acute hemorrhagic encephalomyelitis after SARS-CoV-2 vaccination. Frontiers in neurology 2022, 12, 820049. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.; Seo, J.-W.; Kim, M.-j.; Jeon, Y.H.; Park, J.H.; Lee, J.K.; Yeo, N.S. Myocarditis-induced sudden death after BNT162b2 mRNA COVID-19 vaccination in Korea: case report focusing on histopathological findings. Journal of Korean medical science 2021, 36. [Google Scholar] [CrossRef]
- Sung, J.G.; Sobieszczyk, P.S.; Bhatt, D.L. Acute myocardial infarction within 24 hours after COVID-19 vaccination. The American Journal of Cardiology 2021, 156, 129–131. [Google Scholar] [CrossRef]
- Baumeier, C.; Aleshcheva, G.; Harms, D.; Gross, U.; Hamm, C.; Assmus, B.; Westenfeld, R.; Kelm, M.; Rammos, S.; Wenzel, P. Intramyocardial inflammation after COVID-19 vaccination: an endomyocardial biopsy-proven case series. International Journal of Molecular Sciences 2022, 23, 6940. [Google Scholar] [CrossRef]
- Walter, A.; Kraemer, M. A neurologist’s rhombencephalitis after comirnaty vaccination. A change of perspective. Neurological Research and Practice 2021, 3, 56. [Google Scholar] [CrossRef]
- Bensaidane, M.R.; Picher-Martel, V.; Émond, F.; De Serres, G.; Dupré, N.; Beauchemin, P. Case report: acute necrotizing encephalopathy following COVID-19 vaccine. Frontiers in Neurology 2022, 13, 872734. [Google Scholar] [CrossRef] [PubMed]
- Pongpitakmetha, T.; Hemachudha, P.; Rattanawong, W.; Thanapornsangsuth, P.; Viswanathan, A.; Hemachudha, T. COVID-19 related acute necrotizing encephalopathy with extremely high interleukin-6 and RANBP2 mutation in a patient with recently immunized inactivated virus vaccine and no pulmonary involvement. BMC Infectious Diseases 2022, 22, 640. [Google Scholar] [CrossRef]
- Rezvani, M.; Mahmoodkhani, M.; Sourani, A.; Sharafi, M.; Foroughi, M.; Mahdavi, S.B.; Sourani, A.; Khah, R.N.; Veisi, S. Treatment refractory acute necrotizing myelitis after COVID-19 vaccine injection: a case report. Annals of Medicine and Surgery 2024, 86, 1185–1190. [Google Scholar] [PubMed]
- Mikami, S.; Ishii, M.; Yano, T.; Hirayama, I.; Hayashi, Y.; Shiomi, T.; Tominaga, Y.; Ishida, T. Multifocal meningoencephalitis after vaccination against COVID-19. Pathology International 2024, 74, 697–703. [Google Scholar] [CrossRef]
- Siriratnam, P.; Buzzard, K.; Yip, G. Acute haemorrhagic necrotizing encephalopathy in the setting of SARS-CoV-2 vaccination: a case report and literature review. Acta neurologica Belgica 2023, 123, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Senda, J.; Ashida, R.; Sugawara, K.; Kawaguchi, K. Acute meningoencephalitis after COVID-19 vaccination in an adult patient with rheumatoid vasculitis. Internal Medicine 2022, 61, 1609–1612. [Google Scholar] [CrossRef]
- Permezel, F.; Borojevic, B.; Lau, S.; de Boer, H.H. Acute disseminated encephalomyelitis (ADEM) following recent Oxford/AstraZeneca COVID-19 vaccination. Forensic Science, Medicine and Pathology 2022, 18, 74–79. [Google Scholar]
- Zanin, L.; Saraceno, G.; Renisi, G.; Signorini, L.; Battaglia, L.; Ferrara, M.; Rasulo, F.A.; Panciani, P.P.; Fontanella, M.M. Delayed onset of fatal encephalitis in a COVID-19 positive patient. International Journal of Neuroscience 2023, 133, 77–80. [Google Scholar]
- Ayuningtyas, T.; Natadidjaja, R.I.; Octaviani, C.; Sahli, F.; Adlani, H. Confirmed severe acute respiratory syndrome coronavirus 2 encephalitis in cerebrospinal fluid: a case report. Journal of Medical Case Reports 2022, 16, 154. [Google Scholar] [CrossRef]
- Benameur, K.; Agarwal, A.; Auld, S.C.; Butters, M.P.; Webster, A.S.; Ozturk, T.; Howell, J.C.; Bassit, L.C.; Velasquez, A.; Schinazi, R.F. Encephalopathy and encephalitis associated with cerebrospinal fluid cytokine alterations and coronavirus disease, Atlanta, Georgia, USA, 2020. Emerging infectious diseases 2020, 26, 2016. [Google Scholar] [CrossRef]
- Bernard-Valnet, R.; Pizzarotti, B.; Anichini, A. Two patients with acute meningo-encephalitis concomitant to SARS-CoV-2 infection. medRxiv. 2020; 20060251. Publisher Full Text.
- Bodro, M.; Compta, Y.; Llansó, L.; Esteller, D.; Doncel-Moriano, A.; Mesa, A.; Rodríguez, A.; Sarto, J.; Martínez-Hernandez, E.; Vlagea, A. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2–associated encephalitis. Neurology: Neuroimmunology & Neuroinflammation 2020, 7, e821. [Google Scholar]
- Cao, A.; Rohaut, B.; Le Guennec, L.; Saheb, S.; Marois, C.; Altmayer, V.; Carpentier, V.T.; Nemlaghi, S.; Soulie, M.; Morlon, Q. Severe COVID-19-related encephalitis can respond to immunotherapy. Brain 2020, 143, e102–e102. [Google Scholar] [CrossRef]
- Cheraghali, F.; Tahamtan, A.; Hosseini, S.A.; Gharib, M.H.; Moradi, A.; Razavi Nikoo, H.; Tabarraei, A. Case report: detection of SARS-CoV-2 from cerebrospinal fluid in a 34-month-old child with encephalitis. Frontiers in pediatrics 2021, 9, 565778. [Google Scholar] [CrossRef]
- Dahshan, A.; Abdellatef, A.A. Autoimmune encephalitis as a complication of COVID-19 infection: a case report. The Egyptian Journal of Internal Medicine 2022, 34, 32. [Google Scholar] [CrossRef]
- Dono, F.; Carrarini, C.; Russo, M.; De Angelis, M.V.; Anzellotti, F.; Onofrj, M.; Bonanni, L. New-onset refractory status epilepticus (NORSE) in post SARS-CoV-2 autoimmune encephalitis: a case report. Neurological Sciences 2021, 42, 35–38. [Google Scholar] [CrossRef]
- Elmouhib, A.; Benramdane, H.; zohra Ahsayen, F.; El Haddad, I.A.; El Ghalet, A.; Laaribi, I.; Bkiyar, H.; Nasri, S.; Skiker, I.; Housni, B. A case of limbic encephalitis associated with severely COVID-19 infection. Annals of Medicine and Surgery 2022, 74, 103274. [Google Scholar] [CrossRef] [PubMed]
- Etemadifar, M.; Salari, M.; Murgai, A.A.; Hajiahmadi, S. Fulminant encephalitis as a sole manifestation of COVID-19. Neurological Sciences 2020, 41, 3027–3029. [Google Scholar] [CrossRef]
- Gunawardhana, C.; Nanayakkara, G.; Gamage, D.; Withanage, I.; Bandara, M.; Siriwimala, C.; Senaratne, N.; Chang, T. Delayed presentation of postinfectious encephalitis associated with SARS-CoV-2 infection: a case report. Neurological Sciences 2021, 42, 3527–3530. [Google Scholar] [CrossRef] [PubMed]
- Khoo, A.; McLoughlin, B.; Cheema, S.; Weil, R.S.; Lambert, C.; Manji, H.; Zandi, M.S.; Morrow, J.M. Postinfectious brainstem encephalitis associated with SARS-CoV-2. Journal of Neurology, Neurosurgery & Psychiatry 2020, 91, 1013–1014. [Google Scholar] [CrossRef]
- Koh, S.; Kim, Y.S.; Kim, M.H.; Choi, Y.H.; Choi, J.Y.; Kim, T.-J. Encephalitis with status epilepticus and stroke as complications of non-severe COVID-19 in a young female patient: a case report. BMC neurology 2022, 22, 253. [Google Scholar] [CrossRef]
- Mekheal, E.; Mekheal, M.; Roman, S.; Mikhael, D.; Mekheal, N.; Manickam, R. A case report of autoimmune encephalitis: could post-COVID-19 autoimmunity become a lethal health issue? Cureus 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Meshref, M.; Hewila, I.M.; Mageed, S.A.; Morra, M.E. COVID-19 associated with encephalitis: case report and review of literature. The neurologist 2021, 26, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Pizzanelli, C.; Milano, C.; Canovetti, S.; Tagliaferri, E.; Turco, F.; Verdenelli, S.; Nesti, L.; Franchi, M.; Bonanni, E.; Menichetti, F. Autoimmune limbic encephalitis related to SARS-CoV-2 infection: case-report and review of the literature. Brain, behavior, & immunity-health 2021, 12, 100210. [Google Scholar]
- Sangare, A.; Dong, A.; Valente, M.; Pyatigorskaya, N.; Cao, A.; Altmayer, V.; Zyss, J.; Lambrecq, V.; Roux, D.; Morlon, Q. Neuroprognostication of consciousness recovery in a patient with COVID-19 related encephalitis: preliminary findings from a multimodal approach. Brain Sciences 2020, 10, 845. [Google Scholar] [CrossRef]
- Sarmast, S.T.; Mohamed, A.S.; Amar, Z.; Sarwar, S.; Ahmed, Z. A case of acute encephalitis in COVID-19 patient: a rare complication. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Sattar, S.B.A.; Haider, M.A.; Zia, Z.; Niazi, M.; Iqbal, Q.Z.; Niazi, M.R.K. Clinical, radiological, and molecular findings of acute encephalitis in a COVID-19 patient: a rare case report. Cureus 2020, 12. [Google Scholar] [CrossRef]
- Sharma, R.; Nalleballe, K.; Shah, V.; Haldal, S.; Spradley, T.; Hasan, L.; Mylavarapu, K.; Vyas, K.; Kumar, M.; Onteddu, S. Spectrum of hemorrhagic encephalitis in COVID-19 patients: a case series and review. Diagnostics 2022, 12, 924. [Google Scholar] [CrossRef]
- Sofijanova, A.; Bojadzieva, S.; Duma, F.; Superlishka, E.; Murtezani, A.; Jordanova, O. Severe encephalitis in infant with COVID-19: a case report. Open Access Macedonian Journal of Medical Sciences 2020, 8, 514–517. [Google Scholar] [CrossRef]
- Wettervik, T.S.; Kumlien, E.; Rostami, E.; Howells, T.; von Seth, M.; Velickaite, V.; Lewén, A.; Enblad, P. Intracranial pressure dynamics and cerebral vasomotor reactivity in coronavirus disease 2019 patient with acute encephalitis. Critical care explorations 2020, 2, e0197. [Google Scholar] [CrossRef] [PubMed]
- Tee, T.Y.; Thabit, A.A.M.; Khoo, C.S.; Shahrom, H.M.; Chan, E.Z.; Marzukie, M.M.; Kamaruddin, Z.A.C.; Thayan, R.; Chidambaram, S.K. Acute encephalitis associated with SARS-CoV-2 confirmed in cerebrospinal fluid: First case in Malaysia. Journal of Clinical Neurology (Seoul, Korea) 2021, 17, 490. [Google Scholar]
- Woldie, I.L.; Brown, I.G.; Nwadiaro, N.F.; Patel, A.; Jarrar, M.; Quint, E.; Khokhotva, V.; Hugel, N.; Winger, M.; Briskin, A. Autoimmune hemolytic anemia in a 24-year-old patient with COVID-19 complicated by secondary cryptococcemia and acute necrotizing encephalitis: a case report and review of literature. Journal of medical cases 2020, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Ren, Y.; Lv, T. Encephalitis as a clinical manifestation of COVID-19. Brain, behavior, and immunity 2020, 88, 945. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Distefano, M.G.; Cambula, G.; Colomba, A.I.; Nuzzo, D.; Picone, P.; Giacomazza, D.; Sicurella, L. The case of encephalitis in a COVID-19 pediatric patient. Neurological Sciences 2022, 43, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Vandervorst, F.; Guldolf, K.; Peeters, I.; Vanderhasselt, T.; Michiels, K.; Berends, K.J.; Van Laethem, J.; Pipeleers, L.; Vincken, S.; Seynaeve, L. Encephalitis associated with the SARS-CoV-2 virus: a case report. Interdisciplinary Neurosurgery 2020, 22, 100821. [Google Scholar] [CrossRef]
- Zambreanu, L.; Lightbody, S.; Bhandari, M.; Hoskote, C.; Kandil, H.; Houlihan, C.F.; Lunn, M.P. A case of limbic encephalitis associated with asymptomatic COVID-19 infection. Journal of Neurology, Neurosurgery & Psychiatry 2020, 91, 1229–1230. [Google Scholar] [CrossRef]
- Zandifar, S.; Zandifar, Z. Acute viral encephalitis associated with SARS-CoV-2. Ann Clin Case Rep 2020, 5, 1845. [Google Scholar]
- Lersy, F.; Anheim, M.; Willaume, T.; Chammas, A.; Brisset, J.-C.; Cotton, F.; Kremer, S. Cerebral vasculitis of medium-sized vessels as a possible mechanism of brain damage in COVID-19 patients. Journal of Neuroradiology 2021, 48, 141–146. [Google Scholar] [CrossRef]
- Zuhorn, F.; Omaimen, H.; Ruprecht, B.; Stellbrink, C.; Rauch, M.; Rogalewski, A.; Klingebiel, R.; Schäbitz, W.-R. Parainfectious encephalitis in COVID-19:“the claustrum sign”. Journal of neurology 2021, 268, 2031–2034. [Google Scholar]
- Tan, S.; Cui, C.; Song, E.-P.; Shan, Y.; Chang, Y.; Qiu, W.; Lu, Z. Acute haemorrhagic necrotizing encephalopathy and inflammatory demyelinating encephalopathy associated with COVID-19 in adults in Southern China. BMC Infectious Diseases 2025, 25, 1–10. [Google Scholar] [CrossRef]
- Ghosh, R.; Dubey, S.; Finsterer, J.; Chatterjee, S.; Ray, B.K. SARS-CoV-2-associated acute hemorrhagic, necrotizing encephalitis (AHNE) presenting with cognitive impairment in a 44-year-old woman without comorbidities: a case report. The American journal of case reports 2020, 21, e925641–925641. [Google Scholar] [CrossRef]
- Mierzewska-Schmidt, M.; Baranowski, A.; Szymanska, K.; Ciaston, M.; Kuchar, E.; Ploski, R.; Kosinska, J.; Pagowska-Klimek, I. The case of fatal acute hemorrhagic necrotizing encephalitis in a two-month-old boy with Covid-19. International Journal of Infectious Diseases 2022, 116, 151–153. [Google Scholar] [CrossRef]
- Morvan, A.-C.; Kerambrun, H. Fatal necrotizing encephalitis associated with COVID-19. Neurology: Clinical Practice 2021, 11, e214–e215. [Google Scholar] [CrossRef]
- Mullaguri, N.; Sivakumar, S.; Battineni, A.; Anand, S.; Vanderwerf, J. COVID-19 related acute hemorrhagic necrotizing encephalitis: a report of two cases and literature review. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Massari, M.; Spila Alegiani, S.; Morciano, C.; Spuri, M.; Marchione, P.; Felicetti, P.; Belleudi, V.; Poggi, F.R.; Lazzeretti, M.; Ercolanoni, M. Postmarketing active surveillance of myocarditis and pericarditis following vaccination with COVID-19 mRNA vaccines in persons aged 12 to 39 years in Italy: A multi-database, self-controlled case series study. PLoS Medicine 2022, 19, e1004056. [Google Scholar] [CrossRef]
- Ling, R.R.; Ramanathan, K.; Tan, F.L.; Tai, B.C.; Somani, J.; Fisher, D.; MacLaren, G. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. The Lancet Respiratory Medicine 2022, 10, 679–688. [Google Scholar] [CrossRef]
- Stowe, J.; Miller, E.; Andrews, N.; Whitaker, H.J. Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARS-CoV-2 infection: a self-controlled case series analysis in England. PLoS Medicine 2023, 20, e1004245. [Google Scholar]
- Abue, M.; Mochizuki, M.; Shibuya-Takahashi, R.; Ota, K.; Wakui, Y.; Iwai, W.; Kusaka, J.; Saito, M.; Suzuki, S.; Sato, I. Repeated COVID-19 Vaccination as a Poor Prognostic Factor in Pancreatic Cancer: A Retrospective, Single-Center Cohort Study. Cancers 2025, 17, 2006. [Google Scholar] [CrossRef]
- Ota, N.; Itani, M.; Aoki, T.; Sakurai, A.; Fujisawa, T.; Okada, Y.; Noda, K.; Arakawa, Y.; Tokuda, S.; Tanikawa, R. Expression of SARS-CoV-2 spike protein in cerebral Arteries: Implications for hemorrhagic stroke Post-mRNA vaccination. Journal of Clinical Neuroscience 2025, 136, 111223. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nature reviews genetics 2012, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Clausen, L.; Abildgaard, A.B.; Gersing, S.K.; Stein, A.; Lindorff-Larsen, K.; Hartmann-Petersen, R. Protein stability and degradation in health and disease. Advances in protein chemistry and structural biology 2019, 114, 61–83. [Google Scholar] [PubMed]
- Li, W.; Kitsios, G.D.; Bain, W.; Wang, C.; Li, T.; Fanning, K.V.; Deshpande, R.; Qin, X.; Morris, A.; Lee, J.S. Stability of SARS-CoV-2-encoded proteins and their antibody levels correlate with interleukin 6 in COVID-19 patients. Msystems 2022, 7, e00058–00022. [Google Scholar] [CrossRef]
- Moderbacher, C.R.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020, 183, 996–1012. e1019. [Google Scholar] [CrossRef]
- Rong, Z.; Mai, H.; Ebert, G.; Kapoor, S.; Puelles, V.G.; Czogalla, J.; Hu, S.; Su, J.; Prtvar, D.; Singh, I. Persistence of spike protein at the skull-meninges-brain axis may contribute to the neurological sequelae of COVID-19. Cell Host & Microbe 2024. [Google Scholar]
- Olsen, J.V.; Ong, S.-E.; Mann, M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Molecular & cellular proteomics 2004, 3, 608–614. [Google Scholar]
- Miller, N.L.; Clark, T.; Raman, R.; Sasisekharan, R. Insights on the mutational landscape of the SARS-CoV-2 Omicron variant. BioRxiv 2021. [Google Scholar]
- Zuo, W.; He, D.; Liang, C.; Du, S.; Hua, Z.; Nie, Q.; Zhou, X.; Yang, M.; Tan, H.; Xu, J. The persistence of SARS-CoV-2 in tissues and its association with long COVID symptoms: a cross-sectional cohort study in China. The Lancet Infectious Diseases 2024, 24, 845–855. [Google Scholar] [CrossRef]
- Gould, A.L. Unified screening for potential elevated adverse event risk and other associations. Statistics in Medicine 2018, 37, 2667–2689. [Google Scholar] [CrossRef] [PubMed]
- Broudic, K.; Laurent, S.; Perkov, V.; Simon, C.; Garinot, M.; Truchot, N.; Latour, J.; Désert, P. Nonclinical safety assessment of an mRNA Covid-19 vaccine candidate following repeated administrations and biodistribution. Journal of Applied Toxicology 2024, 44, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Molecular Therapy-Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reichmuth, A.M.; Oberli, M.A.; Jaklenec, A.; Langer, R.; Blankschtein, D. mRNA vaccine delivery using lipid nanoparticles. Therapeutic delivery 2016, 7, 319–334. [Google Scholar] [CrossRef]
- Röltgen, K.; Nielsen, S.C.; Silva, O.; Younes, S.F.; Zaslavsky, M.; Costales, C.; Yang, F.; Wirz, O.F.; Solis, D.; Hoh, R.A. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell 2022, 185, 1025–1040. e1014. [Google Scholar] [CrossRef]
- Lindsay, K.E.; Bhosle, S.M.; Zurla, C.; Beyersdorf, J.; Rogers, K.A.; Vanover, D.; Xiao, P.; Araínga, M.; Shirreff, L.M.; Pitard, B. Visualization of early events in mRNA vaccine delivery in non-human primates via PET–CT and near-infrared imaging. Nature biomedical engineering 2019, 3, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Krauson, A.J.; Casimero, F.V.C.; Siddiquee, Z.; Stone, J.R. Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. npj Vaccines 2023, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Spanevello, A.; Reboldi, G.; Visca, D.; Verdecchia, P. SARS-CoV-2 vaccines: Lights and shadows. European journal of internal medicine 2021, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mead, M.N.; Rose, J.; Seneff, S.; Rogers, C.; Craven, B.; Hulscher, N.; Cosgrove, K.; Marik, P.; McCullough, P.A. Compound Adverse Effects of COVID-19 mRNA Vaccination and Coronavirus Infection: A Convergence of Extensive Spike Protein Harms to the Human Body. 2025.
- Patterson, B.K.; Yogendra, R.; Francisco, E.B.; Guevara-Coto, J.; Long, E.; Pise, A.; Osgood, E.; Bream, J.; Kreimer, M.; Jeffers, D. Detection of S1 spike protein in CD16+ monocytes up to 245 days in SARS-CoV-2-negative post-COVID-19 vaccine syndrome (PCVS) individuals. Human Vaccines & Immunotherapeutics 2025, 21, 2494934. [Google Scholar]
- Bhattacharjee, B.; Lu, P.; Monteiro, V.S.; Tabachnikova, A.; Wang, K.; Hooper, W.B.; Bastos, V.; Greene, K.; Sawano, M.; Guirgis, C. Immunological and antigenic signatures associated with chronic illnesses after COVID-19 vaccination. medRxiv 2025, 2025.2002. 2018.25322379.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).