1. Introduction
African swine fever virus (ASFV) is a complex, enveloped, double-stranded DNA virus belonging to the
Asfarviridae family and is the only known DNA arbovirus, capable of being transmitted by soft ticks of the genus
Ornithodoros in certain endemic regions [
1,
2]. The virus primarily infects domestic pigs and wild boars, causing a highly contagious and often fatal disease characterized by high fever, hemorrhages, lymphoid depletion, and multi-organ failure [
3]. Mortality rates can reach up to 100% in naïve populations infected with highly virulent strains, making ASF one of the most devastating swine diseases worldwide [
4].
The socioeconomic impact of ASF outbreaks extends far beyond animal health, posing a severe threat to global food security. Since its initial identification in Kenya in 1921, ASFV has expanded beyond Africa, spreading into Europe, Asia, and more recently the Americas, with large-scale outbreaks reported in countries such as China, Vietnam, India, and the Dominican Republic [1,5-7]. This transcontinental movement of ASFV highlights the key prevention and control technologies, including strengthened biosecurity, animal movement control, and early detection.
Development of a safe and effective ASF vaccine remains a major challenge in the veterinary field due to its large genome, sophisticated replication process, host immune evasion, genetic variability and unknown functions of novel proteins [8-11]. Among various vaccine development strategies towards ASF, live attenuated vaccines (LAVs)—typically generated through serial passage in cell culture or targeted deletion of virulence-associated genes—have shown promise [12-14]. LAVs mimic natural infection without causing severe disease, thereby stimulating both humoral and cell-mediated immune responses. Several experimental LAVs have demonstrated partial to complete protection against homologous and, in some cases, heterologous challenges in laboratory settings. Field trials in endemic areas have also provided encouraging evidence of efficacy in the context of AVAC ASF LIVE® and NAVET-ASFVAC®, two ASFV LAVs licensed and commercialized in Vietnam, although concerns are rising regarding residual virulence, genetic stability, and potential reversion to virulence [
15,
16]
In recent years, various LAV vaccine candidates have been developed by deleting single or multiple genes, including members of the multigene family (MGF), such as MGF360 and MGF505 [
17], I177L [
18], 9GL and UK [
19]. These vaccine candidate strains have exhibited varying levels of protective efficacy
in vivo. Our previous study identified MGF505-2R as a potent inhibitor of innate immunity
in vitro. Very recently, deletion of MGF505-2R gene activates the cGAS-STING pathway
in vitro and leads to attenuation and protection against lethal challenge in the context of ASFV strain Arm/07/CBM/C2 (LR812933.1) [
20]. However, how dose deletion of MGF505-2R affect the pathogenicity and virulence of highly pathogenic Eurasian genotype II strain is unknown. We aim to evaluate the pathogenicity and virulence of ASFV-ΔMGF505-2R in piglets and explore its potential as a live attenuated vaccine candidate.
2. Materials and Methods
2.1 Animals
Twelve clinically healthy, five-week-old crossbred pigs weighing approximately 10 kg were obtained from a commercial pig farm. All pigs tested negative for ASFV, classical swine fever virus (CSFV), porcine circovirus type 2 (PCV2), pseudorabies virus (PRV), and porcine reproductive and respiratory syndrome virus (PRRSV) antigens and antibodies. The experiments were conducted in a Biosafety Level 3 laboratory (BSL-3) at Lanzhou Veterinary Research Institute (LVRI), Chinese Academy of Agricultural Sciences (CAAS).
2.2 Virus and Cells
The parental virus strain, ASFV CN/GS 2018 strain, was preserved in our laboratory. The MGF505-2R gene deletion strain, ASFV-ΔMGF505-2R, was constructed by homologous recombination as described previously [
21]. Porcine bone marrow-derived macrophages (BMDMs) were obtained and maintained in RPMI 1640 medium containing 20% fetal bovine serum (FBS) with 1% penicillin-streptomycin (P/S) and 2 mM L-glutamine, and 10 ng/mL of recombinant porcine granulocyte-macrophage colony-stimulating factor (GM-CSF) was added to maintain optimal BMDMs viability and physiological state [
22].
2.3 Pathogenicity Evaluation of ASFV-ΔMGF505-2R
Twelve piglets negative of ASFV antigen and antibody were randomly split into two groups. Group of 8 piglets received an intramuscular (IM) injection of 10² HAD₅₀ of ASFV-ΔMGF505-2R, while the other group of 4 piglets were administered with the same dose of parental ASFV CN/GS 2018. Daily body temperature measurements were taken, and clinical manifestations were systematically observed during the entire experimental period. Blood and fecal swabs were gathered at every other days after inoculation [
23]. Viremia and virus shedding were assessed using quantitative PCR. At necropsy, tissue specimens including the heart, liver, spleen, lung, kidney, submandibular lymph nodes, hepatogastric lymph nodes, and mesenteric lymph nodes were harvested and prepared for histopathological examination.
2.4 Anesthesia Procedure
For pigs which reached the predefined human endpoint or undertook all the experiments, they were securely restrained and injected IM with an overdose of Zoletil®50 (Virbac, China) at 16 mg per kilogram of body weight to induce anesthesia. The depth of anesthesia was monitored every 5 to 10 minutes by assessing muscle relaxation, cessation of voluntary movement, absence of palpebral reflex, and loss of consciousness [
24]. Later, animals were humanely euthanized. All procedures involving anesthesia and euthanasia were carried out in strict compliance with animal welfare regulations and by following the "Standard Operating Procedures for Anesthesia and Euthanasia of Laboratory Animals" issued by the Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences.
2.5 Immune Response Analysis
Serum specimens were tested for the presence of p30 antibodies with an ASFV p30 blocking ELISA kit (IDvet, France). The concentrations of IFN-β and IL-1β were determined using commercial ELISA kits.
2.6 The q-PCR assay
Genomic DNA of ASFV was isolated from BMDMs or tissue samples using the E.Z.N.A.® Tissue DNA Kit (OMEGA, USA) following the manufacturer’s instructions. Quantitative PCR was performed on a QuantStudio system (Applied Biosystems, USA) with the Pro taq HS premix probe qPCR kit (ACCURATE BIOLOGY AG, China) to measure the copy numbers of ASFV P72 genes in blood, swabs, and tissues.
2.7 Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Group comparisons were conducted using the t-test in GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA). A p value below 0.05 was considered statistically significant (**, P < 0.01; ***, P < 0.001).
3. Results
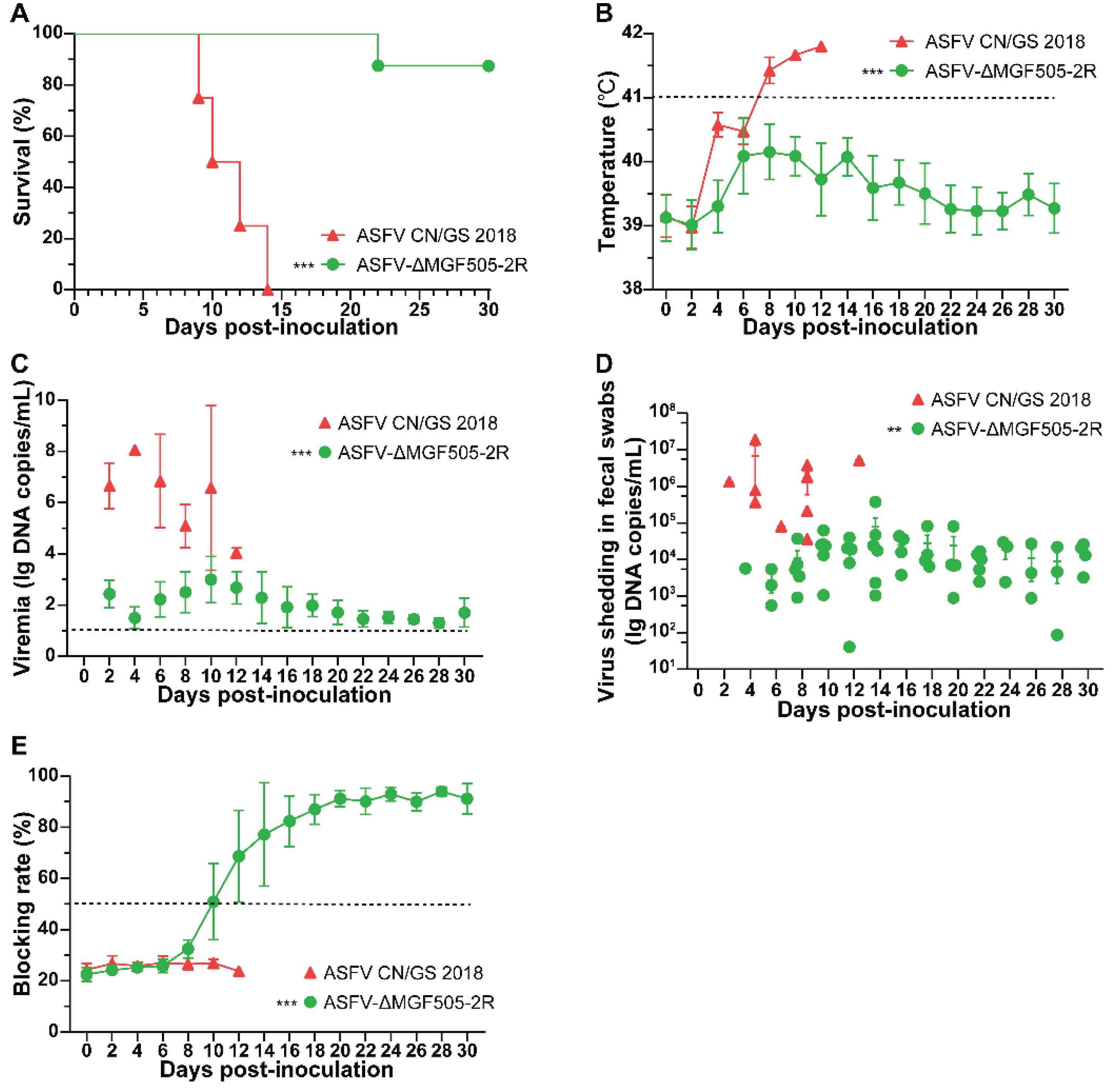
3.1 Pathogenicity of ASFV-ΔMGF505-2R in piglets
All twelve piglets were divided randomly into two groups, with one group of 8 being IM injected with 10
2 HAD
50 of ASFV-ΔMGF505-2R while the other group of 4 being IM injected with same dose of parental ASFV CN/GS 2018. ASFV CN/GS 2018-inoculated piglets exhibited high fever (over 41.0 ℃) by 6-8 days post-inoculation (dpi), followed by ASF-compatible clinical manifestations, then died within 14 dpi (
Figure 1 A and B). In contrast, piglets inoculated with ASFV-ΔMGF505-2R showed transient fever peaking at 6-10 dpi. One pig started to show clinical signs of disease (anorexia, depression, and fever) and died at 22 dpi, while the remaining seven animals in the group were clinically healthy throughout the entire experiment, except for the transient increase in body temperature (
Figure 1 A and B). Therefore, deletion of MGF505-2R led to attenuation of ASFV CN/GS 2018 strain.
The viremia and viral sheddings in fecal swabs of both groups were also verified and compared. The ASFV CN/GS 2018 group had higher levels of viremia and more viral shedding in swabs (10
6 to 10
8 copies/mL) by 2 dpi, remaining the peak titers until death. However, ASFV-ΔMGF505-2R group showed a slight increase in viremia at 10 dpi and declined to almost undetectable levels. Viral shedding was observed in fecal samples, however, at a relatively low level (
Figure 1 C and D). Therefore, ASFV-ΔMGF505-2R is highly attenuated in piglets.
To explore whether the attenuated strain could exert antibody response, we found that ASFV-ΔMGF505-2R was capable of inducing P30 antibodies even at 10 dpi. The antibody titers increased, peaked at 20 dpi and remained at plateau levels for the rest of days. Those data rationalize the strong immunogenicity of ASFV-ΔMGF505-2R (
Figure 1 E).
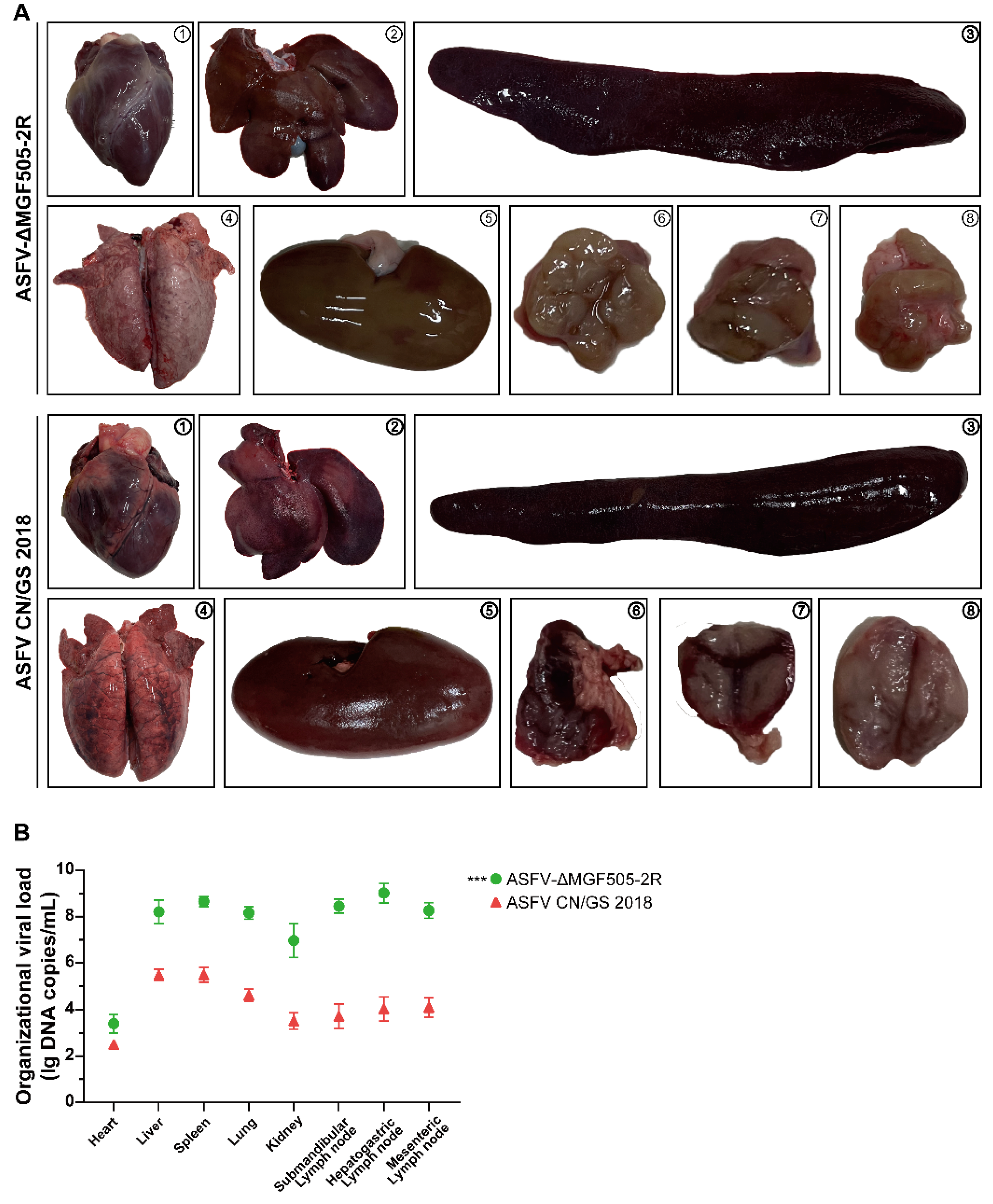
3.2 ASFV-ΔMGF505-2R induces Slight Pathological Damages in Organs
Organs were systematically examined for macroscopic and pathological changes, with a focus on indicators such as organ swelling, discoloration, and vascular congestion [
25]. Gross examination revealed that the spleen and lung from ASFV CN/GS 2018-infected pigs displayed a markedly enlarged size and a uniform dark red to nearly black appearance, consistent with severe splenic congestion and infarction. Widespread areas of hemorrhagic necrosis were evident across the parenchyma, indicating extensive tissue damage due to uncontrolled viral replication and associated inflammatory responses. The kidney and liver also showed pronounced abnormalities, being swollen and discolored with a dull, dark brownish-red surface, which is characteristic of renal congestion and possible microvascular thrombosis. These macroscopic findings suggest impaired blood flow and potential ischemic injury in renal tissues. Additionally, the submandibular, hepatogastric and mesenteric lymph nodes were significantly enlarged and exhibited intense reddening due to marked vascular engorgement and inflammatory cell infiltration, further supporting systemic infection of the virus (
Figure 2 A).
In contrast, pigs from ASFV-ΔMGF505-2R group exhibited considerably slight clinical and pathological manifestations. Macroscopic evaluation of their organs revealed only moderate enlargement and minimal discoloration, with no evidence of widespread hemorrhage or necrosis (
Figure 2 A).
Moreover, quantitative assessment of viral loads demonstrated significantly lower levels of viral DNA in the tissues of ASFV-ΔMGF505-2R group when compared to the ASFV CN/GS 2018 group (
Figure 2 B). This reduction in viral replication efficiency correlates directly with the observed attenuation in clinical symptoms and organ pathology. These findings highlight the importance of the MGF505-2R gene in viral pathogenesis and support its potential as a target for the development of live-attenuated vaccines against ASF.
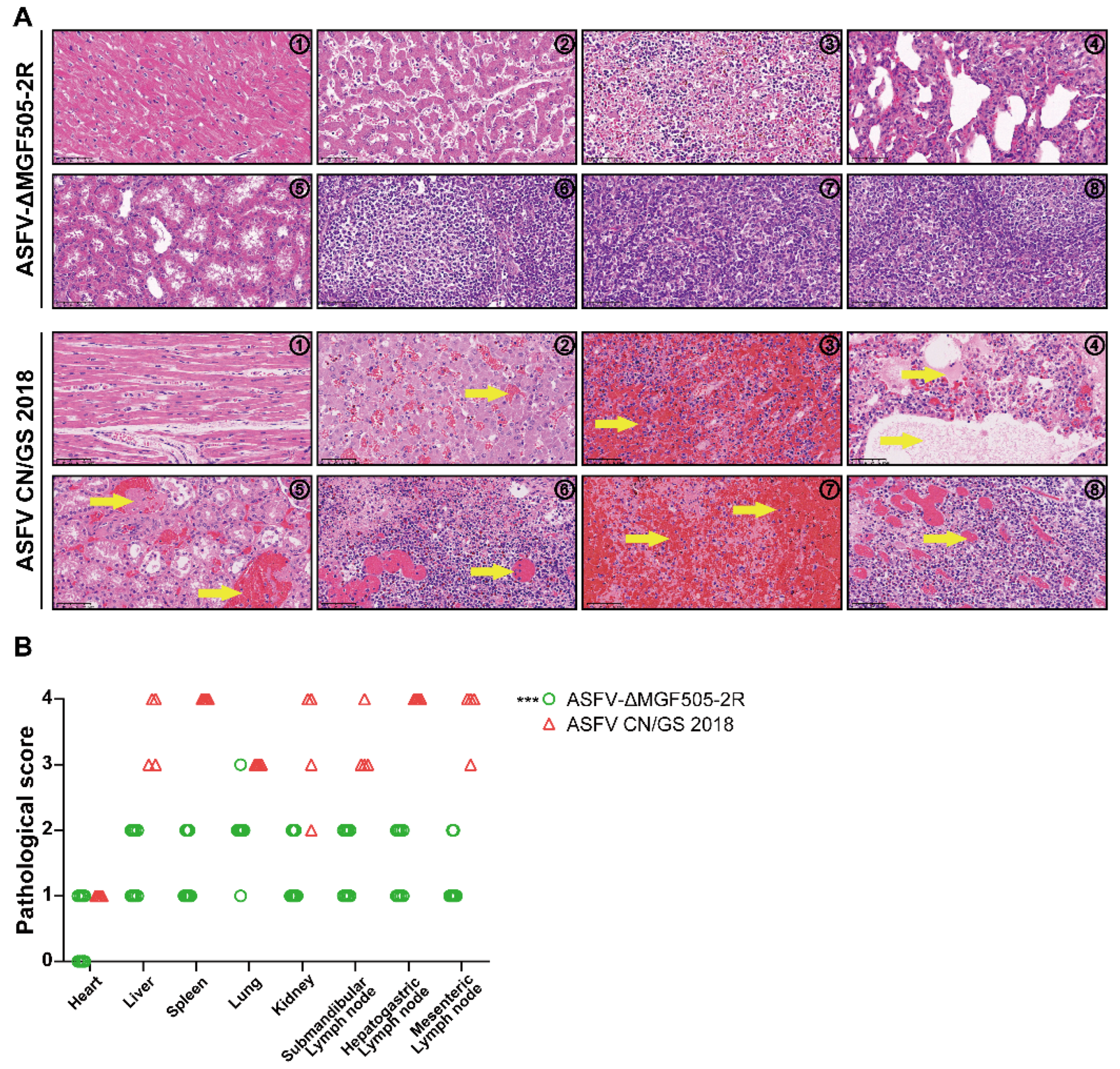
3.3. ASFV-ΔMGF505-2R Group Showed Only Mild to Moderate Histological Changes
To further characterize the extent of pathological damages, tissue samples were collected from both groups at the end of the experiment [
26]. Based on the scoring criteria outlined [
27], a comprehensive histopathological evaluation indicated that control group demonstrated severe pathological damages. The spleen exhibited extensive hemorrhage characterized by red pulp congestion and widespread necrosis of lymphocytes, consistent with the acute phase of ASFV infection. The lungs revealed marked shedding of bronchial epithelial cells, disruption of the respiratory epithelium, and the presence of serous exudates within both blood vessels and bronchioles. Additionally, diffuse infiltration of inflammatory cells—predominantly macrophages and neutrophils—was evident within pulmonary lobules, accompanied by alveolar wall thickening and parenchymal destruction. Renal tissues displayed notable interstitial hemorrhage and focal areas of tubular epithelial cell necrosis, suggestive of impaired renal function and possible toxin-mediated or ischemic injury secondary to systemic viremia. The liver and associated lymphoid tissues also showed significant pathological damages. Specifically, three lymph nodes demonstrated disintegration of lymphoid nodules, loss of normal follicular architecture, and extensive lymphocyte depletion with nuclear fragmentation and cytoplasmic eosinophilia—morphological hallmarks of apoptosis and necrosis (
Figure 3 A). These changes are indicative of systemic vascular damage and immune system collapse, which are hallmark features of highly pathogenic ASFV infections.
In stark contrast, pigs that survived from ASFV-ΔMGF505-2R strain exhibited markedly reduced clinical severity and minimal tissue damage. Histopathological analysis revealed either the absence of detectable lesions or only mild, localized pathological changes in the spleen, kidney, lung, submandibular lymph node, hepatogastric lymph node and mesenteric lymph node. Notably, the structural integrity of lymphoid tissues was largely preserved, with minimal inflammatory infiltration and no evidence of widespread necrosis (
Figure 3 A).
Quantitative assessment of lesion scores demonstrated that the pathological damages in the ASFV-ΔMGF505-2R is more slightly when compared to the control group (
Figure 3 B). This data strongly indicates that the deletion of the MGF505-2R gene plays a critical role in attenuating ASFV. Collectively, these results demonstrate that the ASFV-ΔMGF505-2R mutant strain induces significantly slight histopathological changes, highlighting its potential as a candidate for further development in vaccine research.
3.4 Immune Response Analysis
Viral infection can trigger a robust immune response in the host. MGF505-2R was previously identified as a potent inhibitor of innate immunity
in vitro [
20,
21,
28]. ELISA analysis revealed that pigs inoculated with ASFV-ΔMGF505-2R produced significantly higher levels of IFN-β and IL-1β compared to those infected with the ASFV CN/GS 2018, suggestive of the immunosuppressive properties of MGF505-2R
in vivo (
Figure 4 A and B).
4. Discussion
4.1 Progress in Live Attenuated Vaccine Development
LAVs have emerged as a promising strategy for combating ASFV. Prior research has established that targeted deletion of specific Multigene Family (MGF) genes can effectively attenuate ASFV and elicit protective immunity. For example, the deletion of MGF360 and MGF505 genes has been demonstrated to mitigate viral pathogenicity and confer protection in pigs against lethal challenge [29-31]. Consistent with this, the deletion of the MGF505-7R gene has also been reported to attenuate the virus and induce a protective response [32-34]. Sunwoo et al. have recently corroborated the pivotal role of the MGF505-2R gene in modulating the cGAS-STING signaling pathway through a series of
in vitro and
in vivo experiments, thereby confirming that the deletion of MGF505-2R results in reduced viral virulence [
20]. The current study gives more insights into how the ASFV-ΔMGF505-2R induce high level of antibody response during infection. These findings underscore the significant impact of the MGF505-2R gene on ASFV virulence and validate that its deletion markedly diminishes the pathogenic potential of the virus. Collectively, our research further substantiates the potential utility of MGF gene deletions in the development of LAVs for ASFV.
4.2 Analysis of Data from ASFV-ΔMGF505-2R Inoculation
Regarding the immune response, Sunwoo et al. elucidated the molecular mechanism by which the MGF505-2R protein inhibited the cGAS-STING signaling pathway, thereby modulating the production of IFN-β via in vitro experimental approaches, which is consistent with our findings [
20]. In our study, deletion of MGF505-2R in ASFV enhanced immune response in pigs, characterized by elevated levels of IFN-β and IL-1β, further supporting the potential of ASFV-ΔMGF505-2R as a inhibitor of innate immunity. These findings indicate that the deletion of the MGF505-2R gene not only weakens the virulence of the virus but also enhances the host's immune response. This study highlights the importance of the MGF505-2R gene in immune regulation at multiple levels and provides a more comprehensive perspective for understanding the immune evasion mechanisms of ASFV.
4.3 Correlation Between Antibody Levels and Survival Rates
The elevated antibody levels and high survival rate observed in the ASFV-ΔMGF505-2R group indicate a positive correlation between antibody production and protection against ASFV, aligning with prior research that demonstrates LAVs can elicit robust immune responses and protect pigs from lethal ASFV challenge. Sunwoo et al. previously confirmed the partial protective efficacy of ASFV-ΔMGF505-2R through animal experiments, achieving protection rates of up to 75%, while also noting the limited protection and the need for further optimization [
20].
Our study reinforces the evidence that targeted deletion of MGF genes can facilitate the development of effective LAVs for ASFV. The recombinant virus lacking the MGF505-2R gene emerges as a promising candidate for a modified live vaccine. This investigation underscores that the recombinant virus significantly attenuates virulence in animals and induces a potent immune response, thereby providing a theoretical foundation for the commercialization of modified live vaccines. However, current LAV research faces certain limitations, including incomplete protection and potential risks of virulence reversion. Future endeavors should focus on exploring additional virulence factors, refining vaccine design, enhancing protective efficacy, and ensuring vaccine safety.
5. Conclusions
The deletion of the MGF505-2R gene in ASFV significantly reduces its virulence and enhances the host immune response in piglets. The recombinant virus ASFV-ΔMGF505-2R shows promise as a live attenuated vaccine candidate. Future studies should focus on further characterizing the immune mechanisms involved and evaluating the long-term efficacy and safety of ASFV-ΔMGF505-2R in larger cohorts and under field conditions.
Author Contributions
Conceptualization, F.X., H.H., W.D. and H.Z.; methodology, F.X., H.H., M.D. and T.L.; validation, Y.D., H.L., Z.S. H.T. and J.H.; writing—original draft preparation, F.X. and H.H.; writing—review and editing, W.D. and H.Z.; supervision, J.H.; funding acquisition, H.Z., M.D. and F.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the open competition program of top ten critical priorities of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province, grant number 2024KJ14; the National Key R&D Program of China, grant number 2021YFD1800100; the Fundamental Research Funds for the Central Universities, China Agriculture Research System of Ministry of Finance and Ministry of Agriculture and Rural Affairs, grant number CARS-35; Project of National Center of Technology Innovation for Pigs, grant number NCTIP-XD/C03; Innovation Program of Chinese Academy of Agricultural Sciences, grant number CAAS-CSLPDCP-202302 and CAAS-ASTIP-2024-LVRI; Science and Technology Program of Gansu Province, grant number 24JRRA01; the China Postdoctoral Science Foundation, grant number 2023M733817 and the Postdoctoral Fellowship Program (Grade B) of China Postdoctoral Science Foundation, grant number GZB20230857.
Institutional Review Board Statement
All animal experiments related to ASFV were conducted in compliance with the recommendation in the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology (China). The protocols were approved by the Committee on Animal Research and Ethics of Lanzhou Veterinary Research Institute (LVRI) (approval code: SYXK 2020-0010) on 1 June 2022, Chinese Academy of Agricultural Sciences (CAAS) and Ethics Committee for Animal Experimentation of Gansu Province, China. All anesthesia and euthanasia procedures in this study were in compliance with animal welfare requirements and were strictly conducted in accordance with the "Standard Operating Procedures for Anesthesia and Euthanasia of Laboratory Animals" (Document No.: LVRI/HL/SY024-03-03), issued by the Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We gratefully acknowledge the support and assistance provided by all staff members of the BSL-3 facilities in LVRI throughout the course of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ASFV |
African swine fever virus |
| LAVs |
Live attenuated vaccines |
| MGF |
multigene family |
| dpi |
days post-inoculation |
| IFN-β |
Interferon beta |
| ISGs |
interferon-stimulated genes |
| IL-1β |
Interleukin 1β |
| ELISA |
Enzyme Linked Immunosorbent Assay |
References
- Spinard, E.; O'Donnell, V.; Vuono, E.; Rai, A.; Davis, C.; Ramirez-Medina, E.; Espinoza, N.; Valladares, A.; Borca, M.V.; Gladue, D.P. Full genome sequence for the African swine fever virus outbreak in the Dominican Republic in 1980. Sci Rep 2023, 13, 1024. [Google Scholar] [CrossRef]
- Hubálek, Z.; Rudolf, I. Tick-borne viruses in Europe. Parasitol Res 2012, 111, 9–36. [Google Scholar] [CrossRef] [PubMed]
- Rajko-Nenow, P.; Batten, C. Genotyping of African Swine Fever Virus. Methods Mol Biol 2022, 2503, 119–132. [Google Scholar] [CrossRef]
- Galindo, I.; Alonso, C. African Swine Fever Virus: A Review. Viruses 2017, 9. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Conraths, F.J.; Probst, C.; Blohm, U.; Schulz, K.; Sehl, J.; Fischer, M.; Forth, J.H.; Zani, L.; Depner, K.; et al. African Swine Fever in Wild Boar in Europe-A Review. Viruses 2021, 13. [Google Scholar] [CrossRef]
- Li, M.; Zheng, H. Insights and progress on epidemic characteristics, pathogenesis, and preventive measures of African swine fever virus: A review. Virulence 2025, 16, 2457949. [Google Scholar] [CrossRef]
- Mai, N.T.A.; Vu, X.D.; Nguyen, T.T.H.; Nguyen, V.T.; Trinh, T.B.N.; Kim, Y.J.; Kim, H.J.; Cho, K.H.; Nguyen, T.L.; Bui, T.T.N.; et al. Molecular profile of African swine fever virus (ASFV) circulating in Vietnam during 2019-2020 outbreaks. Arch Virol 2021, 166, 885–890. [Google Scholar] [CrossRef]
- Chathuranga, K.; Lee, J.S. African Swine Fever Virus (ASFV): Immunity and Vaccine Development. Vaccines (Basel) 2023, 11. [Google Scholar] [CrossRef]
- Wu, Q.; Lei, Y.; Zuo, Y.; Zhang, J.; Guo, F.; Xu, W.; Xie, T.; Wang, D.; Peng, G.; Wang, X.; et al. Interactome between ASFV and host immune pathway proteins. mSystems 2023, 8, e0047123. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, N.; Xue, Y.; Wang, Z.; Fang, Z.; An, H.; Liu, S.; Weng, C.; Huang, L.; Wang, G. From hemorrhage to apoptosis: understanding the devastating impact of ASFV on piglets. Microbiol Spectr 2025, 13, e0290224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sun, E.; Huang, L.; Ding, L.; Zhu, Y.; Zhang, J.; Shen, D.; Zhang, X.; Zhang, Z.; Ren, T.; et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat Commun 2023, 14, 3096. [Google Scholar] [CrossRef]
- Vu, H.L.X.; McVey, D.S. Recent progress on gene-deleted live-attenuated African swine fever virus vaccines. NPJ Vaccines 2024, 9, 60. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci China Life Sci 2020, 63, 623–634. [Google Scholar] [CrossRef]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Velazquez-Salinas, L.; Gay, C.G.; Gladue, D.P. ASFV-G-∆I177L as an Effective Oral Nasal Vaccine against the Eurasia Strain of Africa Swine Fever. Viruses 2021, 13. [Google Scholar] [CrossRef]
- van den Born, E.; Olasz, F.; Mészáros, I.; Göltl, E.; Oláh, B.; Joshi, J.; van Kilsdonk, E.; Segers, R.; Zádori, Z. African swine fever virus vaccine strain Asfv-G-∆I177l reverts to virulence and negatively affects reproductive performance. NPJ Vaccines 2025, 10, 46. [Google Scholar] [CrossRef]
- Diep, N.V.; Duc, N.V.; Ngoc, N.T.; Dang, V.X.; Tiep, T.N.; Nguyen, V.D.; Than, T.T.; Maydaniuk, D.; Goonewardene, K.; Ambagala, A.; et al. Genotype II Live-Attenuated ASFV Vaccine Strains Unable to Completely Protect Pigs against the Emerging Recombinant ASFV Genotype I/II Strain in Vietnam. Vaccines (Basel) 2024, 12. [Google Scholar] [CrossRef]
- O'Donnell, V.; Holinka, L.G.; Gladue, D.P.; Sanford, B.; Krug, P.W.; Lu, X.Q.; Arzt, J.; Reese, B.; Carrillo, C.; Risatti, G.R.; et al. African Swine Fever Virus Georgia Isolate Harboring Deletions of MGF360 and MGF505 Genes Is Attenuated in Swine and Confers Protection against Challenge with Virulent Parental Virus. Journal of Virology 2015, 89, 6048–6056. [Google Scholar] [CrossRef]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a Highly Effective African Swine Fever Virus Vaccine by Deletion of the I177L Gene Results in Sterile Immunity against the Current Epidemic Eurasia Strain. J Virol 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- O'Donnell, V.; Risatti, G.R.; Holinka, L.G.; Krug, P.W.; Carlson, J.; Velazquez-Salinas, L.; Azzinaro, P.A.; Gladue, D.P.; Borca, M.V. Simultaneous Deletion of the 9GL and UK Genes from the African Swine Fever Virus Georgia 2007 Isolate Offers Increased Safety and Protection against Homologous Challenge. J Virol 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, S.Y.; García-Belmonte, R.; Walczak, M.; Vigara-Astillero, G.; Kim, D.M.; Szymankiewicz, K.; Kochanowski, M.; Liu, L.; Tark, D.; Podgórska, K.; et al. Deletion of MGF505-2R Gene Activates the cGAS-STING Pathway Leading to Attenuation and Protection against Virulent African Swine Fever Virus. Vaccines (Basel) 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.G.; Dang, W.; Shi, Z.W.; Ding, M.Y.; Xu, F.; Li, T.; Feng, T.; Zheng, H.X.; Xiao, S.Q. Identification of African swine fever virus MGF505-2R as a potent inhibitor of innate immunity. Virol Sin 2023, 38, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.Y.; Scheenstra, M.R.; van Dijk, A.; Veldhuizen, E.J.A.; Haagsman, H.P. A new and efficient culture method for porcine bone marrow-derived M1-and M2-polarized macrophages. Vet Immunol Immunop 2018, 200, 7–15. [Google Scholar] [CrossRef]
- Weesendorp, E.; Stegeman, A.; Loeffen, W. Dynamics of virus excretion via different routes in pigs experimentally infected with classical swine fever virus strains of high, moderate or low virulence. Veterinary Microbiology 2009, 133, 9–22. [Google Scholar] [CrossRef]
- Costea, R.; Ene, I.; Pavel, R. Pig Sedation and Anesthesia for Medical Research. Animals (Basel) 2023, 13. [Google Scholar] [CrossRef]
- Fan, W.; Cao, Y.; Jiao, P.; Yu, P.; Zhang, H.; Chen, T.; Zhou, X.; Qi, Y.; Sun, L.; Liu, D.; et al. Synergistic effect of the responses of different tissues against African swine fever virus. Transbound Emerg Dis 2022, 69, e204–e215. [Google Scholar] [CrossRef]
- Sehl-Ewert, J.; Friedrichs, V.; Carrau, T.; Deutschmann, P.; Blome, S. Pathology of African Swine Fever in Reproductive Organs of Mature Breeding Boars. Viruses 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- King, K.; Chapman, D.; Argilaguet, J.M.; Fishbourne, E.; Hutet, E.; Cariolet, R.; Hutchings, G.; Oura, C.A.; Netherton, C.L.; Moffat, K.; et al. Protection of European domestic pigs from virulent African isolates of African swine fever virus by experimental immunisation. Vaccine 2011, 29, 4593–4600. [Google Scholar] [CrossRef] [PubMed]
- Hakobyan, S.; Bayramyan, N.; Karalyan, Z.; Izmailyan, R.; Avetisyan, A.; Poghosyan, A.; Arakelova, E.; Vardanyan, T.; Avagyan, H. The Involvement of MGF505 Genes in the Long-Term Persistence of the African Swine Fever Virus in Gastropods. Viruses 2025, 17. [Google Scholar] [CrossRef]
- Koltsov, A.; Sukher, M.; Krutko, S.; Belov, S.; Korotin, A.; Rudakova, S.; Morgunov, S.; Koltsova, G. Construction of the First Russian Recombinant Live Attenuated Vaccine Strain and Evaluation of Its Protection Efficacy Against Two African Swine Fever Virus Heterologous Strains of Serotype 8. Vaccines (Basel) 2024, 12. [Google Scholar] [CrossRef]
- Zheng, L.; Yan, Z.; Qi, X.; Ren, J.; Ma, Z.; Liu, H.; Zhang, Z.; Li, D.; Pei, J.; Xiao, S.; et al. The Deletion of the MGF360-10L/505-7R Genes of African Swine Fever Virus Results in High Attenuation but No Protection Against Homologous Challenge in Pigs. Viruses 2025, 17. [Google Scholar] [CrossRef]
- O'Donnell, V.; Holinka, L.G.; Gladue, D.P.; Sanford, B.; Krug, P.W.; Lu, X.; Arzt, J.; Reese, B.; Carrillo, C.; Risatti, G.R.; et al. African Swine Fever Virus Georgia Isolate Harboring Deletions of MGF360 and MGF505 Genes Is Attenuated in Swine and Confers Protection against Challenge with Virulent Parental Virus. J Virol 2015, 89, 6048–6056. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, W.P.; Li, L.L.; Li, P.; Ma, Z.; Zhang, J.; Qi, X.L.; Ren, J.J.; Ru, Y.; Niu, Q.L.; et al. African Swine Fever Virus MGF-505-7R Negatively Regulates cGAS-STING-Mediated Signaling Pathway. J Immunol 2021, 206, 1844–1857. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, J.; Zheng, J.; Li, D.; Weng, C. Multifunctional pMGF505-7R Is a Key Virulence-Related Factor of African Swine Fever Virus. Front Microbiol 2022, 13, 852431. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, J.; Kang, L.; Huang, L.; Zhou, S.; Hu, L.; Zheng, J.; Li, C.; Zhang, X.; He, X.; et al. pMGF505-7R determines pathogenicity of African swine fever virus infection by inhibiting IL-1β and type I IFN production. PLoS Pathog 2021, 17, e1009733. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).