Submitted:
22 September 2025
Posted:
23 September 2025
You are already at the latest version
Abstract
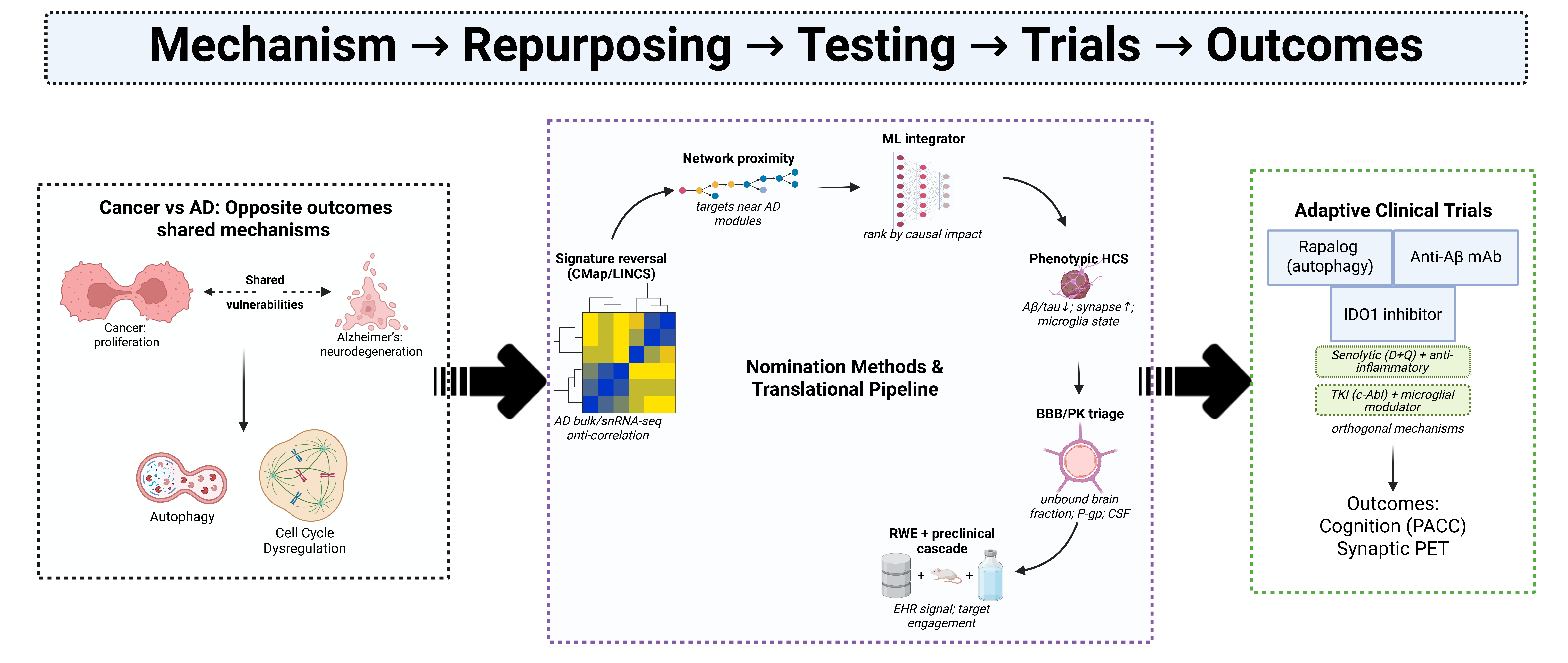
Keywords:
1. Introduction
2. Cross-Disciplinary Biology: Where Cancer and Alzheimer’s Intersect
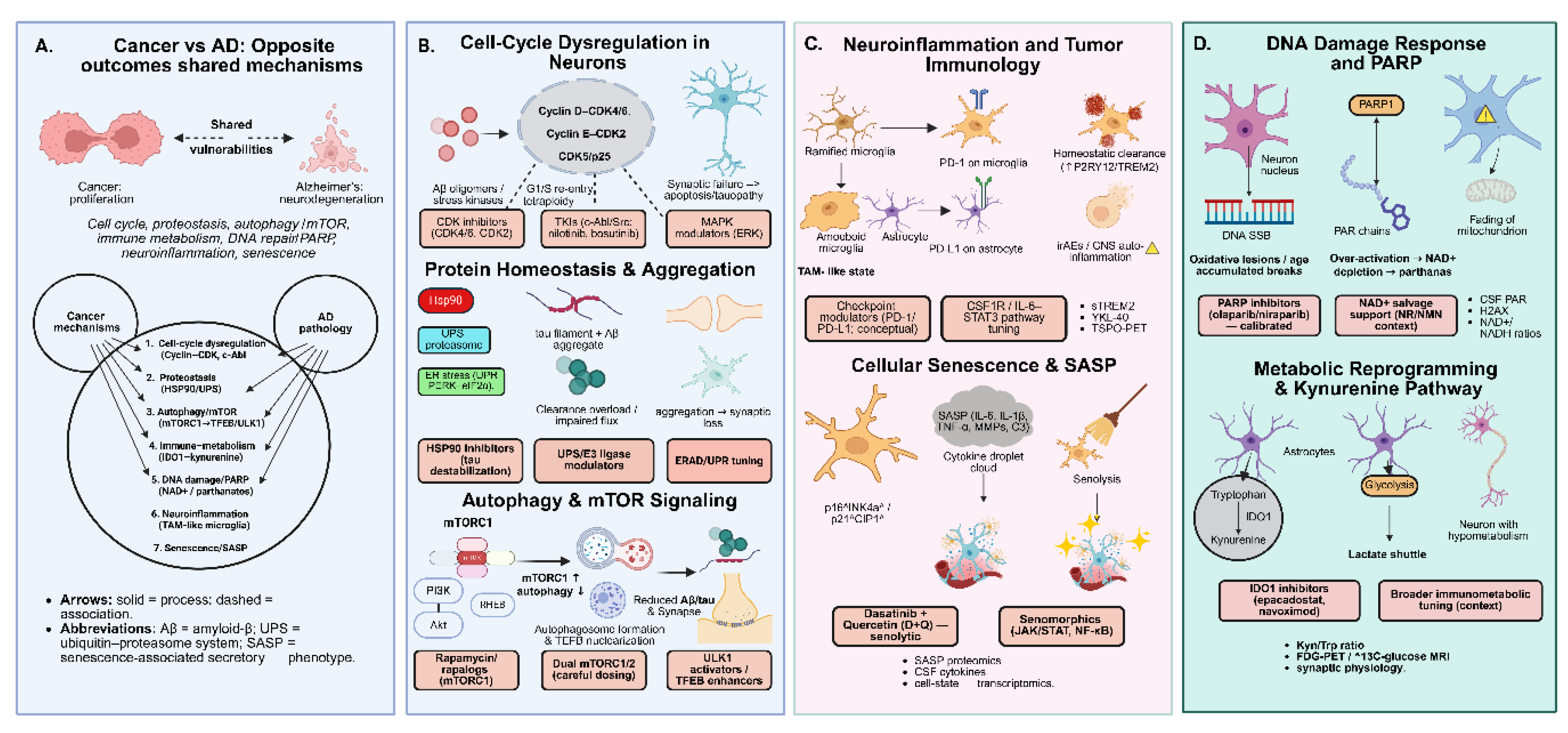
2.1. Cell-Cycle Dysregulation in Neurons
2.2. Protein Homeostasis and Aggregation
2.3. Autophagy and mTOR Signaling
2.4. Metabolic Reprogramming and the Kynurenine Pathway
2.5. DNA Damage Response and PARP
2.6. Neuroinflammation and Tumor Immunology
2.7. Cellular Senescence and SASP
3. Oncology Drug Classes with Mechanistic/Translational Promise in AD
3.1. Tyrosine Kinase Inhibitors (TKIs): Nilotinib, Bosutinib, etc.
3.3. Retinoid X Receptor Agonists (Bexarotene) – A Cautionary Case
3.4. Immune–Metabolism Targets: IDO1 Inhibitors and the Kynurenine Pathway
3.5. Cytotoxic Agents Identified by Transcriptomic Signature Reversal (Letrozole + Irinotecan)
3.6. PARP Inhibitors and DNA Damage Modulators
3.7. Epigenetic Modulators (HDAC Inhibitors)
3.8. Immune Checkpoint Modulators
3.9. Other Oncology Agents (Anti-Angiogenics, Immunomodulators, Metabolic)
4. Methods and Approaches Used to Nominate Oncology–AD Repurposing Candidates
4.1. Transcriptomic Signature Reversal (CMap/LINCS)
4.2. Network Pharmacology and Knowledge Graph
4.3. Cheminformatics, Docking, and Ligand-Based Screens
4.4. AI/Machine-Learning Integrators
4.5. Single-Cell and Multi-Omics Integration
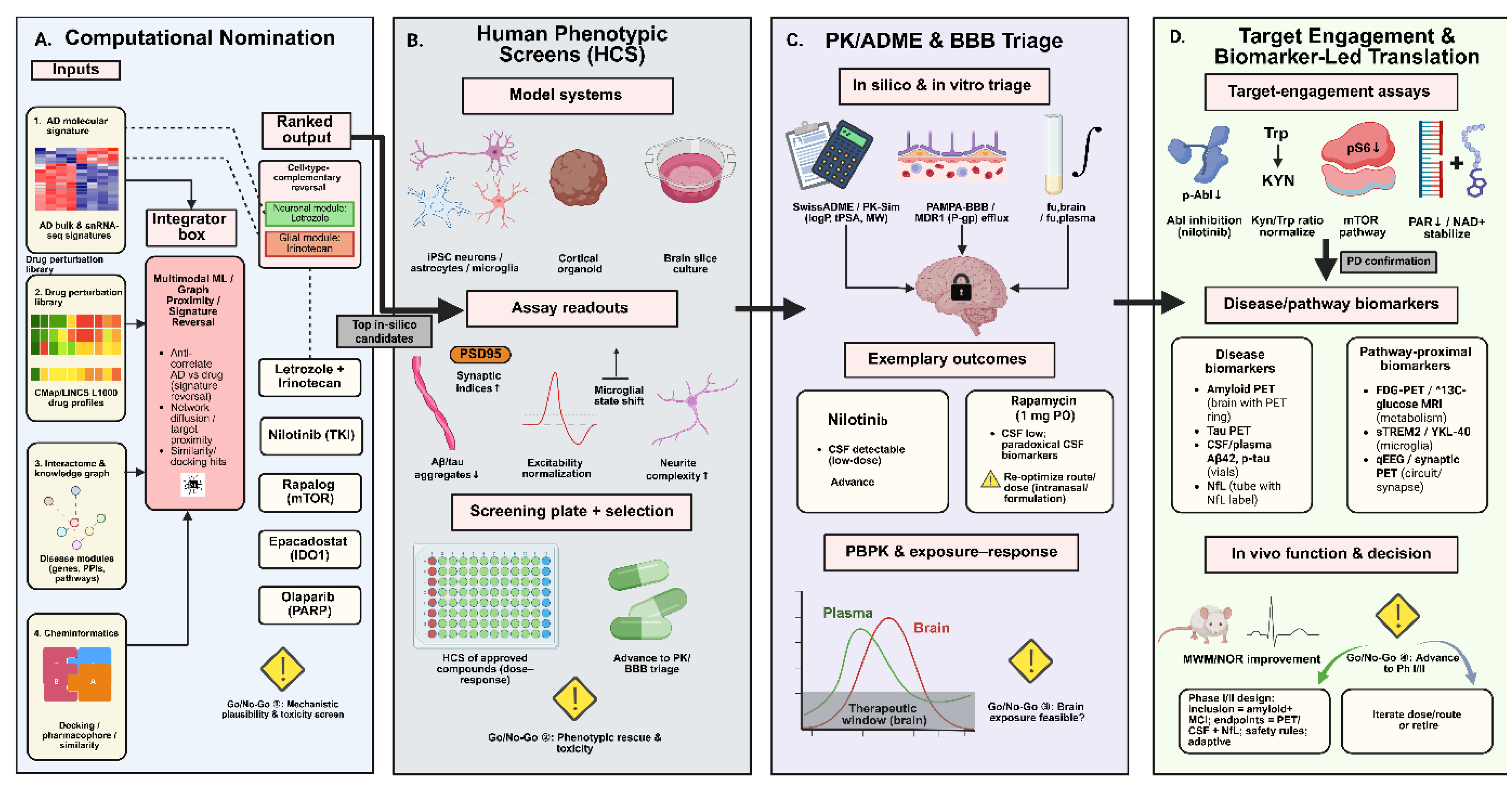
4.6. Network Pharmacology and Knowledge Graph
4.7. Phenotypic High-Content Screening (iPSC, Organoids, Slices)
4.8. PK/ADME and BBB Triage
4.9. Preclinical Validation Cascades and Translational Readouts
| Drug (Class) | Proposed mechanism in AD | Preclinical evidence | Trial (Phase; NCT) | Endpoints / Biomarkers | Key clinical status / comments |
|---|---|---|---|---|---|
| Nilotinib (TKI) | c-Abl/Src inhibition; ↑autophagy → Aβ/tau clearance | In AD mice: lowers Aβ and tau; cognition improved | Phase II; NCT02921477 [69] | Primary: ADAS-Cog; Secondary: amyloid PET, CSF Aβ/p-tau | Biomarker movement observed (↓brain amyloid; ↓CSF Aβ, p-tau) [18]. 150 mg tolerated; 300 mg linked to agitation. |
| Masitinib (TKI) | c-Kit/PDGFR/Fyn inhibition; mast-cell/microglial modulation | AD rodents: ↓inflammation, ↓plaques, memory rescue | Phase 2B/3; NCT00976118 [132] | ADAS-Cog, ADCS-ADL; MRI, CSF | 4.5 mg/kg qd significantly slowed cognitive/ADL decline (p=0.0003). Confirmatory Phase 3 ongoing. |
| Rapamycin (mTOR inhibitor) | mTORC1 inhibition → autophagy ↑; ↓Aβ/τ pathology [133] | Multiple AD rodent studies: rescue of memory; pathology reduction | Phase I; NCT04200911 [134] | CSF p-tau, GFAP; MRI volume | Safe, not detectable in CSF at tested dose; paradoxical ↑CSF p-tau, GFAP. Dose/route optimization needed. |
| Bexarotene (RXR agonist) | ↑ApoE/ABCA1; lipid transport; proposed Aβ clearance | Initial 2012 report of rapid plaque clearance not replicated across labs | Phase II (BEAT-AD, completed); NCT02061878 [135] | Amyloid PET; ADAS-Cog | Marked hypertriglyceridemia; no significant cognitive or PET benefit; tolerability limits CNS-relevant dosing. |
| Dasatinib + Quercetin (Senolytic) | Senescent cell clearance; ↓neuroinflammation | AD mice: preserved cognitive function; reduced senescent cells | Phase 1; NCT04063124 [136]; Phase 2 ongoing | Primary: Safety, CNS penetration; Secondary: CSF biomarkers | Dasatinib detected in CSF in most participants; signals for amyloid clearance and ↓inflammation; intermittent dosing well-tolerated. |
| Temsirolimus (mTOR inhibitor) | mTOR inhibition; ↑autophagy → Aβ clearance | APP/PS1 mice: ↓Aβ burden, ↓apoptosis, cognition improved | Preclinical only [137] | N/A | Promoted autophagic Aβ clearance; efficacy likely greatest in early disease windows. |
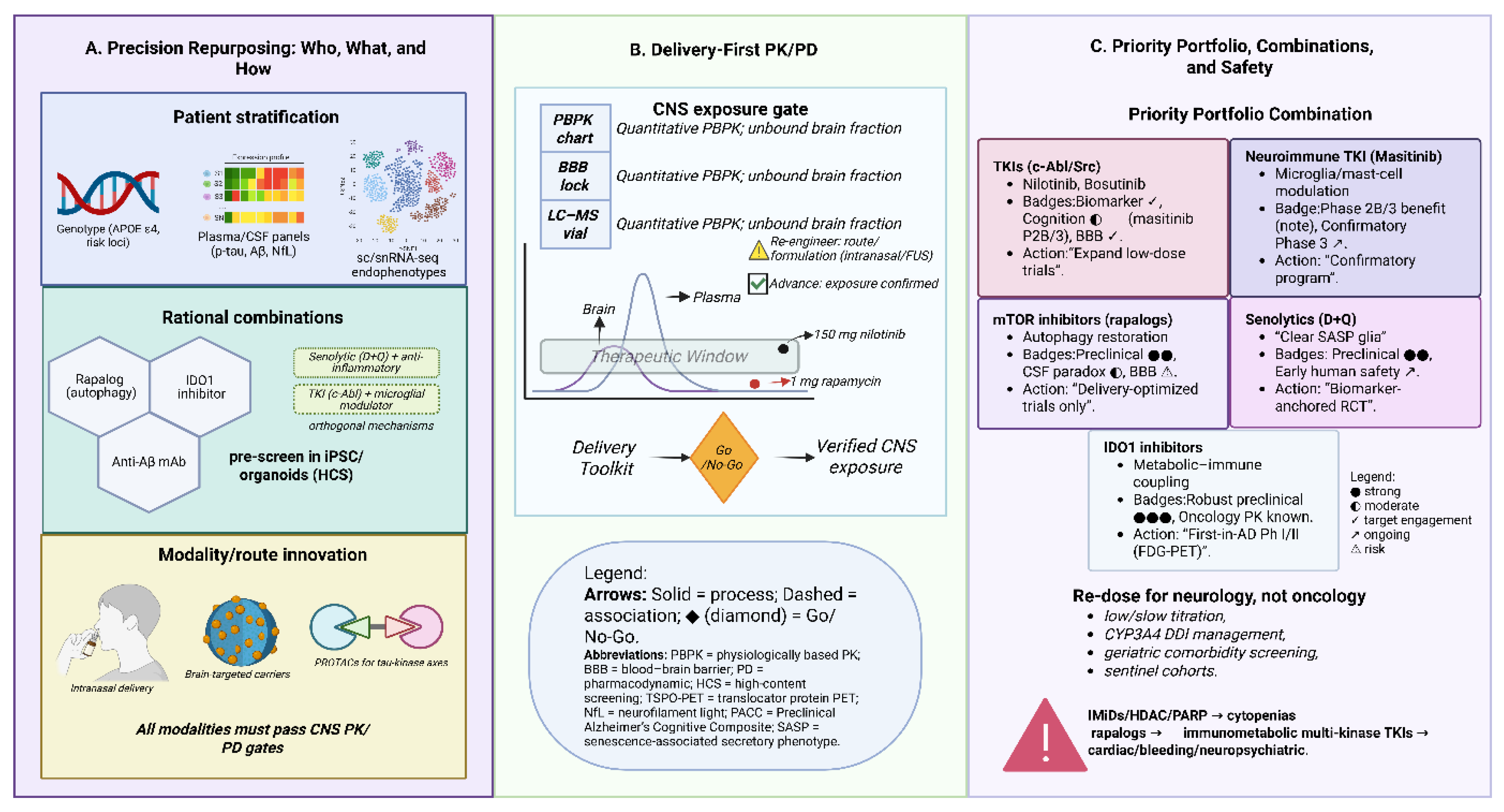
5. Clinical Evidence, Ongoing Trials, and Negative Studies
5.1. Summary of Trials
5.2. Lessons from Negative or Irreproducible Results
5.2.1. Clinical Trial Design Recommendations
6. Conclusions and Future Directions
Author Contributions
Funding
Declaration of generative AI and AI-assisted technologies in the writing process
Ethical Statement
Acknowledgments
Conflicts of Interest
References
- Mamun M, Bin Shawkat S, Ahammed MS, Uddin MM, Mahmud MI, Islam AM. Deep Learning Based Model for Alzheimer’s Disease Detection Using Brain MRI Images. In: 2022 IEEE 13th Annual Ubiquitous Computing, Electronics & Mobile Communication Conference (UEMCON) [Internet]. New York, NY, NY, USA: IEEE; 2022 [cited 2025 Sept 2]. p. 0510–6. Available from: https://ieeexplore.ieee.org/document/9965730/.
- Rosende-Roca M, García-Gutiérrez F, Cantero-Fortiz Y, Alegret M, Pytel V, Cañabate P, González-Pérez A, De Rojas I, Vargas L, Tartari JP, Espinosa A, Ortega G, Pérez-Cordón A, Moreno M, Preckler S, Seguer S, Gurruchaga MJ, Tárraga L, Ruiz A, Valero S, Boada M, Marquié M. Exploring sex differences in Alzheimer’s disease: a comprehensive analysis of a large patient cohort from a memory unit. Alzheimers Res Ther. 2025 Jan 22;17(1):27. [CrossRef]
- 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025 Apr;21(4):e70235.
- C. Vickers J, Mitew S, Woodhouse A, M. Fernandez-Martos C, T. Kirkcaldie M, J. Canty A, H. McCormack G, E. King A. Defining the earliest pathological changes of Alzheimer’s disease. Curr Alzheimer Res. 2016 Feb 12;13(3):281–7.
- Wimo A, Seeher K, Cataldi R, Cyhlarova E, Dielemann JL, Frisell O, Guerchet M, Jönsson L, Malaha AK, Nichols E, Pedroza P, Prince M, Knapp M, Dua T. The worldwide costs of dementia in 2019. Alzheimers Dement. 2023 July;19(7):2865–73. [CrossRef]
- 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023 Apr;19(4):1598–695.
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary Costs of Dementia in the United States. N Engl J Med. 2013 Apr 4;368(14):1326–34. [CrossRef]
- 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. 2024 May;20(5):3708–821.
- Paduvilan AK, Livingston GAL, Kuppuchamy SK, Dhanaraj RK, Subramanian M, Al-Rasheed A, Getahun M, Soufiene BO. Attention-driven hybrid deep learning and SVM model for early Alzheimer’s diagnosis using neuroimaging fusion. BMC Med Inform Decis Mak. 2025 July 1;25(1):219. [CrossRef]
- Das U, Chandramouli L, Uttarkar A, Kumar J, Niranjan V. Discovery of natural compounds as novel FMS-like tyrosine kinase-3 (FLT3) therapeutic inhibitors for the treatment of acute myeloid leukemia: An in-silico approach. Asp Mol Med. 2025 June;5:100058. [CrossRef]
- Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther. 2014 July 3;6(4):37. [CrossRef]
- Birks JS, Harvey RJ. Donepezil for dementia due to Alzheimer’s disease. Cochrane Dementia and Cognitive Improvement Group, editor. Cochrane Database Syst Rev [Internet]. 2018 June 18 [cited 2025 Sept 2];2018(6). [CrossRef]
- Van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, Kanekiyo M, Li D, Reyderman L, Cohen S, Froelich L, Katayama S, Sabbagh M, Vellas B, Watson D, Dhadda S, Irizarry M, Kramer LD, Iwatsubo T. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. 2023 Jan 5;388(1):9–21.
- Gandy S, DeKosky ST. Toward the Treatment and Prevention of Alzheimer’s Disease: Rational Strategies and Recent Progress. Annu Rev Med. 2013 Jan 14;64(1):367–83. [CrossRef]
- Kim CK, Lee YR, Ong L, Gold M, Kalali A, Sarkar J. Alzheimer’s Disease: Key Insights from Two Decades of Clinical Trial Failures. J Alzheimers Dis. 2022 May 3;87(1):83–100. [CrossRef]
- Das U. Generative AI for drug discovery and protein design: the next frontier in AI-driven molecular science. Med Drug Discov. 2025 Sept;27:100213. [CrossRef]
- DeTure MA, Dickson DW. The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener. 2019 Dec;14(1):32.
- Das U, Chanda T, Kumar J, Peter A. Discovery of natural MCL1 inhibitors using pharmacophore modelling, QSAR, docking, ADMET, molecular dynamics, and DFT analysis. Comput Biol Chem. 2025 Aug;117:108427. [CrossRef]
- Grabowska ME, Huang A, Wen Z, Li B, Wei WQ. Drug repurposing for Alzheimer’s disease from 2012–2022—a 10-year literature review. Front Pharmacol. 2023 Sept 7;14:1257700.
- Das U, Uttarkar A, Kumar J, Niranjan V. In silico exploration natural compounds for the discovery of novel dnmt3a inhibitors as potential therapeutic agents for acute myeloid leukaemia. Silico Res Biomed. 2025 Apr;100006. [CrossRef]
- Weth FR, Hoggarth GB, Weth AF, Paterson E, White MPJ, Tan ST, Peng L, Gray C. Unlocking hidden potential: advancements, approaches, and obstacles in repurposing drugs for cancer therapy. Br J Cancer. 2024 Mar 23;130(5):703–15. [CrossRef]
- Xia Y, Sun M, Huang H, Jin WL. Drug repurposing for cancer therapy. Signal Transduct Target Ther. 2024 Apr 19;9(1):92.
- Zhang W, Xiao D, Mao Q, Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther. 2023 July 12;8(1):267. [CrossRef]
- Zabłocka A, Kazana W, Sochocka M, Stańczykiewicz B, Janusz M, Leszek J, Orzechowska B. Inverse Correlation Between Alzheimer’s Disease and Cancer: Short Overview. Mol Neurobiol. 2021 Dec;58(12):6335–49. [CrossRef]
- Driver JA, Beiser A, Au R, Kreger BE, Splansky GL, Kurth T, Kiel DP, Lu KP, Seshadri S, Wolf PA. Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ. 2012 Mar 12;344(mar12 1):e1442–e1442.
- Okereke OI, Meadows ME. More Evidence of an Inverse Association Between Cancer and Alzheimer Disease. JAMA Netw Open. 2019 June 21;2(6):e196167. [CrossRef]
- Mezencev R, Chernoff YO. Risk of Alzheimer’s Disease in Cancer Patients: Analysis of Mortality Data from the US SEER Population-Based Registries. Cancers. 2020 Mar 26;12(4):796. [CrossRef]
- Nandakumar S, Rozich E, Buttitta L. Cell Cycle Re-entry in the Nervous System: From Polyploidy to Neurodegeneration. Front Cell Dev Biol. 2021 June 24;9:698661. [CrossRef]
- Seo J, Park M. Molecular crosstalk between cancer and neurodegenerative diseases. Cell Mol Life Sci. 2020 July;77(14):2659–80. [CrossRef]
- Stevenson M, Algarzae NK, Moussa C. Tyrosine kinases: multifaceted receptors at the intersection of several neurodegenerative disease-associated processes. Front Dement. 2024 Aug 16;3:1458038.
- Valiukas Z, Tangalakis K, Apostolopoulos V, Feehan J. Microglial activation states and their implications for Alzheimer’s Disease. J Prev Alzheimers Dis. 2025 Jan;12(1):100013. [CrossRef]
- Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015 Feb;14(2):130–46.
- Gérard C, Goldbeter A. Temporal self-organization of the cyclin/Cdk network driving the mammalian cell cycle. Proc Natl Acad Sci. 2009 Dec 22;106(51):21643–8. [CrossRef]
- Denechaud M, Geurs S, Comptdaer T, Bégard S, Garcia-Núñez A, Pechereau LA, Bouillet T, Vermeiren Y, De Deyn PP, Perbet R, Deramecourt V, Maurage CA, Vanderhaegen M, Vanuytven S, Lefebvre B, Bogaert E, Déglon N, Voet T, Colin M, Buée L, Dermaut B, Galas MC. Tau promotes oxidative stress-associated cycling neurons in S phase as a pro-survival mechanism: Possible implication for Alzheimer’s disease. Prog Neurobiol. 2023 Apr;223:102386.
- Newton TM, Duce JA, Bayle ED. The proteostasis network provides targets for neurodegeneration. Br J Pharmacol. 2019 Sept;176(18):3508–14.
- Wellman R, Jacobson D, Secrier M, Labbadia J. Distinct patterns of proteostasis network gene expression are associated with different prognoses in melanoma patients. Sci Rep. 2024 Jan 2;14(1):198. [CrossRef]
- Tseng CS, Chao YW, Liu YH, Huang YS, Chao HW. Dysregulated proteostasis network in neuronal diseases. Front Cell Dev Biol. 2023 Feb 24;11:1075215. [CrossRef]
- Cai Z, Zhou Y, Xiao M, Yan LJ, He W. Activation of mTOR: a culprit of Alzheimer’s disease? Neuropsychiatr Dis Treat. 2015 Apr;1015.
- Panwar V, Singh A, Bhatt M, Tonk RK, Azizov S, Raza AS, Sengupta S, Kumar D, Garg M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct Target Ther. 2023 Oct 2;8(1):375.
- Xie PL, Zheng MY, Han R, Chen WX, Mao JH. Pharmacological mTOR inhibitors in ameliorating Alzheimer’s disease: current review and perspectives. Front Pharmacol. 2024 May 30;15:1366061. [CrossRef]
- De D, Mukherjee I, Guha S, Paidi RK, Chakrabarti S, Biswas SC, Bhattacharyya SN. Rheb-mTOR activation rescues Aβ-induced cognitive impairment and memory function by restoring miR-146 activity in glial cells. Mol Ther - Nucleic Acids. 2021 June;24:868–87.
- Davoody S, Asgari Taei A, Khodabakhsh P, Dargahi L. mTOR signaling and Alzheimer’s disease: What we know and where we are? CNS Neurosci Ther. 2024 Apr;30(4):e14463. [CrossRef]
- Alzheimer’s: Novel cancer drug restores cognition, memory loss in mice [Internet]. 2024 [cited 2025 Sept 3]. Available from: https://www.medicalnewstoday.com/articles/cancer-drug-targets-brain-metabolism-alzheimers-treatment-study.
- Minhas PS, Jones JR, Latif-Hernandez A, Sugiura Y, Durairaj AS, Wang Q, Mhatre SD, Uenaka T, Crapser J, Conley T, Ennerfelt H, Jung YJ, Liu L, Prasad P, Jenkins BC, Ay YA, Matrongolo M, Goodman R, Newmeyer T, Heard K, Kang A, Wilson EN, Yang T, Ullian EM, Serrano GE, Beach TG, Wernig M, Rabinowitz JD, Suematsu M, Longo FM, McReynolds MR, Gage FH, Andreasson KI. Restoring hippocampal glucose metabolism rescues cognition across Alzheimer’s disease pathologies. Science. 2024 Aug 23;385(6711):eabm6131.
- Murata MM, Kong X, Moncada E, Chen Y, Imamura H, Wang P, Berns MW, Yokomori K, Digman MA. NAD+ consumption by PARP1 in response to DNA damage triggers metabolic shift critical for damaged cell survival. Mol Biol Cell. 2019 Sept 15;30(20):2584–97. [CrossRef]
- Salech F, Ponce DP, Paula-Lima AC, SanMartin CD, Behrens MI. Nicotinamide, a Poly [ADP-Ribose] Polymerase 1 (PARP-1) Inhibitor, as an Adjunctive Therapy for the Treatment of Alzheimer’s Disease. Front Aging Neurosci. 2020 Aug 13;12:255.
- Gupta R, Kumar P. Computational Analysis Indicates That PARP1 Acts as a Histone Deacetylases Interactor Sharing Common Lysine Residues for Acetylation, Ubiquitination, and SUMOylation in Alzheimer’s and Parkinson’s Disease. ACS Omega. 2021 Mar 2;6(8):5739–53.
- Ispirjan M, Marx S, Freund E, Fleck SK, Baldauf J, Roessler K, Schroeder HWS, Bekeschus S. Markers of tumor-associated macrophages and microglia exhibit high intratumoral heterogeneity in human glioblastoma tissue. Oncoimmunology. 13(1):2425124. [CrossRef]
- Wang G, Zhong K, Wang Z, Zhang Z, Tang X, Tong A, Zhou L. Tumor-associated microglia and macrophages in glioblastoma: From basic insights to therapeutic opportunities. Front Immunol. 2022 July 27;13:964898. [CrossRef]
- Zhao W, Zhang Z, Xie M, Ding F, Zheng X, Sun S, Du J. Exploring tumor-associated macrophages in glioblastoma: from diversity to therapy. Npj Precis Oncol. 2025 May 2;9(1):126. [CrossRef]
- Park T, Chang L, Chung SW, Lee S, Bae S, Condello C, Kim YH, Lee J, Kim HJ, Kwon HK, Suh M. Targeting glial PD-1/PD-L1 restores microglial homeostasis and attenuates neuronal hyperactivity in an Alzheimer’s disease model [Internet]. bioRxiv; 2025 [cited 2025 Sept 3]. p. 2024.09.13.612389. Available from: https://www.biorxiv.org/content/10.1101/2024.09.13.612389v2.
- Liu Y, Wu J, Najem H, Lin Y, Pang L, Khan F, Zhou F, Ali H, Heimberger AB, Chen P. Dual targeting macrophages and microglia is a therapeutic vulnerability in models of PTEN-deficient glioblastoma. J Clin Invest [Internet]. 2024 Nov 15 [cited 2025 Sept 3];134(22). Available from: https://www.jci.org/articles/view/178628.
- Rosenzweig N, Dvir-Szternfeld R, Tsitsou-Kampeli A, Keren-Shaul H, Ben-Yehuda H, Weill-Raynal P, Cahalon L, Kertser A, Baruch K, Amit I, Weiner A, Schwartz M. PD-1/PD-L1 checkpoint blockade harnesses monocyte-derived macrophages to combat cognitive impairment in a tauopathy mouse model. Nat Commun. 2019 Jan 28;10(1):465. [CrossRef]
- Mortada I, Farah R, Nabha S, Ojcius DM, Fares Y, Almawi WY, Sadier NS. Immunotherapies for Neurodegenerative Diseases. Front Neurol. 2021 June 7;12:654739.
- Immunotherapy of brain cancers and neurological disorders - MedCrave online [Internet]. [cited 2025 Sept 3]. Available from: https://medcraveonline.com/JCPCR/immunotherapy-of-brain-cancers-and-neurological-disorders.html.
- The Role of Glial Cell Senescence in Alzheimer’s Disease - Alshaebi - 2025 - Journal of Neurochemistry - Wiley Online Library [Internet]. [cited 2025 Sept 3]. [CrossRef]
- Han X, Zhang T, Liu H, Mi Y, Gou X. Astrocyte Senescence and Alzheimer’s Disease: A Review. Front Aging Neurosci. 2020 June 9;12:148. [CrossRef]
- Behfar Q, Ramirez Zuniga A, Martino-Adami PV. Aging, Senescence, and Dementia. J Prev Alzheimers Dis. 2022 July 1;9(3):523–31.
- Hudson HR, Sun X, Orr ME. Senescent brain cell types in Alzheimer’s disease: Pathological mechanisms and therapeutic opportunities. Neurotherapeutics. 2025 Apr 1;22(3):e00519. [CrossRef]
- Franco A de O, Schlickmann TH, Ferrari-Souza JP, de Bastiani MA, Castilhos RM, Zimmer ER. Senescence-associated secretory phenotype (SASP) index in individuals across the Alzheimer’s disease continuum. Alzheimers Dement. 2024;20(S2):e092727. [CrossRef]
- Yang J, Zhou Y, Wang T, Li N, Chao Y, Gao S, Zhang Q, Wu S, Zhao L, Dong X. A multi-omics study to monitor senescence-associated secretory phenotypes of Alzheimer’s disease. Ann Clin Transl Neurol. 2024 Apr 11;11(5):1310–24.
- Barrio-Alonso E, Hernández-Vivanco A, Walton CC, Perea G, Frade JM. Cell cycle reentry triggers hyperploidization and synaptic dysfunction followed by delayed cell death in differentiated cortical neurons. Sci Rep. 2018 Sept 25;8(1):14316.
- Luo W, Rodina A, Chiosis G. Heat shock protein 90: translation from cancer to Alzheimer’s disease treatment? BMC Neurosci. 2008 Dec;9(S2):S7.
- Li VOK, Han Y, Kaistha T, Zhang Q, Downey J, Gozes I, Lam JCK. DeepDrug as an expert guided and AI driven drug repurposing methodology for selecting the lead combination of drugs for Alzheimer’s disease. Sci Rep. 2025 Jan 15;15(1):2093. [CrossRef]
- Das V, Miller JH, Alladi CG, Annadurai N, De Sanctis JB, Hrubá L, Hajdúch M. Antineoplastics for treating Alzheimer’s disease and dementia: Evidence from preclinical and observational studies. Med Res Rev. 2024 Sept;44(5):2078–111. [CrossRef]
- Ancidoni A, Bacigalupo I, Remoli G, Lacorte E, Piscopo P, Sarti G, Corbo M, Vanacore N, Canevelli M. Anticancer drugs repurposed for Alzheimer’s disease: a systematic review. Alzheimers Res Ther. 2021 May 5;13:96. [CrossRef]
- Eshraghi M, Ahmadi M, Afshar S, Lorzadeh S, Adlimoghaddam A, Rezvani Jalal N, West R, Dastghaib S, Igder S, Torshizi SRN, Mahmoodzadeh A, Mokarram P, Madrakian T, Albensi BC, Łos MJ, Ghavami S, Pecic S. Enhancing autophagy in Alzheimer’s disease through drug repositioning. Pharmacol Ther. 2022 Sept 1;237:108171. [CrossRef]
- Masitinib for the Treatment of Alzheimer’s Disease: Neurodegenerative Disease Management: Vol 11, No 4 [Internet]. [cited 2025 Sept 3]. [CrossRef]
- Jordan S. Open Label Study for the Use of Tyrosine Kinase Inhibitors for Treatment of Cognitive Decline Due to Degenerative Dementias [Internet]. clinicaltrials.gov; 2022 Sept [cited 2025 Sept 3]. Report No.: NCT02921477. Available from: https://clinicaltrials.gov/study/NCT02921477.
- AB Science. A Multicenter, Double-blind, Placebo-controlled, Randomized, Parallel-group Phase 3 Study to Evaluate the Safety and Efficacy of Masitinib in Patients With Mild to Moderate Alzheimer's Disease [Internet]. clinicaltrials.gov; 2023 Sept [cited 2025 Sept 3]. Report No.: NCT01872598. Available from: https://clinicaltrials.gov/study/NCT01872598.
- Dubois B, López-Arrieta J, Lipschitz S, Doskas T, Spiru L, Moroz S, Venger O, Vermersch P, Moussy A, Mansfield CD, Hermine O, Tsolaki M, AB09004 Study Group Investigators. Masitinib for mild-to-moderate Alzheimer’s disease: results from a randomized, placebo-controlled, phase 3, clinical trial. Alzheimers Res Ther. 2023 Feb 28;15(1):39.
- Shafei MA, Harris M, Conway ME. Divergent Metabolic Regulation of Autophagy and mTORC1—Early Events in Alzheimer’s Disease? Front Aging Neurosci [Internet]. 2017 June 2 [cited 2025 Sept 3];9. Available from: https://www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2017.00173/full.
- Xie PL, Zheng MY, Han R, Chen WX, Mao JH. Pharmacological mTOR inhibitors in ameliorating Alzheimer’s disease: current review and perspectives. Front Pharmacol [Internet]. 2024 May 30 [cited 2025 Sept 3];15. Available from: https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2024.1366061/full. [CrossRef]
- Subramanian A, Tamilanban T, Alsayari A, Ramachawolran G, Wong LS, Sekar M, Gan SH, Subramaniyan V, Chinni SV, Izzati Mat Rani NN, Suryadevara N, Wahab S. Trilateral association of autophagy, mTOR and Alzheimer’s disease: Potential pathway in the development for Alzheimer’s disease therapy. Front Pharmacol. 2022 Dec 22;13:1094351. [CrossRef]
- Zhang W, Xu C, Sun J, Shen HM, Wang J, Yang C. Impairment of the autophagy–lysosomal pathway in Alzheimer’s diseases: Pathogenic mechanisms and therapeutic potential. Acta Pharm Sin B. 2022 Mar 1;12(3):1019–40. [CrossRef]
- Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by Rapamycin Abolishes Cognitive Deficits and Reduces Amyloid-β Levels in a Mouse Model of Alzheimer’s Disease. PLOS ONE. 2010 Apr 1;5(4):e9979.
- Tousi B. The emerging role of bexarotene in the treatment of Alzheimer’s disease: current evidence. Neuropsychiatr Dis Treat. 2015 Feb 5;11:311–5.
- Cummings JL, Zhong K, Kinney JW, Heaney C, Moll-Tudla J, Joshi A, Pontecorvo M, Devous M, Tang A, Bena J. Double-blind, placebo-controlled, proof-of-concept trial of bexarotene Xin moderate Alzheimer’s disease. Alzheimers Res Ther. 2016 Jan 29;8:4.
- The Cleveland Clinic. A Double Blind Placebo Controlled Randomized Study to Evaluate the Efficacy and Safety of Bexarotene in Patients With Mild to Moderate Alzheimer's Disease [Internet]. clinicaltrials.gov; 2016 Feb [cited 2025 Sept 3]. Report No.: NCT01782742. Available from: https://clinicaltrials.gov/study/NCT01782742.
- Johnson LA, Macauley SL. Alzheimer’s and metabolism wed with IDO1. Science. 2024 Aug 23;385(6711):826–7.
- Crunkhorn S. Targeting the kynurenine pathway. Nat Rev Drug Discov. 2024 Sept 27;23(11):816–816.
- Duan Z, Shi L, He ZNT, Kuang C, Han T, Yang Q. The Protective Effect of IDO1 Inhibition in Aβ-Treated Neurons and APP/PS1 Mice. Am J Alzheimers Dis Other Demen. 2023 Nov 9;38:15333175231214861. [CrossRef]
- INCB24360 (Epacadostat), a Highly Potent and Selective Indoleamine-2,3-dioxygenase 1 (IDO1) Inhibitor for Immuno-oncology | ACS Medicinal Chemistry Letters [Internet]. [cited 2025 Sept 3]. Available from: https://pubs.acs.org/doi/10.1021/acsmedchemlett.6b00391.
- Jiang K, Wang Q, Chen XL, Wang X, Gu X, Feng S, Wu J, Shang H, Ba X, Zhang Y, Tang K. Nanodelivery Optimization of IDO1 Inhibitors in Tumor Immunotherapy: Challenges and Strategies. Int J Nanomedicine. 2024 Aug 28;19:8847–82. [CrossRef]
- Jiang S, Li H, Piao L, Jin Z, Liu J, Chen S, Liu LL, Shao Y, Zhong S, Wu B, Li W, Ren J, Zhang Y, Wang H, Jin R. Computational study on new natural compound inhibitors of indoleamine 2,3-dioxygenase 1. Aging. 2020 June 22;12(12):11349–63. [CrossRef]
- Savonije K, Meek A, Weaver DF. Indoleamine 2,3-Dioxygenase as a Therapeutic Target for Alzheimer’s Disease and Geriatric Depression. Brain Sci. 2023 May 24;13(6):852. [CrossRef]
- Li Y, Serras CP, Blumenfeld J, Xie M, Hao Y, Deng E, Chun YY, Holtzman J, An A, Yoon SY, Tang X, Rao A, Woldemariam S, Tang A, Zhang A, Simms J, Lo I, Oskotsky T, Keiser MJ, Huang Y, Sirota M. Data-driven discovery of cell-type-directed network-correcting combination therapy for Alzheimer’s disease. bioRxiv. 2024 Dec 13;2024.12.09.627436.
- Li Y, Pereda Serras C, Blumenfeld J, Xie M, Hao Y, Deng E, Chun YY, Holtzman J, An A, Yoon SY, Tang X, Rao A, Woldemariam S, Tang A, Zhang A, Simms J, Lo I, Oskotsky T, Keiser MJ, Huang Y, Sirota M. Cell-type-directed network-correcting combination therapy for Alzheimer’s disease. Cell. 2025 July 15;S0092-8674(25)00737-8. [CrossRef]
- Alzheimer’s Mouse Model Shows Memory Boost from Anticancer Drug Combo [Internet]. [cited 2025 Sept 3]. Available from: https://www.genengnews.com/topics/drug-discovery/alzheimers-mouse-model-shows-memory-boost-from-anticancer-drug-combo/.
- Do These Two Cancer Drugs Have What It Takes to Beat Alzheimer’s? | UC San Francisco [Internet]. [cited 2025 Sept 3]. Available from: https://www.ucsf.edu/news/2025/07/430386/do-these-two-cancer-drugs-have-what-it-takes-beat-alzheimers.
- Alzheimer’s treatment: Cancer drug combo shows promise in mice [Internet]. [cited 2025 Sept 3]. Available from: https://www.medicalnewstoday.com/articles/might-a-combination-of-2-cancer-drugs-help-treat-alzheimers-disease.
- Cancer Drugs Could Treat Alzheimer’s: Drug Repurposing Win [Internet]. Medscape. [cited 2025 Sept 3]. Available from: https://www.medscape.com/viewarticle/cancer-drugs-could-treat-alzheimers-drug-repurposing-win-2025a1000kv4.
- Ordaz DA, Gupta K, Bota DA. The role of Poly-ADP ribose polymerase (PARP) enzymes in chemotherapy-induced cognitive impairments – parallels with other neurodegenerative disorders. Front Pharmacol. 2025 June 9;16:1615843. [CrossRef]
- Mao K, Zhang G. The role of PARP1 in neurodegenerative diseases and aging. FEBS J. 2022;289(8):2013–24. [CrossRef]
- Zeng J, Libien J, Shaik F, Wolk J, Hernández AI. Nucleolar PARP-1 Expression Is Decreased in Alzheimer’s Disease: Consequences for Epigenetic Regulation of rDNA and Cognition. Neural Plast. 2016;2016:8987928. [CrossRef]
- Martire S, Mosca L, d’Erme M. PARP-1 involvement in neurodegeneration: A focus on Alzheimer’s and Parkinson’s diseases. Mech Ageing Dev. 2015 Mar 1;146–148:53–64.
- Hu ML, Pan YR, Yong YY, Liu Y, Yu L, Qin DL, Qiao G, Law BYK, Wu JM, Zhou XG, Wu AG. Poly (ADP-ribose) polymerase 1 and neurodegenerative diseases: Past, present, and future. Ageing Res Rev. 2023 Nov 1;91:102078. [CrossRef]
- Abeti R, Abramov AY, Duchen MR. β-amyloid activates PARP causing astrocytic metabolic failure and neuronal death. Brain. 2011 June 1;134(6):1658–72. [CrossRef]
- Shukla S, Tekwani BL. Histone Deacetylases Inhibitors in Neurodegenerative Diseases, Neuroprotection and Neuronal Differentiation. Front Pharmacol [Internet]. 2020 Apr 24 [cited 2025 Sept 3];11. Available from: https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2020.00537/full.
- Joshi N, Khadse P, Jadhav S, Alavala RR. Histone Deacetylase Inhibition in Alzheimer’s Disease: Molecular Mechanisms, Therapeutic Potential, and Future Perspectives. Curr Top Med Chem. 2025 June 16; [CrossRef]
- Xu K, Dai XL, Huang HC, Jiang ZF. Targeting HDACs: A Promising Therapy for Alzheimer’s Disease. Oxid Med Cell Longev. 2011;2011:143269. [CrossRef]
- Kilgore M, Miller CA, Fass DM, Hennig KM, Haggarty SJ, Sweatt JD, Rumbaugh G. Inhibitors of Class 1 Histone Deacetylases Reverse Contextual Memory Deficits in a Mouse Model of Alzheimer’s Disease. Neuropsychopharmacology. 2010 Mar;35(4):870–80.
- Pereira M, Cruz MT, Fortuna A, Bicker J. Restoring the epigenome in Alzheimer’s disease: advancing HDAC inhibitors as therapeutic agents. Drug Discov Today. 2024 July 1;29(7):104052. [CrossRef]
- Zhang LY, Zhang SY, Wen R, Zhang TN, Yang N. Role of histone deacetylases and their inhibitors in neurological diseases. Pharmacol Res. 2024 Oct 1;208:107410. [CrossRef]
- Kummer MP, Ising C, Kummer C, Sarlus H, Griep A, Vieira-Saecker A, Schwartz S, Halle A, Brückner M, Händler K, Schultze JL, Beyer M, Latz E, Heneka MT. Microglial PD-1 stimulation by astrocytic PD-L1 suppresses neuroinflammation and Alzheimer’s disease pathology. EMBO J. 2021 Dec 15;40(24):e108662.
- Manenti S, Orrico M, Masciocchi S, Mandelli A, Finardi A, Furlan R. PD-1/PD-L Axis in Neuroinflammation: New Insights. Front Neurol [Internet]. 2022 June 9 [cited 2025 Sept 3];13. Available from: https://www.frontiersin.org/journals/neurology/articles/10.3389/fneur.2022.877936/full. [CrossRef]
- Linnerbauer M, Beyer T, Nirschl L, Farrenkopf D, Lößlein L, Vandrey O, Peter A, Tsaktanis T, Kebir H, Laplaud D, Oellinger R, Engleitner T, Alvarez JI, Rad R, Korn T, Hemmer B, Quintana FJ, Rothhammer V. PD-L1 positive astrocytes attenuate inflammatory functions of PD-1 positive microglia in models of autoimmune neuroinflammation. Nat Commun. 2023 Sept 9;14(1):5555. [CrossRef]
- Ito T, Vien PTX, Inaba M, Yoshimura H, Hotta M, Nakanishi T, Fujita S, Nakaya A, Satake A, Ishii K, Nomura S. Immunomodulatory Drugs (IMiDs), Lenalidomide and Pomalidomide, Suppress Th1-Inducing Capacity of Dendritic Cells but Enhance Th2-Mediated Allergic Response. Blood. 2016 Dec 2;128(22):2520. [CrossRef]
- Kotla V, Goel S, Nischal S, Heuck C, Vivek K, Das B, Verma A. Mechanism of action of lenalidomide in hematological malignancies. J Hematol OncolJ Hematol Oncol. 2009 Dec;2(1):36. [CrossRef]
- Decourt B, Wilson J, Ritter A, Dardis C, DiFilippo FP, Zhuang X, Cordes D, Lee G, Fulkerson ND, St Rose T, Hartley K, Sabbagh MN. MCLENA-1: A Phase II Clinical Trial for the Assessment of Safety, Tolerability, and Efficacy of Lenalidomide in Patients with Mild Cognitive Impairment Due to Alzheimer’s Disease. Open Access J Clin Trials. 2020;12:1–13. [CrossRef]
- Deng MY, Ahmad KA, Han QQ, Wang ZY, Shoaib RM, Li XY, Wang YX. Thalidomide alleviates neuropathic pain through microglial IL-10/β-endorphin signaling pathway. Biochem Pharmacol. 2021 Oct;192:114727. [CrossRef]
- Shortt J, Hsu AK, Johnstone RW. Thalidomide-analogue biology: immunological, molecular and epigenetic targets in cancer therapy. Oncogene. 2013 Sept;32(36):4191–202.
- Das U, Banerjee S, Sarkar M, Muhammad L F, Soni TK, Saha M, Pradhan G, Chatterjee B. Circular RNA vaccines: Pioneering the next-gen cancer immunotherapy. Cancer Pathog Ther. 2024 Dec;S2949713224000892. [CrossRef]
- Das U, Banerjee S, Sarkar M. Bibliometric analysis of circular RNA cancer vaccines and their emerging impact. Vacunas. 2025 Mar;500391.
- Team TB. Repurposing Cancer Drugs for Alzheimer’s Disease | Rutgers BHI [Internet]. BHI. 2025 [cited 2025 Sept 10]. Available from: https://brainhealthinstitute.rutgers.edu/2025/08/07/repurposing-cancer-drugs-for-alzheimers-insights/.
- Malesu VK. Dual cancer drugs restore memory and rewire brain cells in Alzheimer’s mouse models [Internet]. News-Medical. 2025 [cited 2025 Sept 10]. Available from: https://www.news-medical.net/news/20250722/Dual-cancer-drugs-restore-memory-and-rewire-brain-cells-in-Alzheimere28099s-mouse-models.aspx.
- Ganamurali N, Sabarathinam S. Network pharmacology-guided systems biology reveals β-Sitosterol’s multi-target role in reversing 7-ketocholesterol-induced oxidative and inflammatory stress. J Steroid Biochem Mol Biol. 2026 Jan;255:106863. [CrossRef]
- Ou YN, Tan L, Yu JT. Identification of Novel Drug Targets for Alzheimer’s Disease by Integrating Genetics and Proteomes from Brain and Blood (P7-9.012). Neurology. 2024 Apr 14;102(7_supplement_1):107.
- Podder A, Pandit M, Narayanan L. Drug Target Prioritization for Alzheimer’s Disease Using Protein Interaction Network Analysis. OMICS J Integr Biol. 2018 Oct;22(10):665–77.
- Funari A, De Smaele E, Paci P, Fiscon G. Prioritizing repurposable drugs for Alzheimer’s disease using network-based analysis with concurrent assessment of Long QT syndrome risk. Biotechnol Rep. 2025 July 29;47:e00909. [CrossRef]
- Peng Y, Yuan M, Xin J, Liu X, Wang J. Screening novel drug candidates for Alzheimer’s disease by an integrated network and transcriptome analysis. Bioinformatics. 2020 Nov 1;36(17):4626–32.
- Advani D, Kumar P. Therapeutic Targeting of Repurposed Anticancer Drugs in Alzheimer’s Disease: Using the Multiomics Approach. ACS Omega. 2021 May 19;6(21):13870–87. [CrossRef]
- Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L, Abdurrob F, Jiang X, Martorell AJ, Ransohoff RM, Hafler BP, Bennett DA, Kellis M, Tsai LH. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019 June;570(7761):332–7.
- Lin H, Su L, Mao D, Yang G, Huang Q, Lan Y, Zeng J, Song W, Liang G, Wei Q, Zou D, Li R, Zou C. Identification of altered immune cell types and molecular mechanisms in Alzheimer’s disease progression by single-cell RNA sequencing. Front Aging Neurosci [Internet]. 2024 Nov 14 [cited 2025 Sept 11];16. Available from: https://www.frontiersin.org/journals/aging-neuroscience/articles/10.3389/fnagi.2024.1477327/full. [CrossRef]
- Das U. Emerging trends and research landscape of the tumor microenvironment in head-and-neck cancer: A comprehensive bibliometric analysis. Cancer Plus. 2025 Apr 8;0(0):025060008.
- Cai Y, Kanyo J, Wilson R, Bathla S, Cardozo PL, Tong L, Qin S, Fuentes LA, Pinheiro-de-Sousa I, Huynh T, Sun L, Mansuri MS, Tian Z, Gan HR, Braker A, Trinh HK, Huttner A, Lam TT, Petsalaki E, Brennand KJ, Nairn AC, Grutzendler J. Subcellular proteomics and iPSC modeling uncover reversible mechanisms of axonal pathology in Alzheimer’s disease. Nat Aging. 2025 Mar;5(3):504–27.
- Penney J, Ralvenius WT, Tsai LH. Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol Psychiatry. 2020 Jan;25(1):148–67. [CrossRef]
- Pharmacokinetic (PK) Considerations For Central Nervous System (CNS) Drugs - Featured Stories - News [Internet]. [cited 2025 Sept 11]. Available from: https://www.prisysbiotech.com/news/pharmacokinetic-pk-considerations-for-centra-81209739.html.
- Caban A, Pisarczyk K, Kopacz K, Kapuśniak A, Toumi M, Rémuzat C, Kornfeld A. Filling the gap in CNS drug development: evaluation of the role of drug repurposing. J Mark Access Health Policy. 2017 Apr 10;5(1):1299833. [CrossRef]
- Pascoal TA, Aguzzoli CS, Lussier FZ, Crivelli L, Suemoto CK, Fortea J, Rosa-Neto P, Zimmer ER, Ferreira PCL, Bellaver B. Insights into the use of biomarkers in clinical trials in Alzheimer’s disease. eBioMedicine [Internet]. 2024 Oct 1 [cited 2025 Sept 11];108. Available from: https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(24)00358-X/fulltext. [CrossRef]
- Cummings J. The Role of Biomarkers in Alzheimer’s Disease Drug Development. Adv Exp Med Biol. 2019;1118:29–61.
- AB Science. A Multicenter, Double-blind, Placebo-controlled, Randomized, Parallel-group Study to Evaluate the Efficacy of Oral AB1010 in Adults Patients With Mild to Moderate Alzheimer-type Disease. [Internet]. clinicaltrials.gov; 2018 Dec [cited 2025 Sept 11]. Report No.: NCT00976118. Available from: https://clinicaltrials.gov/study/NCT00976118.
- Gonzales MM, Garbarino VR, Kautz TF, Song X, Lopez-Cruzan M, Linehan L, Van Skike CE, De Erausquin GA, Galvan V, Orr ME, Musi N, He Y, Bateman RJ, Wang CP, Seshadri S, Kraig E, Kellogg D. Rapamycin treatment for Alzheimer’s disease and related dementias: a pilot phase 1 clinical trial. Commun Med. 2025 May 20;5(1):189. [CrossRef]
- Trial | NCT04200911 [Internet]. [cited 2025 Sept 11]. Available from: https://cdek.pharmacy.purdue.edu/trial/NCT04200911/.
- Ghosal K, Haag M, Verghese PB, West T, Veenstra T, Braunstein JB, Bateman RJ, Holtzman DM, Landreth GE. A randomized controlled study to evaluate the effect of bexarotene on amyloid-β and apolipoprotein E metabolism in healthy subjects. Alzheimers Dement Transl Res Clin Interv. 2016 June;2(2):110–20. [CrossRef]
- Gonzales M. Pilot Study to Investigate the Safety and Feasibility of Senolytic Therapy to Modulate Progression of Alzheimer's Disease (SToMP-AD) [Internet]. clinicaltrials.gov; 2023 Feb [cited 2025 Sept 11]. Report No.: NCT04063124. Available from: https://clinicaltrials.gov/study/NCT04063124.
- Maiese K. Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol. 2016 Nov;82(5):1245–66. [CrossRef]
- Cummings J, Lee G, Zhong K, Fonseca J, Taghva K. Alzheimer’s disease drug development pipeline: 2021. Alzheimers Dement N Y N. 2021;7(1):e12179.
- Folch J, Petrov D, Ettcheto M, Pedrós I, Abad S, Beas-Zarate C, Lazarowski A, Marin M, Olloquequi J, Auladell C, Camins A. Masitinib for the treatment of mild to moderate Alzheimer’s disease. Expert Rev Neurother. 2015 June;15(6):587–96. [CrossRef]
- New AD drug development pipeline report is out. 2025 Aug 31 [cited 2025 Sept 11]; Available from: https://www.alzheimer-europe.org/news/new-ad-drug-development-pipeline-report-out?language_content_entity=en.
- Alzheimer’s Drug Development Pipeline: Promising Therapies, Pharma Investment Drive Momentum in Clinical Trials | UNLV [Internet]. 2023 [cited 2025 Sept 11]. Available from: https://www.unlv.edu/news/release/alzheimers-drug-development-pipeline-promising-therapies-pharma-investment-drive.
- van Bokhoven P, de Wilde A, Vermunt L, Leferink PS, Heetveld S, Cummings J, Scheltens P, Vijverberg EGB. The Alzheimer’s disease drug development landscape. Alzheimers Res Ther. 2021 Nov 11;13:186. [CrossRef]
- Mahdavi KD, Jordan SE, Barrows HR, Pravdic M, Habelhah B, Evans NE, Blades RB, Iovine JJ, Becerra SA, Steiner RA, Chang M, Kesari S, Bystritsky A, O’Connor E, Gross H, Pereles FS, Whitney M, Kuhn T. Treatment of Dementia With Bosutinib. Neurol Clin Pract. 2021 June;11(3):e294–302.
- Newton W. Alzheimer’s disease drug development: three major questions for 2023 [Internet]. Clinical Trials Arena. 2022 [cited 2025 Sept 11]. Available from: https://www.clinicaltrialsarena.com/features/alzheimers-2023/.
- Das U. Transforming Precision Medicine through Generative AI: Advanced Architectures and Tailored Therapeutic Design for Patient-Specific Drug Discovery. ChemistrySelect. 2025 Sept;10(36):e02448.
- Rajendran A, Rajan RA, Balasubramaniyam S, Elumalai K. Nano delivery systems in stem cell therapy: Transforming regenerative medicine and overcoming clinical challenges. Nano TransMed. 2025 Dec;4:100069. [CrossRef]
| Hallmark | Cancer Context | AD Context | Therapeutic Implications |
|---|---|---|---|
| Cell cycle control | Loss of cell-cycle checkpoints, uncontrolled proliferation | Aberrant re-entry of neurons into S-phase, leading to apoptosis [62] | Inhibitors of cell-cycle kinases (e.g. CDK/Abl inhibitors) |
| Proteostasis/chaperones | High demand on chaperones (HSPs) & UPS to stabilize mutated oncoproteins | Impaired UPS/autophagy, protein aggregates (Aβ, tau). Chaperones (Hsp90) stabilize toxic species [63] | HSP90 inhibitors, proteasome modulators, autophagy enhancers |
| Autophagy/mTOR pathway | mTORC1 often overactive (promoting growth), variable autophagy | mTORC1 hyperactivation blocks autophagy, contributing to tau/Aβ accumulation [40] | mTOR inhibitors (rapamycin/rapalogs) to restore autophagy |
| Metabolism (Warburg effect) | Aerobic glycolysis in tumors; high glutamine use | Neuronal glucose hypometabolism; astrocytic glycolysis deficits; tryptophan→kynurenine shift [44] | IDO1 inhibitors (e.g. epacadostat) to restore glucose metabolism |
| DNA damage/repair | DNA repair machinery often mutated; PARP inhibitors exploit BRCA-deficiency | Accumulation of DNA damage; PARP1 hyperactivation depletes NAD+ [64] | PARP inhibitors (e.g. niraparib) – potential neuroprotection, but needs caution |
| Chronic inflammation | Tumor microenvironment: TAMs, Tregs, immunosuppression | Microglial/astrocytic activation, elevated cytokines (IL-1β, TNFα); complement activation | Immune checkpoint blockade (PD-1/PD-L1), anti-inflammatory agents |
| Cellular senescence/SASP | Oncogenic stress leads to senescence; SASP fuels tumor progression | Age-induced senescence of neurons/glia; SASP factors contribute to neurodegeneration | Senolytic drugs (dasatinib+quercetin) to clear senescent cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





