1. Introduction
Accurate and ultrasensitive detection of disease-related biomolecules such as proteins, nucleic acids, and metabolites has become increasingly crucial for personalized medicine and precision diagnostics. Single-molecule detection (SMD) technologies are central to achieving this objective [
1]. Conventional assays, including enzyme-linked immunosorbent assays (ELISA), polymerase chain reaction (PCR), mass spectrometry, chromatography, and electrochemical methods, are extensively used to quantify biomarker levels in clinical and laboratory settings. However, despite being effective, these techniques typically fall short in detecting low-abundance biomarkers, particularly in complex biological fluids, such as peripheral blood [
2,
3]. Such limitations have spurred the development of advanced imaging approaches, particularly single-molecule and superresolution microscopy (SRM) techniques, enabling the visualization and quantification of individual molecules with nanometer precision [
4].
Various SMD assays, both labeled and label-free, have been developed to achieve nanometer-scale resolution of individual molecules. Labeled approaches include single-molecule localization microscopy, single-particle tracking, single-molecule fluorescence resonance energy transfer (smFRET), and single-molecule polarization imaging. These techniques reveal structural and kinetic heterogeneity typically obscured by ensemble-based methods, providing transformative insights into molecular biology, electrochemistry, materials science, and pharmaceutical research [
4,
5,
6,
7]. Alongside these fluorescence-based assays, label-free plasmonic methods such as surface plasmon resonance (SPR), localized SPR (LSPR), plasmonic scattering microscopy, and surface-enhanced Raman spectroscopy (SERS) enable real-time SMD and kinetic analysis at metal interfaces. These complement fluorescence by allowing mass sensing (LSPR/SPR) and molecular fingerprinting (SERS) [
8,
9,
10].
In parallel, nanobiosensors, which are analytical devices integrating nanotechnology with biological recognition, have demonstrated remarkable advantages over conventional sensing methods for detecting diverse biological and chemical entities [
11,
12,
13]. By leveraging the high surface-to-volume ratio, quantum confinement effects, and enhanced reactivity of nanomaterials such as nanoparticles (NPs), nanotubes, nanowires, and nanopores, these sensors achieve outstanding sensitivity and specificity. Their applications span from environmental monitoring to clinical diagnostics, with particular importance for the early detection of cancer, infectious diseases, and neurodegenerative disorders [
14,
15,
16]. The integration of nanobiosensors with SRM platforms enables spatially resolved interrogation of biomolecular interactions beyond the diffraction limit of conventional optics.
In recent years, numerous reviews have addressed nanobiosensors or SRM techniques individually. However, there remains a lack of comprehensive summaries focusing on their integration. In this review, we first provide an overview of nanobiosensors, including their design principles, components, and detection strategies. Then, we examine SMD approaches and highlight how their convergence with SRM techniques, including stochastic optical reconstruction microscopy (STORM), photoactivated localization microscopy (PALM), stimulated emission depletion (STED), minimal photon fluxes (MINFLUX), and DNA points accumulation for imaging in nanoscale topography (DNA-PAINT), has advanced biomolecular diagnostics. Next, we discuss representative applications, such as plasmonic–SRM hybrids, electrochemical–optical correlatives, and SRM-enabled immunoassays, focusing on their implications for early disease detection and precision medicine. Finally, we evaluate current challenges, including reproducibility, multiplexing, and clinical translation, and outline emerging directions, such as scalable fabrication, photostable probes, artificial intelligence (AI)-assisted image reconstruction, microfluidic integration, and regulatory considerations. Unlike previous reviews that have primarily focused on nanobiosensors or SRM individually, this study provides an integrated perspective, highlighting the transformative potential of nanobiosensor–SRM synergy for next-generation molecular diagnostics.
2. Nanobiosensors: Overview
2.1. Biosensor
Biosensors are analytical devices that detect and quantify specific biological and molecular compounds from biological samples. Their core function is to convert molecular recognition or binding events into measurable physical signals. Biosensors provide rapid, accurate, real-time, and reliable information about the analyte of interest. The first biosensor was introduced by Leland C. Clark, Jr. and Champ Lyons in 1962 [
17,
18]. Since then, the field has witnessed remarkable progress, with the development of innovative biosensor designs and continual improvements in sensitivity, selectivity, and portability.
2.2. Nanobiosensors
Nanobiosensors, which are developed using advanced nanotechnology techniques, are a class of sensors for observing, measuring, and analyzing biological events at the molecular level. These sensors incorporate various engineered nanomaterials, such as quantum dots (QDs), metallic and oxide NPs, carbon-based nanowires, and ultra-thin nanofilms, as functional components to improve signal generation and amplification. Nanoscale engineering of biosensors leverages the unique physicochemical properties of materials, including enhanced electron transport, plasmonic resonance, fluorescence yield, and surface interaction dynamics, to considerably improve performance. These enhancements are pivotal for detecting rare or low-abundance biomolecular targets with high sensitivity [
11,
19,
20]. In addition to material design, the performance of nanobiosensors is typically evaluated using key analytical metrics, including limit of detection (LOD), sensitivity, selectivity, dynamic range, response time, and reproducibility. These parameters are strongly influenced by the choice of nanomaterial, surface functionalization strategy, and the transduction method employed, thereby determining the clinical and point-of-care (POC) applicability of the sensor.
2.2.1. Working Principle
Nanobiosensors operate via specific interactions between the target analytes and bioreceptors, which induce detectable changes in the physicochemical, electrical, optical, thermal, or mechanical properties of the sensor. Nanomaterials serve as high-performance interfaces between the recognition elements and the transducer, improving sensitivity through their large surface-to-volume ratio, tunable optical/electronic characteristics, and quantum confinement effects. The transducer then converts these interactions into measurable signals such as electrical fluctuations, optical shifts, or resonance frequency changes, which are subsequently amplified, processed, and displayed by the readout system [
21,
22,
23].
2.2.2. Nanomaterials
Nanostructures, including nanoclusters, nanorods, nanotubes, and nanowires, typically range from 1 to 100 nm. Their synthesis depends on parameters such as the precursor concentration, temperature, and processing time. The synthesis can follow either a top-down approach (e.g., laser ablation, arc discharge, physical vapor deposition, and ball milling) or a bottom-up approach (e.g., hydrothermal processing, chemical vapor deposition, sol–gel, and co-precipitation) [
24,
25]. They can also be fabricated biologically using eco-friendly agents such as plants, bacteria, fungi, algae, and biomimetic materials to yield biocompatible and low-toxicity nanostructures [
26,
27].
Common nanomaterials used in biosensors include gold NPs (AuNPs), graphene, and metal oxides, owing to their excellent conductivity, optical tunability, and catalytic properties. AuNPs are valued for their biocompatibility, and carbon nanotubes (CNTs) exhibit superior electrical conductivity [
28,
29]. Advances in materials science continue to drive progress in nanobiosensor technology. For enhanced specificity, NPs are typically functionalized with enzymes, antibodies, or nucleic acids to enhance molecular recognition.
In addition to these well-established materials, emerging two-dimensional (2D) nanomaterials, such as molybdenum disulfide and MXenes, and DNA origami-based nanostructures have expanded the toolbox of nanobiosensor platforms. These next-generation materials provide tunable surface chemistry, strong plasmonic or catalytic activity, and excellent biocompatibility, enabling ultrasensitive and selective biosensing applications. Nanomaterials can also be categorized by dimensionality: zero-dimensional structures such as fullerenes, one-dimensional structures such as CNTs, 2D materials such as graphene, and three-dimensional (3D) structures such as graphite [
31,
32]. Nanobiosensors provide a versatile platform that bridges nanotechnology and molecular biology, providing unprecedented opportunities for early disease detection, environmental monitoring, and precision diagnostics.
2.3. Microfluidics and Nanobiosensors
Recent advances have successfully integrated nanobiosensors with portable analytical platforms, particularly microfluidic devices, to enhance on-site detection capabilities. Microfluidic systems offer several advantages, including simultaneous processing of multiple samples, high throughput, short analysis times, and minimal reagent and specimen consumption [
32].
Microfluidic platforms allow highly controlled sensing processes in lab-on-a-chip (LOC) configurations by enabling precise manipulation of minute fluid volumes within lithographically fabricated microchannels. These systems can also replicate cell culture microenvironments and facilitate the isolation of microparticles such as extracellular vesicles (EVs). For example, a microfluidic-integrated biosensor was developed for breast cancer diagnostics by targeting EV-associated microRNA biomarkers. This LOC device exhibited an impressive LOD of 84 aM, with a dynamic range of 1 fM–1 nM [
33]. Similar approaches have been extended to infectious disease diagnostics, such as SARS-CoV-2 antigen and nucleic acid detection, and to single-cell analysis platforms in which microfluidics provides rapid cell sorting and high-throughput molecular profiling [
34,
35]. Therefore, the integration of nanobiosensors with microfluidic technologies not only enhances analytical sensitivity and throughput but also enables sample preprocessing, multiplexing, and automation within a single device. Such platforms are emerging as promising candidates for POC testing. They can be further advanced by coupling with smartphone-based readout systems and wearable LOC devices, highlighting their transformative potential for clinical translation and personalized diagnostics.
2.4. Surface Functionalization
Surface functionalization considerably influences biosensor performance by enabling the selective and stable immobilization of bioreceptors onto nanomaterials. Functional groups such as −COOH, −NH
2, and −SH, along with polymer coatings such as polyethylene glycol (PEG) and polydopamine, are extensively used to improve biocompatibility, minimize nonspecific binding, and support ultrasensitive detection. Molecularly imprinted polymers (MIPs) have also been used to provide synthetic recognition sites with high stability and low production costs [
36,
37].
A critical challenge in nanomaterial-based biosensors is the tendency of NPs to aggregate or lose dispersibility under physiological or environmental stress. Recent studies have demonstrated effective strategies to overcome this limitation. For example, carboxylate-terminated ligands have been used to stabilize AuNPs under diverse pH conditions and high ionic strength while enabling facile conjugation with antibodies for pathogen detection [
38]. Similarly, dual functionalization approaches, such as combining aptamers with PEG layers on graphene field-effect transistors (GFETs), have achieved both high specificity and reduced nonspecific adsorption, enabling picomolar-level detection of cytokines in complex physiological media [
39].
In addition to these conventional approaches, emerging functionalization strategies have been used to exploit advanced nanostructures and bio interfaces. Zwitterionic coatings and click-chemistry-based linkers provide enhanced antifouling properties, improving sensor performance in whole-blood or serum samples [
40]. DNA origami nanostructures and peptide-based linkers provide programmable biomolecule spacing and orientation, optimizing recognition efficiency and signal reproducibility [
41]. In addition, hybrid interfaces that integrate inorganic nanomaterials with biomimetic membranes are gaining traction for applications requiring long-term stability and real-time monitoring in physiological environments [
42].
Surface functionalization is a cornerstone in nanobiosensor design. The continuous development of robust, antifouling, and multifunctional coatings is expected to accelerate the clinical translation of these devices, ensuring reproducibility and reliability across diverse biomedical and environmental applications.
2.5. Nanobiosensor Components
Nanobiosensors typically comprise four major components: (i) bioreceptors, (ii) transducers, (iii) electronic systems, and (iv) display/readout modules. Each component plays a critical role in determining the sensitivity, selectivity, and overall performance of the biosensor.
Bioreceptors provide the molecular recognition element that imparts specificity toward the target analyte. Conventional recognition elements include antibodies, enzymes, nucleic acids, and aptamers [
43]. More recently, synthetic recognition platforms such as MIPs, nanobodies, and whole-cell systems have emerged as promising alternatives because of their enhanced stability, reproducibility, and cost-effectiveness [
11,
44]. Transducers convert the recognition event into a measurable physical signal. Depending on the modality, transducers may operate via optical (e.g., fluorescence, plasmonic resonance, and interferometry), electrochemical (e.g., amperometry, impedance, and field-effect transistors (FETs)), thermal, acoustic, or magnetic mechanisms [
45,
46,
47]. Optical transducers are favored for label-free, real-time monitoring; electrochemical systems enable miniaturization and low-cost POC platforms; thermal and acoustic designs suit enzyme activity or mass-based sensing; magnetic approaches provide excellent performance in complex matrices with reduced background noise. Electronic circuits amplify, process, and filter the raw signals generated by transducers to ensure accurate and high-precision readouts [
48]. The miniaturization of electronic systems has allowed nanobiosensors to be integrated into portable and wearable devices. Recent progress in wireless electronics and smartphone-based interfaces has further enabled real-time monitoring and remote healthcare applications. Display and readout systems translate processed signals into user-friendly formats, enabling clinicians and end-users to rapidly interpret results. Conventional formats include optical density readings and electrical output values. However, modern platforms increasingly employ digital interfaces, cloud-based storage, and AI-driven data analysis pipelines to improve decision making and clinical utility [
49].
Beyond their structural components, nanobiosensors can be classified according to their transduction mechanisms, which convert biochemical recognition events into measurable physical signals. The most commonly employed approaches include optical, electrochemical, thermal, mechanical, and magnetic modalities, each providing distinct advantages and limitations [
50,
51]. Optical transduction, encompassing fluorescence, LSPR, SERS, and SPR, provides high sensitivity, label-free detection, and strong multiplexing ability. However, this approach can be hindered by photobleaching and background interference. Electrochemical platforms, including amperometric, impedimetric, and FET-based sensors, are attractive for their miniaturization, portability, and low cost. However, they are susceptible to fouling in complex biological fluids [
46]. Thermal and mechanical systems, such as calorimetric sensors, microcantilevers, and quartz crystal microbalance, enable direct mass or energy detection; however, they are vulnerable to environmental noise and require careful calibration [
52]. Magnetic transduction, typically employing magnetic NPs or giant magnetoresistance devices, is effective for turbid samples with high signal-to-noise ratios; however, its performance depends on external magnetic fields and limited probe diversity [
53].
3. SMD Biosensors
In response to the demand for highly sensitive biomedical diagnostics, the latest generation of nanobiosensors has advanced to achieve biomarker SMD. These platforms leverage nanoscale materials (e.g., NPs and 2D materials) and confined reaction volumes to convert individual binding events into detectable signals. The following sections summarize recent experimental progress across various nanobiosensor classes, including electrochemical, optical, plasmonic, SERS-based, hybrid, and nanoimmunosensors, highlighting their detection mechanisms, material platforms, and analytical performance in terms of sensitivity and LOD in biomedical applications [
54].
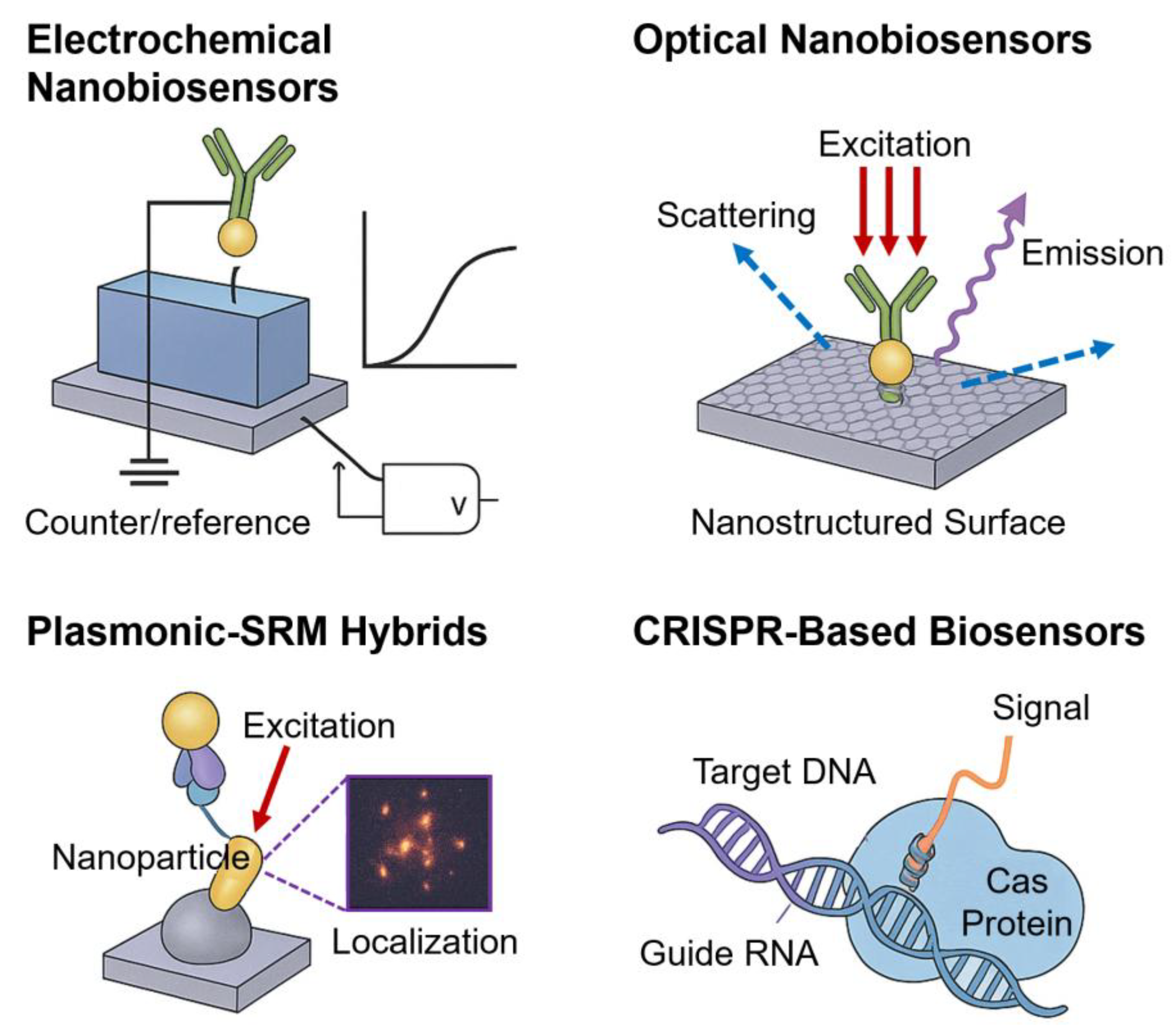
Figure 1 shows a comparative schematic overview of the four representative classes of nanobiosensors discussed in this section. Electrochemical nanobiosensors transduce biomolecular binding into electrical signals via FETs or organic electrochemical transistors (OECTs). Optical nanobiosensors exploit photonic structures such as photonic crystals (PCs) and whispering-gallery mode (WGM) resonators to amplify fluorescence for single-molecule readout. Plasmonic–SRM hybrids integrate noble metal nanostructures and superresolution imaging to achieve nanoscale mapping and enhanced localization precision. CRISPR-based biosensors use Cas12a-mediated trans-cleavage coupled with nanomaterial-assisted FRET for programmable nucleic acid detection. These platforms exemplify the diverse strategies by which nanobiosensors achieve single-molecule diagnostics. Although only four representative nanobiosensor classes (electrochemical, optical, plasmonic–SRM, and CRISPR-based) are shown in
Figure 1, additional emerging modalities such as fluorescent nanoprobe–SRM platforms (
Section 3.5) and hybrid/multimodal devices (
Section 3.6) further expand the landscape of single-molecule diagnostics.
3.1. Electrochemical Nanobiosensors
Electrochemical nanobiosensors operate by monitoring changes in electrical properties, such as current, voltage, and impedance, that are triggered by biomolecular recognition events on electrode surfaces. This strategy offers several advantages: label-free detection, straightforward instrumentation, high sensitivity, miniaturization potential, and seamless integration with portable devices [
55,
56]. The incorporation of nanostructured electrodes (e.g., CNTs, graphene, and metal NPs) enhances electron transfer kinetics and the signal-to-noise ratio, thereby enabling detection down to the level of single binding events [
57,
58]. Notable examples include single-molecule electrochemical assays and FET-based biosensors for DNA and protein detection [
59,
60].
Recent advances have considerably expanded the capabilities of electrochemical nanobiosensors. For example, OECTs functionalized with nanobody probes enabled the direct detection of viral antigens for COVID-19 and MERS in unprocessed saliva and serum samples. This label-free OECT immunosensor achieved attomolar-level LODs, with a dynamic range spanning 8–10 orders of magnitude, and exhibited clinical performance comparable to RT-PCR [
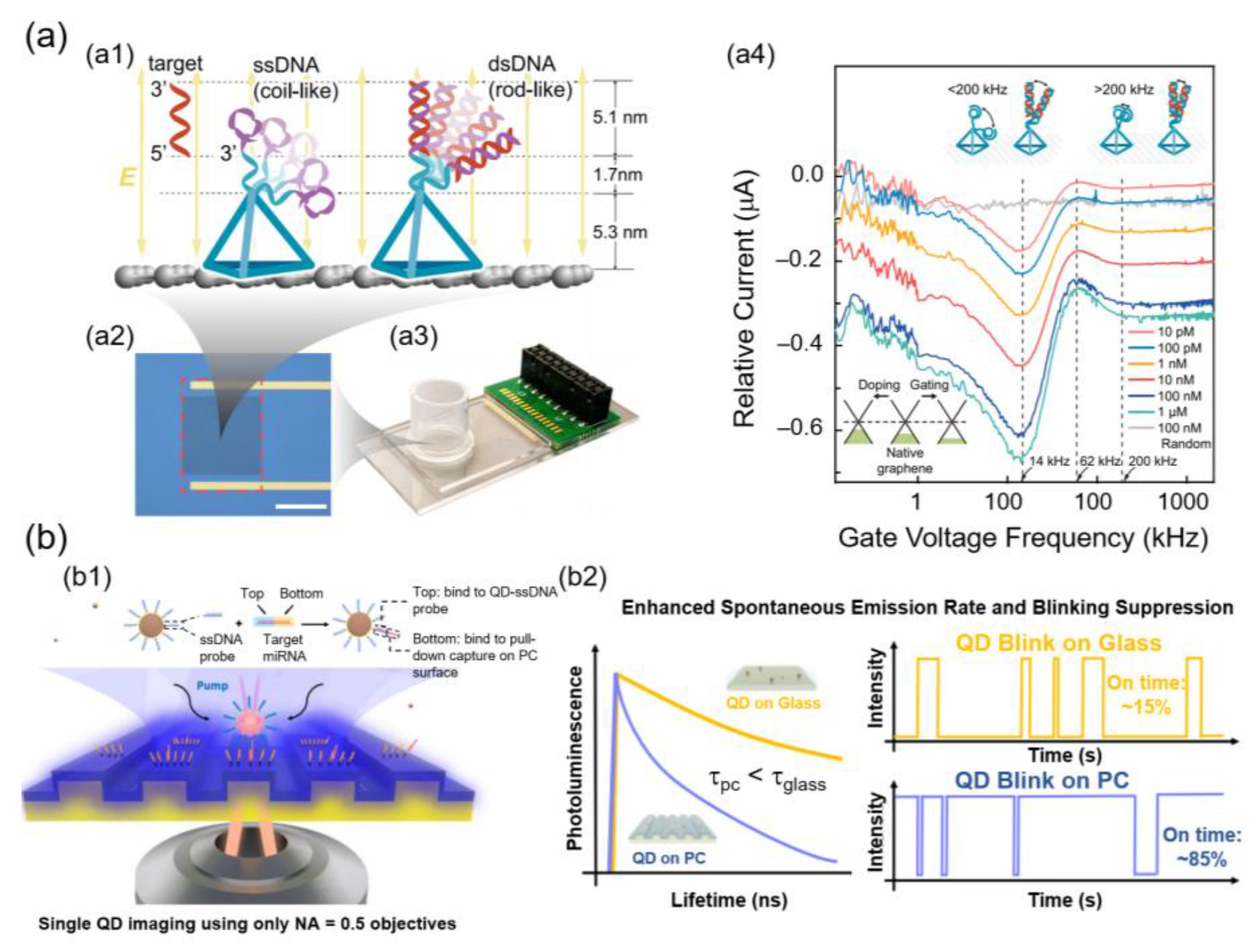
61]. Similarly, GFETs have driven DNA analysis into the single-molecule range. In a previous study, DNA probes were immobilized onto a GFET and subjected to an alternating electric field, thereby inducing oscillatory strand motion. The distinct oscillation spectra allowed the discrimination of the single hybridization events, thereby improving sensitivity by two orders of magnitude compared to conventional FETs. As shown in
Figure 2a, the tetrahedral DNA nanostructure anchored ssDNA probes on the graphene surface, and upon hybridization with complementary strands, these probes formed rigid dsDNA that oscillated differently under the applied field. This produced obvious frequency shifts in the current spectra, enabling femtomolar to sub-femtomolar quantification via direct electronic readout [
62].
Electrochemical nanobiosensors provide a versatile and scalable platform for single-molecule diagnostics. Their ability to combine ultra-high sensitivity and miniaturized, low-cost architectures makes them particularly well suited for POC applications, viral diagnostics, and genetic screening. In addition, the integration of electrochemical nanobiosensors and SRM techniques can provide correlative information by combining electrical readouts and nanoscale spatial localization of molecular events, thereby improving mechanistic insights and assay reproducibility.
3.2. Optical Nanobiosensors
Optical nanobiosensors detect molecular interactions by monitoring changes in the refractive index, fluorescence emissions, or light scattering that are triggered when biomolecules interact with a nanostructured surface or photonic device. These systems are highly attractive because they provide exceptional sensitivity, real-time monitoring, and the possibility of label-free operation [
63,
64]. Advanced optical strategies, including WGM resonators [
65], PC sensors [
66], and interferometric scattering microscopy (iSCAT) [
67], have enabled the detection of single biomolecular binding events by capturing resonance shifts or discrete fluorescence bursts.
A notable example is the study of Yu et al. (2016), who developed a label-free optical nano-sensing strategy that exploited the optical spring effect in a high-Q coherent optomechanical oscillator. In this system, light circulates within a micro/nano-photonic cavity coupled to mechanical vibrations; molecular binding induces measurable resonance shifts amplified by changes in optomechanical stiffness. This approach substantially enhanced sensitivity compared with conventional cavity resonance sensing and enabled the SMD of bovine serum albumin (BSA, 66 kDa) with a signal-to-noise ratio of 16.8, without the need for fluorescent labeling [
68].
PC platforms provide another powerful approach for ultrasensitive single-molecule analysis. By amplifying fluorescence emissions within confined optical modes, PC devices enable digital counting of single molecules. For example, a PC chip reported in 2022 improved the QD emission by approximately 3,000-fold, thereby enabling high-fidelity single-molecule imaging. The chip also achieved a LOD of 10 aM for cancer-associated miRNA biomarkers, along with the observation of altered QD surface motion trajectories upon sequence variation. As shown in
Figure 2b, the target miRNA hybridized a QD–ssDNA probe to a capture probe on a PC surface. The PC substrate enhanced spontaneous emissions and suppressed blinking. This resulted in shorter fluorescence lifetimes and markedly higher on-time ratios (~85%) compared with QDs on glass (~15%), providing reliable single-molecule resolution for ultra-low-concentration miRNA detection [
69].
Optical nanobiosensors, including WGM resonators, PC devices, and iSCAT, provide powerful label-free or fluorescence-enhanced approaches for SMD. Importantly, their integration with SRM combines high-sensitivity optical sensing and nanoscale spatial resolution, creating a powerful route for quantitative and spatially resolved single-molecule diagnostics.
For example, STORM and PALM combined with nanostructured sensing substrates have enabled nanoscale mapping of protein–DNA interactions, revealing spatial heterogeneity at the single-molecule level [
70]. Similarly, DNA-PAINT coupled with QD-based probes has been used to achieve multiplexed detection of nucleic acids with nanometer precision [
71]. Thus, these SRM-enabled optical nanobiosensors provide both quantitative and spatially resolved insights, overcoming the limitations of ensemble fluorescence assays.
3.3. Plasmonic–SRM Hybrids
Beyond nucleic acids and proteins, plasmonic nanostructures have also been employed for exosome biosensing, enabling the highly sensitive detection of extracellular vesicles with direct relevance to cancer diagnostics [
72]. Plasmonic nanobiosensors exploit the interactions between light and metallic nanostructures to generate collective electron oscillations known as surface plasmons, which are highly sensitive to changes in the local dielectric environment [
73]. Depending on the geometry and excitation mode, these phenomena manifest as LSPR in metallic NPs or propagating SPR on planar metal films. Both effects have been extensively used in biosensing, providing real-time, label-free analysis with single-molecule sensitivity [
74].
LSPR-based biosensors detect spectral shifts in NP scattering or absorption upon biomolecular binding. Their strong electromagnetic confinement enables attomolar detection of nucleic acids and proteins in complex media. Architectures such as plasmonic rulers, nanoholes, and nanopores further expand their utility, allowing precise interrogation of binding events and molecular translocation [
75].
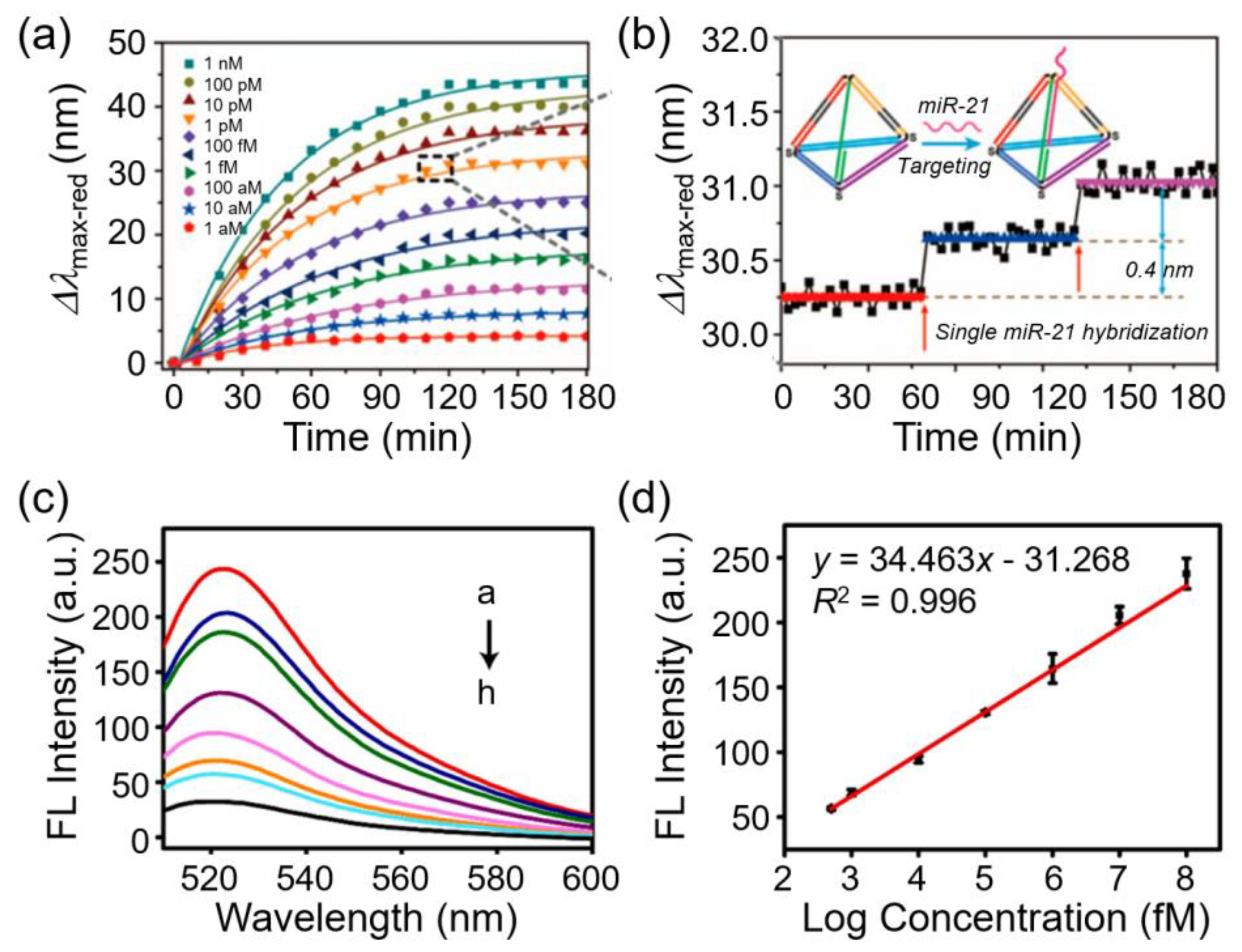
A key example was demonstrated by Zhang et al. by developing a smart plasmonic nanobiosensor using individual Au@Ag core–shell nanocubes (Au@Ag NCs) functionalized with tetrahedron-structured DNA for detecting microRNA-21 (miR-21) at the single-molecule level. Each hybridization event produced an average LSPR scattering spectral shift of ~0.4 nm, confirming detection at single-molecule resolution. The sensing principle was further confirmed using 3D finite-difference time-domain simulations. As shown in Figures 3a and b, this sensor exhibited time-dependent spectral shifts with increasing miR-21 concentration and stepwise spectral jumps corresponding to single hybridization events, thereby demonstrating its SMD ability. In addition, the system enabled the real-time monitoring of miR-21 with attomolar sensitivity across a broad dynamic range (1 aM–1 nM) and was further extended to perform DNA-based logic operations and biomemory assays using miR-21, KpnI, and StuI as inputs [
76].
Another powerful plasmonic phenomenon is surface-enhanced Raman scattering, which relies on localized “hot spots” at nanogaps or roughened metallic surfaces to amplify Raman signals by factors up to 10
12. This enables the acquisition of molecular vibrational fingerprints at the single-molecule level, thereby allowing the precise detection of proteins, DNA, and metabolites. In parallel, metal-enhanced fluorescence (MEF) has been employed to increase the excitation and emission rates of nearby fluorophores, thereby improving the fluorescence intensity and photostability. This is particularly advantageous for single-molecule fluorescence detection and multiplexed bioimaging [
77,
78]. Emerging approaches such as plasmonic optical tweezers and nanopore–plasmon hybrids further expand sensing capabilities by combining plasmonic near-fields and mechanical or ionic readouts for single-molecule manipulation, sequencing, and conformational analysis.
Beyond fundamental principles, several experimental studies have demonstrated the diagnostic potential of plasmonic nanobiosensors. For example, Fu et al. (2023) developed a plasmonic tweezer platform that created a dynamic AgNP nanocavity for single-molecule SERS, thereby enabling the real-time observation of intrinsically disordered proteins under physiological conditions [
79]. Zhao et al. (2023) introduced a bowl-shaped plasmonic nanopore that concentrated near-infrared excitation into a 3-nm hotspot, allowing the Raman-based identification of the DNA strands and the discrimination of nucleotide sequences during translocation [
80]. More recently, Macchia et al. (2025) demonstrated a plasmonic affinity SPR biosensor capable of detecting proteins and nucleic acids at ~10
-20 M, equivalent to a single molecule in 0.1 mL of human serum, within 1 h [
81].
Plasmonic nanobiosensors, including LSPR, SPR, SERS, MEF, and hybrid designs, provide a powerful way to probe biomolecular interactions at the single-molecule level. Their plasmon-enhanced near-fields make them well suited for coupling with SRM, where fluorescence amplification is combined with nanoscale localization. Demonstrations such as STED with gold nanorods and MINFLUX with plasmonic substrates [
82,
83] have primarily focused on imaging, highlighting the synergistic potential of plasmonic sensing and SRM. Exploring these approaches to nanobiosensor platforms can open new ways for ultrasensitive, spatially resolved, and dynamic SMD.
3.4. CRISPR-based Biosensors
CRISPR/Cas systems show high potential in biosensing because of their inherent nonspecific collateral cleavage properties upon target sequence recognition [
84,
85,
86,
87]. Recently, these systems have been integrated with nanomaterials for improved performance. For example, MnO
2 nanoflowers were incorporated in CRISPR/Cas12a to examine intracellular monitoring via miRNA detection [
88]. In another study, a colorimetric biosensor for African swine fever virus (ASFV) was developed by integrating AuNPs and magnetic beads with CRISPR/Cas12a [
89]. Moreover, a Mn
2+-activated CRISPR/Cas12a system was advanced, where metal ions improved fluorescent signal amplification for carbaryl insecticide detection [
90].
3.4.1. CRISPR/Cas-based Fluorescent Biosensors
The sensitivity of CRISPR/Cas-based nanobiosensors can be enhanced by integrating CRISPR/Cas systems with plasmonic nanomaterials, which generate fluorescent or optical signals upon biomarker recognition. In a previous study, a CRISPR/Cas12a fluorescent nanobiosensor was fabricated using the plasmonic quenching properties of graphene oxide (GO) and AuNPs [
76]. FAM-labeled DNA probes, which were designed as ssDNA, dsDNA with a single-stranded overhang, or hairpin structures, were immobilized on the nanomaterial surfaces. In the absence of the target DNA, fluorescence was quenched via FRET. Upon hybridization and target recognition, Cas12a was activated and cleaved the ssDNA segment of the probe, releasing the fluorophore and restoring fluorescence. As shown in Figures 3c and d, the biosensor achieved an impressive LOD of 134 fM for nucleic acid detection. The GO exhibited stronger quenching and higher fluorescence recovery than the AuNPs, and the staggered dsDNA probes improved the cleavage efficiency by reducing steric hindrance [
91]. The fluorescence spectra demonstrated recovery upon target recognition, with the emission intensity increasing proportionally with the DNA concentration; the calibration curve exhibited excellent linearity (
R2 = 0.996) and reproducibility across multiple replicates. These results highlight the strong potential of CRISPR/Cas-based fluorescent nanobiosensors for highly sensitive and quantitative nucleic acid diagnostics.
3.4.2. CRISPR/Cas-based Non-fluorescent Biosensor
Alongside fluorescent biosensor research, highly sensitive SERS-based biosensors have been developed by combining nanomaterials such as Au-coated magnetic NPs and Au core–satellite nanoclusters. In a recent study, Choi et al. developed a CRISPR/Cas12a-based SERS biosensor for viral DNA detection, eliminating the need for amplification by combining a SERS-active nanoarray with AuNPs compactly loaded with Raman probes. Specifically, a triangular Au nanoflower array with strong SERS activity was used to enhance the Raman signals many times via electromagnetic and chemical enhancement mechanisms. Raman probe-labeled AuNPs were immobilized on the nanoflower array using ssDNA linkers. Upon recognition of the target viral DNAs (hepatitis B virus (HPV), HPV16, and HPV18), activated CRISPR/Cas12a cleaved the ssDNA, thereby releasing the probe-modified AuNPs and considerably reducing the Raman intensity. The SERS biosensor achieved attomolar sensitivity without relying on nucleic acid amplification [
92].
Figure 3.
Plasmonic and CRISPR/Cas-based nanobiosensors for SMD. (a) Time-dependent red shift of Au@Ag nanocubes functionalized with DNA probes in response to increasing concentrations of miR-21, showing larger and faster shifts at higher concentrations. (b) Zoomed-in trace highlighting discrete step-like shifts from single miR-21 hybridization events, demonstrating the SMD ability of the sensor. Reprinted with permission from [
76]. Copyright (2018) American Chemical Society. (c) Fluorescence (FL) spectra of CRISPR/Cas12a-based biosensor at various target DNA concentrations (10
8–0 fM), showing increased emission intensity with higher DNA levels detected on Cas12a fluorescence biosensor. (d) Calibration curve of fluorescence intensity versus log concentration, demonstrating excellent linearity (
R2 = 0.996) and reproducibility across three replicates. Reprinted with permission from [
91]. Copyright (2021) Elsevier Science SA.
Figure 3.
Plasmonic and CRISPR/Cas-based nanobiosensors for SMD. (a) Time-dependent red shift of Au@Ag nanocubes functionalized with DNA probes in response to increasing concentrations of miR-21, showing larger and faster shifts at higher concentrations. (b) Zoomed-in trace highlighting discrete step-like shifts from single miR-21 hybridization events, demonstrating the SMD ability of the sensor. Reprinted with permission from [
76]. Copyright (2018) American Chemical Society. (c) Fluorescence (FL) spectra of CRISPR/Cas12a-based biosensor at various target DNA concentrations (10
8–0 fM), showing increased emission intensity with higher DNA levels detected on Cas12a fluorescence biosensor. (d) Calibration curve of fluorescence intensity versus log concentration, demonstrating excellent linearity (
R2 = 0.996) and reproducibility across three replicates. Reprinted with permission from [
91]. Copyright (2021) Elsevier Science SA.
3.5. Fluorescent Nanoprobe–SRM Platforms
Advances in fluorescence imaging techniques such as STED, PALM, and STORM have overcome the diffraction limit of conventional optics, enabling nanometer-scale resolution of biological nanostructures. Along with confocal and total internal reflection fluorescence (TIRF) microscopy, these techniques have become fundamental tools for single-molecule biosensing [
94].
Although we briefly discuss key SRM techniques (e.g., STORM, PALM, STED, MINFLUX, and DNA-PAINT), an in-depth description of their principles is beyond the scope of this review. Comprehensive reviews are available in the literature [
94,
95]. Importantly, fluorescence-based assays are particularly compatible with SRM platforms, because they allow direct visualization of molecular interactions with sub-diffraction accuracy, making them one of the most potent modalities for integrating nanobiosensors with advanced imaging.
QDs, which are extensively used in DNA-PAINT strategies as discussed in
Section 3.2, also serve as versatile fluorescent nanoprobes for SRM-enabled immunoassays. Their high brightness, photobleaching resistance, and tunable emission spectra make them ideal for SMD, multiplexing, and long-term tracking in complex biological environments [
95].
3.5.1. Conventional Single-Molecule Immunoassays with SRM
Within the broad category of nanobiosensors, nanoimmunosensors form a vital subclass that exploits the specific binding between antibodies and antigens to detect biomolecules such as hormones, drugs, and proteins [
96]. Immunoassays have long been used for protein quantification in clinical diagnostics and research. They have been adapted to diverse detection platforms, including optical and electrochemical systems. By incorporating nanopores or SRM, several studies have pushed LODs down to the zeptoliter (10
−21 L) scale.
For example, Lu et al. developed an immunobiosensor to monitor anti-BSA IgG binding to BSA on an indium tin oxide surface. Using dSTORM imaging, they visualized individual antibody–antigen binding events with superior spatial resolution and sensitivity compared with conventional microscopy [
9].
3.5.2. Advanced Nanoimmunosensors
A leading contributor to the integration of immune biosensors and SRM is Kang and colleagues, who have developed a series of platforms combining nanostructured chips, QD probes, and advanced optical imaging for ultrasensitive biomarker detection. Their work illustrates how coupling nanoscale architectures and SRM enables precise spatial localization, exceptional sensitivity, and high multiplexing ability.
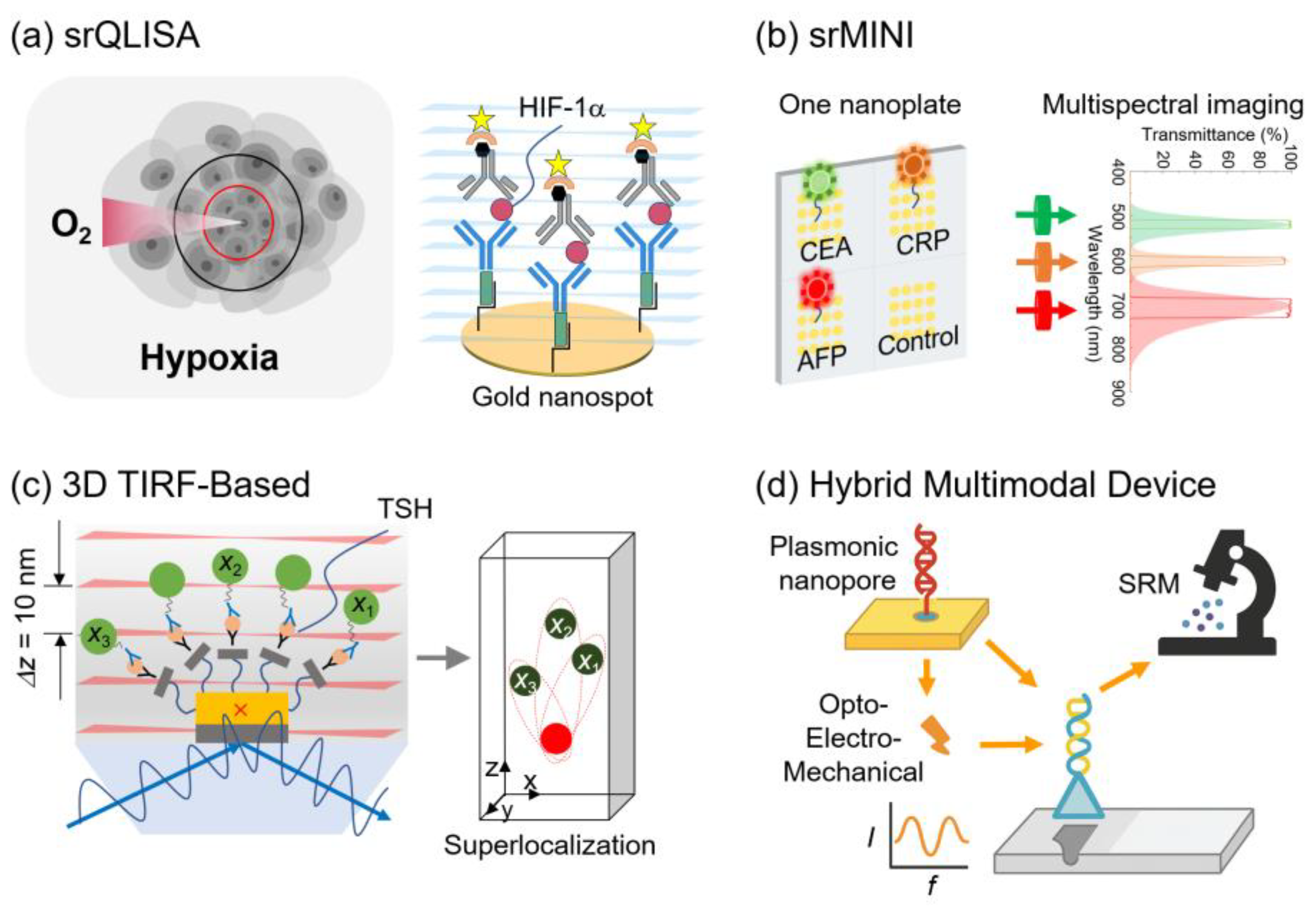
Representative examples are as follows: (1) Superresolution QD-linked immunosorbent sandwich assay (srQLISA), which enabled the ultrasensitive detection of HIF-1
α with a LOD of 16 zM, approximately 10
6-fold more sensitive than conventional ELISA [
97].
(2) Superresolution multispectral nanoimmunosensor (srMINI), which enabled the simultaneous detection of carcinoembryonic antigen (CEA), C-reactive protein (CRP), and alpha-fetoprotein (AFP) at zeptomolar concentrations, achieving over 10
8-fold higher sensitivity than commercial ELISA kits [
98].
(3) 3D TIRF-based superlocalization platform, which quantified thyroid-stimulating hormone (TSH) with yoctomole sensitivity (~54 molecules); the result was validated in human serum [
99].
(4) Beyond cancer biomarkers, these SRM-enabled platforms have been applied to food safety (biogenic amines) and small-molecule sensing (capsaicin), demonstrating zepto- to yoctomolar LODs and extending the applicability of nanoimmunosensors to broader biomedical and environmental domains [
100].
Fluorescence-based nanobiosensors integrated with SRM demonstrate the merging of sub-diffraction imaging and nanoscale sensing to achieve single-molecule resolution, multiplexing, and quantitative analysis. This integration not only advances our fundamental understanding of biomolecular interactions but also accelerates the development of clinically relevant diagnostic technologies.
Figure 4 summarizes representative examples of SRM-enabled nanoimmunoassays and hybrid multimodal devices. In particular, the srQLISA platform uses gold nanospot substrates for ultrasensitive detection of hypoxia biomarkers such as HIF-1
α, achieving LODs approximately six orders of magnitude lower than those of conventional ELISA. The srMINI system further extends this ability to multiplexed profiling, enabling simultaneous quantification of tumor markers, including CEA, CRP, and AFP, on a single nanoplate. In the 3D domain, a 3D TIRF-based superlocalization assay achieved the yoctomole-level quantification of TSH with an axial resolution of ~10 nm, validating its clinical utility in human serum. Beyond immunoassays, hybrid multimodal platforms that integrate plasmonic nanopores, opto-electro-mechanical transduction, and SRM readouts have been proposed to improve sensitivity, reduce false positives, and provide orthogonal confirmation of single-molecule events. These platforms demonstrate how coupling nanostructured immunoassays and multimodal devices with SRM can unlock unprecedented sensitivity, multiplexing, and reliability in molecular diagnostics.
3.6. Hybrid and Multimodal Devices
To improve both sensitivity and specificity, researchers have developed hybrid nanobiosensors that integrate multiple sensing principles within a single platform. A promising direction involves merging optical and electrical modalities. For example, plasmonic nanopore sensors can concurrently monitor plasmonic resonance shifts and ionic current changes, thereby providing dual-modality confirmation of single-molecule events. This dual readout considerably improves confidence in signal assignment by reducing false positives and enabling more robust kinetic analysis [
101].
As discussed in
Section 3.2, mechanical motion can be linked to electronic transduction. In a recent design, DNA probes were immobilized on a GFET and driven by an alternating electric field, converting hybridization events into frequency-domain electrical signatures. This opto-electro-mechanical fusion strategy achieved sub-femtomolar sensitivity and ultra-high specificity in DNA detection, demonstrating the potential of hybrid modalities to expand beyond conventional current–voltage readouts [
62]. Similarly, a one-shot dual-detection SR imaging method was developed to monitor spatiotemporal catalytic activity variations on plasmonic gold NPs, providing real-time insights into nanoscale reaction dynamics [
102].
Hybridization of distinct sensor platforms can also produce synergistic effects. For example, embedding a plasmonic LSPR chip in a photonic microcavity dramatically enhances optical signal strength for on-site pathogen and EV detection in complex biological samples [
65]. Similarly, combining microfluidic confinement and plasmonic or electrochemical transducers enables real-time monitoring of rare analytes under controlled reaction conditions [
32].
Looking forward, the incorporation of microfluidics plays a vital role in many single-molecule devices, facilitating precise sample delivery, digital compartmentalization, and efficient analyte trapping. The next generation of multimodal platforms is expected to integrate AI-driven data fusion to simultaneously analyze optical, electrical, and mechanical signals, thereby unlocking richer information from single events. These multimodal and microfluidic-enhanced platforms are advancing toward real-world POC single-molecule diagnostics, combining the strengths of complementary modalities to achieve unparalleled sensitivity, reproducibility, and clinical utility [
103].
Importantly, hybrid biosensors also provide unique opportunities for SRM integration. For example, correlative SRM–electrochemical platforms directly align STORM-based fluorescence maps with electrochemical readouts at nanostructured electrodes. Similarly, plasmonic nanopore–SRM hybrids enable simultaneous ionic current measurements and super-resolved optical detection, providing dual confirmation of single-molecule translocation events [
104,
105]. These multimodal SRM-integrated systems not only reduce false positives but also improve reproducibility and provide a comprehensive view of biomolecular dynamics at the single-molecule level.
4. Integration of Nanobiosensors and SRM
The convergence of nanobiosensors and SRM represents a transformative step in single-molecule diagnostics. Although modality-specific examples of electrochemical, optical, plasmonic, and hybrid nanobiosensors have been introduced in
Section 3, a cross-cutting synthesis is presented in this section. We detail how these platforms benefit from SRM integration and the unique opportunities this combination creates.
4.1. Synergistic Signal Amplification and Spatial Resolution
SRM methods such as STORM, PALM, STED, MINFLUX, and DNA-PAINT overcome the diffraction limit of conventional optics, achieving resolutions down to 10–20 nm or even below 5 nm. When coupled with the ultra-sensitivity of nanobiosensors, SRM approaches enable the direct observation, precise localization, and multiplex quantification of biomolecular events that were previously undetectable.
For example, plasmonic near-fields not only improve the fluorescence intensity but also complement the ability of SRM to localize single fluorophores with nanometer precision. Similarly, electrochemical biosensors gain new dimensions of correlative analysis when paired with SRM, allowing simultaneous mapping of molecular interactions and electrical signals at the nanoscale. This dual readout reduces false positives and provides mechanistic insights that cannot be achieved using either technique alone.
4.2. Multiplexing and Spatiotemporal Profiling
Another important benefit lies in multiplexing and spatiotemporal profiling. SRM techniques such as DNA-PAINT and multicolor STORM enable nanometer-level discrimination of multiple biomarkers, and nanobiosensors extend this ability by providing zepto- to yoctomolar LODs. This integration has already enabled ultrasensitive assays such as srQLISA and srMINI, which outperform conventional ELISA by several orders of magnitude. This approach has further been extended to a fourplex nanoimmunosensor for the simultaneous quantification of thyroid hormones at yoctomole sensitivity, highlighting the multiplexing potential of SRM-enabled nanoimmunosensors [
106]. In addition, current 3D TIRF- and MINFLUX-based nanobiosensors allow real-time tracking of biomolecules in live-cell environments, bridging the gap between in vitro detection and physiologically relevant conditions. To address these limitations, a four-dimensional cuboid multiangle light-sheet SR imaging approach has been introduced, which minimizes phototoxicity and imaging artifacts while maintaining nanoscale resolution in live-cell environments [
107]. Beyond these approaches, six-dimensional tracking of anisotropic NPs in live cells has also been demonstrated using multifunctional light-sheet nanoscopy, further expanding the spatiotemporal profiling capabilities of SRM-enabled Nanobiosensors [
108].
4.3. Nanostructure-Assisted SRM
In addition to direct nanobiosensor–SRM hybrids, recent advances in nanostructure-assisted SRM provide valuable insights into how engineered nanomaterials can improve imaging performance even though they do not constitute true biosensors. As summarized in
Table 1, nanobiosensor modalities, including electrochemical, optical, plasmonic, hybrid, and immunoassay-based systems, demonstrate unique opportunities for SRM integration, collectively outlining a diverse toolbox for single-molecule diagnostics. Beyond these modality-specific examples, nanostructure-assisted SRM illustrates how material engineering can push the resolution limits of imaging techniques, thereby complementing biosensor development.
Recent studies have shown that nanostructures can substantially enhance localization precision and generate novel near-field patterns. For example, pulsed MINFLUX combined with graphene energy transfer and DNA-PAINT achieved <2-nm localization precision in three dimensions, directly resolving DNA origami docking strands only 3 nm apart [
109]. Similarly, lanthanide nanocrystals employing surface-migration emission depletion reached sub-20-nm resolution at saturation intensities three orders of magnitude lower than those of conventional STED dyes [
110]. In another approach, plasmonic-structured illumination microscopy achieved ~60-nm resolution using self-assembled AuNP substrates [
111]. In addition, intracellular orientation of anisotropic plasmonic aggregates at nuclear indentation sites has been directly resolved using integrated light-sheet SRM, highlighting how engineered nanostructures can enhance imaging precision in complex cellular environments [
112]. Although these methods are not diagnostic assays, they expand the SRM toolbox and inform the design of next-generation biosensor platforms by overcoming fundamental resolution and photostability barriers.
4.4. Technical Challenges and Opportunities in SRM Integration
Despite the obvious benefits of combining nanobiosensors and SRM, several technical challenges remain. SRM techniques require highly controlled sample preparation and precise calibration to avoid artifacts, which become even more demanding when nanostructured sensors are involved. In addition, multiplexing remains difficult. Although spatial separation strategies, such as the srMINI platform, help reduce spectral overlap, modified nanostructures can still cause crosstalk. Furthermore, prolonged SRM illumination induces photobleaching and phototoxicity, thereby limiting long-term tracking.
On the biosensor side, reproducibility is a persistent issue, because nanomaterial variability typically affects performance across experiments. However, these challenges create opportunities for innovation. Highly photostable fluorophores and hybrid plasmonic–fluor nanoprobes may reduce photobleaching, and deep learning-based SRM reconstruction can enhance resolution and suppress artifacts. In addition, integrating SRM-enabled nanobiosensors and automated microfluidics provides standardized sample handling and higher throughput, facilitating the development of additional reproducible assays. Addressing these integration-level challenges is essential for reliable SMD.
5. Applications in Biomolecular Diagnostics
Nanobiosensors have emerged as powerful tools for the early and precise diagnosis of various diseases, because of their exceptional sensitivity, specificity, and compatibility with POC platforms. Their applications include infectious, neurodegenerative, oncological, viral, metabolic, and renal diseases, as summarized below.
(1) Infectious diseases: Nanobiosensors enable ultrasensitive detection of pathogen-specific biomarkers for diseases such as malaria, leishmaniasis, echinococcosis, schistosomiasis, and taeniasis. Platforms incorporating advanced nanomaterials, including AuNPs, CNTs, QDs, and GO, have achieved superior sensitivity compared with ELISA and PCR while supporting multiplexed LOC systems for broad-spectrum pathogen screening and rapid POC testing [
113]. In addition, plasmonic scattering-based immunosensors have demonstrated ultrasensitive pathogen detection, such as a 3D TIR scattering platform enabling single-molecule level identification of norovirus particles [
114].
(2) Neurodegenerative diseases: For disorders such as Alzheimer’s and Parkinson’s disease, nanobiosensors provide early diagnostic ability by detecting biomarkers such as amyloid-
β and hyperphosphorylated tau. By leveraging the unique optical, electrical, and surface properties of nanomaterials, these platforms can be functionalized with molecular beacons to enable noninvasive, rapid, and personalized diagnostic approaches, with significant potential to revolutionize disease monitoring and clinical management [
115,
116].
(3) Oncological applications: Nano-enhanced biosensors based on AuNPs and other nanomaterials have facilitated the detection of key cancer biomarkers, including HER2 (breast cancer), prostate-specific antigen (PSA, prostate cancer), and AFP (liver cancer). With sub-nanogram LODs, these systems enable early cancer screening, prognosis monitoring, and more effective personalized treatment planning [
117,
118]. In addition, plasmonic scattering-based nanoimmunosensors have been developed for ultrasensitive detection of CEA, achieving enhanced sensitivity through transmission grating-based total internal reflection scattering microscopy [
119].
(4) Viral infections: QD- or CNT-based nanobiosensors have proven particularly effective for detecting viral RNA or antigens from pathogens such as HIV, HBV, and SARS-CoV-2. These platforms deliver rapid results with high sensitivity and can be integrated into portable devices, making them highly suitable for on-site testing and epidemic control [
120,
121,
122].
(5) Metabolic and renal diseases: For diabetes management, biosensors detecting HbA1c or insulin levels enable accurate assessment of glycemic control and insulin resistance. In chronic kidney disease (CKD), biosensors targeting creatinine and cystatin C provide timely diagnostic information, supporting early intervention strategies to slow disease progression [
123,
124]. More recently, multidimensional spatiotemporal tracking of intracellular fucoidan using plasmon-enhanced dark-field SR imaging has been demonstrated, offering new insights into cellular metabolism and intracellular molecular interactions at the single-molecule level [
125].
Although these applications demonstrate the remarkable promise of nanobiosensors and, in particular, the combination of nanoimmunosensors and SRM for single-molecule analysis, significant challenges remain before their widespread clinical translation can be realized. Overcoming issues such as reproducibility, standardization, and integration into user-friendly platforms will define the next phase of innovation in this field.
Table 2 summarizes representative nanobiosensor applications in major disease areas, including the target biomarkers, nanomaterial platforms, and diagnostic advantages. These examples demonstrate the translational potential of nanobiosensors, which extend beyond proof-of-concept demonstrations toward clinically relevant diagnostics. Beyond these disease-specific applications, SRM-enabled nanobiosensors broaden the diagnostic landscape by providing unprecedented sensitivity and spatial resolution, thereby paving the way for clinical translation.
6. Clinical Translation Challenges and Future Directions
Beyond integration-specific issues, broader challenges must be addressed for the advancement of nanobiosensor–SRM platforms toward clinical translation. Standardization and reproducibility across laboratories remain critical obstacles, and the high costs associated with advanced nanomaterials and SRM instrumentation hinder scalability. Bridging the gap between proof-of-concept prototypes and deployable diagnostic devices requires not only cost-effective fabrication but also rigorous validation using physiologically relevant samples and in large-scale clinical trials. Regulatory hurdles further complicate translation, necessitating early engagement with agencies and well-defined manufacturing protocols.
Future directions point to several promising solutions. Advances in scalable nanomaterial synthesis and robust sensor fabrication will be crucial for enhancing reproducibility and reducing costs. Industrial–academic partnerships may accelerate technology transfer, and AI-assisted multimodal data fusion and cloud-based workflows can streamline analysis of increasingly complex datasets. Importantly, regulatory alignment and early-stage clinical evaluation are necessary to ensure safety, efficacy, and adoption in precision medicine. In parallel, deep learning has also been applied to nanobiosensor platforms themselves, as exemplified by nanozyme-based biosensors enhanced with AI algorithms to achieve improved diagnostic sensitivity and robustness [
126].
These challenges and strategies are systematically summarized in
Table 3. The table categorizes the barriers into technical, reproducibility, economic, regulatory, and data-related domains. It also outlines potential solutions such as standardized protocols, photostable probe design, scalable fabrication, industrial partnerships, and AI-driven data analysis. These approaches highlight a roadmap for translating nanobiosensor–SRM platforms from laboratory research into practical, clinically viable diagnostic technologies.
7. Conclusions
The convergence of nanoscale biosensing architectures and advanced imaging has considerably broadened the scope of molecular diagnostics, enabling the direct visualization and quantitative analysis of biomolecular events at the single-molecule level. By leveraging the structural tunability and reactivity of engineered nanomaterials, nanobiosensors achieve remarkable gains in terms of sensitivity, selectivity, and spatiotemporal resolution. When integrated with SRM, these systems overcome the diffraction barrier, thereby enabling the precise tracking of biomolecular dynamics in complex environments with zepto- to yoctomolar LODs. Such capabilities highlight their transformative potential for early disease detection, therapeutic monitoring, and precision health strategies.
Beyond sensitivity, SRM-enabled nanobiosensors provide multiplexed profiling, real-time monitoring, and 3D mapping of molecular interactions. However, challenges such as fluorophore photostability, fabrication complexity, and the gap between laboratory prototypes and clinically deployable systems remain. Addressing these challenges requires close interdisciplinary collaboration among materials scientists, optical engineers, computational modelers, and clinicians.
Looking forward, advances in next-generation QDs, hybrid plasmonic–fluor probes, and photostable fluorophores are expected to mitigate photobleaching and extend observation times. AI-driven image reconstruction and multimodal data fusion will improve resolution, suppress artifacts, and accelerate analysis, and integration with automated microfluidic platforms will support high-throughput, multiplexed clinical assays. Along with standardized fabrication protocols, scalable manufacturing, early regulatory engagement, and cross-laboratory benchmarking, these advances will pave the way for clinically viable diagnostic solutions.
Importantly, this review provides a perspective not fully addressed in previous studies: the systematic integration of nanobiosensors and SRM. By consolidating progress across electrochemical, optical, plasmonic, hybrid, and immunosensing modalities and by mapping their SRM-enabled applications through comparative tables, we outline a roadmap for next-generation single-molecule diagnostic platforms that are both technically advanced and clinically translatable.
Author Contributions
Conceptualization, S.L. and S.H.K.; writing—original draft preparation, S.R.; writing—review and editing, S.L. and S.H.K.; supervision, S.H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2023R1A2C2002623 and RS-2025-00519455).
Data Availability Statement
Not applicable.
Acknowledgments
Some of the illustrations in this study were partially generated or refined using ChatGPT (OpenAI, 2025) under the authors’ supervision.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
AgNP, silver nanoparticle
AFP, alpha-fetoprotein
AI, artificial intelligence
AuNP, gold nanoparticle
BAs, biogenic amines
BSA, bovine serum albumin
CNTs, carbon nanotubes
CEA, carcinoembryonic antigen
CRP, C-reactive protein
DNA-PAINT, DNA points accumulation for imaging in nanoscale topography
dSTORM, direct stochastic optical reconstruction microscopy
ELISA, enzyme-linked immunosorbent assay
EV, extracellular vesicle
FET, field-effect transistor
GFET, graphene field-effect transistor
GO, graphene oxide
HBV, hepatitis B virus
HER2, human epidermal growth factor receptor 2
HIV, human immunodeficiency virus
IgG, Immunoglobulin G
iSCAT, interferometric scattering microscopy
ITO, indium tin oxide
LOD, limit of detection
LOC, lab-on-a-chip
LSPR, localized surface plasmon resonance
MEF, metal-enhanced fluorescence
MERS, Middle East respiratory syndrome
MIPs, molecularly imprinted polymers
miRNA, microRNA
MINFLUX, minimal photon fluxes
MXenes, transition metal carbides/nitrides (2D nanomaterials)
NPs, nanoparticles
OECTs, organic electrochemical transistors
PALM, photoactivated localization microscopy
PC, photonic crystal
PCR, polymerase chain reaction
PEG, polyethylene glycol
POC, point-of-care
QDs, quantum dots
RT-PCR, reverse transcription polymerase chain reaction
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
SERS, surface-enhanced Raman spectroscopy
SMD, single-molecule detection
smFRET, single-molecule fluorescence resonance energy transfer
SMLM, single-molecule localization microscopy
SPR, surface plasmon resonance
srQLISA, superresolution quantum dot-linked immunosorbent assay
SRM, superresolution microscopy
srMINI, superresolution multispectral imaging nanoimmunosensor
STED, stimulated emission depletion microscopy
STORM, stochastic optical reconstruction microscopy
TIRF, total internal reflection fluorescence
TSH, thyroid-stimulating hormone
WGM, whispering-gallery mode
References
- Herkert, E.K.; Bermeo Alvaro, D.R.; Recchia, M.; Langbein, W.; Borri, P.; Garcia-Parajo, M.F. Hybrid Plasmonic Nanostructures for Enhanced Single-Molecule Detection Sensitivity. ACS Nano 2023, 17, 8453–8464. [Google Scholar] [CrossRef]
- Shin, S.; Kim, J.; Song, E.; Han, S.; Hohng, S. Analytical Techniques for Nucleic Acid and Protein Detection with Single-Molecule Sensitivity. Exp. Mol. Med. 2025, 57, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xu, Y.; Cao, M.; Chen, N.; Zeng, Q.; Lai, M.K.P.; Fan, D.; Sethi, G.; Cao, Y. Fluid-Based Biomarkers for Neurodegenerative Diseases. Ageing Res. Rev. 2025, 108, 102739. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, Y.; Cui, Y.; Yuan, J.; Fang, X. Deep Learning in Single-Molecule Imaging and Analysis: Recent Advances and Prospects. Chem. Sci. 2022, 13, 11964–11980. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Cheng, L.; Li, Y.; Wang, R.; Wang, J. Advancements in Single-Molecule Fluorescence Detection Techniques and Their Expansive Applications in Drug Discovery and Neuroscience. Biosensors 2025, 15, 283. [Google Scholar] [CrossRef]
- Prakash, K.; Baddeley, D.; Eggeling, C.; Fiolka, R.; Heintzmann, R.; Manley, S.; Radenovic, A.; Shroff, H.; Smith, C.; Schermelleh, L. Resolution in Super-Resolution Microscopy – Facts, Artifacts, Technological Advancements and Biological Applications. J. Cell Sci. 2025, 138, jcs263567. [Google Scholar] [CrossRef]
- Werner, C.; Sauer, M.; Geis, C. Super-Resolving Microscopy in Neuroscience. Chem. Rev. 2021, 121, 11971–12015. [Google Scholar] [CrossRef]
- Lin, C.; Li, Y.; Peng, Y.; Zhao, S.; Xu, M.; Zhang, L.; Huang, Z.; Shi, J.; Yang, Y. Recent Development of Surface-Enhanced Raman Scattering for Biosensing. J. Nanobiotechnol. 2023, 21, 149. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Cao, Y.; An, C.; Kang, S.H. Nanomaterial-Based Single-Molecule Optical Immunosensors for Supersensitive Detection. Biosens. Bioelectron. X 2022, 11, 100191. [Google Scholar] [CrossRef]
- Dey, S.; Dolci, M.; Zijlstra, P. Single-Molecule Optical Biosensing: Recent Advances and Future Challenges. ACS Phys. Chem. Au 2023, 3, 143–156. [Google Scholar] [CrossRef]
- Hemdan, M.; Abuelhaded, K.; Shaker, A.A.S.; Ashour, M.M.; Abdelaziz, M.M.; Dahab, M.I.; Nassar, Y.A.; Sarguos, A.M.M.; Zakaria, P.S.; Fahmy, H.A.; et al. Recent Advances in Nano-Enhanced Biosensors: Innovations in Design, Applications in Healthcare, Environmental Monitoring, and Food Safety, and Emerging Research Challenges. Sens. Bio-Sens. Res. 2025, 48, 100783. [Google Scholar] [CrossRef]
- Singh, N.; Dkhar, D.S.; Chandra, P.; Azad, U.P. Nanobiosensors Design Using 2D Materials: Implementation in Infectious and Fatal Disease Diagnosis. Biosensors 2023, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Ma, S.; Hara, T.O.; Singh, S. Nanomaterials-Based Biosensors for the Detection of Prostate Cancer Biomarkers: Recent Trends and Future Perspective. Adv. Mater. Technol. 2023, 8, 2201860. [Google Scholar] [CrossRef]
- Darwish, M.A.; Abd-Elaziem, W.; Elsheikh, A.; Zayed, A.A. Advancements in Nanomaterials for Nanosensors: A Comprehensive Review. Nanoscale Adv. 2024, 6, 4015–4046. [Google Scholar] [CrossRef] [PubMed]
- Conte, R.; Foggia, R.; Valentino, A.; Salle, A.D.; Kandsi, F.; Calarco, A. Nanotechnology Advancements Transforming Molecular Diagnostics: Applications in Precision Healthcare. Int. J. Nano Dimens. 2024, 15. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Rebelo, R.; Reis, R.L.; Bhattacharya, M.; Correlo, V.M. Current Nanotechnology Advances in Diagnostic Biosensors. Med. Devices Sens. 2021, 4, e10156. [Google Scholar] [CrossRef]
- Banerjee, A.; Maity, S.; Mastrangelo, C.H. Nanostructures for Biosensing, with a Brief Overview on Cancer Detection, IoT, and the Role of Machine Learning in Smart Biosensors. Sensors 2021, 21, 1253. [Google Scholar] [CrossRef]
- Gulati, S.; Yadav, R.; Kumari, V.; Nair, S.; Gupta, C.; Aishwari, M. Nanosensors in Healthcare: Transforming Real-Time Monitoring and Disease Management with Cutting-Edge Nanotechnology. RSC Pharm. 2025. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors 2023, 13, 40. [Google Scholar] [CrossRef]
- Hassan, R.Y.A. Advances in Electrochemical Nano-Biosensors for Biomedical and Environmental Applications: From Current Work to Future Perspectives. Sensors 2022, 22, 7539. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, Y.; Kianfar, E. Nano Biosensors: Properties, Applications and Electrochemical Techniques. J. Mater. Res. Technol. 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Naresh, Varnakavi.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [CrossRef]
- Lutomia, D.; Poria, R.; Kala, D.; Kumar Singh, A.; K Gupta, M.; Kumar, D.; Kaushal, A.; Gupta, S. Unlocking the Potential of 2D Nanomaterial-Based Biosensors in Biomarker-Based Detection of Helicobacter Pylori. Mater. Adv. 2025, 6, 117–142. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of Nanomaterials Using Various Top-down and Bottom-up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Abd-Elrahman, N.K. Physical Methods for Preparation of Nanomaterials, Their Characterization and Applications: A Review. J. Umm Al-Qura Univ. Appl. Sci. 2025, 11, 356–377. [Google Scholar] [CrossRef]
- Dhaka, A.; Chand Mali, S.; Sharma, S.; Trivedi, R. A Review on Biological Synthesis of Silver Nanoparticles and Their Potential Applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Gupta, D.; Boora, A.; Thakur, A.; Gupta, T.K. Green and Sustainable Synthesis of Nanomaterials: Recent Advancements and Limitations. Environ. Res. 2023, 231, 116316. [Google Scholar] [CrossRef] [PubMed]
- Karnwal, A.; Kumar Sachan, R.S.; Devgon, I.; Devgon, J.; Pant, G.; Panchpuri, M.; Ahmad, A.; Alshammari, M.B.; Hossain, K.; Kumar, G. Gold Nanoparticles in Nanobiotechnology: From Synthesis to Biosensing Applications. ACS Omega 2024, 9, 29966–29982. [Google Scholar] [CrossRef]
- Hughes, K.J.; Iyer, K.A.; Bird, R.E.; Ivanov, J.; Banerjee, S.; Georges, G.; Zhou, Q.A. Review of Carbon Nanotube Research and Development: Materials and Emerging Applications. ACS Appl. Nano Mater. 2024, 7, 18695–18713. [Google Scholar] [CrossRef]
- Heydari-Bafrooei, E.; Ensafi, A.A. Nanomaterials-Based Biosensing Strategies for Biomarkers Diagnosis, a Review. Biosens. Bioelectron. X 2023, 13, 100245. [Google Scholar] [CrossRef]
- Banerjee, A.; Maity, S.; Mastrangelo, C.H. Nanotechnology for Biosensors: A Review. 2021, arXiv:2101.02430. [CrossRef]
- Zhang, J.; Zhang, X.; Zhang, Y.; Yang, X.; Guo, L.; Man, C.; Jiang, Y.; Zhang, W.; Zhang, X. Emerging Biosensors Integrated with Microfluidic Devices: A Promising Analytical Tool for on-Site Detection of Mycotoxins. Npj Sci. Food 2025, 9, 84. [Google Scholar] [CrossRef]
- Huang, C.-C.; Kuo, Y.-H.; Chen, Y.-S.; Huang, P.-C.; Lee, G.-B. A Miniaturized, DNA-FET Biosensor-Based Microfluidic System for Quantification of Two Breast Cancer Biomarkers | Microfluidics and Nanofluidics. Microfluid. Nanofluidics 2021, 25, 33. [Google Scholar] [CrossRef]
- Jamiruddin, Mohd.R.; Meghla, B.A.; Islam, D.Z.; Tisha, T.A.; Khandker, S.S.; Khondoker, M.U.; Haq, Md.A.; Adnan, N.; Haque, M. Microfluidics Technology in SARS-CoV-2 Diagnosis and Beyond: A Systematic Review. Life 2022, 12, 649. [CrossRef]
- Sun, Y.; Yu, N.; Zhang, J.; Yang, B. Advances in Microfluidic Single-Cell RNA Sequencing and Spatial Transcriptomics. Micromachines 2025, 16, 426. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Peng, G.; Ma, Z. Surface-Functionalizing Strategies for Multiplexed Molecular Biosensing: Developments Powered by Advancements in Nanotechnologies. Nanomaterials 2024, 14, 2014. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Bonilla-Cruz, J. Review on Healthcare Biosensing Nanomaterials. ACS Appl. Nano Mater. 2023, 6, 5042–5074. [Google Scholar] [CrossRef]
- Ngernpimai, S.; Puangmali, T.; Kopwitthaya, A.; Tippayawat, P.; Chompoosor, A.; Teerasong, S. Enhanced Stability of Gold Nanoparticles with Thioalkylated Carboxyl-Terminated Ligands for Applications in Biosensing. ACS Appl. Nano Mater. 2024, 7, 13124–13133. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, W.; Zhang, Z.; Wang, H. Aptamer-Based Graphene Field-Effect Transistor Biosensor for Cytokine Detection in Undiluted Physiological Media for Cervical Carcinoma Diagnosis. Biosensors 2025, 15, 138. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, T.; Pan, Q.; Zhang, S.; Zhang, S.; Geng, Z.; Zhu, B. A Universal and Versatile Zwitterionic Coating for Blood-Contacting Catheters with Long Lengths and Complex Geometries. Adv. Sci. 2025, 12, 2502411. [Google Scholar] [CrossRef]
- Zhan, P.; Peil, A.; Jiang, Q.; Wang, D.; Mousavi, S.; Xiong, Q.; Shen, Q.; Shang, Y.; Ding, B.; Lin, C.; et al. Recent Advances in DNA Origami-Engineered Nanomaterials and Applications | Chemical Reviews. Chem. Rev. 2023, 123, 3976–4050. [Google Scholar] [CrossRef]
- Lee, J.-C.; Kim, S.Y.; Song, J.; Jang, H.; Kim, M.; Kim, H.; Choi, S.Q.; Kim, S.; Jolly, P.; Kang, T.; et al. Micrometer-Thick and Porous Nanocomposite Coating for Electrochemical Sensors with Exceptional Antifouling and Electroconducting Properties. Nat. Commun. 2024, 15, 711. [Google Scholar] [CrossRef]
- Manoharan Nair Sudha Kumari, S.; Thankappan Suryabai, X. Sensing the Future─Frontiers in Biosensors: Exploring Classifications, Principles, and Recent Advances. ACS Omega 2024, 9, 48918–48987. [Google Scholar] [CrossRef]
- Mpofu, K.; Chauke, S.; Thwala, L.; Mthunzi-Kufa, P. Aptamers and Antibodies in Optical Biosensing. Discov. Chem. 2025, 2, 23. [Google Scholar] [CrossRef]
- Roy, S.S.; Raj, D. Sensors and Modern Transducers. In Emerging Sensors for Environmental Monitoring; Elsevier, 2025; pp. 43–55.
- Polat, E.O.; Cetin, M.M.; Tabak, A.F.; Bilget Güven, E.; Uysal, B.Ö.; Arsan, T.; Kabbani, A.; Hamed, H.; Gül, S.B. Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors 2022, 12, 385. [Google Scholar] [CrossRef]
- Hemdan, M.; Ali, M.A.; Doghish, A.S.; Mageed, S.S.A.; Elazab, I.M.; Khalil, M.M.; Mabrouk, M.; Das, D.B.; Amin, A.S. Innovations in Biosensor Technologies for Healthcare Diagnostics and Therapeutic Drug Monitoring: Applications, Recent Progress, and Future Research Challenges. Sensors 2024, 24, 5143. [Google Scholar] [CrossRef] [PubMed]
- Qazi, R.A.; Aman, N.; Ullah, N.; Jamila, N.; Bibi, N. Recent Advancement for Enhanced e. Coli Detection in Electrochemical Biosensors. Microchem. J. 2024, 196, 109673. [Google Scholar] [CrossRef]
- Yun, J.; Keerthana, S.; Kwon, S.-R. Miniaturized Power-Integrated and Self-Powered Sensor Systems for Advanced Biomedical Applications. Sens. Actuators Rep. 2025, 9, 100260. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, S.; Li, H.; Yang, T.; Zheng, K.; Guo, Z.M.; Shi, J.; Huang, X.; Zou, X.; Picchetti, P.; et al. Design Principles of Nanosensors for Multiplex Detection of Contaminants in Food. Small 2025, 21, 2412271. [Google Scholar] [CrossRef] [PubMed]
- Varadharajan, S.; Gadre, M.; Mathur, V.; Vasanthan, K.S. Sustainable Integration of Nanobiosensors in Biomedical and Civil Engineering: A Comprehensive Review. ACS Omega 2025, 10, 25120–25157. [Google Scholar] [CrossRef]
- Sans, J.; Azevedo Gonçalves, I.; Quintana, R. Establishing Quartz Crystal Microbalance with Dissipation (QCM-D) Coupled with Spectroscopic Ellipsometry (SE) as an Advantageous Technique for the Characterization of Ultra-Thin Film Hydrogels. Small 2024, 20, 2312041. [Google Scholar] [CrossRef]
- Tan, X.; Li, Z.; Gao, X.; Liu, W.; Zhao, M.; Ren, Y.; Ding, Q.; Li, B.; Song, Y.; Zheng, B.; et al. Enhancing Magnetic Bead Detection: Structural Innovations in GMR Sensors. IEEE Sens. J. 2025, 25, 13048–13054. [Google Scholar] [CrossRef]
- Akkilic, N.; Geschwindner, S.; Höök, F. Single-Molecule Biosensors: Recent Advances and Applications. Biosens. Bioelectron. 2020, 151, 111944. [Google Scholar] [CrossRef]
- Sumitha, M.S.; Xavier, T.S. Recent Advances in Electrochemical Biosensors – A Brief Review. Hybrid Adv. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, J.; Ruan, G.; Zhou, Y.; Feng, J. Quantitative Single-Molecule Electrochemiluminescence Bioassay. Angew. Chem. Int. Ed Engl. 2023, 62, e202214419. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, F.J.; Shah, A.; Wali, Q.; Kokab, T. Advancements in Nanofiber-Based Electrochemical Biosensors for Diagnostic Applications - ProQuest. Biosensors 2023, 13, 416. [Google Scholar] [CrossRef]
- Atış, H.E.; Turan, K.; Aydoğdu Tığ, G. Development of a Cu@ERGO-p(L-Lys) Modified GCE Electrochemical Sensor for the Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid. J. Electrochem. Soc. 2024, 171, 077503. [Google Scholar] [CrossRef]
- Hwang, M.T.; Heiranian, M.; Kim, Y.; You, S.; Leem, J.; Taqieddin, A.; Faramarzi, V.; Jing, Y.; Park, I.; van der Zande, A.M.; et al. Ultrasensitive Detection of Nucleic Acids Using Deformed Graphene Channel Field Effect Biosensors. Nat. Commun. 2020, 11, 1543. [Google Scholar] [CrossRef] [PubMed]
- Zabitler, D.; Ülker, E.; Turan, K.; Erdoğan, N.Ö.; Aydoğdu Tığ, G. Electrochemical Sensor for Biological Samples Monitoring. Top. Catal. 2025. [Google Scholar] [CrossRef]
- Guo, K.; Wustoni, S.; Koklu, A.; Díaz-Galicia, E.; Moser, M.; Hama, A.; Alqahtani, A.A.; Ahmad, A.N.; Alhamlan, F.S.; Shuaib, M.; et al. Rapid Single-Molecule Detection of COVID-19 and MERS Antigens via Nanobody-Functionalized Organic Electrochemical Transistors. Nat. Biomed. Eng. 2021, 5, 666–677. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Bao, H.; Ping, J. Nanomechanoelectrical Approach to Highly Sensitive and Specific Label-Free DNA Detection. Proc. Natl. Acad. Sci. U. S. A. 2023, 120, e2306130120. [Google Scholar] [CrossRef]
- Mostufa, S.; Rezaei, B.; Ciannella, S.; Yari, P.; Gómez-Pastora, J.; He, R.; Wu, K. Advancements and Perspectives in Optical Biosensors. ACS Omega 2024, 9, 24181–24202. [Google Scholar] [CrossRef]
- Palounek, D.; Vala, M.; Bujak, Ł.; Kopal, I.; Jiříková, K.; Shaidiuk, Y.; Piliarik, M. Surpassing the Diffraction Limit in Label-Free Optical Microscopy. ACS Photonics 2024, 11, 3907–3921. [Google Scholar] [CrossRef]
- Houghton, M.C.; Kashanian, S.V.; Derrien, T.L.; Masuda, K.; Vollmer, F. Whispering-Gallery Mode Optoplasmonic Microcavities: From Advanced Single-Molecule Sensors and Microlasers to Applications in Synthetic Biology. ACS Photonics 2024, 11, 892–903. [Google Scholar] [CrossRef]
- Li, T.; Liu, G.; Kong, H.; Yang, G.; Wei, G.; Zhou, X. Recent Advances in Photonic Crystal-Based Sensors. Coord. Chem. Rev. 2023, 475, 214909. [Google Scholar] [CrossRef]
- Velasco, L.; Islam, A.N.; Kundu, K.; Oi, A.; Reinhard, B.M. Two-Color Interferometric Scattering (iSCAT) Microscopy Reveals Structural Dynamics in Discrete Plasmonic Molecules. Nanoscale 2024, 16, 11696–11704. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Jiang, W.C.; Lin, Q.; Lu, T. Cavity Optomechanical Spring Sensing of Single Molecules. Nat. Commun. 2016, 7, 12311. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Huang, Q.; Canady, T.D.; Barya, P.; Liu, S.; Arogundade, O.H.; Race, C.M.; Che, C.; Wang, X.; Zhou, L.; et al. Photonic Crystal Enhanced Fluorescence Emission and Blinking Suppression for Single Quantum Dot Digital Resolution Biosensing. Nat. Commun. 2022, 13, 4647. [Google Scholar] [CrossRef]
- Chang, Y.; Kim, D.-H.; Zhou, K.; Jeong, M.G.; Park, S.; Kwon, Y.; Hong, T.M.; Noh, J.; Ryu, S.H. Improved Resolution in Single-Molecule Localization Microscopy Using QD-PAINT. Exp. Mol. Med. 2021, 53, 384–392. [Google Scholar] [CrossRef]
- Eerqing, N.; Subramanian, S.; Rubio, J.; Lutz, T.; Wu, H.-Y.; Anders, J.; Soeller, C.; Vollmer, F. Comparing Transient Oligonucleotide Hybridization Kinetics Using DNA-PAINT and Optoplasmonic Single-Molecule Sensing on Gold Nanorods. ACS Photonics 2021, 8, 2882–2888. [Google Scholar] [CrossRef]
- Lee, S.; Moussa, N.A.M.; Kang, S.H. Plasmonic Nanostructures for Exosome Biosensing: Enabling High-Sensitivity Diagnostics. Nanomaterials 2025, 15, 1153. [Google Scholar] [CrossRef]
- Liu, J.; Jalali, M.; Mahshid, S.; Wachsmann-Hogiu, S. Are Plasmonic Optical Biosensors Ready for Use in Point-of-Need Applications? Analyst 2020, 145, 364–384. [Google Scholar] [CrossRef]
- Mcoyi, M.P.; Mpofu, K.T.; Sekhwama, M.; Mthunzi-Kufa, P. Developments in Localized Surface Plasmon Resonance. Plasmonics 2025, 20, 5481–5520. [Google Scholar] [CrossRef]
- Kim, D.M.; Park, J.S.; Jung, S.-W.; Yeom, J.; Yoo, S.M. Biosensing Applications Using Nanostructure-Based Localized Surface Plasmon Resonance Sensors. Sensors 2021, 21, 3191. [Google Scholar] [CrossRef]
- Zhang, Y.; Shuai, Z.; Zhou, H.; Luo, Z.; Liu, B.; Zhang, Y.; Zhang, L.; Chen, S.; Chao, J.; Weng, L.; et al. Single-Molecule Analysis of MicroRNA and Logic Operations Using a Smart Plasmonic Nanobiosensor. J. Am. Chem. Soc. 2018, 140, 3988–3993. [Google Scholar] [CrossRef]
- Jeong, Y.; Kook, Y.-M.; Lee, K.; Koh, W.-G. Metal Enhanced Fluorescence (MEF) for Biosensors: General Approaches and a Review of Recent Developments. Biosens. Bioelectron. 2018, 111, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, S.H. Wavelength-Dependent Metal-Enhanced Fluorescence Biosensors via Resonance Energy Transfer Modulation. Biosensors 2023, 13, 376. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Chi, H.; Dai, X.; Zhu, H.; Mesias, V.S.D.; Liu, W.; Huang, J. Efficient Optical Plasmonic Tweezer-Controlled Single-Molecule SERS Characterization of pH-Dependent Amylin Species in Aqueous Milieus. Nat. Commun. 2023, 14, 6996. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hubarevich, A.; De Fazio, A.F.; Iarossi, M.; Huang, J.-A.; De Angelis, F. Plasmonic Bowl-Shaped Nanopore for Raman Detection of Single DNA Molecules in Flow-Through. Nano Lett. 2023, 23, 4830–4836. [Google Scholar] [CrossRef]
- Macchia, E.; Franco, C.D.; Scandurra, C.; Sarcina, L.; Piscitelli, M.; Catacchio, M.; Caputo, M.; Bollella, P.; Scamarcio, G.; Torsi, L. Plasmonic Single-Molecule Affinity Detection at 10−20 Molar. Adv. Mater. 2025, 37, 2418610. [Google Scholar] [CrossRef]
- Cortés, E.; Huidobro, P.A.; Sinclair, H.G.; Guldbrand, S.; Peveler, W.J.; Davies, T.; Parrinello, S.; Görlitz, F.; Dunsby, C.; Neil, M.A.A.; et al. Plasmonic Nanoprobes for Stimulated Emission Depletion Nanoscopy. ACS Nano 2016, 10, 10454–10461. [Google Scholar] [CrossRef]
- Balzarotti, F.; Eilers, Y.; Gwosch, K.C.; Gynnå, A.H.; Westphal, V.; Stefani, F.D.; Elf, J.; Hell, S.W. Nanometer Resolution Imaging and Tracking of Fluorescent Molecules with Minimal Photon Fluxes. Science 2017, 355, 606–612. [Google Scholar] [CrossRef]
- Wani, A.K.; Akhtar, N.; Mir, T. ul G.; Chopra, C.; Singh, R.; Hong, J.C.; Kadam, U.S. CRISPR/Cas12a-Based Biosensors for Environmental Monitoring and Diagnostics. Environ. Technol. Innov. 2024, 34, 103625. [Google Scholar] [CrossRef]
- Liu, X.; Hussain, M.; Dai, J.; Li, Y.; Zhang, L.; Yang, J.; Ali, Z.; He, N.; Tang, Y. Programmable Biosensors Based on RNA-Guided CRISPR/Cas Endonuclease. Biol. Proced. Online 2022, 24, 2. [Google Scholar] [CrossRef]
- He, Y.; Hu, Q.; San, S.; Kasputis, T.; Splinter, M.G.D.; Yin, K.; Chen, J. CRISPR-Based Biosensors for Human Health: A Novel Strategy to Detect Emerging Infectious Diseases. Trends Anal. Chem. TRAC 2023, 168, 117342. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Yoon, J.; Chen, M.; Shin, M.; Goldston, L.L.; Lee, K.-B.; Choi, J.-W. CRISPR/Cas-Based Nanobiosensor Using Plasmonic Nanomaterials to Detect Disease Biomarkers. BioChip J. 2025, 19, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-M.; Kang, Y.-F.; He, J.-W.; Tang, H.-W.; Liu, D.; Li, C.-Y. Near-Infrared Light Activated and Hybridization Chain Reaction Cascaded CRISPR/Cas12a System under the Enhancement of Mn2+ for Intracellular Biosensing. Sens. Actuators B Chem. 2024, 398, 134777. [Google Scholar] [CrossRef]
- Zhu, D.; Su, T.; Sun, T.; Qin, X.; Su, S.; Bai, Y.; Li, F.; Zhao, D.; Shao, G.; Chao, J.; et al. Enhancing Point-of-Care Diagnosis of African Swine Fever Virus (ASFV) DNA with the CRISPR-Cas12a-Assisted Triplex Amplified Assay. Anal. Chem. 2024, 96, 5178–5187. [Google Scholar] [CrossRef]
- Tian, F.; Jiang, L.; Wang, Z.; Peng, L.; Zhang, Z.; Huang, Y. Mn2+-Activated CRISPR-Cas12a Strategy for Fluorescence Detection of the Insecticide Carbaryl. Sens. Actuators B Chem. 2024, 398, 134695. [Google Scholar] [CrossRef]
- Cheng, X.; Yan, Y.; Chen, X.; Duan, J.; Zhang, D.; Yang, T.; Gou, X.; Zhao, M.; Ding, S.; Cheng, W. CRISPR/Cas12a-Modulated Fluorescence Resonance Energy Transfer with Nanomaterials for Nucleic Acid Sensing. Sens. Actuators B Chem. 2021, 331, 129458. [Google Scholar] [CrossRef]
- Choi, J.-H.; Shin, M.; Yang, L.; Conley, B.; Yoon, J.; Lee, S.-N.; Lee, K.-B.; Choi, J.-W. Clustered Regularly Interspaced Short Palindromic Repeats-Mediated Amplification-Free Detection of Viral DNAs Using Surface-Enhanced Raman Spectroscopy-Active Nanoarray. ACS Nano 2021, 15, 13475–13485. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Gockel, N.; Arizono, M.; Dembitskaya, Y.; Nägerl, U.V.; Pennacchietti, F.; Damenti, M.; Testa, I.; Willig, K.I. Super-Resolution Microscopy Opens New Doors to Life at the Nanoscale. J. Neurosci. 2022, 42, 8488–8497. [Google Scholar] [CrossRef]
- Valli, J.; Garcia-Burgos, A.; Rooney, L.M.; Oliveira, B.V. de M. e; Duncan, R.R.; Rickman, C. Seeing beyond the Limit: A Guide to Choosing the Right Super-Resolution Microscopy Technique. J. Biol. Chem. 2021, 297. [Google Scholar] [CrossRef]
- Li, W.; Kaminski Schierle, G.S.; Lei, B.; Liu, Y.; Kaminski, C.F. Fluorescent Nanoparticles for Super-Resolution Imaging | Chemical Reviews. Chem. Rev. 2022, 122, 12495–12543. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Shaddel, R.; Lee, C.-C.; Pourjafar, H.; Ansari, F.; Alizadeh Sani, M.; Ajili, N.; Assadpour, E.; Zhang, F.; Jafari, S.M. Nano-Immunosensors for the Rapid and Sensitive Detection of Foodborne Toxins; Recent Advances. Ind. Crops Prod. 2025, 228, 120879. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Batjikh, I.; Yu, H.; Kang, S.H. Ultrasensitive Hypoxia Sensing at the Single-Molecule Level via Super-Resolution Quantum Dot-Linked Immunosandwich Assay. ACS Sens. 2022, 7, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Kang, S.H. Super-Resolution Multispectral Imaging Nanoimmunosensor for Simultaneous Detection of Diverse Early Cancer Biomarkers. ACS Sens. 2024, 9, 3652–3659. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Kang, S.H. Wavelength-Dependent Three-Dimensional Single-Molecule Superlocalization Imaging for Yoctomole Detection of Thyroid-Stimulating Hormone on a Quantum Dot Nanobiosensor. Chin. Chem. Lett. 2023, 34, 108383. [Google Scholar] [CrossRef]
- Chakkarapani, S.K.; Lee, S.; Kang, S.H. Ultrasensitive Capsaicin Sensor Based on Endogenous Single-Molecule Fluorophore Enhancement and Quenching Interface on Gold Nanoislands. Bull. Korean Chem. Soc. 2021, 42, 1319–1326. [Google Scholar] [CrossRef]
- Liu, H.-L.; Zhan, K.; Wang, K.; Xia, X.-H. Recent Advances in Nanotechnologies Combining Surface-Enhanced Raman Scattering and Nanopore. TrAC Trends Anal. Chem. 2023, 159, 116939. [Google Scholar] [CrossRef]
- Cao, Y.; Lee, D.; Lee, S.; Lin, J.-M.; Kang, S.H. One-Shot Dual-Detection-Based Single-Molecule Super-Resolution Imaging Method for Real-Time Observation of Spatiotemporal Catalytic Activity Variations on the Plasmonic Gold Nanoparticle Surface. Anal. Chem. 2024, 96, 1957–1964. [Google Scholar] [CrossRef]
- Mauriz, E.; Lechuga, L.M. Plasmonic Biosensors for Single-Molecule Biomedical Analysis. Biosensors 2021, 11, 123. [Google Scholar] [CrossRef]
- Verschueren, D.V.; Pud, S.; Shi, X.; De Angelis, L.; Kuipers, L.; Dekker, C. Label-Free Optical Detection of DNA Translocations through Plasmonic Nanopores. ACS Nano 2019, 13, 61–70. [Google Scholar] [CrossRef]
- Spitzberg, J.D.; Zrehen, A.; van Kooten, X.F.; Meller, A. Plasmonic-Nanopore Biosensors for Superior Single-Molecule Detection. Adv. Mater. 2019, 31, 1900422. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Lee, G.; Kang, S.H. Simultaneous Quantification of Thyroid Hormones Using an Ultrasensitive Single-Molecule Fourplex Nanoimmunosensor in an Evanescent Field. Biosens. Bioelectron. 2023, 220, 114894. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lee, S.; Kim, K.; Kang, S.H. Minimizing the Optical Illusion of Nanoparticles in Single Cells Using Four-Dimensional Cuboid Multiangle Illumination-Based Light-Sheet Super-Resolution Imaging. Anal. Chem. 2022, 94, 17877–17884. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lee, S.; Kim, K.; Kwak, J.-Y.; Kang, S.H. Real-Time Six-Dimensional Spatiotemporal Tracking of Single Anisotropic Nanoparticles in Live Cells by Integrated Multifunctional Light-Sheet Nanoscopy | Microchimica Acta. Microchim. Acta 2023, 190, 54. [Google Scholar] [CrossRef] [PubMed]
- Zähringer, J.; Cole, F.; Bohlen, J.; Steiner, F.; Kamińska, I.; Tinnefeld, P. Combining pMINFLUX, Graphene Energy Transfer and DNA-PAINT for Nanometer Precise 3D Super-Resolution Microscopy. Light Sci. Appl. 2023, 12, 70. [Google Scholar] [CrossRef]
- Pu, R.; Zhan, Q.; Peng, X.; Liu, S.; Guo, X.; Liang, L.; Qin, X.; Zhao, Z.W.; Liu, X. Super-Resolution Microscopy Enabled by High-Efficiency Surface-Migration Emission Depletion. Nat. Commun. 2022, 13, 6636. [Google Scholar] [CrossRef]
- Guan, Y.; Masui, S.; Kadoya, S.; Michihata, M.; Takahashi, S. Super-Resolution by Localized Plasmonic Structured Illumination Microscopy Using Self-Assembled Nanoparticle Substrates. Nanomanufacturing Metrol. 2024, 7, 14. [Google Scholar] [CrossRef]
- Chakkarapani, S.K.; Sun, Y.; Lee, S.; Fang, N.; Kang, S.H. Three-Dimensional Orientation of Anisotropic Plasmonic Aggregates at Intracellular Nuclear Indentation Sites by Integrated Light Sheet Super-Resolution Microscopy. ACS Nano 2018, 12, 4156–4163. [Google Scholar] [CrossRef]
- Sadr, S.; Hajjafari, A.; Sazmand, A.; Santucciu, C.; Masala, G.; Soroushianfar, M.; Nazemian, S.; Rahdar, A.; Pandey, S.; Guettari, M.; et al. Nanobiosensors for Revolutionizing Parasitic Infections Diagnosis: A Critical Review to Improve Global Health with an Update on Future Challenges Prospect. Eur. J. Med. Res. 2025, 30, 484. [Google Scholar] [CrossRef]
- Lee, S.; Ahn, S.; Chakkarapani, S.K.; Kang, S.H. Supersensitive Detection of the Norovirus Immunoplasmon by 3D Total Internal Reflection Scattering Defocus Microscopy with Wavelength-Dependent Transmission Grating. ACS Sens. 2019, 4, 2515–2523. [Google Scholar] [CrossRef]
- Dhahi, T.S.; Yousif Dafhalla, A.K.; Al-Mufti, A.W.; Elobaid, M.E.; Adam, T.; Gopinath, S.C.B. Application of Nanobiosensor Engineering in the Diagnosis of Neurodegenerative Disorders. Results Eng. 2024, 24, 102790. [Google Scholar] [CrossRef]
- Yadav, S.; Bukke, S.P.N.; Prajapati, S.; Singh, A.P.; Chettupalli, A.K.; Nicholas, B. Nanobiosensors in Neurodegenerative Disease Diagnosis: A Promising Pathway for Early Detection. Digit. Health 2025, 11, 20552076251342457. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, G.; Yang, X. Electrochemical Immunosensor Based on Fe3O4/MWCNTs-COOH/AuNPs Nanocomposites for Trace Liver Cancer Marker Alpha-Fetoprotein Detection. Talanta 2023, 259, 124492. [Google Scholar] [CrossRef] [PubMed]
- Makableh, Y.; Athamneh, T.; Ajlouni, M.; Hijazi, S.; Alnaimi, A. Enhanced Response and Selective Gold Nanoparticles/Carbon Nanotubes Biosensor for the Early Detection of HER2 Biomarker. Sens. Actuators Rep. 2023, 5, 100158. [Google Scholar] [CrossRef]
- Ahn, S.; Yu, H.; Kang, S.H. Enhanced Detection Sensitivity of Carcinoembryonic Antigen on a Plasmonic Nanoimmunosensor by Transmission Grating-Based Total Internal Reflection Scattering Microscopy. Biosens. Bioelectron. 2017, 96, 159–166. [Google Scholar] [CrossRef]
- Meskher, H.; Mustansar, H.C.; Thakur, A.K.; Sathyamurthy, R.; Lynch, I.; Singh, P.; Han, T.K.; Saidur, R. Recent Trends in Carbon Nanotube (CNT)-Based Biosensors for the Fast and Sensitive Detection of Human Viruses: A Critical Review. Nanoscale Adv. 2023, 5, 992–1010. [Google Scholar] [CrossRef]
- Curulli, A. Functional Nanomaterials Enhancing Electrochemical Biosensors as Smart Tools for Detecting Infectious Viral Diseases. Molecules 2023, 28, 3777. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, M.; Xie, J.; Li, J.; Zhang, Y.; Liu, H.; Zhang, Y.; He, J.; Zhang, G.; Wei, N.; et al. Amplification-Free Detection of SARS-CoV-2 Down to Single Virus Level by Portable Carbon Nanotube Biosensors - Liang - 2023 - Small - Wiley Online Library. Small 2023, 19, 2208198. [Google Scholar] [CrossRef]
- Shoaib, A.; Darraj, A.; Khan, M.E.; Azmi, L.; Alalwan, A.; Alamri, O.; Tabish, M.; Khan, A.U. A Nanotechnology-Based Approach to Biosensor Application in Current Diabetes Management Practices. Nanomater. Basel Switz. 2023, 13, 867. [Google Scholar] [CrossRef]
- Saddique, Z.; Faheem, M.; Habib, A.; UlHasan, I.; Mujahid, A.; Afzal, A. Electrochemical Creatinine (Bio)Sensors for Point-of-Care Diagnosis of Renal Malfunction and Chronic Kidney Disorders. Diagnostics 2023, 13, 1737. [Google Scholar] [CrossRef]
- Lee, S.; Cao, Y.; Kwak, J.-Y.; Kang, S.H. Multidimensional Spatiotemporal Tracking of Intracellular Fucoidan via Plasmon-Enhanced Dark-Field Superresolution Imaging. Sens. Actuators B Chem. 2026, 446, 138647. [Google Scholar] [CrossRef]
- Lee, S.; Moussa, N.A.M.; Kang, S.H. Deep Learning-Enhanced Nanozyme-Based Biosensors for next-Generation Medical Diagnostics. Biosensors 2025, 15, 571. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).