Submitted:
15 September 2025
Posted:
16 September 2025
You are already at the latest version
Abstract
The increasing prevalence of antimicrobial resistance (AMR) among foodborne pathogens has emerged as a critical global health concern, undermining the efficacy of conventional antimicrobial agents and threatening the safety and integrity of the food supply chain. In response, probiotics, prebiotics, and their combinations as synbiotics are increasingly recognised as sustainable, health-oriented strategies to mitigate AMR across the food chain. Probiotics—live microorganisms that, when administered in adequate amounts, confer health benefits to the host—contribute to AMR mitigation through multiple mechanisms, including competitive exclusion of resistant pathogens, production of antimicrobial metabolites (e.g., bacteriocins and organic acids), modulation of host immunity, and restoration of gut microbial balance. Prebiotics, defined as non-digestible food ingredients, selectively stimulate the growth and/or metabolic activity of beneficial bacteria such as Lactobacillus and Bifidobacterium spp., thereby reinforcing colonisation resistance. When combined as synbiotics, these agents may exert synergistic effects, enhancing microbial resilience, promoting gut health, and reducing the colonisation and persistence of AMR-related pathogens. The integration of these bio-based approaches into food systems—particularly in the development of fermented and functional foods—supports broader One Health objectives by reducing the need for antibiotics and contributing to global AMR containment efforts. This review summarises current scientific insights, explores practical applications, and outlines future perspectives on the role of probiotics, prebiotics, and synbiotics in combating AMR throughout the food chain.
Keywords:
1. Introduction
2. Materials and Methods
3. Antimicrobial Resistance and the Food Chain
3.1. Historical Context of Antibiotic Discovery and AMR Emergence
3.2. Drivers of AMR in Medicine and Animal Agriculture
3.3. Global Trends and One Health Challenges
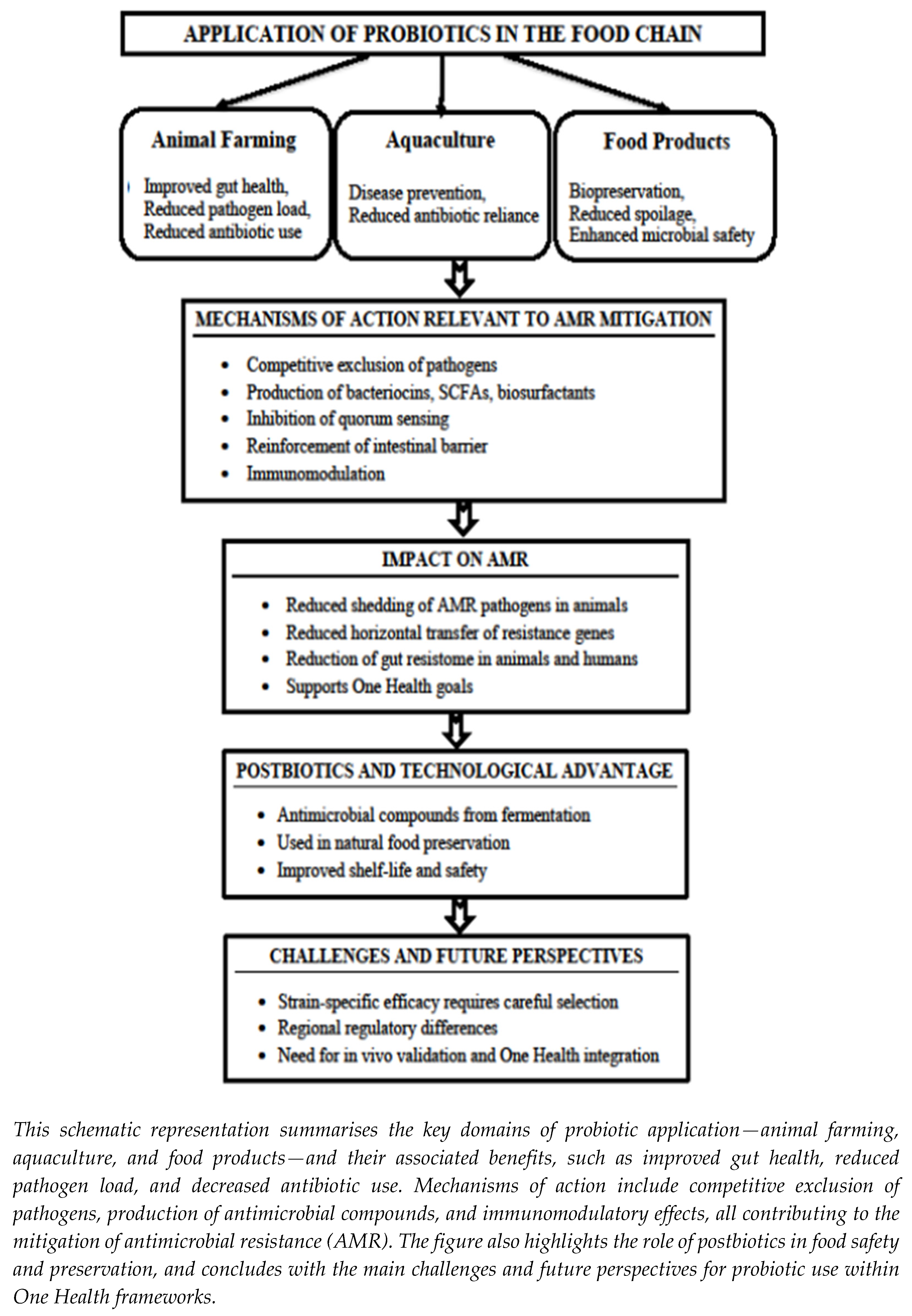
4. Probiotics, Prebiotics and Synbiotics for Combating Antimicrobial Resistance in the Food Chain
4.1. Probiotics for Combating AMR in the Food Chain
4.1.1. Definition, Diversity, and Health Effects
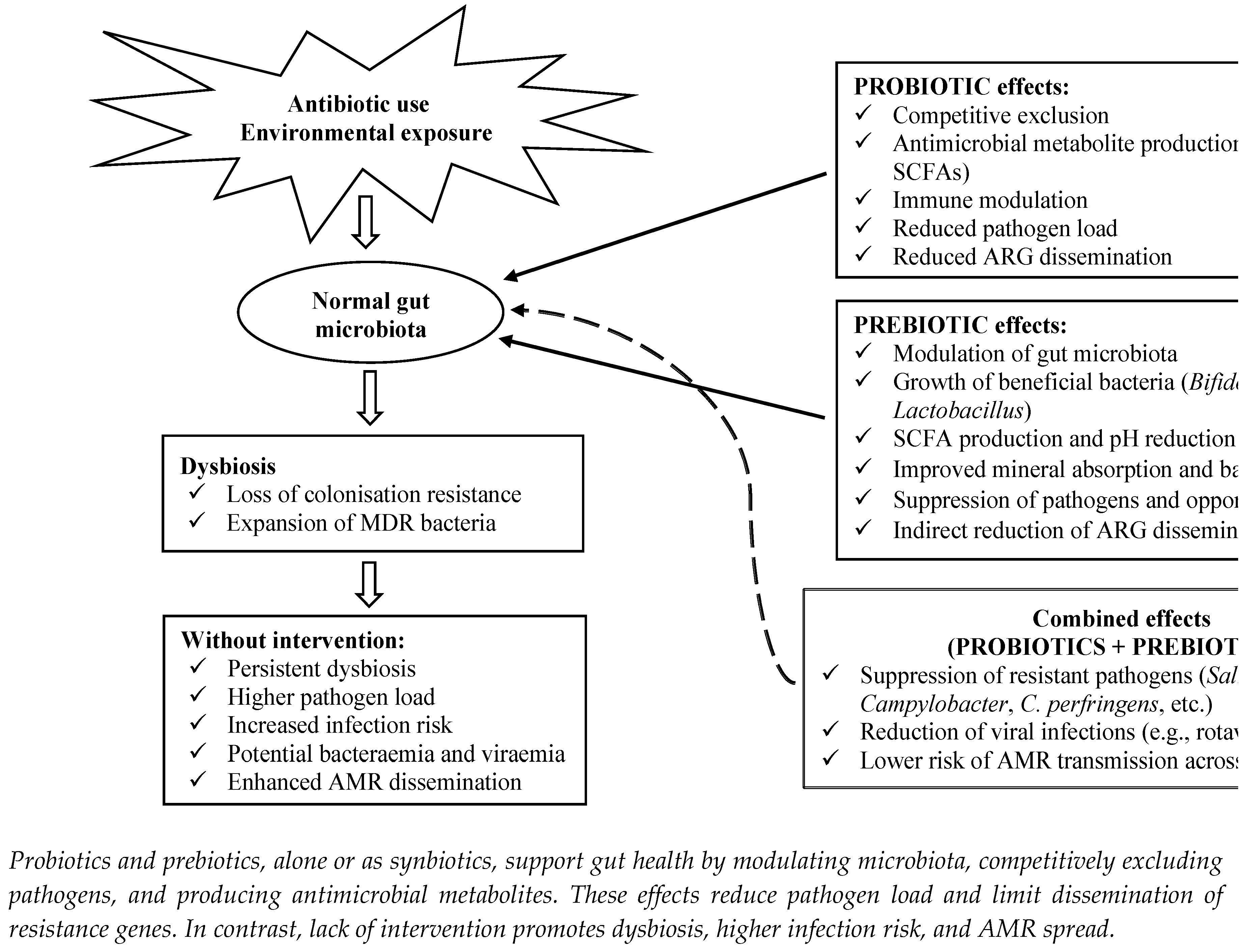
4.1.2. Sustainable Strategies for Combating AMR
4.2. Prebiotics for Combating AMR in the Food Chain
4.2.1. Definition, Types, and Health Effects
4.2.2. Mechanisms of Prebiotic Action in AMR Mitigation
4.3. Synbiotics for Combating AMR in the Food Chain
4.3.1. Definition and Relevance in AMR Mitigation
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Antimicrobial Resistance. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 26 May 2025).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; Browne, A.J.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655.
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Resistance in the EU/EEA: Annual Epidemiological Report 2023. ECDC: Stockholm, Sweden, 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-ears-net-annual-epidemiological-report-2023 (accessed on 12 May 2025).
- Organisation for Economic Co-operation and Development (OECD); World Health Organization (WHO). (2022). Addressing the burden of infections and antimicrobial resistance associated with health care. Available online: https://www.oecd.org/health/Addressing-burden-of-infections-and-AMR-associated-with-health-care.pdf (accessed on 12 May 2025).
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance, 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 12 May 2025).
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food-Producing Animals: 2020 to 2030. PLOS Glob. Public Health 2023, 3, e0001305. [CrossRef]
- Barroso-Arévalo, S.; Re, M.; San Miguel Ayanz, J.M.; Peralta Val, E.; Alvarado-Piqueras, A.; Fernández-Valeriano, R.; Blanco-Murcia, J. Prevalence of Bacteria Involved in Bovine Respiratory Disease in Dairy Heifers in Spain: Influence of Environmental Factors. Front. Vet. Sci. 2025, 12, 1605045. [CrossRef]
- European Commission (EC). Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect. Report No. IP/05/1687, 2005. Available online: https://ec.europa.eu/commission/presscorner/detail/en/IP_05_1687 (accessed on 18 May 2025).
- Lees, P.; Pelligand, L.; Giraud, E.; Toutain, P.L. A History of Antimicrobial Drugs in Animals: Evolution and Revolution. J. Vet. Pharmacol. Ther. 2021, 44(2), 137–171. [CrossRef]
- European Union (EU). Regulation (EU) 2019/6 of the European Parliament and of the Council of 11 December 2018 on Veterinary Medicinal Products and Repealing Directive 2001/82/EC. Off. J. Eur. Union 2019, L4, 43–167. Available online: http://data.europa.eu/eli/reg/2019/6/oj (accessed on 8 May 2025).
- Milijašević, M.; Vesković-Moračanin, S.; Babić Milijašević, J.; Petrović, J.; Nastasijević, I. Antimicrobial Resistance in Aquaculture: Risk Mitigation within the One Health Context. Foods 2024, 13(15), 2448. [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and Prebiotics in Intestinal Health and Disease: From Biology to the Clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; Verbeke, K.; Reid, G. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10(Suppl. 1), S49–S66. [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [CrossRef]
- Habteweld, H.A.; Asfaw, T. Novel dietary approach with probiotics, prebiotics, and synbiotics to mitigate antimicrobial resistance and subsequent out marketplace of antimicrobial agents: A review. Infect. Drug Resist. 2023, 16, 3191–3211. [CrossRef]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial resistance and recent alternatives to antibiotics for the control of bacterial pathogens with an emphasis on foodborne pathogens. Antibiotics 2023, 12, 274. [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; Sanders, M.E. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [CrossRef]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug resistant and extensively drug resistant bacteria: A study. J. Pathog. 2016, 2016, 4065603. [CrossRef]
- Gaynes, R. The discovery of penicillin—new insights after more than 75 years of clinical use. Emerg. Infect. Dis. 2017, 23, 849–853. [CrossRef]
- Fleming, A. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. influenzæ. Br. J. Exp. Pathol. 1929, 10, 226–236.
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [CrossRef]
- Paul, M.; Daikos, G.L.; Durante-Mangoni, E.; Yahav, D.; Carmeli, Y.; Benattar, Y.D.; Skiada, A.; Andini, R.; Eliakim-Raz, N.; Nutman, A.; Zusman, O.; Antoniadou, A.; Pafundi, P.C.; Adler, A.; Dickstein, Y.; et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 391–400. [CrossRef]
- Nation, R.L.; Li, J.; Cars, O.; Couet, W.; Dudley, M.N.; Kaye, K.S.; Mouton, J.W.; Paterson, D.L.; Tam, V.H.; Theuretzbacher, U.; Tsuji, B.T.; Turnidge, J.D. Framework for optimisation of the clinical use of colistin and polymyxin B: The Prato polymyxin consensus. Lancet Infect. Dis. 2015, 15, 225–234. [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Tackling Antimicrobial Use and Resistance in Food-Producing Animals—Lessons Learned in the United Kingdom of Great Britain and Northern Ireland; FAO: Rome, Italy, 2022. [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [CrossRef]
- Aidara-Kane, A.; Angulo, F.J.; Conly, J.M.; Minato, Y.; Silbergeld, E.K.; McEwen, S.A.; Collignon, P.J.; WHO Guideline Development Group. World Health Organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control 2018, 7, 7. [CrossRef]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D.; Ghali, W.A. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [CrossRef]
- Price, L.B.; Stegger, M.; Hasman, H.; Aziz, M.; Larsen, J.; Andersen, P.S.; Pearson, T.; Waters, A.E.; Foster, J.T.; Schupp, J.; Gillece, J.; Driebe, E.; Liu, C.M.; Springer, B.; Zdovc, I.; Battisti, A.; Franco, A.; Żmudzki, J.; Schwarz, S.; … Aarestrup, F.M. Staphylococcus aureus CC398: Host adaptation and emergence of methicillin resistance in livestock. mBio 2012, 3, e00305–11. [CrossRef]
- Ašanin, J.; Mišić, D.; Aksentijević, K.; Tambur, Z.; Rakonjac. B.; Kovačević, I.; Spergser, J.; Lončarić I. Genetic profiling and comparison of human and animal methicillin-resistant Staphylococcus aureus (MRSA) isolates from Serbia. Antibiotics 2019 8(1), 26. [CrossRef]
- Sasaki, Y., Yonemitsu, K., Uema, M., Asakura, H., Asai, T. Prevalence and antimicrobial resistance of Campylobacter and Salmonella in layer flocks in Honshu, Japan. J. Vet. Med. Sci. 2022, 84, 11. [CrossRef]
- Soubai, Z.; Ziyate, N.; Darkaoui, S.; Rais, R.; Fellahi, S.; Attarassi, B.; Auajjar, N. Antimicrobial susceptibility profiles of Campylobacter jejuni and Campylobacter coli isolated from processed chickens and turkeys in Morocco. Poultry 2025, 4, 23. [CrossRef]
- Ramatla, T.; Mafokwane, T.; Lekota, K.; Khumalo, K.; Matle, I.; Adesiyun, A.A. “One Health” perspective on prevalence of co-existing extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella pneumoniae: A comprehensive systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 88. [CrossRef]
- Hadi, H.A.; Al-Hail, H.; Aboidris, L.E.; Al-Orphaly, M.; Sid Ahmed, M.A.; Samuel, B.G.; Mohamed, H.A.; Sultan, A.A.; Skariah, S. Prevalence and genetic characterization of clinically relevant extended-spectrum β-lactamase-producing Enterobacterales in the Gulf Cooperation Council countries. Front. Antibiot. 2023, 2, 1177954. [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2018–2019. EFSA J. 2021, 19, e06490. [CrossRef]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [CrossRef]
- Muloi, D.; Ward, M.J.; Pedersen, A.B.; Fevre, E.M.; Woolhouse, M.E.J.; van Bunnik, B.A.D.; Global Burden of Animal Diseases (GBADs) Consortium. Are food animals responsible for transfer of antimicrobial-resistant Escherichia coli or their resistance determinants to human populations? A systematic review. Foodborne Pathog. Dis. 2018, 15, 467–474. [CrossRef]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [CrossRef]
- World Organisation for Animal Health (WOAH). Annual Report on Antimicrobial Agents Intended for Use in Animals. WOAH: Paris, France, 2023. Available online: https://www.woah.org/en/document/annual-report-antimicrobial-agents-2023/ (accessed on 26 May 2025).
- European Medicines Agency (EMA). Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2022. Trends from 2010 to 2022. Thirteenth ESVAC Report. 2023. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2022-trends-2010-2022-thirteenth-esvac-report_en.pdf (accessed on 26 May 2025).
- World Organisation for Animal Health (WOAH). Sixth Annual Report on Antimicrobial Agents Intended for Use in Animals. WOAH: Paris, France, 2025. Available online: https://www.woah.org/en/document/annual-report-antimicrobial-agents-2025/ (accessed on 26 May 2025).
- Matheou, A.; Abousetta, A.; Pascoe, A.P.; Papakostopoulos, D.; Charalambous, L.; Panagi, S.; Panagiotou, S.; Yiallouris, A.; Filippou, C.; Johnson, E.O. Antibiotic Use in Livestock Farming: A Driver of Multidrug Resistance? Microorganisms 2025, 13, 779. [CrossRef]
- U.S. Food and Drug Administration (FDA). Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. Center for Veterinary Medicine: Silver Spring, MD, USA, 2024. Available online: https://www.fda.gov/media/174667/download (accessed on 26 May 2025).
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2019, 357(6358), 1350–1352. [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; Calder, P.C.; Sanders, M.E. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO). Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. FAO Food and Nutrition Paper 85; FAO/WHO: Rome, Italy, 2006. Available online: https://www.fao.org/food/food-safety-quality/a-z-index/probiotics/en/ (accessed on 26 May 2025).
- Thomas, S. Döderlein’s Bacillus: Lactobacillus acidophilus. J. Infect. Dis. 1928, 43, 218–227.
- Vinayamohan, P.; Joseph, D.; Viju, L.S.; Baskaran, S.A.; Venkitanarayanan, K. Efficacy of Probiotics in Reducing Pathogenic Potential of Infectious Agents. Fermentation 2024, 10(12), 599. [CrossRef]
- Yépez, L.; Tenea, G. N. Genetic diversity of lactic acid bacteria strains towards their potential probiotic application. Rom. Biotech. Lett. 2015, 20(2), 10191–10200.
- Sarita, B.; Samadhan, D.; Hassan, M. Z.; Kovaleva, E. G. A comprehensive review of probiotics and human health: Current prospective and applications. Front. Microbiol. 2025, 15, 1487641. [CrossRef]
- Vesković, S. Natural Food Preservation: Controlling Loss, Advancing Safety 1st ed. Cham: Springer Nature Switzerland, 2025 . [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61(2), 160–174. [CrossRef]
- Sar, T.; Bogović Matijačić, B.; Danilović, B.; Gamero, A.; Gandía, M.; Krausova, G.; Martínez-Villaluenga, C.; Peñas, E.; Bagherzadehsurbagh, E.; Cemali, Ö.; Santa, D.; Künili, I.E.; Kesenkas, H.; Chassard, C.; Pracer, S.; Vergères, G.; Ergün, B.G. A systematic review of health promoting effects of consumption of whey-based fermented products on adults. Front. Nutr. 2025, 12, 1651365. doi: 10.3389/fnut.2025.1651365.
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C. M. A. P.; Harris, H. M. B.; Mattarelli, P.; O’Toole, P. W.; Pot, B.; Vandamme, P.; Walter, J.; Watanabe, K.; Wuyts, S.; Felis, G. E.; Gänzle, M. G.; Lebeer, S. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70 (4), 2782–2858. [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 12: Suitability of taxonomic units notified to EFSA until March 2020. EFSA J. 2020, 18 (7), 6174. [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Update of the list of qualified presumption of safety (QPS) recommended microbiological agents intentionally added to food or feed as notified to EFSA 17: Suitability of taxonomic units notified to EFSA until September 2022. EFSA J. 2023, 21 (1), 7746. [CrossRef]
- Ashaolu, T.J.; Greff, B.; Varga, L. Action and immunomodulatory mechanisms, formulations, and safety concerns of probiotics. Biosci. Microbiota Food Health 2025, 44 (1), 4–15. [CrossRef]
- Vesković, S.; Đukić, D. Application of Bioprotectors in Food Production; Agronomy Faculty in Čačak: Čačak, Serbia, 2015; p. 377, ISBN 978-86-87611-34-4.
- Nataraj, B.H.; Mallappa, R.H. Antibiotic resistance crisis: an update on antagonistic interactions between probiotics and methicillin-resistant Staphylococcus aureus (MRSA). Curr. Microbiol. 2021, 78, 2194–2211. [CrossRef]
- Anjana; Tiwari, S.K. Bacteriocin-producing probiotic lactic acid bacteria in controlling dysbiosis of the gut microbiota. Front. Cell. Infect. Microbiol. 2022, 12, 851140. [CrossRef]
- Zavišić, G.; Ristić, S.; Petričević, S.; Janković, D.; Petković, B. Microbial contamination of food: Probiotics and postbiotics as potential biopreservatives. Foods 2024, 13 (16), 2487. [CrossRef]
- Leistikow, R.L.; Ramachandran, R.; Johnson, M.; Straight, P.D. A Bacillus subtilis–derived peptide disrupts biofilms and enhances antibiotic susceptibility in pathogenic bacteria. mSystems 2024, 9 (1), e00712-24. [CrossRef]
- Adnan, M.; Siddiqui, A.J.; Noumi, E.; Ashraf, S.A.; Awadelkareem, A.M.; Hadi, S.; Snoussi, M.; Badraoui, R.; Bardakci, F.; Sachidanandan, M.; Patel, M. Biosurfactant derived from probiotic Lactobacillus acidophilus exhibits broad-spectrum antibiofilm activity and inhibits the quorum sensing-regulated virulence. Biomol Biomed. 2023, 23(6), 1051–1068. [CrossRef]
- Patel, M.; Siddiqui, A.J.; Ashraf, S.A.; Surti, M.; Awadelkareem, A.M.; Snoussi, M.; Hamadou, W.S.; Bardakci, F.; Jamal, A.; Jahan, S.; Sachidanandan, M.; Adnan, M. Lactiplantibacillus plantarum-derived biosurfactant attenuates quorum sensing-mediated virulence and biofilm formation in Pseudomonas aeruginosa and Chromobacterium violaceum. Microorganisms 2022, 10 (5), 1026. [CrossRef]
- Redweik, G.A.J.; Stromberg, Z.R.; Van Goor, A.; Mellata, M. Protection against avian pathogenic Escherichia coli and Salmonella Kentucky exhibited in chickens given both probiotics and live Salmonella vaccine. Poult. Sci. 2020, 99 (1), 752–762. [CrossRef]
- Šikić Pogačar, M.; Langerholc, T.; Mičetić-Turk, D.; Matijašić, B.B. Lactobacillus rhamnosus GG and other probiotic strains reduce adhesion and invasion of Campylobacter jejuni in pig and chicken intestinal epithelial cells in vitro. BMC Vet. Res. 2020, 16 (1), 310. [CrossRef]
- de Oliveira, L.I.G.; de Araujo, A.R.R.; Pimentel, T.C.; Capozzi, V.; Bezerra, T.K.A.; Magnani, M. Probiotics and prebiotics in foodborne illness: Mechanisms, applications, and future directions. J. Food Prot. 2025, 88 (9), 100584 . [CrossRef]
- Huang, F.; Zhao, Y.; Hou, Y.; Yang, Y.; Yue, B.; Zhang, X. Unraveling the antimicrobial potential of Lactiplantibacillus plantarum strains TE0907 and TE1809 sourced from Bufo gargarizans: Advancing the frontier of probiotic-based therapeutics. Front. Microbiol. 2024, 15, 1347830. [CrossRef]
- Fusco, W.; Bernabeu Lorenzo, M.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; Cammarota, G.; Ianiro, G. Short-chain fatty-acid-producing bacteria: Key components of the human gut microbiota. Nutrients 2023, 15, 2211. [CrossRef]
- De Bruyn, F.; James, K.; Cottenet, G.; Maes, D.; Johnson, K. Combining Bifidobacterium longum subsp. infantis and human milk oligosaccharides synergistically increases short chain fatty acid production ex vivo. Commun. Biol. 2024, 7, 943. [CrossRef]
- Salazar-Parra, M.A.; Cruz-Neri, R.U.; Trujillo-Trujillo, X.A.; Dominguez-Mora, J.J.; Cruz-Neri, H.I.; Guzmán-Díaz, J.M.; Guzmán-Ruvalcaba, M.J.; Vega-Gastelum, J.O.; Ascencio-Díaz, K.V.; Zarate-Casas, M.F.; et al. Effectiveness of Saccharomyces boulardii CNCM I-745 probiotic in acute inflammatory viral diarrhoea in adults: Results from a single-centre randomized trial. BMC Gastroenterol. 2023, 23, 229. [CrossRef]
- Gao, H.; Li, Y.; Xu, J.; Zuo, X.; Yue, T.; Xu, H.; Sun, J.; Wang, M.; Ye, T.; Yu, Y.; Yao, Y. Saccharomyces boulardii protects against murine experimental colitis by reshaping the gut microbiome and its metabolic profile. Front. Microbiol. 2023, 14, 1204122. [CrossRef]
- Liu, Y.; Nawazish, H.; Farid, M.S.; Qadoos, K.A.; Habiba, U.E.; Muzamil, M.; Tanveer, M.; Sienkiewicz, M.; Lichota, A.; Łopusiewicz, Ł. Health-promoting effects of Lactobacillus acidophilus and its technological applications in fermented food products and beverages. Fermentation 2024, 10, 380. [CrossRef]
- Ma, L.; Tian, G.; Pu, Y.; Qin, X.; Zhang, Y.; Wang, H.; You, L.; Zhang, G.; Fang, C.; Liang, X.; Wei, H.; Tan, L.; Jiang, L. Bacillus coagulans MF-06 alleviates intestinal mucosal damage and reduces Salmonella pullorum colonization in chicks. Front. Microbiol. 2024, 15, 1492035. [CrossRef]
- Lee, E.B.; Lee, K. Woodfordia fruticosa fermented with lactic acid bacteria impact on foodborne pathogens adhesion and cytokine production in HT-29 cells. Front. Microbiol. 2024, 15, 1346909. [CrossRef]
- Singh, S. Use of probiotics in swine nutrition: A review. Int. J. Agric. Extension Social Dev. 2024, 7, 422–429. [CrossRef]
- Vesković-Moračanin, S.; Djukic, D.; Kurćubić, V.S.; Maskovic, P. Natural antimicrobial compounds and biopreservation of food. Tehnol. Mesa 2015, 56, 16–25. [CrossRef]
- Chen, L.; Song, Z.; Tan, S.Y.; Zhang, H.; Yuk, H.G. Application of bacteriocins produced from lactic acid bacteria for microbiological food safety. Curr. Top. Lactic Acid Bact. Probiot. 2020, 6, 1–8. [CrossRef]
- George, F.; Daniel, C.; Thomas, M.; Singer, E.; Guilbaud, A.; Tessier, F.J.; Revol-Junelles, A.M.; Borges, F.; Foligné, B. Occurrence and dynamism of lactic acid bacteria in distinct ecological niches: A multifaceted functional health perspective. Front. Microbiol. 2018, 9, 2899. [CrossRef]
- Milićević, B.; Danilović, B.; Kocić, M.; Džinić, N.; Milosavljević, N.; Savić, D. The production and antimicrobial activity of bacteriocin produced by Lactobacillus paracasei. In Industrial, Medical and Environmental Applications of Microorganisms: Current Status and Trends; Méndez-Vilas, A., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2014; pp. 385–390.
- Hills, R.D.; Pontefract, B.A.; Mishcon, H.R.; Black, C.A.; Sutton, S.C.; Theberge, C.R. Gut microbiome: profound implications for diet and disease. Nutrients 2019, 11, 1613. [CrossRef]
- Panesar, P.S.; Anal, A.K.; Kaur, R. Probiotics, prebiotics and synbiotics: Opportunities, health benefits and industrial challenges. In Probiotic Functional Foods; Panesar, P.S., Anal, A.K., Eds.; Wiley: Hoboken, NJ, USA, 2022; Chapter 1. [CrossRef]
- Isacco, C.G.; Ballini, A.; De Vito, D.; Inchingolo, A.M.; Cantore, S.; Paduanelli, G.; Nguyen, K.C.D.; Inchingolo, A.D.; Dipalma, G.; Inchingolo, F. Probiotics in health and immunity: A first step toward understanding the importance of microbiota system in translational medicine. In Prebiotics and Probiotics—Potential Benefits in Nutrition and Health; Franco-Robles, E., Ramírez-Emiliano, J., Eds.; IntechOpen: London, UK, 2019. [CrossRef]
- Tong, Y.; Abbas, Z.; Zhang, J.; Wang, J.; Zhou, Y.; Si, D.; Wei, X.; Zhang, R. Antimicrobial activity and mechanism of novel postbiotics against foodborne pathogens. LWT 2025, 217, 117464. [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond pre- and probiotics. Nutrients 2020, 12, 2189. [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem MLemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; Turroni, F.; van Sinderen, D.; Varmanen, P.; Ventura, M.; Zúñiga, M.; Tsakalidou, E,; Kok, J. Stress physiology of lactic acid bacteria. Microbiol. Mol. Biol. Rev. 2015, 80, 837–890. [CrossRef]
- Milićević, B.; Danilović, B.; Zdolec, N.; Kozačinski, L.; Dobranić, V.; Savić, D. Microbiota of the fermented sausages: influence to product quality and safety. Bulgar. J. Agric. Sci. 2014, 20, 1061–1078.
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory effects of probiotics during pathogenic infections with emphasis on immune regulation. Front. Immunol. 2021, 12, 616713. [CrossRef]
- Du, R.; Jiao, S.; Dai, Y.; An, J.; Lv, J.; Yan, X.; Wang, J.; Han, B. Probiotic Bacillus amyloliquefaciens C-1 improves growth performance, stimulates GH/IGF-1, and regulates the gut microbiota of growth-retarded beef calves. Front. Microbiol. 2018, 9, 2006. [CrossRef]
- Li, Z.; Dai, X.; Yang, F.; Zhao, W.; Xiong, Z.; Wan, W.; Wu, G.; Xu, T.; Cao, H. Compound probiotics promote the growth of piglets through activating the JAK2/STAT5 signaling pathway. Front. Microbiol. 2025, 16, 1480077. [CrossRef]
- Xie, G.; Chen, X.; Feng, Y.; Yu, Z.; Lu, Q.; Li, M.; Ye, Z.; Lin, H.; Yu, W.; Shu, H. Effects of dietary multi-strain probiotics on growth performance, antioxidant status, immune response, and intestinal microbiota of hybrid groupers (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Microorganisms 2024, 12, 1358. [CrossRef]
- Kuebutornye, F.K.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol. 2019, 87, 820–828. [CrossRef]
- Al-Shawi, S.G.; Dang, D.S.; Yousif, A.Y.; Al-Younis, Z.K.; Najm, T.A.; Matarneh, S.K. The potential use of probiotics to improve animal health, efficiency, and meat quality: A review. Agriculture 2020, 10, 452. [CrossRef]
- Sharifuzzaman, S.; Austin, B. Introduction. In Probiotics in Aquaculture; Austin, B., Sharifuzzaman, S., Eds.; Springer: Cham, Switzerland, 2022. [CrossRef]
- Feng, Z.; Song, X.; Zhao, L.; Zhu, W. Isolation of probiotics and their effects on growth, antioxidant and non-specific immunity of sea cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2020, 106, 1087–1094. [CrossRef]
- Banerjee, G.; Ray, A.K. The advancement of probiotics research and its application in fish farming industries. Res. Vet. Sci. 2017, 115, 66–77. [CrossRef]
- Monier, M.N.; Kabary, H.; Elfeky, A.; Saadony, S.; Abd El-Hamed, N.N.B.; Eissa, M.E.H.; Eissa, E.-S.H. The effects of Bacillus species probiotics (Bacillus subtilis and B. licheniformis) on the water quality, immune responses, and resistance of whiteleg shrimp (Litopenaeus vannamei) against Fusarium solani infection. Aquac. Int. 2023, 31, 3437–3455. [CrossRef]
- Wu, Y.S.; Chu, Y.T.; Chen, Y.Y.; Chang, C.S.; Lee, B.H.; Nan, F.H. Effects of dietary Lactobacillus reuteri and Pediococcus acidilactici on the cultured water qualities, the growth and non-specific immune responses of Penaeus vannamei. Fish Shellfish Immunol. 2022, 127, 176–186. [CrossRef]
- Du, S.; Chen, W.; Yao, Z.; Huang, X.; Chen, C.; Guo, H.; Zhang, D. Enterococcus faecium are associated with the modification of gut microbiota and shrimp post-larvae survival. Anim. Microbiome 2021, 3, 88. [CrossRef]
- Islam, S.M.M.; Rohani, M.F.; Shahjahan, M. Probiotic yeast enhances growth performance of Nile tilapia (Oreochromis niloticus) through morphological modifications of intestine. Aquac. Rep. 2021, 21, 100800. [CrossRef]
- Rahayu, S.; Amoah, K.; Huang, Y.; Cai, J.; Wang, B.; Shija, V.M.; Jin, X.; Anokyewaa, M.A.; Jiang, M. Probiotics application in aquaculture: Its potential effects, current status in China and future prospects. Front. Mar. Sci. 2024, 11, 1455905. [CrossRef]
- Gadhiya, A.; Katariya, S.; Khapandi, K.; Chhatrodiya, D. Probiotics as a sustainable alternative to antibiotics in aquaculture: A review of the current state of knowledge. Microbe 2025, 8, 100426. [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAO Animal Production and Health: Annual Report 2023; FAO: Rome, Italy, 2024. Available online: https://openknowledge.fao.org/handle/20.500.14283/cd1311en (accessed on 22 August 2025).
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [CrossRef]
- Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Prebiotics: definitions, types, functions and emerging trends. Foods 2024, 13, 446. [CrossRef]
- Deehan, E.C.; Al Antwan, S.; Witwer, R.S.; Guerra, P.; John, T.; Monheit, L. Revisiting the concepts of prebiotic and prebiotic effect in light of scientific and regulatory progress—A consensus paper from the Global Prebiotic Association. Adv. Nutr. 2024, 15, 100329. [CrossRef]
- Bode, L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [CrossRef]
- Iribarren, C.; Magnusson, M.K.; Vigsnæs, L.K.; Aziz, I.; Amundsen, I.D.; Šuligoj, T.; Juge, N.; Patel, P.; Sapnara, M.; Johnsen, L.; Sørensen, N.; Sundin, J.; Törnblom, H.; Simrén, M.; Öhman, L. The effects of human milk oligosaccharides on gut microbiota, metabolite profiles and host mucosal response in patients with irritable bowel syndrome. Nutrients 2021, 13, 3836. [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24(8), 1415-22. [CrossRef]
- Cherry, P.; O’Hara, C.; Magee, P.J.; McSorley, E.M.; Allsopp, P.J. Risks and benefits of consuming edible seaweeds. Nutr Rev. 2019, 77, 307–329. [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [CrossRef]
- Carrasqueira, J.; Bernardino, S.; Bernardino, R.; Afonso, C. Marine-derived polysaccharides and their potential health benefits in nutraceutical applications. Mar. Drugs 2025, 23, 60. [CrossRef]
- Peres Fabbri, L.; Cavallero, A.; Vidotto, F.; Gabriele, M. Bioactive peptides from fermented foods: Production approaches, sources, and potential health benefits. Foods 2024, 13, 3369. [CrossRef]
- EFSA NDA Panel. Scientific opinion on the substantiation of a health claim related to “native chicory inulin” and maintenance of normal defecation by increasing stool frequency. EFSA J. 2015, 13, 3951. [CrossRef]
- Rigo-Adrover, M.; Saldaña-Ruíz, S.; van Limpt, K.; Knipping, K.; Garssen, J.; Pérez-Cano, F.J. A combination of scGOS/lcFOS with Bifidobacterium breve M-16V protects suckling rats from rotavirus gastroenteritis. Eur. J. Nutr. 2017, 56, 1657–1670. [CrossRef]
- Kishino, E.; Takemura, N.; Masaki, H.; Ito, T.; Nakazawa, M. Dietary lactosucrose suppresses influenza A (H1N1) virus infection in mice by enhancing the innate immune response. Biosci. Microbiota Food Health 2015, 34, 67–76. [CrossRef]
- Pineiro, M.; Asp, N.-G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO technical meeting on prebiotics. J. Clin. Gastroenterol. 2008, 42 (Suppl. 3 Pt. 2), S156–S159. [CrossRef]
- Health Canada. List of dietary fibres reviewed and accepted by Health Canada’s Food Directorate. 2021. Available online: https://www.canada.ca/en/health-canada/services/publications/food-nutrition/list-reviewed-accepted-dietary-fibres.html (accessed on 22 August 2025).
- Food Standards Australia New Zealand (FSANZ). Qualifying criteria for nutrition content claims about dietary fibre in Standard 1.2.7—Nutrition, Health and Related Claims. 2016. Available online: https://www.foodstandards.gov.au/consumer/labelling/nutrition (accessed on 22 August 2025).
- ANVISA, Agência Nacional de Vigilância Sanitária. Resolução RDC nº 54, de 12 de novembro de 2012. Regulamento técnico sobre informação nutricional complementar; Brasília, DF, Brazil, 2012.
- Akhavan, N.; Hrynkiewicz, K.; Thiem, D.; Randazzo, C.; Walsh, A.M.; Guinan, K.J.; O’Sullivan, J.T.; Stadnicka, K. Evaluation of probiotic growth stimulation using prebiotic ingredients to optimise compounds for in ovo delivery. Front. Microbiol. 2023, 14, 1242027. [CrossRef]
- Abdel-Shafi, S.; Abd El-Hack, M.E.; Amen, S.; Helmi, A.; Swelum, A.A.; Tellez-Isaias, G.; Enan, G. The efficacy of some probiotics and prebiotics on the prevalence of E. coli and the immune response of chickens. Poult. Sci. 2023, 102, 103219. [CrossRef]
- Froebel, L.K.; Jalukar, S.; Lavergne, T.A.; Lee, J.T.; Duong, T. Administration of dietary prebiotics improves growth and reduces Clostridium perfringens in poultry. Poult. Sci. 2019, 98, 6753–6762. [CrossRef]
- Rahman, M.N.; Barua, N.; Tin, M.C.F.; Dharmaratne, P.; Wong, S.H.; Ip, M. The use of probiotics and prebiotics in decolonizing pathogenic bacteria from the gut; a systematic review and meta-analysis of clinical outcomes. Gut Microbes 2024, 16, 2356279. [CrossRef]
- Olveira, G.; González-Molero, I. An update on probiotics, prebiotics and symbiotics in clinical nutrition. Endocrinol. Nutr. 2016, 63, 482–494. [CrossRef]
- Sáez-Lara, M.J.; Robles-Sanchez, C.; Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Gil, A. Effects of probiotics and synbiotics on obesity, insulin resistance syndrome, type 2 diabetes and non-alcoholic fatty liver disease: A review of human clinical trials. Int. J. Mol. Sci. 2016, 17, 928. [CrossRef]
- McFarland, L.V.; Karakan, T.; Karatas, A. Strain-specific and outcome-specific efficacy of probiotics for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. EClinicalMedicine 2021, 41, 101154. [CrossRef]
- Zhang, M.M.; Qian, W.; Qin, Y.Y.; He, J.; Zhou, Y.H. Probiotics in Helicobacter pylori eradication therapy: A systematic review and meta-analysis. World J. Gastroenterol. 2019, 21, 4345–4357. [CrossRef]
- Veziant, J.; Bonnet, M.; Occean, B.V.; Dziri, C.; Pereira, B.; Slim, K. Probiotics/synbiotics to reduce infectious complications after colorectal surgery: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2022, 14, 3066. [CrossRef]
- Sharma, M.; Shukla, G. Metabiotics: One step ahead of probiotics; an insight into mechanisms involved in anticancerous effect in colorectal cancer. Front. Microbiol. 2016, 7, 1940. [CrossRef]
- Liu, P.C.; Yan, Y.K.; Ma, Y.J.; Wang, X.W.; Geng, J.; Wang, M.C.; Wei, F.X.; Zhang, Y.W.; Xu, X.D.; Zhang, Y.C. Probiotics reduce postoperative infections in patients undergoing colorectal surgery: A systematic review and meta-analysis. Gastroenterol. Res. Pract. 2017, 2017, 6029075. [CrossRef]
- Skonieczna-Żydecka, K.; Kaczmarczyk, M.; Łoniewski, I.; Lara, L.F.; Koulaouzidis, A.; Misera, A.; Maciejewska, D.; Marlicz, W. A systematic review, meta-analysis, and meta-regression evaluating the efficacy and mechanisms of action of probiotics and synbiotics in the prevention of surgical site infections and surgery-related complications. J. Clin. Med. 2018, 7, 556. [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics: A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [CrossRef]
- Jiang, H.; Cai, M.; Shen, B.; Wang, Q.; Zhang, T.; Zhou, X. Synbiotics and gut microbiota: New perspectives in the treatment of type 2 diabetes mellitus. Foods 2022, 11, 2438. [CrossRef]
- Işlek, A.; Sayar, E.; Yılmaz, A.; Bö, B.; Mutlu, D.; Artan, R. The role of Bifidobacterium lactis B94 plus inulin in the treatment of acute infectious diarrhea in children. Turk. J. Gastroenterol. 2014, 25, 628–633. [CrossRef]
- Panigrahi, P.; Parida, S.; Nanda, N.C.; Satpathy, R.; Pradhan, L.; Tripathy, R.; Jena, P.K.; Sethi, N.; Baccaglini, L.; Mohapatra, A.; Mohapatra, S.S.; Misra, P.R.; Chaudhry, R.; Chen, H.H.; Johnson, J.A.; Taneja, V.; Morris, J.G.; Paneth, N.; Gewolb, I.H. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017, 548, 407–412. [CrossRef]
| Carnobacterium divergens | Lactobacillus johnsonii | Ligilactobacillus (formerly Lactobacillus) animalis |
| Companilactobacillus (formerly Lactobacillus) alimentarius | Lactobacillus kefiranofaciens | Ligilactobacillus (formerly Lactobacillus) aviarius |
| Companilactobacillus (formerly Lactobacillus) farciminis | Lactococcus lactis | Ligilactobacillus (formerly Lactobacillus) salivarius |
| Fructilactobacillus (formerly Lactobacillus) sanfranciscensis | Lapidilactobacillus (formerly Pediococcus) dextrinicus | Limosilactobacillus (formerly Lactobacillus) fermentum |
| Lacticaseibacillus (formerly Lactobacillus) casei | Latilactobacillus (formerly Lactobacillus) curvatus | Limosilactobacillus (formerly Lactobacillus) mucosae |
| Lacticaseibacillus (formerly Lactobacillus) paracasei | Latilactobacillus (formerly Lactobacillus) sakei | Limosilactobacillus (formerly Lactobacillus) panis |
| Lacticaseibacillus (formerly Lactobacillus) rhamnosus | Lentilactobacillus (formerly Lactobacillus) buchneri | Limosilactobacillus (formerly Lactobacillus) pontis |
| Lactiplantibacillus (formerly Lactobacillus) pentosus | Lentilactobacillus (formerly Lactobacillus) diolivorans | Limosilactobacillus (formerly Lactobacillus) reuteri |
| Lactiplantibacillus (formerly Lactobacillus) plantarum | Lentilactobacillus (formerly Lactobacillus) hilgardii | Loigolactobacillus (formerly Lactobacillus) coryniformis |
| Lactobacillus acidophilus | Lentilactobacillus (formerly Lactobacillus) kefiri | Oenococcus oeni |
| Lactobacillus amylolyticus | Lentilactobacillus (formerly Lactobacillus) parafarraginis | Pediococcus acidilactici |
| Lactobacillus amylovorus | Lentilactobacillus (formerly Lactobacillus) paraplantarum | Pediococcus parvulus |
| Lactobacillus crispatus | Leuconostoc citreum | Pediococcus pentosaceus |
| Lactobacillus delbrueckii | Leuconostoc lactis | Secundilactobacillus (formerly Lactobacillus) collinoides |
| Lactobacillus gallinarum | Leuconostoc mesenteroides | Streptococcus thermophilus |
| Lactobacillus gasseri | Leuconostoc pseudomesenteroides | |
| Lactobacillus helveticus | Levilactobacillus (formerly Lactobacillus) brevis |
|
Probiotic strains |
Target foodborne pathogens |
Reported effects | Mechanisms of action | References |
| L. rhamnosus GG | C. jejuni | Reduced adhesion and invasion |
Competitive exclusion, immunomodulation | [68] |
| L. plantarum strains |
Salmonella spp., E. coli, C. jejuni |
Growth inhibition, reduced adhesion |
Organic acids, bacteriocins, competition for nutrients |
[69,70] |
| Bifidobacterium longum |
S. enterica, E. coli |
Reduced colonisation | SCFA production, epithelial barrier enhancement | [71,72] |
| Saccharomyces boulardii |
C. difficile, E. coli, Salmonella spp. |
Protection from diarhoea; modulation of inflammation | Toxin neutralisation, anti-inflammatory activity |
[73,74] |
| L. acidophilus |
L. monocytogenes, E. coli |
In vitro and in vivo pathogen reduction |
Bacteriocin production, inhibition of quorum sensing |
[50,75] |
|
Heyndrickxia coagulans (formerly B. coagulans) |
E. coli, Salmonella spp. |
Growth inhibition; gut barrier enhancement |
Spore formation, SCFA production |
[76] |
| Fermented Woodfordia fruticosa (with L. plantarum and L. rhamnosus) |
L. monocytogenes, Vibrio parahaemolyticus |
Reduced epithelial adhesion; immunostimulation | Interference with adhesion; ↑ IL-6 production (immunomodulation) |
[77] |
|
L. fermentum and L. salivarius |
S. Typhi | Reduced virulence gene expression | Quorum sensing interfeence, inhibition of biofilm formation |
[77] |
| Class | Examples | Sources | Reported effects | References |
| Carbohydrate-based (traditional) | Inulin, GOS, FOS | Chicory root, onion, garlic, banana, legumes, human milk | Selective stimulation of bifidobacteria and lactobacilli; improved gut health; enhanced mineral absorption | [13,107] |
| Human milk oligosaccharides | 2′-fucosyllactose, lacto-N-neotetraose |
Human milk | Bifidogenic effect; immune modulation; pathogen protection | [110,111] |
| Non-carbohydrate substrates | Conjugated linoleic acid, polyunsaturated fatty acids | Dairy, meat, plant oils | Anti-inflammatory activity; immunomodulation; microbiota modulation | [108] |
| Plant-derived polyphenols | Flavonoids (catechins, anthocyanins), stibenes (resveratrol) | Berries, grapes, tea, cocoa | Fermentation by gut microbiota; antioxidant and anti-inflammatory effects; modulation of microbial composition | [108,112] |
| Marine- and fungal-derived polysaccharides | Fucoidan, laminarin, alginate oligosaccharides, chitosan oligosaccharides, β-glucans | Seaweeds, shellfish, yeast, mushrooms | Immunomodulation; antioxidant activity; stimulation of beneficial bacteria | [113,114,115] |
| Proteins and peptides | Bioactive peptides (milk- and soy-derived) | Dairy, legumes, cereals | Microbiota modulation; enhanced mineral bioavailability; immune stimulation | [116] |
| Minerals as prebiotic co-factors | Calcium, magnesium, zinc | Dairy products, cereals, vegetables | Synergistic effects with fibers; support for microbiota and host health | [109,117] |
| Carbohydrate-based oligosaccharides with antiviral activity | GOS, FOS, lactosucrose | Infant formula, human milk, synthetic oligosaccharides | GOS+FOS: reduced rotavirus shedding; improved stool consistency; alleviated gastroenteritis symptoms; improved immune responses. Lactosucrose: enhanced innate immune responses; increased survival against influenza A virus infection |
[17,118,119] |
| Mechanism of Action | Effects | References |
| Enhanced probiotic survival and implantation | Improved viability and colonisation of Lactobacillus and Bifidobacterium during gastrointestinal transit | [136,137] |
| Synergistic fermentation of prebiotics by co-administered probiotics | Higher SCFA production (acetate, butyrate, propionate); reduced colonic pH; inhibition of pathogens | [19,128] |
| Immune modulation | Increased IgA secretion, enhanced antimicrobial peptide production, reduced systemic inflammation | [14,129] |
| Suppression of resistant pathogens | Reduced colonisation by Salmonella spp., E. coli, C. perfringens, Campylobacter spp. |
[126,131] |
| Antiviral protection | Attenuated rotavirus gastroenteritis and influenza A infection in vivo | [118,119] |
| Reduction of clinical infections and antimicrobial use | Decreased incidence of surgical site infections, sepsis, diarrhea, pneumonia; shortened hospital stay and reduced antibiotic therapy | [132,133,134] |
| Synbiotic composition | Target pathogens / conditions | Effects | References |
| L. fermentum CECT5716 + GOS | Rotavirus, respiratory infections | Inhibition of rotavirus; fewer gastrointestinal infections in infants | [106,130] |
| B. lactis B94 + inulin | Salmonella, Shigella, C. difficile, adenovirus, Campylobacter | Reduced duration of diarrhea; protection against multiple enteric pathogens | [138] |
| L. rhamnosus + inulin / FOS | Vancomycin-resistant Enterococcus | Significant inhibition of VRE growth | [20] |
| L. plantarum ATCC-202195 + FOS | Infant sepsis, respiratory infections | Reduced sepsis incidence and respiratory tract infections | [139] |
| Multi-strain mix (e.g., L. acidophilus, L. rhamnosus, B. bifidum + FOS) |
Surgical site infections | Reduced postoperative infections and shortened antibiotic therapy | [133,134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





