Submitted:
15 September 2025
Posted:
16 September 2025
You are already at the latest version
Abstract
Based on the results of pot experiments, a small-scale field trial was designed to test the efficacy of two methanotrophs on rice plant growth. A methanotroph, Methylomonas strain Kb3, and Methylomagnum ishizawai strain KRF4 were found to be promising in promoting rice plant growth in our earlier experiments done on an Indian rice cultivar, Indrayani. The same methanotrophs were used in a small-scale field trial, where the individual methanotrophs and in combination were used. Methylomonas strain Kb3 was helpful in enhancing the plant growth height and yield in the field experiment, compared to the control plants. Methylomonas Kb3 and Methylomagnum KRF4 in combination also showed considerable positive effects. Methylomagnum ishizawai KRF4 alone did not show an increase in grain yield. The nitrogen input in this field, as per the farmers' practice, has been considerably low (~50kg N/ha) compared to the normally used fertiliser input (100 or 150 kg N/ha). The treatment with methanotrophs was done by dipping the plants in the inoculum. Methylomonas strain Kb3, an indigenously isolated methanotroph, was reported in 2014 from a field ~25 km away from the region where trials were taken, and the genome analysis was done in our prior studies, indicating that it has a complete nitrogen fixation pathway. Kb3 showed an increase in plant height of ~ 15% and an increase in grain yield of ~17% compared to the control plants, with no methanotroph treatment. Methylomonas Kb3 also induced early flowering in the rice plants, followed by early grain formation and early maturation. Methylomonas-Methylomagnum combination treatment showed ~15% height increase and ~15% yield increase compared to the control, whereas Methylomagnum ishizawai KRF4 showed comparable growth yields to the control plants.
Keywords:
1. Introduction
2. Materials and Methods
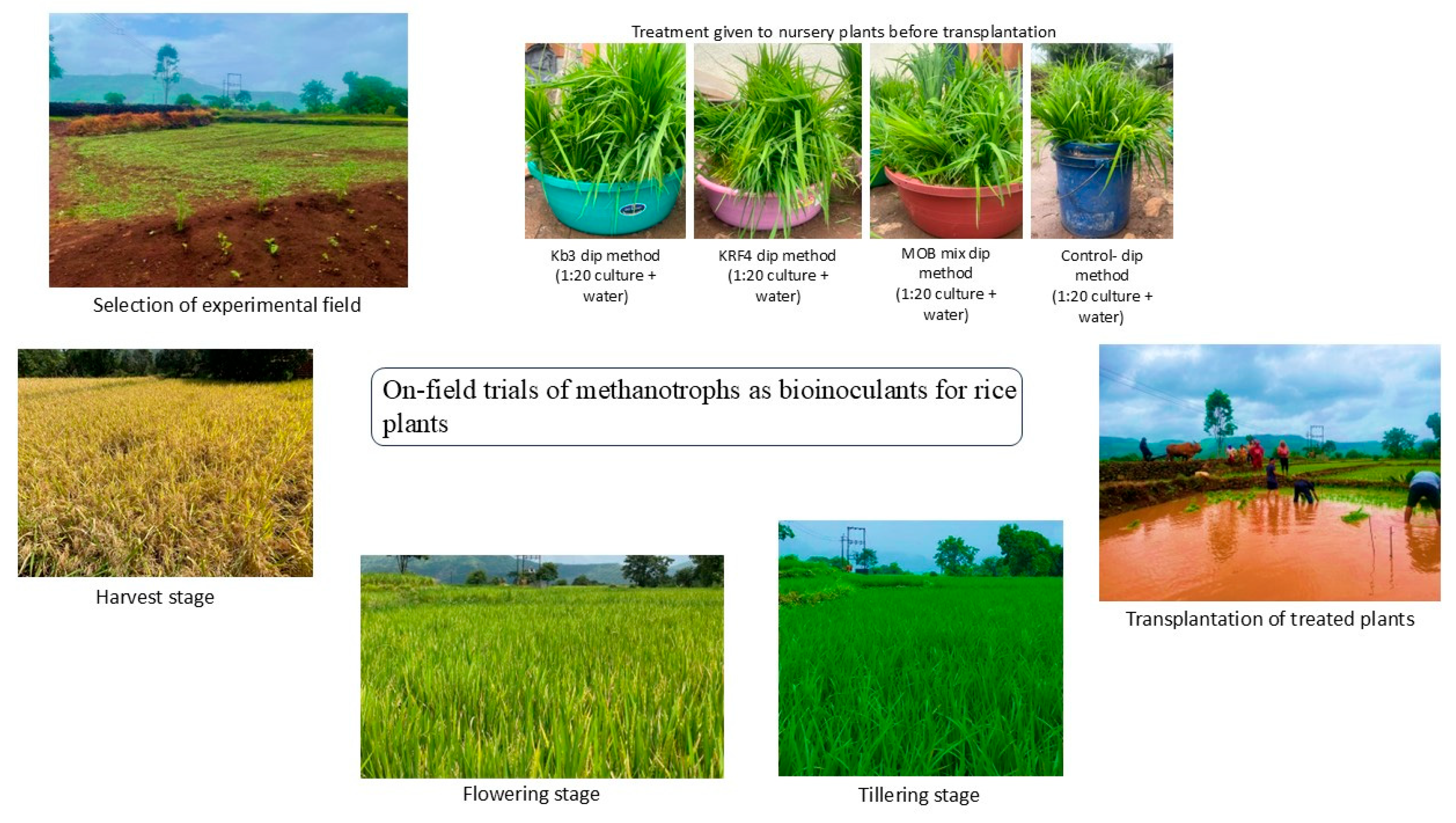
2.1. Selection of a Rice Field for the Field Experiment
2.2. Treatment of Rice Plantlets with Selected Methanotroph Strains
2.3. Data Collection, Farm Visits, and Soil Sampling
2.4. Soil Sampling for Enrichment of Methanotrophs
2.5. Data Analysis
3. Results
3.1. Overall Health of the Plants and Early Flowering Seen in Methylomonas Kb3-Treated Plants
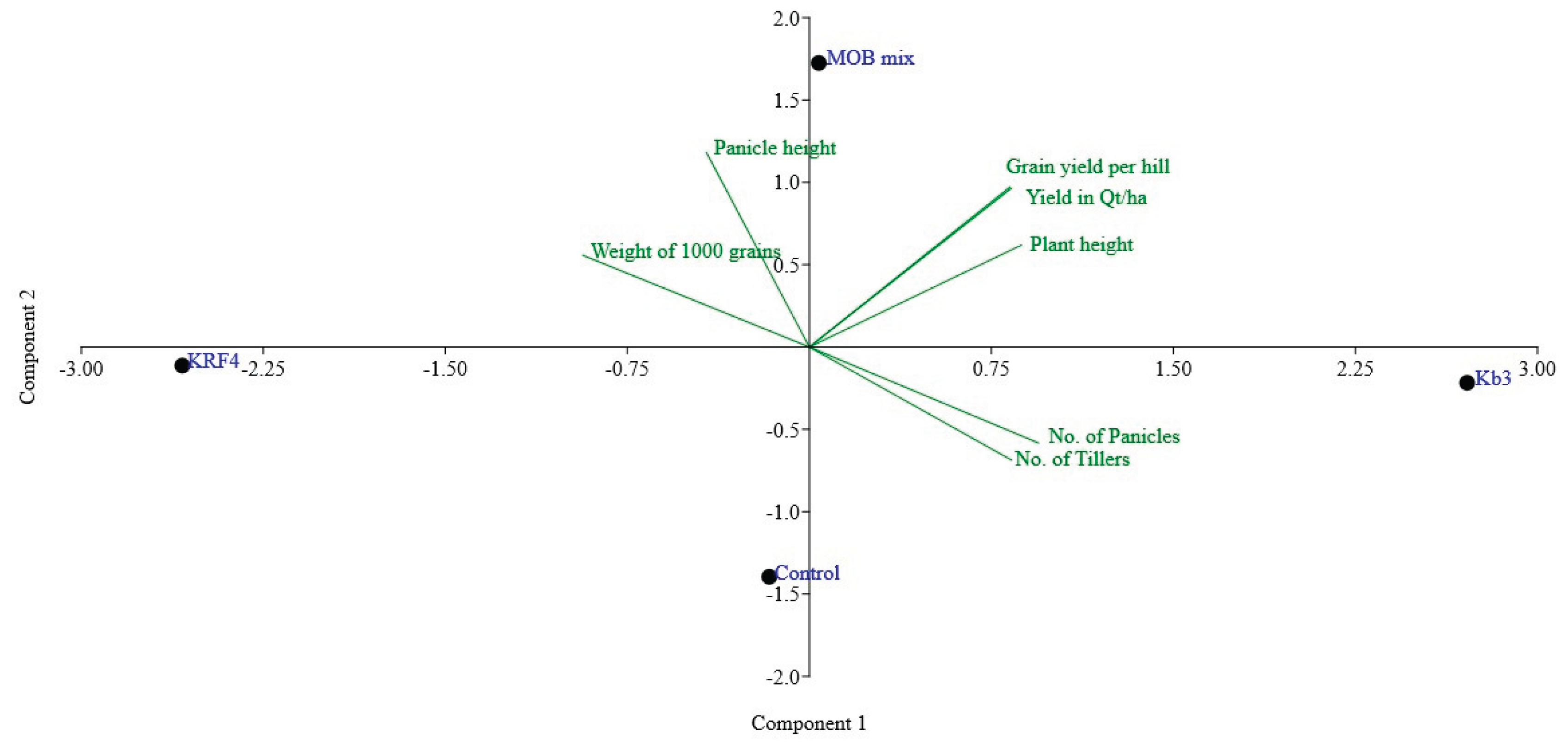
3.2. Enhanced Growth Yield, Plant Height in Methylomonas Kb3-Treated Plants
3.3. Re-Isolation of Methanotrophs
4. Discussion
4.1. Need for Novel Bio-Inoculants in Rice
4.2. Methanotrophs as a New Class of Bio-Inoculants
4.3. Nitrogen Fixation by Methanotrophs
4.4. A Methanotroph, Methylococcus Capsulatus, Has Been Shown to Act as a Bio-Stimulant on Multiple Levels
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Methane-oxidizing bacteria | MOB |
References
- Conrad, R. The global methane cycle: recent advances in understanding the microbial processes involved. Environ. Microbiol. Rep. 2009, 1, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Canadell, J.G.; Monteiro, P.M.S. Global Carbon and Other Biogeochemical Cycles and Feedback; 2021; pp. 673-779.
- Ganesan, A.; Rigby, M.; Lunt, M.F.; Parker, R.J.; Boesch, H.; Goulding, N.; Umezawa, T.; Zahn, A.; Chatterjee, A.; Prinn, R.G.; et al. Atmospheric observations show accurate reporting and little growth in India’s methane emissions. Nature Communications 2017, 8, 836. [Google Scholar] [CrossRef] [PubMed]
- Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiol. Mol. Biol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef]
- Gilbert, B.; Frenzel, P. Rice roots and CH4 oxidation: the activity of bacteria, their distribution and the microenvironment. Soil Biol. Biochem. 1998, 30, 1903–1916. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Andreote, F.D.; Reinhold-Hurek, B.; Sessitsch, A.; van Overbeek, L.S.; van Elsas, J.D. Rice root-associated bacteria: insights into community structures across 10 cultivars. FEMS Microbiology Ecology 2011, 77, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ma, K.; Lu, Y. Rice roots select for type I methanotrophs in rice field soil. Systematic and applied microbiology 2009, 32, 421–428. [Google Scholar] [CrossRef]
- Ferrando, L.; Tarlera, S. Activity and diversity of methanotrophs in the soil-water interface and rhizospheric soil from a flooded temperate rice field. Journal of applied microbiology 2009, 106, 306–316. [Google Scholar] [CrossRef]
- Rahalkar, M.; Khatri, K.; Pandit, P.S.; Bahulikar, R.; Mohite, J.A. Cultivation of Important Methanotrophs From Indian Rice Fields. Front. Microbiol. 2021, 1–15. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Paleontologica Electronica 2001, 4, 9. [Google Scholar]
- Scagliola M; Valentinuzzi F; Mimmo T; Cesco S; C, C.; Y, P. Bioinoculants as Promising Complement of Chemical Fertilizers for a More Sustainable Agricultural Practice. Front. Sustain. Food Syst. 2021, 4, 622169. [Google Scholar] [CrossRef]
- Yuan, J.; Yuan, Y.; Zhu, Y.; Cao, L. Effects of different fertilizers on methane emissions and methanogenic community structures in paddy rhizosphere soil. Science of The Total Environment 2018, 627, 770–781. [Google Scholar] [CrossRef]
- The Fertilizer Association of India, N.D. FAI Annual Seminar – 2019 on New Approach to Fertilizer Sector. 2019.
- Tallapragada, P.; Seshagiri, S. Application of Bioinoculants for Sustainable Agriculture. In Probiotics and Plant Health, Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 473–495.
- Thomas, L.; Singh, I. Microbial Biofertilizers: Types and Applications. In Biofertilizers for Sustainable Agriculture and Environment Giri, B., Prasad, R., Wu, Q., Varma, A., Eds.; Soil Biology; Springer: 2019; Volume 55, pp. 1-19.
- Orozco-Mosqueda, M.D.C.; Rocha-Granados, M.D.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef]
- Pandit, P.S.; Rahalkar, M.; Dhakephalkar, P.; Ranade, D.R.; Pore, S.; Arora, P.; Kapse, N. Deciphering community structure of methanotrophs dwelling in rice rhizospheres of an Indian rice field using cultivation and cultivation independent approaches. Microb. Ecol. 2016, 71, 634–644. [Google Scholar] [CrossRef]
- Pandit, P.S.; Rahalkar, M.C. Renaming of ‘Candidatus Methylocucumis oryzae’ as Methylocucumis oryzae gen. nov., sp. nov., a novel Type I methanotroph isolated from India. Antonie van Leeuwenhoek 2019, 112, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Pandit, P.S.; Ranade, D.R.; Dhakephalkar, P.K.; Rahalkar, M.C. A pmoA-based study reveals dominance of yet uncultured Type I methanotrophs in rhizospheres of an organically fertilized rice field in India. 3 Biotech 2016, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Rahalkar, M.C.; Khatri, K.; Mohite, J.; Pandit, P.; Bahulikar, R. A novel Type I methanotroph Methylolobus aquaticus gen. nov. sp. nov. isolated from a tropical wetland. Antonie van Leeuwenhoek 2020, 113, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Rahalkar, M.C.; Khatri, K.; Pandit, P.; Mohite, J. Polyphasic Characterization of Ca. Methylomicrobium oryzae: A Methanotroph Isolated from Rice Fields. Ind. J. Microbiol. 2024, 1–9. [Google Scholar] [CrossRef]
- Rahalkar, M.C.; Khatri, K.; Pandit, P.S.; Dhakephalkar, P.K. A putative novel Methylobacter member (KRF1) from the globally important Methylobacter clade 2: cultivation and salient draft genome features. Antonie van Leeuwenhoek 2019, 112, 1399–1408. [Google Scholar] [CrossRef]
- Rahalkar, M.C.; Pandit, P. Genome-based insights into a putative novel Methylomonas species (strain Kb3), isolated from an Indian rice field. Gene Rep. 2018, 13, 9–13. [Google Scholar] [CrossRef]
- Mohite, J.; Khatri, K.; Pardhi, K.; Manvi, S.S.; Jadhav, R.; Rathod, S.; Rahalkar, M.C. Exploring the potential of methanotrophs for plant growth promotion in rice agriculture. Methane 2023, 2, 361–371. [Google Scholar] [CrossRef]
- Auman, A.J.; Speake, C.C.; Lidstrom, M.E. nifH Sequences and Nitrogen Fixation in Type I and Type II Methanotrophs. Appl. Environ. Microbiol. 2001, 67, 4009–4016. [Google Scholar] [CrossRef]
- Yuanfeng Cai; Yan Zheng; Paul L. E. Bodelier; Conrad, R.; Jia, Z. Conventional methanotrophs are responsible for atmospheric methane oxidation in paddy soils. Nature Communications 2016, 11728. [Google Scholar] [CrossRef]
- Murrell, J.C.; Dalton, H. Nitrogen-fixation in obligate methanotrophs. Journal of General Microbiology 1983, 129, 3481–3486. [Google Scholar] [CrossRef]
- Bahulikar, R.A.; Chaluvadi, S.R.; Torres-Jerez, I.; Mosali, J.; Bennetzen, J.L.; Udvardi, M. Nitrogen Fertilization Reduces Nitrogen Fixation Activity of Diverse Diazotrophs in Switchgrass Roots. Phytobiomes 2021. [Google Scholar] [CrossRef]
- Kumar, S.R.; David, E.M.; Pavithra, G.J.; Kumar, G.S.; Subbian, E. Methane-derived microbial biostimulant reduces greenhouse gas emissions and improves rice yield. Front. Plant Sci. 2024, 15, 1432460. [Google Scholar] [CrossRef]


| Treatment | Total Plant height cm |
No. of Tillers | No. of Panicles | Panicle height cm |
Weight of 1000 grains g | Average grain weight per hill g | Yield quintal per ha | Increased in yield |
|---|---|---|---|---|---|---|---|---|
| Kb3 | 106.8+/-4.6 | 19+/-3 | 18+/-3 | 22.8+/-1.5 | 20.01 | 38.1 | 61.06 | 17% |
| KRF4 | 96.0+/-6 | 17+/-3 | 15+/-1 | 23.3+/-2.2 | 22.71 | 31.6 | 50.5 | Little decrease (-3%) |
| Kb3 + KRF4 (MOB mix) | 101.8+/-6.4 | 18+/-3 | 16+/-3 | 24.1+/-1.2 | 21.59 | 37.5 | 59.99 | 15% |
| Control | 94.8+/-6.4 | 19+/-3 | 17+/-3 | 23.3+/-1.3 | 20.68 | 32.6 | 52.13 | Control yield |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





