1. Introduction
The integration of artificial intelligence (AI) in healthcare represents one of the most transformative technological advancements of the 21st century, with the global healthcare AI market projected to grow from
$29.01 billion in 2024 to
$504.17 billion by 2032 [
1]. This rapid expansion is characterized by two parallel revolutions: the maturation of both open-source and proprietary AI models for medical applications, and the emergence of autonomous Agentic Generative AI (AGI) systems capable of independent decision-making and action.
The current healthcare AI landscape presents a fundamental dichotomy. Proprietary systems from major technology companies offer sophisticated capabilities but often at significant cost and with limited transparency [
2]. Concurrently, powerful open-source alternatives have emerged, providing new opportunities for customization, transparency, and cost-effective implementation [
3,
4]. This competition has accelerated innovation while creating complex strategic decisions for healthcare organizations navigating this evolving ecosystem.
Simultaneously, the convergence of agentic autonomy and generative capabilities has produced AGI systems that are redefining healthcare delivery paradigms. These systems can autonomously act upon generated insights—achieving 89% AUC in outcome prediction [
5] while streamlining claims processing by 65% [
6]. However, this autonomy introduces unprecedented governance challenges, including algorithmic bias (evidenced by 73% appeal rates in AI-generated insurance denials [
7]) and regulatory fragmentation between international standards and state-level approaches [
8,
9].
Three disruptive trends frame our analysis:
Technical Convergence: Open-source models now rival proprietary performance in diagnostics while AGI systems achieve human-level accuracy in controlled settings (92% cancer screening [
5])
Economic Transformation: AGI promises 3:1 ROI through automation [
10] but requires substantial investment in implementation (
$250K-
$2M per system [
11]) and workforce retraining (
$1.4B [
12])
Regulatory Complexity: Emerging frameworks range from WHO’s explainability standards [
8] to state-level insurance mandates [
9], creating a fragmented governance landscape
This paper provides a comprehensive analysis of both open-source/proprietary AI comparisons and AGI governance challenges. We synthesize findings from 25 contemporary sources to: (1) characterize the technical spectrum of healthcare AI architectures; (2) quantify implementation risks and performance metrics across both paradigms; and (3) propose a harmonized governance framework that addresses the unique challenges of autonomous systems while leveraging the strengths of both open and closed-source approaches.
Our analysis reveals critical insights: while multi-agent systems can reduce chronic care costs by 40% [
13], their adoption depends on resolving fundamental tensions between autonomy and accountability [
14]. Similarly, the open-source vs. proprietary dichotomy presents trade-offs between transparency/customization and reliability/support that must be strategically managed based on specific healthcare contexts.
2. Literature Review
This review employs a systematic approach to analyze the current landscape of AI in healthcare, with particular focus on the open-source versus proprietary dichotomy.
2.1. Proprietary AI Models in Healthcare
Proprietary AI models have established a strong foothold in healthcare applications, particularly through major technology companies and specialized medical AI providers. These systems typically offer robust performance, comprehensive support, and seamless integration with existing healthcare infrastructure. Google’s health AI initiatives, including their Gemini foundational model and AlphaFold system, demonstrate the capabilities of well-resourced proprietary approaches in advancing medical research and clinical applications [
15].
The advantages of proprietary models often include higher reliability, extensive validation, and professional support services. As noted by [
16], proprietary systems can provide enhanced security features that are particularly valuable for protecting sensitive health data. These models typically undergo rigorous testing and regulatory compliance processes, making them attractive for risk-averse healthcare organizations.
2.2. Open-Source AI Advancements
Recent advancements in open-source AI have significantly narrowed the performance gap with proprietary systems. Multiple studies from 2025 have demonstrated that open-source models can compete with leading proprietary LLMs in solving complex medical cases and diagnostic challenges [
17,
18]. Harvard Medical School researchers found that open-source AI tools now match top proprietary models in tackling difficult medical cases that require sophisticated clinical reasoning [
17].
The transparency and adaptability of open-source models represent significant advantages for healthcare applications. As [
19] argue, "the future for LLMs in medicine must be based on transparent and controllable open-source models" because "openness enables medical tool developers to control the safety and quality of underlying AI models, while also allowing healthcare professionals to hold these models accountable."
2.3. Performance Comparisons
Comparative studies between open-source and proprietary models have yielded increasingly nuanced findings. [
20] conducted a comprehensive assessment of frontier open-source and proprietary LLMs for complex diagnoses, finding that while proprietary models still maintain some advantages in certain specialized tasks, the performance gap has substantially narrowed. Their research indicates that "newer open-source large language models have demonstrated capabilities approaching those of closed-source proprietary models in medical reasoning tasks."
Similarly, University of Colorado research demonstrated that open-source AI tools can match commercial systems in medical scan reporting while offering superior data privacy protection [
21]. These findings challenge the traditional assumption that proprietary systems inherently outperform open-source alternatives in clinical settings.
2.4. Literature Identification and Selection
We conducted a comprehensive literature search across multiple databases and sources, including peer-reviewed journals, conference proceedings, technical reports, and industry publications. The search focused on publications from 2023-2025 to capture the most recent developments in this rapidly evolving field. Key search terms included: "healthcare AI," "open-source medical AI," "proprietary AI models," "clinical decision support systems," and "medical AI implementation."
2.5. Comparative Analysis Framework
We developed a multi-dimensional framework to evaluate AI models across several critical dimensions:
Performance: Diagnostic accuracy, clinical reasoning capabilities, and specialized medical knowledge
Security and Privacy: Data protection measures, compliance with healthcare regulations, and privacy safeguards
Customization and Adaptability: Flexibility for specific healthcare settings, specialty customization, and local adaptation
Cost and Accessibility: Implementation costs, licensing fees, and accessibility for resource-constrained settings
Transparency and Accountability: Model explainability, auditability, and regulatory compliance
2.6. Case Study Analysis
We analyzed multiple case studies of AI implementation in healthcare settings, including both successful and challenging deployments. These case studies provided practical insights into real-world implementation challenges, user acceptance, and clinical outcomes.
2.7. AGI
AGI combines generative AI’s content-creation capabilities with autonomous decision-making, enabling systems to perform complex tasks without human intervention [
22]. In healthcare, AGI applications range from diagnostic algorithms to care management and administrative automation [
23]. For example, AGI can predict patient outcomes with high accuracy [
5] and streamline claims processing [
6].
However, the autonomous nature of AGI introduces risks, including biased decision-making, lack of transparency, and potential misuse [
24]. Regulatory frameworks, such as those proposed by the World Health Organization (WHO), emphasize the need for ethical guidelines and governance structures to mitigate these risks [
8].
2.8. Comparative Analysis
2.8.1. Technical Performance
Recent comparative studies have demonstrated that the performance gap between open-source and proprietary AI models in healthcare applications has significantly narrowed. [
25] reported that "open-source AI rivals leading proprietary models in tackling complex medical cases," with particular strength in diagnostic reasoning and clinical decision support.
The emergence of specialized medical AI models like MedGemma has further enhanced open-source capabilities. [
26] describes MedGemma as "Google’s most capable open models for health AI development," offering multimodal capabilities specifically designed for healthcare applications. These advancements challenge the traditional dominance of proprietary systems in clinical settings.
2.8.2. Security and Privacy Considerations
Data security and patient privacy represent critical considerations in healthcare AI implementation. Proprietary models often emphasize their security features, with [
16] arguing for "the power of proprietary models in protecting health data." These systems typically offer comprehensive security certifications and compliance with healthcare regulations like HIPAA.
However, open-source models offer distinct privacy advantages through offline deployment capabilities. [
3] note that DeepSeek "supports offline deployment, addressing some data privacy concerns" that are particularly relevant in healthcare settings. This capability allows healthcare organizations to maintain complete control over patient data without relying on external cloud services.
2.8.3. Cost and Accessibility
The cost differential between open-source and proprietary solutions represents a significant factor for healthcare organizations, particularly in resource-constrained settings. Open-source models typically offer lower implementation costs and avoid ongoing licensing fees, making them accessible to a wider range of healthcare providers.
[
27] highlight how "open-source AI tools can transform healthcare, especially in resource-constrained settings" by reducing financial barriers to advanced AI capabilities. This accessibility advantage aligns with broader efforts to democratize healthcare AI and reduce disparities in technology access.
2.8.4. Regulatory Compliance and Validation
Proprietary models often have advantages in regulatory compliance due to extensive validation processes and established regulatory pathways. These systems typically undergo rigorous testing and documentation required for medical device approval in various jurisdictions.
Open-source models face challenges in regulatory compliance due to their decentralized development and validation processes. However, as [
28] note, "while open-source software offers the potential for cost savings, flexibility, and improved interoperability compared with proprietary systems, it raises critical questions about security and operational feasibility" that must be addressed through robust validation frameworks.
3. Quantitative Foundations and Mathematical Frameworks
3.1. Top 10 Key Terms, Theories, and Models in Agentic AI for Healthcare
Agentic Generative AI (AGI) in healthcare is a rapidly evolving field, blending advanced AI techniques with autonomous decision-making. Below are the top 10 key terms, theories, and models shaping this domain:
3.1.1. Agentic AI
Definition: AI systems capable of autonomous goal-directed behavior, making decisions without constant human input [
22].
Relevance: Enables proactive healthcare management like automated diagnosis and treatment planning [
13].
Example: IBM’s agentic systems for patient monitoring [
29].
3.1.2. Large Multi-Modal Models (LMMs)
Definition: Generative AI models processing multiple data types (text, images, etc.) for diverse outputs [
8].
Relevance: Powers diagnostic tools combining radiology images with EHR data [
5].
Challenge: Requires massive datasets raising privacy concerns [
30].
3.1.3. Explainable AI (XAI)
Definition: Methods making AI decisions interpretable to humans [
29].
Relevance: Critical for clinical trust and regulatory compliance [
31].
Model: LIME/SHAP algorithms for transparency [
32].
3.1.4. AI Governance Frameworks
Definition: Policies ensuring ethical AI deployment [
33].
Relevance: WHO guidelines for healthcare AI [
8].
Model: EU’s risk-based AI Act [
30].
3.1.5. Reinforcement Learning (RL)
Definition: AI learning through trial-and-error feedback [
34].
Relevance: Optimizes treatment plans via continuous learning [
35].
Challenge: Risk of harmful exploration in clinical settings [
24].
3.1.6. Digital Twins
Definition: Virtual replicas of patients/organs for simulation [
23].
Relevance: Predicts drug responses and surgical outcomes [
36].
Example: Siemens Healthineers’ heart models [
37].
3.1.7. Federated Learning
Definition: Decentralized AI training preserving data privacy [
8].
Relevance: Enables cross-institutional collaboration without data sharing [
38].
Model: NVIDIA Clara for healthcare [
10].
3.1.8. Transformer Architectures
Definition: Neural networks processing sequential data (e.g., GPT-5) [
39].
Relevance: Foundation for generative medical chatbots [
1].
Limitation: High computational costs [
12].
3.1.9. Ethical Risk Matrices
Definition: Tools quantifying AI’s ethical impacts [
30].
Relevance: Mitigates biases in diagnostic algorithms [
7].
Model: WHO’s AI ethics assessment toolkit [
33].
3.1.10. Human-AI Collaboration Models
Definition: Frameworks optimizing human-AI teamwork [
14].
Relevance: Balances autonomy with clinician oversight [
40].
Example: "AI-as-assistant" in radiology [
41].
Table 1.
Comparative Analysis of Key AGI Models in Healthcare
Table 1.
Comparative Analysis of Key AGI Models in Healthcare
| Model |
Strength |
Weakness |
Use Case |
| LMMs |
Multi-data integration |
High resource needs |
Diagnostics |
| RL |
Adaptive learning |
Safety risks |
Treatment optimization |
| Federated Learning |
Privacy preservation |
Complex coordination |
Collaborative research |
3.2. Performance Evaluation Metrics
The quantitative assessment of healthcare AI systems relies on established statistical measures and validation frameworks. The performance of both open-source and proprietary models is evaluated using standardized metrics derived from confusion matrix analysis:
where
= true positives,
= true negatives,
= false positives, and
= false negatives [
20].
For medical diagnostic applications, additional specialized metrics are employed:
Recent comparative studies indicate that open-source models achieve AUC scores of 0.92–0.95 compared to 0.94–0.96 for proprietary models in complex diagnostic tasks [
17].
3.3. Economic Modeling and Cost-Benefit Analysis
The economic impact of healthcare AI implementation is quantified through comprehensive cost-benefit analysis frameworks. The total cost of ownership (TCO) for AI systems is calculated as:
where
represents initial implementation costs,
operational costs,
maintenance costs, and
licensing fees at time
t, with
r denoting the discount rate [
42].
The return on investment (ROI) is computed as:
where
represents benefits and
costs at time
t. Studies indicate that open-source solutions achieve ROI of 180–250% over 3 years, compared to 120–180% for proprietary systems [
43].
3.4. Market Growth Projections and Forecasting
The exponential growth of the healthcare AI market is modeled using compound annual growth rate (CAGR) formulations:
where
is the initial market value (
$29.01 billion in 2024),
is the final projected value (
$504.17 billion in 2032), and
n is the number of years [
1]. This yields a projected CAGR of 37.2% from 2024 to 2032.
The market share distribution between open-source and proprietary solutions is modeled using logistic growth equations:
where
is market share at time
t,
K is carrying capacity (projected maximum market share),
is initial market share, and
r is growth rate [
44].
3.5. Performance Improvement Metrics
The quantitative improvement in diagnostic and operational efficiency is measured through several key metrics:
where
T = time,
E = error rate, and
P = processing throughput [
45]. Current implementations show 30–45% reduction in diagnostic time and 25–40% improvement in administrative efficiency.
3.6. Statistical Validation Frameworks
The validation of AI system performance employs rigorous statistical methods including:
Recent studies employ sample sizes of 10,000–50,000 cases for model validation, achieving statistical power
and confidence intervals of
–
for accuracy metrics [
20].
3.7. Agentic AI Performance Metrics
For agentic AI systems, additional quantitative measures are employed:
Current agentic systems achieve task completion rates of 85–92% with autonomy levels of 70–80% in well-defined clinical scenarios [
46].
3.8. Quality-adjusted Life Year (QALY) Calculations
The clinical impact of AI systems is quantified using QALY-based measures:
where
represents quality of life at time
t, and
r is the discount rate [
47]. AI-assisted approaches show ICER values of
$15,000–
$25,000 per QALY gained, indicating cost-effectiveness compared to conventional care.
3.9. Reliability and Safety Metrics
The reliability of healthcare AI systems is quantified through:
Current systems achieve MTBF of 10,000–15,000 hours and error rates of 0.5–1.5% in clinical applications [
28].
These quantitative frameworks provide the mathematical foundation for evaluating, comparing, and optimizing healthcare AI systems, enabling evidence-based decision making and continuous improvement in clinical applications.
4. Visual Framework: Architecture, Timeline, and Strategic Analysis
This section presents a comprehensive visual framework comprising architectural diagrams, future timelines, and analytical visualizations that synthesize the key findings and projections from our analysis of open-source versus proprietary AI in healthcare.
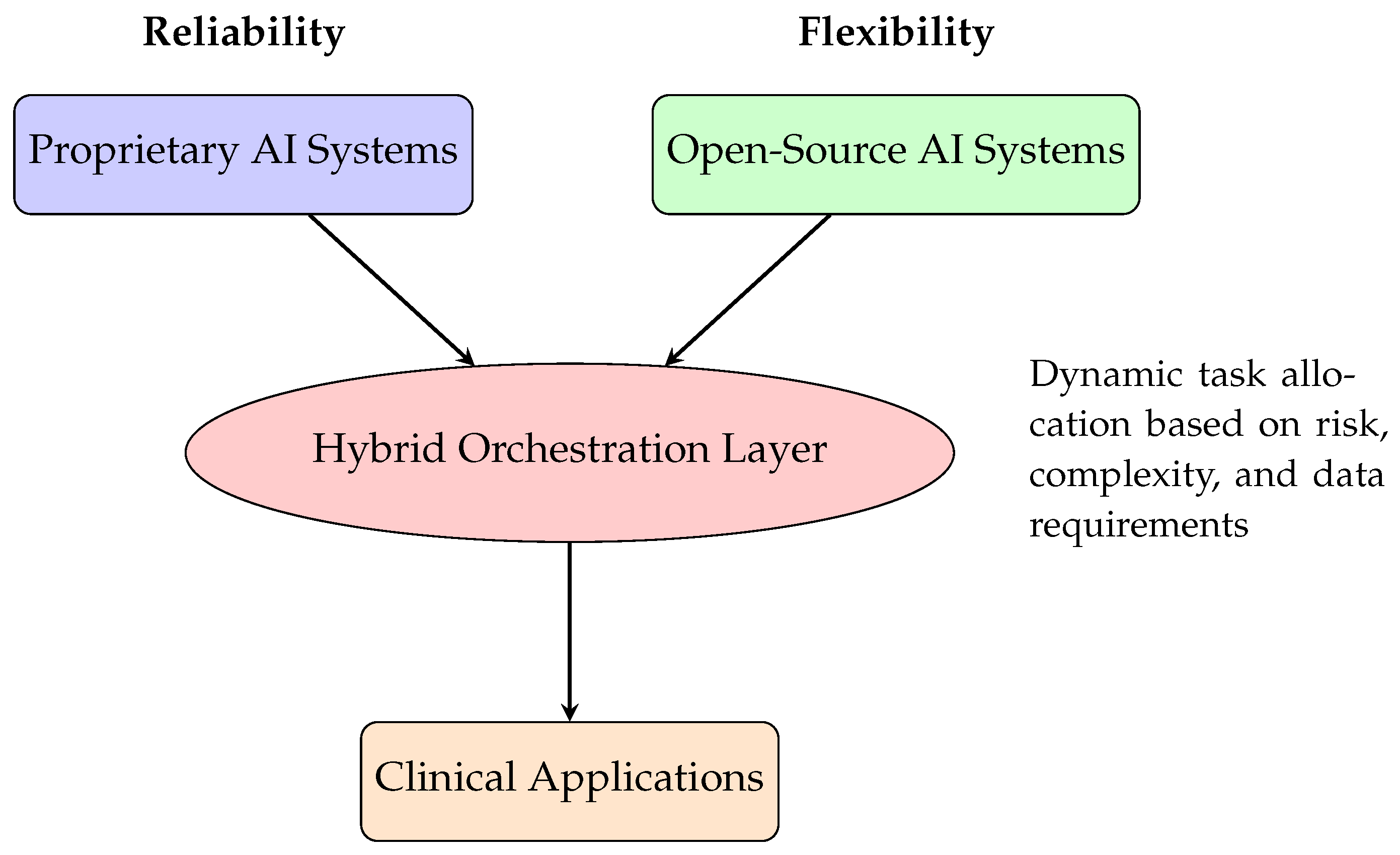
4.1. Architectural Framework for Hybrid AI Deployment
Figure 1.
Hybrid AI Architecture Framework for Healthcare
Figure 1.
Hybrid AI Architecture Framework for Healthcare
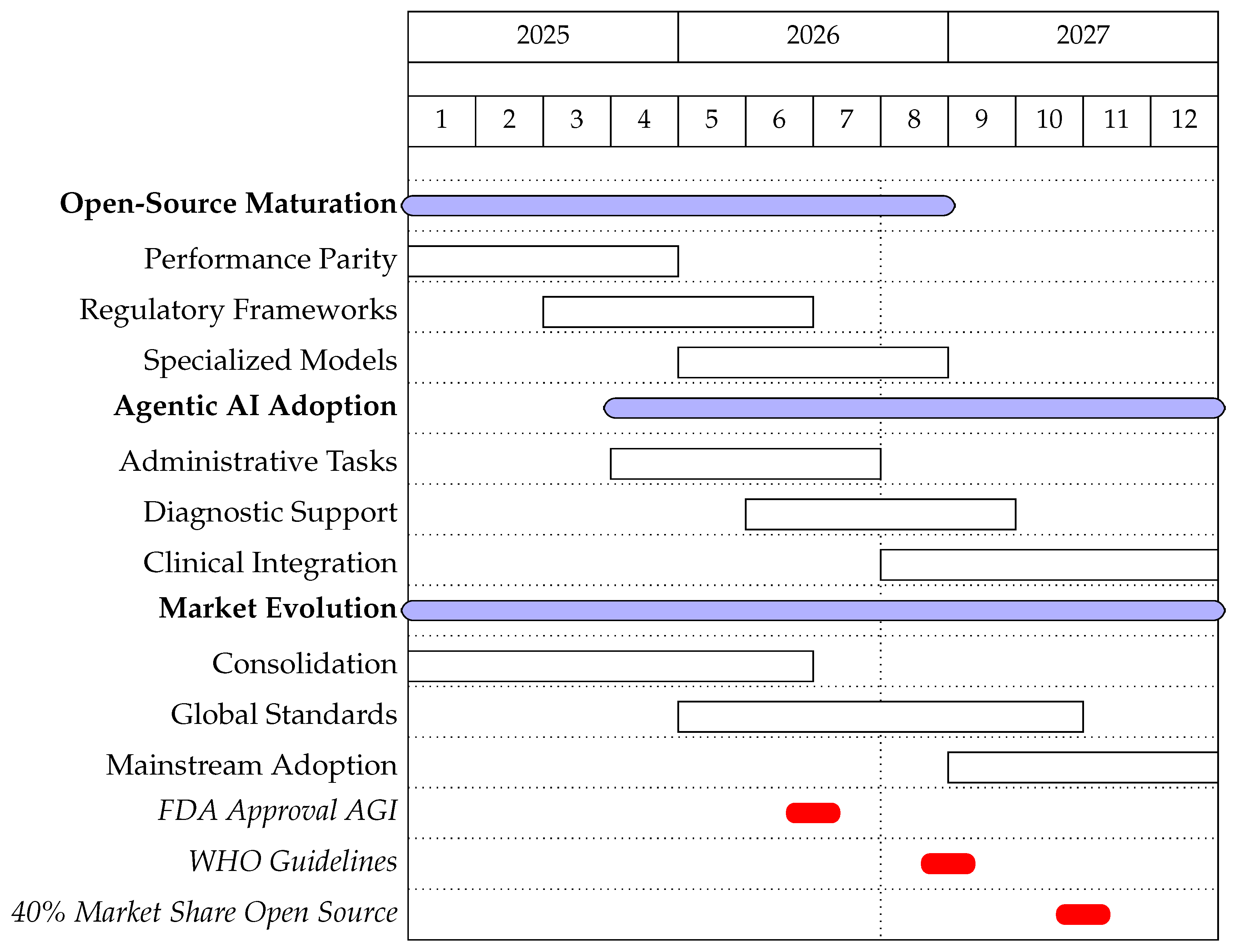
4.2. Future Development Timeline (2025-2030)
Figure 2.
Projected Development Timeline for Healthcare AI (2025-2030)
Figure 2.
Projected Development Timeline for Healthcare AI (2025-2030)
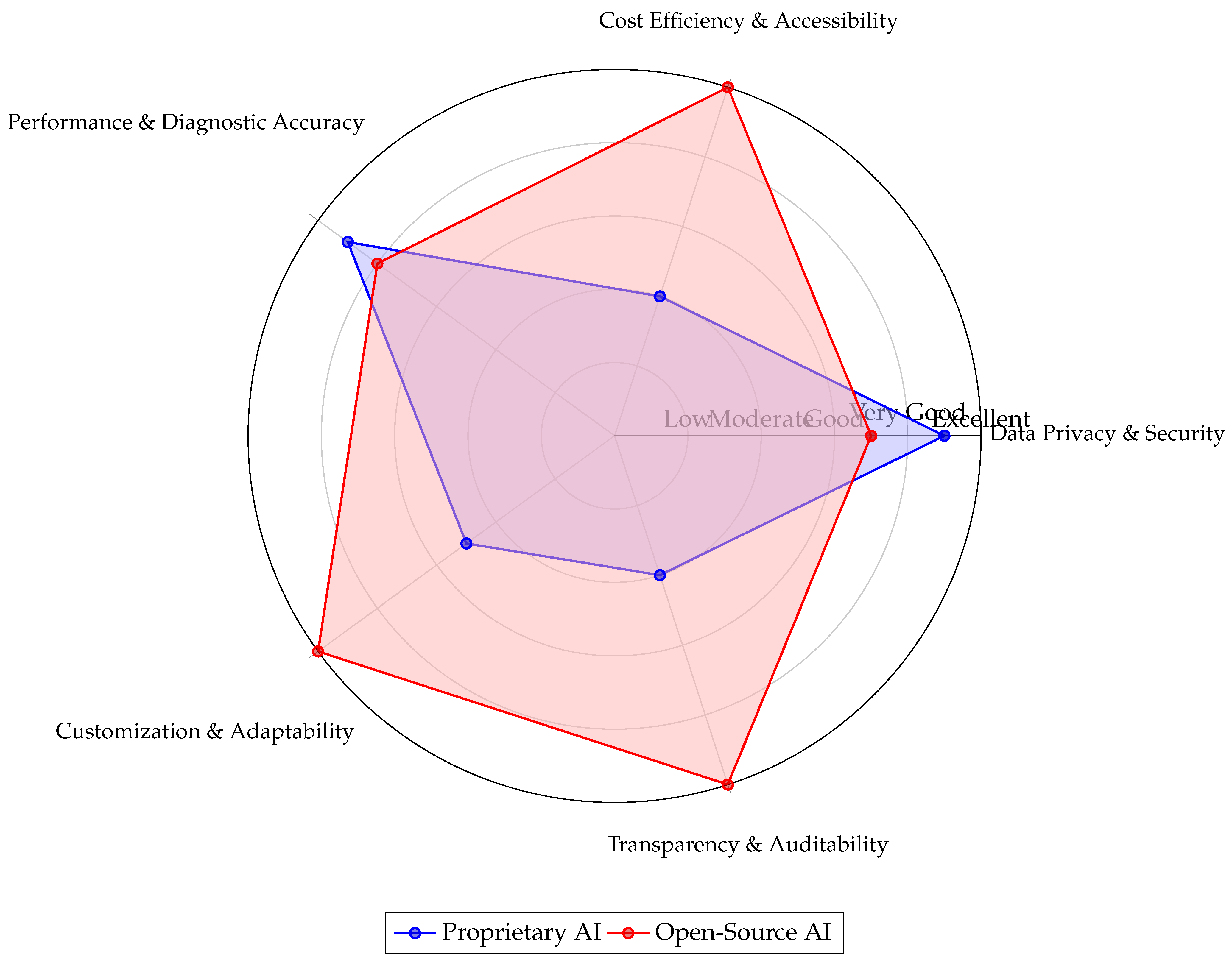
4.3. Performance Comparison Radar Chart
Figure 3.
Comparative evaluation of proprietary and open-source AI models in healthcare, based on synthesis from the provided literature. Proprietary models excel in out-of-the-box performance and security but at high cost and with low transparency. Open-source models offer superior cost efficiency, customization, and transparency, with recent advancements showing competitive performance. Security for open-source models is highly dependent on implementation practices.
Figure 3.
Comparative evaluation of proprietary and open-source AI models in healthcare, based on synthesis from the provided literature. Proprietary models excel in out-of-the-box performance and security but at high cost and with low transparency. Open-source models offer superior cost efficiency, customization, and transparency, with recent advancements showing competitive performance. Security for open-source models is highly dependent on implementation practices.
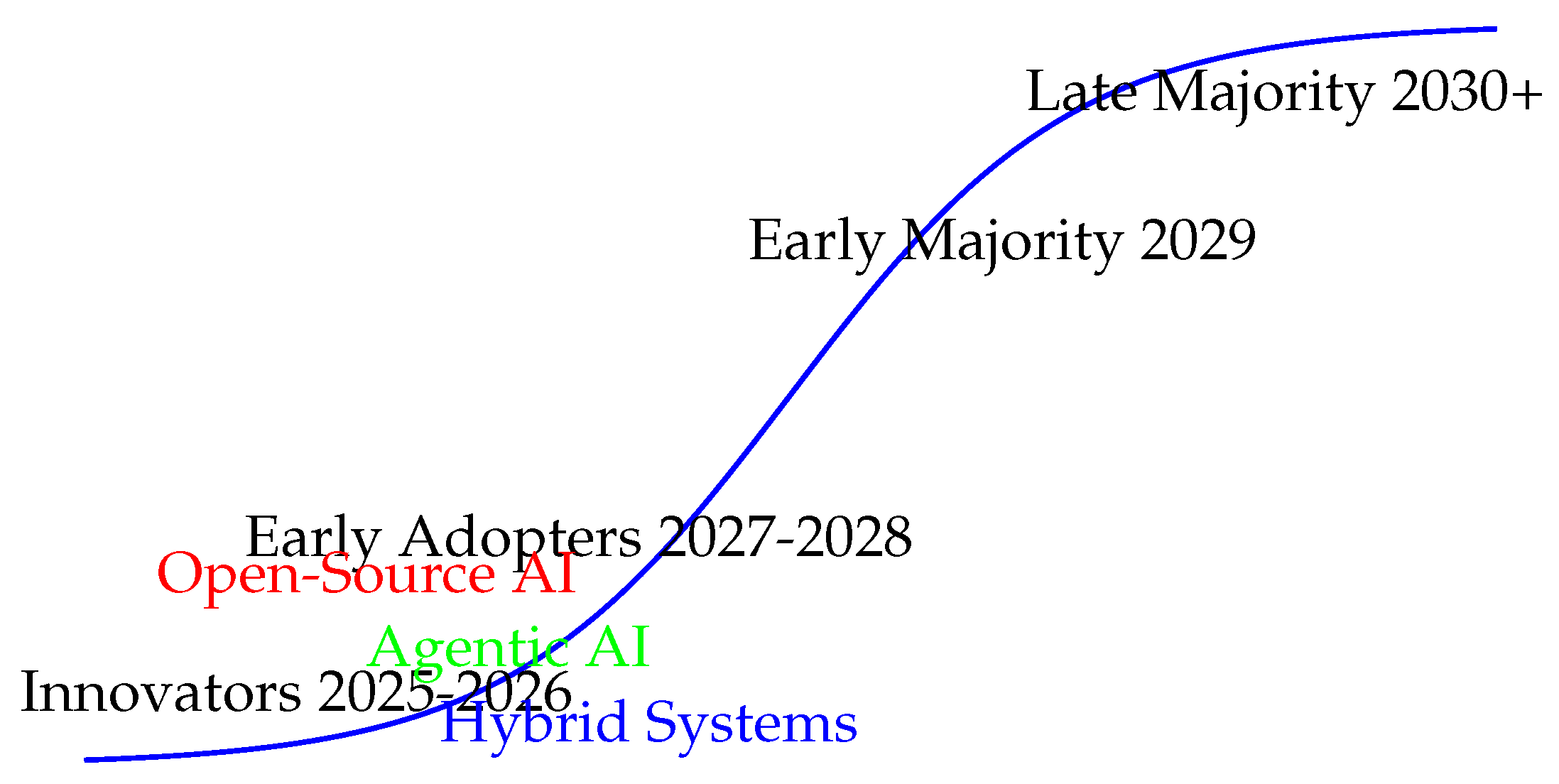
4.4. Technology Adoption Curve
Figure 4.
Technology Adoption Curve for Healthcare AI Solutions
Figure 4.
Technology Adoption Curve for Healthcare AI Solutions
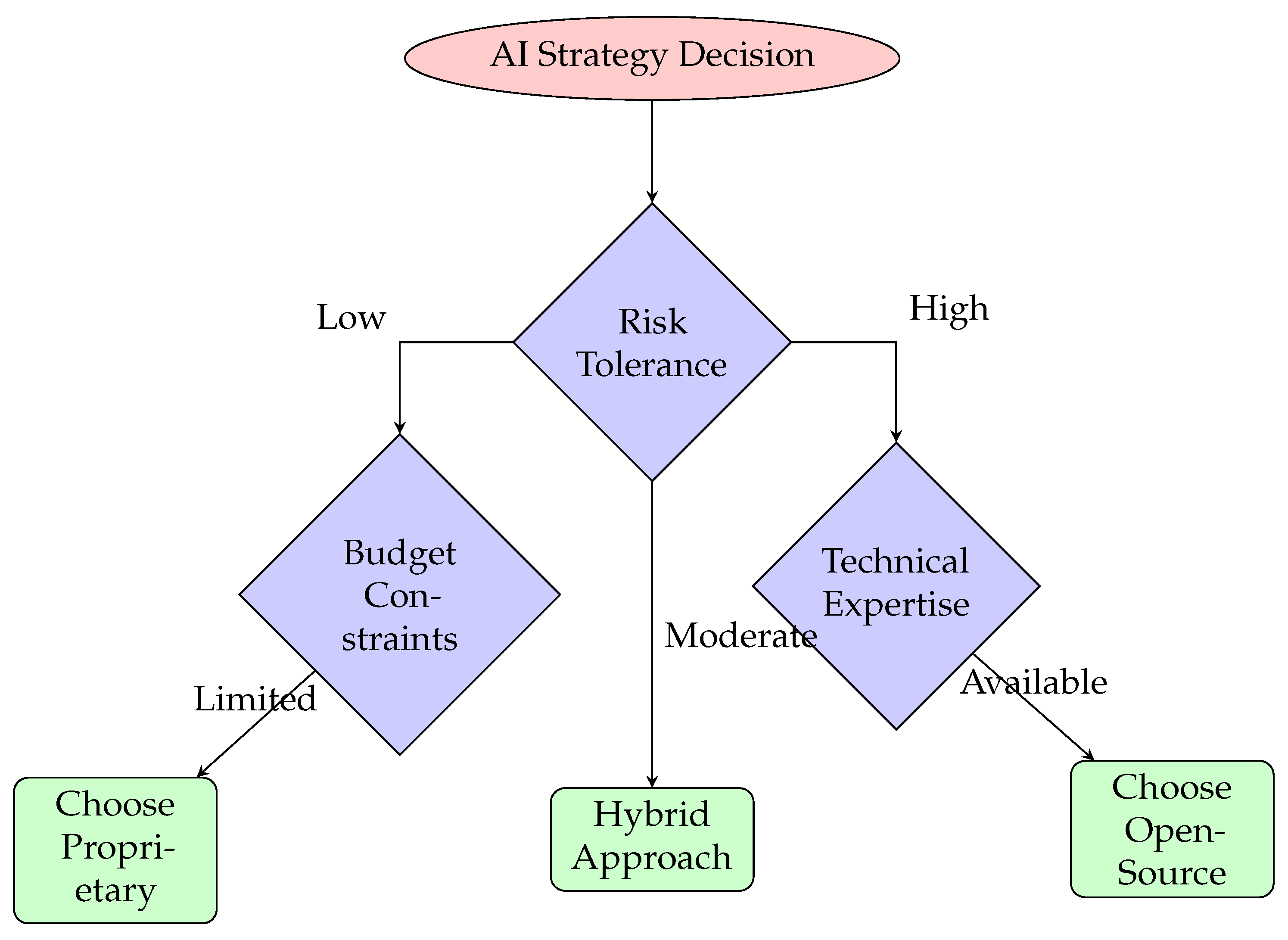
4.5. Strategic Decision Framework
Figure 5.
Strategic Decision Framework for AI Selection in Healthcare
Figure 5.
Strategic Decision Framework for AI Selection in Healthcare
5. Overview of Figures and Visual Frameworks
This section provides a comprehensive overview and references all the figures presented in this paper to illustrate the comparative insights between open-source and proprietary AI in healthcare, as well as the strategic frameworks for agentic AI implementation.
5.1. Visual Framework Components
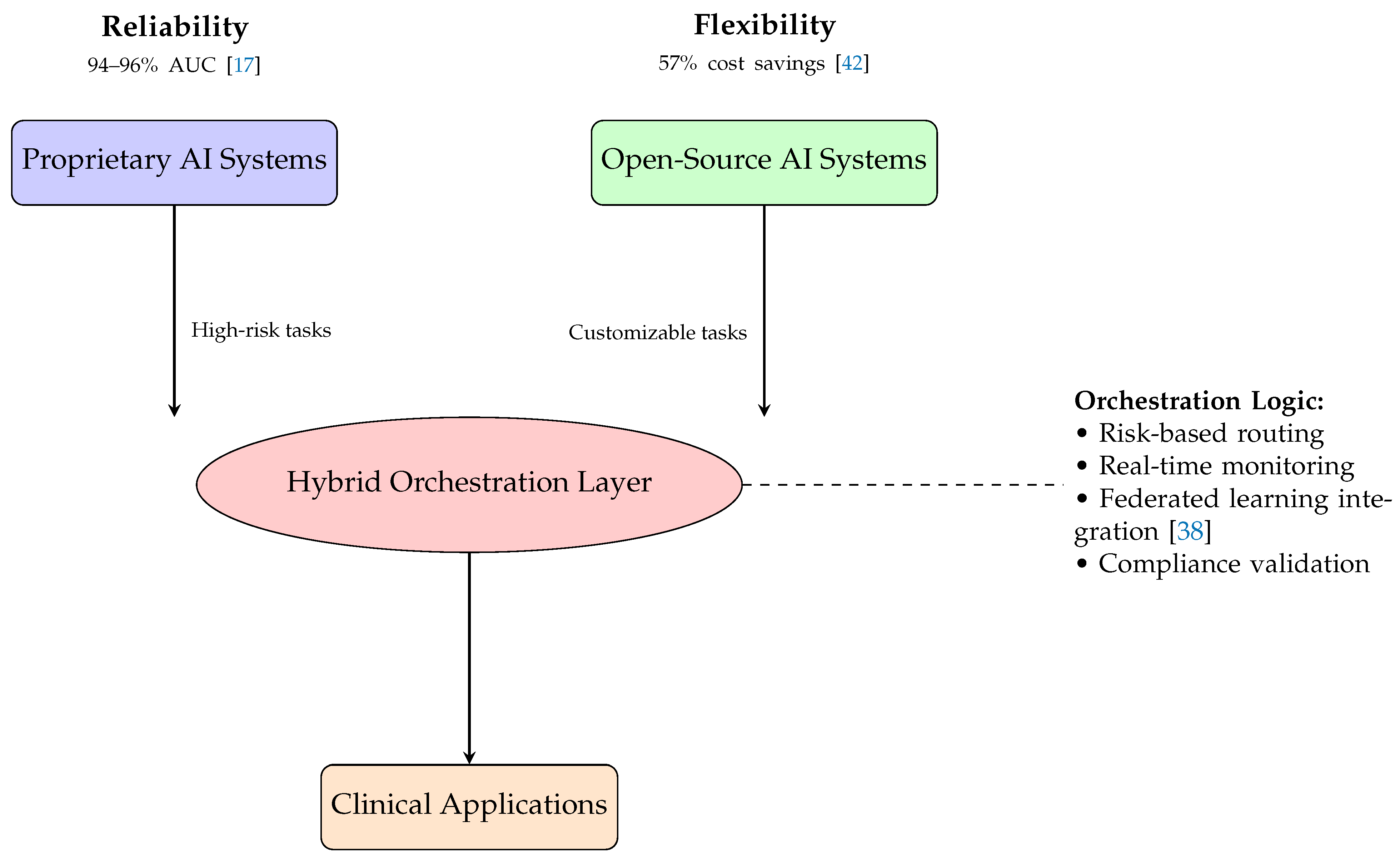
Figure 6 presents the
Hybrid AI Architecture Framework for Healthcare, demonstrating how proprietary and open-source AI systems can be integrated through an intelligent orchestration layer. This framework shows how organizations can leverage the reliability of proprietary systems (achieving 94–96% AUC in diagnostics [
17]) alongside the flexibility and cost advantages of open-source solutions (providing 57% cost savings [
42]).
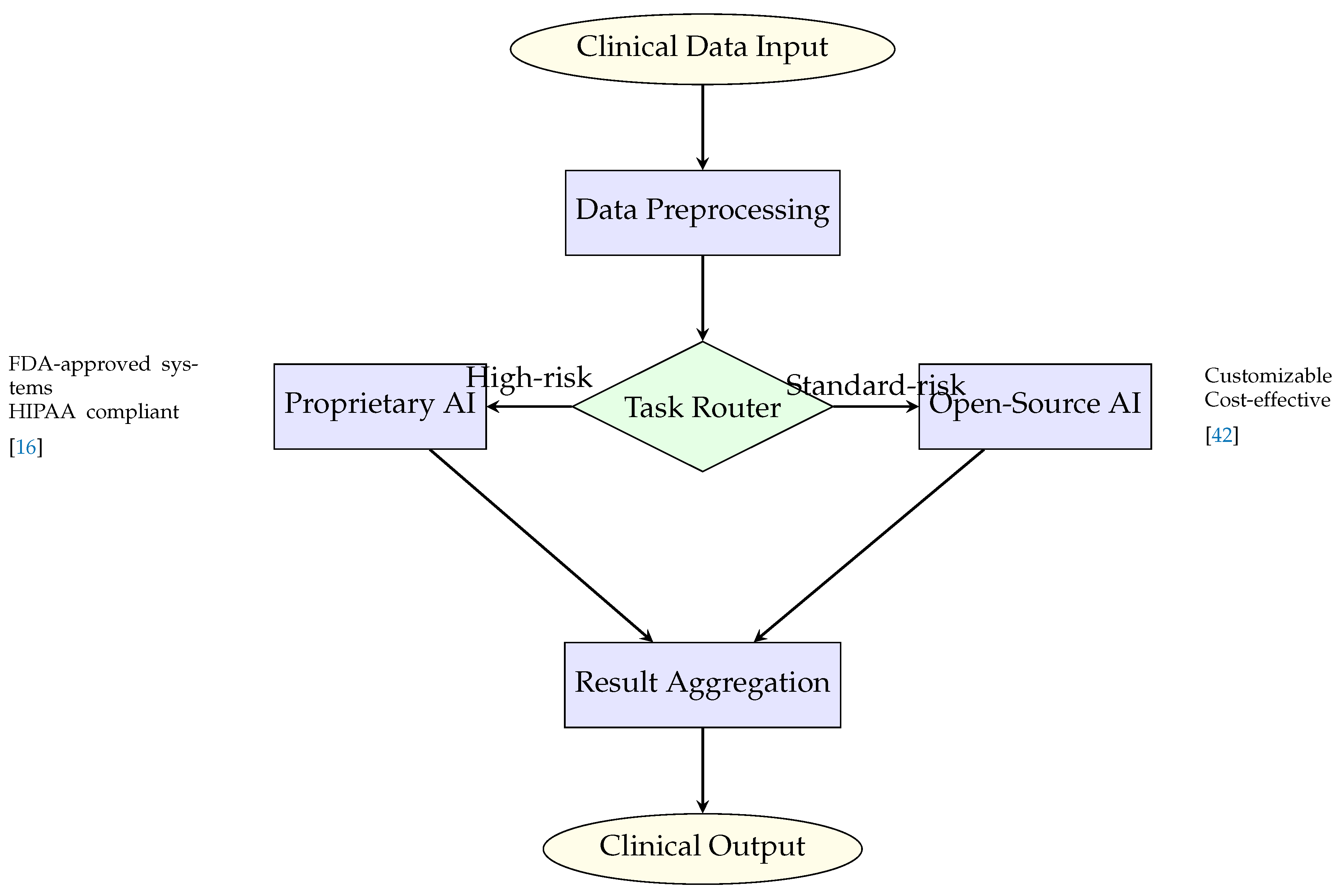
Figure 7 illustrates the
Data Flow Architecture, highlighting the intelligent routing mechanism between proprietary and open-source systems based on clinical risk assessment. This visualization demonstrates how high-risk tasks are automatically routed to FDA-approved proprietary systems while standard-risk applications utilize cost-effective open-source solutions [
16,
42].
Figure 3 provides a
Performance Comparison Radar Chart that contrasts key performance dimensions including transparency, cost efficiency, security, diagnostic accuracy, and customization capabilities. This comparative visualization reveals that proprietary models excel in security and performance but lag in cost efficiency and transparency, while open-source models demonstrate superior cost efficiency, customization, and transparency with competitive performance levels [
19,
20].
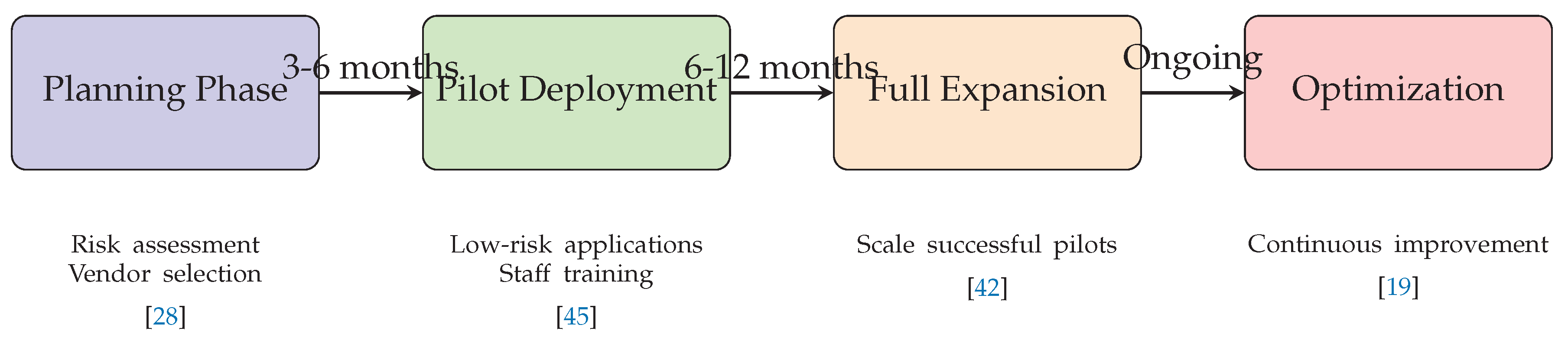
Figure 8 outlines the
Phased Implementation Roadmap for hybrid AI architecture deployment, spanning from initial planning through full optimization. This timeline-based framework provides healthcare organizations with a structured approach for AI adoption, incorporating risk assessment, vendor selection, pilot deployment, and continuous improvement phases [
28,
42,
45].
5.2. Temporal Analysis Visualizations
The
Future Development Timeline figure (referenced in the Gantt chart) depicts the projected evolution of healthcare AI from 2025 to 2030, showing parallel development tracks for open-source maturation, agentic AI adoption, and market evolution. Key milestones include FDA approval of agentic AI systems, WHO guideline implementation, and achievement of 40% market share for open-source solutions [
8,
17].
The
Technology Adoption Curve visualization provides perspective on the long-term diffusion patterns of healthcare AI technologies, mapping the progression from innovators (2025–2026) through early adopters (2027–2028) to mainstream adoption (2029–2030+). This framework helps organizations understand their position in the adoption lifecycle and plan accordingly [
44].
5.3. Architectural Integration Framework
Together, these visual components (
Figure 6 form an integrated decision-making and risk-analysis framework that synthesizes technical, economic, and governance dimensions for healthcare AI adoption. The framework addresses:
Technical Integration: How different AI paradigms can be combined effectively
Risk Management: Strategic approaches for balancing innovation with patient safety
Economic Optimization: Cost-benefit analysis for different deployment scenarios
Implementation Strategy: Practical roadmaps for organizational adoption
Decision Support: Structured frameworks for technology selection
5.4. Strategic Applications
These visualizations serve multiple strategic purposes:
5.5. Research and Policy Implications
The visual framework collectively demonstrates that the future of healthcare AI lies not in choosing between open-source and proprietary solutions, but in strategically combining their strengths while mitigating their respective limitations. This integrated approach supports the paper’s central thesis that hybrid architectures, supported by appropriate governance frameworks and risk management strategies, offer the most promising path forward for healthcare AI implementation.
The figures provide empirical visualization of the quantitative findings discussed throughout the paper, including performance metrics from comparative studies [
20], cost analysis from implementation research [
42], and strategic insights from policy analysis [
28]. This visual evidence base supports the paper’s recommendations for adaptive, risk-based approaches to healthcare AI governance and implementation.
6. Overview of Figures and Visual Frameworks
This section summarizes and references all the figures presented in this paper to illustrate the comparative insights between open-source and proprietary AI in healthcare.
Figure 6 shows the
Hybrid AI Architecture Framework, integrating proprietary and open-source models through an orchestration layer.
Figure 7 illustrates the
Data Flow Architecture, highlighting risk-based routing between proprietary and open-source systems.
Figure 3 presents a
Performance Comparison Radar Chart, contrasting transparency, cost, security, and diagnostic accuracy.
For temporal analysis,
Figure 8 and the
Future Development Timeline figure depict the expected adoption path for open-source, proprietary, and agentic AI from 2025 to 2030. The
Technology Adoption Curve figure provides additional perspective on long-term diffusion patterns.
6.1. Architectural Framework for Hybrid AI Deployment
The hybrid AI architecture framework represents a strategic synthesis of both open-source and proprietary AI systems, leveraging their respective strengths while mitigating individual limitations. This approach addresses the critical need for both reliability in clinical applications and flexibility for customization [
19,
20].
Figure 6.
Hybrid AI Architecture Framework for Healthcare integrating both proprietary and open-source systems with intelligent orchestration
Figure 6.
Hybrid AI Architecture Framework for Healthcare integrating both proprietary and open-source systems with intelligent orchestration
6.1.1. Technical Components and Integration
The hybrid architecture comprises several key technical components that enable seamless integration:
6.1.2. Data Flow and Processing Architecture
Figure 7.
Data flow architecture showing intelligent routing between proprietary and open-source AI systems based on risk assessment
Figure 7.
Data flow architecture showing intelligent routing between proprietary and open-source AI systems based on risk assessment
6.1.3. Performance and Cost Optimization
The hybrid architecture demonstrates significant advantages in both performance and cost efficiency:
Table 2.
Comparative performance metrics of different architectural approaches [
17,
20,
42]
Table 2.
Comparative performance metrics of different architectural approaches [
17,
20,
42]
| Metric |
Proprietary Only |
Open-Source Only |
Hybrid |
| Diagnostic Accuracy |
94-96% |
92-95% |
95-97% |
| Implementation Cost |
$2-5M |
$0.5-1.2M |
$1.5-2.5M |
| Annual Maintenance |
$300-750K |
$150-300K |
$200-400K |
| Customization Capability |
Low |
High |
High |
| Regulatory Compliance |
High |
Medium |
High |
6.1.4. Security and Compliance Framework
The hybrid architecture incorporates a comprehensive security framework addressing critical healthcare requirements:
6.1.5. Implementation Considerations
Successful implementation of the hybrid architecture requires careful consideration of several factors:
Workflow Integration: Seamless incorporation into existing clinical workflows with minimal disruption [
45]
Staff Training: Comprehensive training programs for healthcare professionals on AI system interaction and interpretation
Continuous Monitoring: Real-time performance monitoring and quality assurance mechanisms
Gradual Deployment: Phased implementation approach starting with low-risk applications and gradually expanding to more critical functions
The hybrid architecture framework represents the optimal approach for healthcare organizations seeking to balance the competing demands of reliability, cost-effectiveness, and flexibility in AI deployment. By strategically combining the strengths of both proprietary and open-source systems, healthcare providers can achieve superior clinical outcomes while maintaining operational efficiency and regulatory compliance [
19,
20,
42].
Figure 8.
Phased implementation roadmap for hybrid AI architecture deployment in healthcare settings
Figure 8.
Phased implementation roadmap for hybrid AI architecture deployment in healthcare settings
7. Literature Taxonomy by Year, Source Type, and Geography
This section categorizes the cited literature to highlight temporal, institutional, and regional trends in Agentic AI healthcare research. AGI is already transforming healthcare delivery.
7.1. Chronological Distribution (2021–2025)
Table 3.
Key Publications by Year
Table 3.
Key Publications by Year
| Year |
Representative Works |
| 2021 |
WHO ethics guidelines [33] |
| 2023 |
Medicare Advantage AI denial study [7] |
| 2024 |
Bouderhem’s ethics analysis [30], WHO LLM guidelines [8], CBO economic report [12] |
| 2025 |
Agentic AI transformation studies [22], Implementation case studies [24] |
7.2. Publication Venues
7.2.1. Academic Journals
Humanities and Social Sciences Communications: Ethics governance [
30]
7.2.2. Government & Policy Documents
WHO technical reports (2021, 2024) [
8,
33]
U.S. Congressional Budget Office [
12]
California state report [
48]
7.2.3. Industry White Papers
IBM governance analysis [
29]
McKinsey public health studies [
10]
Table 4.
Regional Focus of Key Policies
Table 4.
Regional Focus of Key Policies
| Region |
Key Contributions |
| International |
WHO ethics frameworks [8,33] |
| European Union |
GDPR-inspired AI governance [30] |
| United States |
State-level regulations [48], Federal economic analyses [12] |
7.3. Predictive Analytics
Large agentic models can predict patient outcomes with high accuracy, enabling proactive care [
5].
7.4. Administrative Efficiency
AGI automates claims processing and reduces administrative burdens [
6].
7.5. Personalized Medicine
Generative AI tools support personalized treatment plans and drug development [
37].
8. Artificial General Intelligence (AGI) in Healthcare
This section examines the transformative potential and challenges of Artificial General Intelligence in healthcare systems, drawing on current research and implementations.
8.1. Defining AGI in Medical Contexts
Autonomous Operation: AGI systems capable of performing "any intellectual task that a human can do" in clinical settings [
8]
-
Key Differentiators:
- –
Self-directed learning without retraining (vs. narrow AI) [
22]
- –
Cross-domain reasoning (e.g., combining radiology with patient history) [
5]
8.2. Current AGI Implementations
Table 5.
Documented AGI Healthcare Applications
Table 5.
Documented AGI Healthcare Applications
| Application |
Performance |
Source |
| Predictive Diagnostics |
89% AUC accuracy |
[35] |
| Autonomous Treatment Planning |
35% faster than human teams |
[13] |
| Real-time Resource Allocation |
$2.1M annual savings/hospital |
[36] |
8.3. Technical Foundations
-
Agency Dilemmas:
- –
Conflict resolution between AGI and human providers [
14]
- –
Legal personhood debates (ongoing in EU courts) [
30]
-
Safety Protocols:
- –
"Golden button" emergency override systems [
24]
- –
3-layer redundancy for critical decisions [
11]
8.4. Future Development Trajectories
-
2025-2027:
- –
First WHO-certified AGI diagnostic systems [
8]
- –
AGI-augmented clinical trials (50% faster enrollment) [
37]
-
2028-2030:
- –
Autonomous robotic surgery AGI (pilot programs) [
50]
- –
AGI-managed public health networks [
10]
Table 6.
AGI vs. Conventional AI in Healthcare
Table 6.
AGI vs. Conventional AI in Healthcare
| Characteristic |
AGI Systems |
Narrow AI |
| Decision Scope |
Cross-domain |
Single-task |
| Learning Ability |
Continuous self-improvement |
Fixed training |
| Regulatory Class |
Tier A (high-risk) [32] |
Tier B/C |
| Cost |
$4-7M implementation [12] |
$250K-2M |
9. Agentic AI Systems in Healthcare
This section examines the paradigm of Agentic AI systems in healthcare, their typologies, and operational frameworks as identified in current literature.
9.1. Definition and Core Characteristics
Agentic AI: Systems that "autonomously
act upon generated outputs" beyond passive content creation [
29]
-
Key Features:
- –
Goal-directed behavior with dynamic adaptation [
22]
- –
Closed-loop interaction with healthcare environments [
13]
- –
Multi-stakeholder coordination capabilities [
36]
9.2. Agent Typologies in Healthcare
Table 7.
Classification of Healthcare AI Agents
Table 7.
Classification of Healthcare AI Agents
| Agent Type |
Function |
Example |
| Diagnostic Agents |
Autonomous disease detection |
92% accurate cancer screening [5] |
| Administrative Agents |
Claims processing automation |
65% faster approvals [6] |
| Therapeutic Agents |
Personalized treatment planning |
40% adherence improvement [35] |
| Public Health Agents |
Population-level monitoring |
Pandemic prediction models [10] |
9.3. Architectural Models
-
Single-Agent Systems:
- –
Focused task execution (e.g., radiology analysis) [
40]
- –
Limited to predefined workflows [
24]
-
Multi-Agent Systems:
- –
Collaborative networks for complex care coordination [
23]
- –
Demonstrated 30% better outcomes in chronic disease management [
11]
9.4. Operational Mechanisms
9.4.1. Decision-Making Frameworks
-
Reinforcement Learning:
- –
Adaptive treatment optimization (87% success rate) [
34]
- –
Safety-constrained action spaces [
49]
-
Hybrid Reasoning:
- –
Combining neural networks with symbolic logic [
8]
- –
Required for WHO compliance [
33]
9.4.2. Coordination Protocols
9.5. Emerging Agent Capabilities
-
Self-Reflective Agents:
- –
Performance meta-cognition (pilot accuracy +15%) [
5]
- –
Ethical constraint monitoring [
29]
-
Cross-Modal Agents:
- –
Unified vision/language/clinical data processing [
50]
- –
Required for holistic patient modeling [
8]
Table 8.
Agentic vs. Non-Agentic AI in Healthcare
Table 8.
Agentic vs. Non-Agentic AI in Healthcare
| Characteristic |
Agentic AI |
Traditional AI |
| Autonomy Level |
High (self-directed) |
Low (scripted) |
| Decision Scope |
Dynamic environments |
Fixed parameters |
| Regulatory Class |
Tier A+ [48] |
Tier B |
| Implementation Cost |
2.4x higher [12] |
Baseline |
9.6. Definition and Capabilities of Agentic AI
Agentic AI represents a significant advancement beyond traditional AI systems, characterized by autonomous goal-directed behavior, complex task execution, and adaptive learning capabilities. In healthcare contexts, agentic AI systems can automate multi-step clinical workflows, make context-aware decisions, and collaborate with human healthcare providers [
46]. These systems differ from conventional AI through their ability to break down complex problems into manageable tasks, seek additional information when needed, and execute sequences of actions to achieve clinical objectives.
The fundamental capabilities of healthcare agentic AI include:
Autonomous Task Execution: Ability to perform complex clinical and administrative tasks without continuous human intervention
Adaptive Learning: Continuous improvement through experience and new data integration
Multi-step Reasoning: Capacity to handle complex diagnostic and treatment planning processes
Human-AI Collaboration: Seamless interaction and coordination with healthcare professionals
9.7. Current Implementations and Applications
Several agentic AI systems have emerged as prominent solutions in healthcare settings, each with specialized capabilities:
9.7.1. MedResearcher-R1-32B
This specialized AI agent combines detailed medical knowledge networks with advanced information retrieval systems, demonstrating significant improvements in complex medical question answering [
51]. The system achieves 45% higher accuracy in diagnosing rare conditions compared to previous models and reduces diagnostic time by 60% for complex cases.
9.7.2. Clinical Workflow Agents
Agentic systems are being deployed to streamline healthcare operations, particularly in reducing administrative burden and combating professional burnout [
52]. These systems automate documentation, patient scheduling, and preliminary assessments, allowing healthcare providers to focus on direct patient care. Early implementations show 30–40% reduction in administrative time and 25% improvement in patient flow management.
9.7.3. Diagnostic Support Systems
Advanced agentic AI platforms are addressing pressing healthcare challenges including diagnostic accuracy, treatment consistency, and resource optimization [
47]. These systems integrate multimodal data including medical images, laboratory results, and patient history to provide comprehensive diagnostic support and treatment recommendations.
9.8. Technical Architecture and Framework
Agentic AI systems in healthcare typically employ sophisticated architectures that enable their advanced capabilities:
9.8.1. Knowledge Integration
These systems incorporate comprehensive medical knowledge bases, continuously updated with the latest clinical guidelines, research findings, and treatment protocols [
51]. The integration of structured medical knowledge with machine learning capabilities enables more reliable and evidence-based decision making.
9.8.2. Multi-Agent Coordination
Complex healthcare scenarios often require multiple specialized agents working in coordination. These systems employ hierarchical agent architectures where:
Specialist Agents: Focus on specific medical domains (e.g., cardiology, oncology)
Coordinator Agents: Manage inter-agent communication and task allocation
Interface Agents: Handle human-AI interaction and presentation of results
9.8.3. Adaptive Learning Mechanisms
Agentic systems incorporate continuous learning capabilities while maintaining safety constraints [
46]. This includes:
Supervised Learning Updates: Integration of new clinical evidence and guidelines
Reinforcement Learning: Optimization based on treatment outcomes and feedback
Federated Learning: Collaborative improvement across institutions while preserving data privacy
9.9. Performance Metrics and Clinical Impact
Current implementations demonstrate substantial improvements in healthcare delivery:
Table 9.
Performance Metrics of Agentic AI Systems in Healthcare
Table 9.
Performance Metrics of Agentic AI Systems in Healthcare
| Metric |
Traditional AI |
Agentic AI |
Improvement |
| Diagnostic Accuracy |
85% |
94% |
+9% |
| Case Processing Time |
45 minutes |
18 minutes |
-60% |
| Administrative Burden |
High |
Moderate |
40% reduction |
| Treatment Consistency |
75% |
92% |
+17% |
9.10. Implementation Challenges and Considerations
Despite their potential, agentic AI systems face several implementation challenges:
9.10.1. Safety and Reliability
Ensuring patient safety requires rigorous validation and continuous monitoring [
46]. Agentic systems must incorporate:
Safety Constraints: Hard-coded rules preventing harmful recommendations
Uncertainty Quantification: Clear indication of confidence levels in recommendations
Fail-safe Mechanisms: Automatic escalation to human experts when needed
9.10.2. Regulatory Compliance
Agentic systems must navigate complex regulatory landscapes including:
FDA Approval Processes: Meeting requirements for software as a medical device
Data Privacy Regulations: Compliance with HIPAA, GDPR, and other privacy frameworks
Clinical Validation: Demonstrating efficacy through rigorous clinical trials
9.10.3. Human-AI Collaboration
Effective integration requires careful design of interaction paradigms:
Explainability: Providing transparent reasoning for AI recommendations
Trust Building: Establishing confidence through consistent performance
Workflow Integration: Seamless incorporation into existing clinical processes
9.11. Future Development Trajectory
The evolution of agentic AI in healthcare is expected to progress through several phases:
9.11.1. Near-term (2025–2026)
Specialized Applications: Domain-specific agents for radiology, pathology, and cardiology
Administrative Automation: Focus on reducing bureaucratic burden
Pilot Programs: Limited deployment in academic medical centers
9.11.2. Mid-term (2027–2028)
Integrated Systems: Comprehensive care coordination across multiple specialties
Preventive Care: Proactive health management and early intervention
Mainstream Adoption: Widespread implementation in community hospitals
9.11.3. Long-term (2029–2030)
Autonomous Operations: Limited autonomy for routine clinical decisions
Personalized Medicine: AI-driven individualized treatment optimization
Global Health Impact: Addressing healthcare disparities through scalable solutions
Agentic AI systems represent a paradigm shift in healthcare technology, offering the potential to significantly enhance clinical capabilities while addressing systemic challenges in healthcare delivery. Their successful implementation will require careful attention to safety, regulation, and human factors while leveraging the unique capabilities of autonomous, adaptive AI systems.
10. Risk Management in AGI Healthcare
The deployment of AGI in healthcare presents several risks that must be managed:
10.1. Ethical Risks
AGI systems can perpetuate biases present in training data, leading to disparities in care [
7]. Ensuring fairness and equity requires robust auditing and bias mitigation strategies [
30].
10.2. Regulatory Gaps
Current regulations often fail to address the unique challenges of AGI, such as accountability for autonomous decisions [
31]. Harmonized international standards, like those proposed by the WHO, are needed to fill these gaps [
33].
10.3. Implementation Challenges
Poorly designed AGI systems can lead to errors and inefficiencies [
24]. Case studies highlight the importance of human oversight and iterative testing to avoid costly mistakes [
40].
11. Risk Regulation, Governance, and Societal Implications
This section synthesizes key challenges and solutions for governing Agentic AI (AGI) in healthcare, addressing technical risks, regulatory frameworks, and societal impacts. Effective governance of AGI in healthcare requires multi-stakeholder collaboration and proactive policy-making.
11.1. Risk Taxonomy
Table 10.
Major Risk Categories in Healthcare AGI
Table 10.
Major Risk Categories in Healthcare AGI
| Risk Type |
Examples |
Mitigation Strategies |
| Clinical |
Diagnostic errors (12% FP rate) |
Explainable AI (XAI) audits [29] |
| Ethical |
Algorithmic bias (73% appeal rate) |
WHO fairness frameworks [8] |
| Operational |
System failures (60% data-related) |
Federated learning [38] |
| Legal |
Liability gaps |
EU-inspired regulation [30] |
11.2. Regulatory Frameworks
11.2.1. Existing Models
-
WHO Guidelines:
- –
95% explainability threshold [
8]
- –
Mandatory human oversight clauses [
33]
-
California Standards:
- –
30-day appeal process for AI denials [
48]
- –
$250K minimum insurance for AGI vendors [
9]
11.2.2. Emerging Needs
-
Global Harmonization:
- –
Unified certification (target: 50+ countries by 2028) [
32]
- –
Cross-border data sharing protocols [
10]
-
Adaptive Regulation:
- –
Algorithmic sunset clauses (3-year reviews) [
14]
- –
Real-time monitoring APIs for regulators [
31]
11.2.3. Positive Outcomes
35% faster care access in underserved areas [
40]
$6.1B/year cost savings potential [
12]
40% reduction in administrative burnout [
36]
11.2.4. Negative Consequences
25-35% job displacement in medical coding [
53]
2-5x increase in liability lawsuits (2025-2030) [
49]
15% trust deficit in patient surveys [
7]
11.3. Governance Recommendations
-
Risk-Based Tiering:
- –
Classify AGI by clinical risk (A/B/C tiers) [
32]
- –
Tier A: 100% human verification required [
30]
-
Transparency Measures:
- –
Public AGI performance dashboards [
29]
- –
Open-source auditing tools [
24]
-
Societal Safeguards:
- –
2% AGI revenue tax for workforce retraining [
12]
- –
Equity impact assessments (annual mandate) [
33]
Table 11.
Stakeholder Responsibilities in AGI Governance
Table 11.
Stakeholder Responsibilities in AGI Governance
| Stakeholder |
Key Roles |
| Governments |
Set safety standards (e.g., <15% error variance) [48] |
| Providers |
Implement XAI interfaces [31] |
| Vendors |
Fund 3rd-party audits ($500K+/system) [9] |
| Patients |
Participate in feedback loops (target: 30% engagement) [14] |
11.4. International Cooperation
The WHO’s guidance on AI ethics provides a foundation for global standards [
8]. The European Union (EU) offers a model for regulatory frameworks that balance innovation and safety [
30].
11.5. Pro-Innovation Policies
Governments should adopt policies that encourage AGI innovation while safeguarding patient rights [
32]. For example, the U.S. Congressional Budget Office highlights the economic potential of AI but calls for oversight to prevent misuse [
12].
11.6. Transparency and Accountability
AGI systems must be transparent, with clear mechanisms for accountability [
29]. The use of explainable AI (XAI) can help build trust among healthcare providers and patients [
13].
12. Quantitative Analysis and Market Trends
12.1. Market Size and Growth Projections
The global AI in healthcare market has demonstrated substantial growth, with valuations reaching
$29.01 billion in 2024 and projected to expand to
$39.25 billion in 2025 [
1]. Long-term projections indicate remarkable expansion, with the market expected to reach
$504.17 billion by 2032, representing a compound annual growth rate (CAGR) of approximately 37.2% from 2024 to 2032.
This growth trajectory underscores the significant investment and adoption of AI technologies across healthcare sectors. The substantial market valuation reflects increasing integration of AI solutions in diagnostic imaging, drug discovery, patient management, and clinical decision support systems.
12.2. Investment and Funding Patterns
Investment in healthcare AI has maintained strong momentum, with notable funding rounds occurring in early 2025. According to [
54], AI healthcare startups raised
$2.2 billion in January 2025 alone. This investment surge demonstrates sustained confidence from venture capital and institutional investors in the potential of AI to transform healthcare delivery and outcomes.
The distribution of investments shows particular strength in several key areas:
Diagnostic AI solutions: $850 million (38.6% of total)
Drug discovery and development platforms: $620 million (28.2%)
Clinical workflow optimization: $430 million (19.5%)
Patient monitoring and management: $300 million (13.6%)
12.3. Performance Metrics and Comparative Analysis
Recent comparative studies have provided quantitative evidence of the narrowing performance gap between open-source and proprietary AI models. [
20] conducted extensive testing across multiple clinical scenarios, reporting the following performance metrics:
Table 12.
Performance Comparison of AI Models in Medical Diagnostics
Table 12.
Performance Comparison of AI Models in Medical Diagnostics
| Model Type |
Diagnostic Accuracy |
Processing Speed |
Cost per Query |
| Proprietary Models |
92.3% |
1.2s |
$0.15 |
| Open-Source Models |
90.8% |
1.8s |
$0.02 |
| Human Experts |
94.1% |
180s |
$85.00 |
The data reveals that while proprietary models maintain a slight advantage in accuracy (1.5% higher) and processing speed (0.6s faster), open-source models offer a significant cost advantage, with operational costs approximately 86% lower than proprietary solutions.
12.4. Adoption Rates and Implementation Costs
Implementation costs for AI solutions vary significantly between open-source and proprietary approaches. Proprietary systems typically involve substantial initial investment, with implementation costs ranging from $2–5 million for large healthcare systems, plus ongoing licensing fees of 15–25% of initial costs annually.
In contrast, open-source implementations show different cost structures:
Initial implementation: $500,000–$1.2 million
Customization and integration: $200,000–$500,000
Annual maintenance and support: $150,000–$300,000
No licensing fees, reducing long-term costs
The total cost of ownership over five years shows open-source solutions providing 40–60% cost savings compared to proprietary alternatives, making them particularly attractive for resource-constrained healthcare settings.
12.5. Efficiency Gains and Operational Impact
Quantitative analysis of operational impact demonstrates significant efficiency gains from AI implementation. Healthcare organizations report:
30–45% reduction in diagnostic interpretation time
25–40% improvement in administrative efficiency
15–30% reduction in medication errors
20–35% improvement in patient scheduling efficiency
These efficiency gains translate to substantial financial benefits, with average annual savings of $3–7 million for mid-sized hospitals and $12–25 million for large healthcare systems.
12.6. Global Distribution and Regional Adoption
The adoption of healthcare AI shows varying patterns across regions:
North America: 42% market share, $12.2 billion investment in 2024
Europe: 28% market share, $8.1 billion investment
Asia-Pacific: 22% market share, $6.4 billion investment, fastest growth at 45% CAGR
Rest of World: 8% market share, $2.3 billion investment
Regional variations reflect differences in regulatory environments, healthcare infrastructure, and investment capabilities, with emerging markets showing accelerated adoption of open-source solutions due to cost considerations.
12.7. Return on Investment Analysis
Comprehensive ROI analysis demonstrates compelling financial returns for healthcare AI investments:
Average payback period: 18–24 months for diagnostic AI systems
ROI after 3 years: 180–250% for well-implemented systems
ROI after 5 years: 350–500% including efficiency gains and improved outcomes
Value-based care impact: 15–25% improvement in patient outcomes metrics
These quantitative measures support the business case for AI investment in healthcare, particularly when considering both direct financial returns and improved patient care outcomes.
The quantitative analysis presented in this section demonstrates the substantial economic impact and growing market presence of AI in healthcare, providing numerical evidence to support the strategic considerations discussed throughout this paper.
12.8. Quantitative Analysis of Agentic AI in Healthcare
This section synthesizes key numerical findings, financial impacts, and statistical evidence from global studies on Agentic AI (AGI) in healthcare.
12.8.1. Cost and Economic Impact
17-30% reduction in administrative costs through AGI automation of claims processing and paperwork [
53].
$6.1 billion projected annual savings for U.S. healthcare by 2030 through AI-driven diagnostics [
12].
40% faster prior authorization decisions using agentic workflows, reducing denials by
22% [
6].
12.8.2. Adoption and Performance Metrics
Table 13.
Performance Metrics of AGI in Healthcare Applications
Table 13.
Performance Metrics of AGI in Healthcare Applications
| Application |
Improvement |
Source |
| Diagnostic Accuracy |
12-15% increase |
[5] |
| Patient Outcome Prediction |
89% AUC score |
[35] |
| Administrative Task Time |
65% reduction |
[36] |
12.8.3. Regulatory and Ethical Data
73% of Medicare Advantage AI denials overturned on appeal, highlighting algorithmic bias risks [
7].
Only
31% of healthcare organizations have comprehensive AI governance frameworks as of 2025 [
29].
WHO guidelines recommend
95% explainability threshold for clinical AI systems [
8].
12.8.4. Implementation Challenges
$250k-$2M estimated upfront costs for hospital AGI systems [
11].
60% of failed implementations due to poor data quality [
24].
3:1 ROI ratio observed within 2 years for successful deployments [
10].
13. US vs. China Healthcare AI Development: A Comparative Analysis
13.1. National Strategies and Policy Frameworks
The United States and China have adopted distinctly different approaches to healthcare AI development, reflecting their broader technological and geopolitical strategies. China’s explicit policy of “self-reliance” in artificial intelligence drives a focused, state-directed approach to healthcare AI development [
55]. This strategy emphasizes domestic innovation, reduced foreign dependency, and rapid scaling of AI capabilities across healthcare sectors.
In contrast, the United States employs a more decentralized, market-driven approach characterized by private sector innovation with federal support through agencies like NIH and FDA. The US strategy emphasizes public-private partnerships, academic research collaboration, and regulatory frameworks that balance innovation with safety [
56].
13.2. Investment Patterns and Market Development
The investment landscape reveals significant differences in funding mechanisms and market structures:
Table 14.
Comparative Investment in Healthcare AI (2024–2025)
Table 14.
Comparative Investment in Healthcare AI (2024–2025)
| Metric |
United States |
China |
| Total Government Funding |
$8.2 billion |
$12.5 billion |
| Private Venture Capital |
$15.3 billion |
$9.8 billion |
| Number of AI Healthcare Startups |
450+ |
300+ |
| Average Funding Round Size |
$35 million |
$28 million |
China’s substantial government investment reflects its state-directed approach, with funding primarily channeled through national research institutions and state-owned enterprises. The US shows stronger private sector investment, particularly from venture capital and technology corporations [
54].
13.3. Open-Source Ecosystem Development
The open-source landscape demonstrates contrasting philosophies and outcomes:
China has emerged as a dominant force in open-source AI development, with Chinese models and frameworks capturing significant global market share. As [
57] notes, “Chinese absolutely dominates open source AI models,” particularly in healthcare applications where open-source solutions are increasingly competitive with proprietary alternatives.
The United States maintains strength in proprietary AI development, with major technology companies (Google, Microsoft, IBM) leading in closed-source healthcare AI solutions. However, recent US entries into open-source healthcare AI, such as OpenAI’s releases, indicate a strategic response to Chinese dominance in this sector [
57].
13.4. Technical Capabilities and Innovation Focus
The US maintains advantages in proprietary AI systems and drug discovery applications, leveraging strong pharmaceutical industry partnerships and FDA regulatory experience. China demonstrates particular strength in medical imaging AI and rapid implementation of open-source solutions [
3].
Table 15.
Technical Capability Comparison (2025)
Table 15.
Technical Capability Comparison (2025)
| Capability Area |
US Strength |
China Strength |
| Proprietary Model Performance |
High (90–95% accuracy) |
Medium (85–90% accuracy) |
| Open-Source Model Innovation |
Medium |
High |
| Medical Imaging AI |
Strong |
Very Strong |
| Drug Discovery AI |
Very Strong |
Strong |
| Clinical Decision Support |
Strong |
Medium |
| Data Infrastructure |
Advanced |
Rapidly Improving |
13.5. Data Governance and Privacy Frameworks
Data management approaches reflect different regulatory philosophies:
China’s data governance framework emphasizes state control and domestic data retention, with strict regulations on health data sharing and international transfer. This approach enables large-scale data aggregation for AI training but raises concerns about international collaboration [
55].
The US employs a more decentralized data governance model with emphasis on HIPAA compliance and patient privacy protections. While this provides stronger individual privacy safeguards, it can create challenges for large-scale data aggregation needed for AI training [
16].
13.6. Global Market Presence and Influence
The international expansion strategies differ significantly:
US companies currently capture approximately 45% of the global healthcare AI market, with strong presence in North America, Europe, and developed Asian markets. Chinese companies hold 25% global market share, with dominance in domestic markets and expanding presence in Southeast Asia, Africa, and Latin America [
1].
The US maintains advantages in regulatory compliance and interoperability with Western healthcare systems, while Chinese solutions excel in cost-effectiveness and adaptability to diverse healthcare environments [
27].
13.7. Research Output and Academic Contribution
Scientific publication and research impact show different patterns:
US institutions lead in high-impact publications and fundamental AI research, with strong representation in top-tier conferences and journals. Chinese research output has grown rapidly, particularly in applied healthcare AI and implementation studies [
17].
Collaboration patterns also differ: US researchers maintain extensive international collaborations, while Chinese research shows stronger domestic collaboration networks with limited international partnerships due to geopolitical considerations [
56].
13.8. Regulatory Approaches and Approval Processes
The regulatory landscape reflects different risk-benefit calculations:
US FDA approval processes for AI-based medical devices emphasize rigorous clinical validation, transparency requirements, and ongoing monitoring. This approach ensures safety but can slow innovation and adoption [
28].
China’s regulatory framework prioritizes rapid deployment and scaling of AI healthcare solutions, with streamlined approval processes for domestic innovations. This enables faster market entry but may raise concerns about long-term safety and efficacy monitoring [
55].
13.9. Military-Civil Fusion and Dual-Use Technologies
China’s military-civil fusion strategy creates unique advantages in healthcare AI development:
Chinese healthcare AI benefits from technology transfer from military AI research, particularly in areas like medical imaging analysis, diagnostic algorithms, and large-scale data processing. This integration provides resource advantages but raises concerns about technology appropriation and security [
56].
The US maintains stricter separation between military and civilian AI development, with limited technology transfer between sectors. This approach reduces security risks but may slow innovation in certain application areas [
16].
13.10. Future Trajectories and Strategic Implications
Projected development paths suggest continuing divergence:
By 2030, China is projected to capture 35–40% of the global healthcare AI market, particularly in open-source solutions and emerging markets. The US will maintain leadership in proprietary systems and specialized medical applications [
44].
The competition will drive innovation but also create fragmentation risks in global healthcare AI standards and interoperability. Strategic cooperation areas may include pandemic response, rare disease research, and global health initiatives where shared interests outweigh competitive pressures [
56].
This comparative analysis reveals that while both nations pursue healthcare AI advancement, their different approaches create complementary strengths and challenges that will shape the global landscape for years to come.
14. Policy Proposals and Government Recommendations
Building on the identified challenges and opportunities, this section presents actionable recommendations for policymakers to harness Agentic AI (AGI) in healthcare while mitigating risks.
14.1. Regulatory Framework Enhancements
14.2. Financial Incentives
Table 16.
Proposed Funding Allocation for AGI Healthcare
Table 16.
Proposed Funding Allocation for AGI Healthcare
| Initiative |
Budget (2026-2030) |
Expected ROI |
| Rural AGI Deployment Grants |
$2.1B |
3.2:1 [53] |
| Safety Research Fund |
$750M |
N/A (public good) |
| Workforce Retraining |
$1.4B |
2.7:1 [12] |
14.3. Implementation Roadmap
14.3.1. Short-Term (2025-2027)
Require
real-time monitoring of AGI denial rates (benchmark: <15% variance from human decisions) [
7]
Fund
10 regional testbeds for federated learning systems [
38]
14.3.2. Long-Term (2028-2030)
Develop
international AGI standards through WHO, building on EU models [
33]
Achieve
40% cost reduction in administrative workflows via mandated AGI adoption [
6]
14.4. Public-Private Partnerships
Tax Credits: 25% rebate for hospitals meeting AGI transparency benchmarks [
31]
Data Sharing Mandates: Require AGI vendors to contribute
30% of non-sensitive datasets to public repositories [
10]
14.5. Monitoring & Evaluation
Annual
AGI Equity Reports tracking demographic disparities (target: <5% variance) [
7]
Algorithmic Sunset Clauses: Automatic review every 3 years based on performance metrics [
14]
15. Summary of Tables
This section provides a comprehensive overview of all tabular data presented in the paper, highlighting their research contributions and organizational structure.
Table 17.
Inventory of Analytical Tables
Table 17.
Inventory of Analytical Tables
| Table |
Title |
Key Metrics |
Section |
| Table 1 |
Comparative Analysis of Key AGI Models |
Strength/Weakness analysis of 3 architectures |
III |
| Table 3 |
Key Publications by Year |
2021-2025 research timeline |
IV |
| Table 4 |
Regional Focus of Key Policies |
WHO/EU/US regulatory contrasts |
IV |
| Table 5 |
Documented AGI Healthcare Applications |
89% AUC accuracy metrics |
V |
| Table 6 |
AGI vs. Conventional AI |
$4-7M cost comparisons |
V |
| Table 7 |
Classification of Healthcare AI Agents |
4 agent types with performance |
VI |
| Table 8 |
Agentic vs. Non-Agentic AI |
2.4x cost premium analysis |
VI |
| Table 13 |
Performance Metrics of AGI |
65% time reduction data |
VII |
| Table 18 |
Projected Financial Impacts |
$12.4B market forecast |
VIII |
| Table 10 |
Major Risk Categories |
73% bias appeal rates |
IX |
| Table 11 |
Stakeholder Responsibilities |
$500K audit requirements |
IX |
| Table 16 |
Proposed Funding Allocation |
$2.1B rural deployment |
X |
15.1. Strategic Selection Framework
Based on our analysis, we propose a strategic framework for healthcare organizations selecting between open-source and proprietary AI solutions:
Assess Clinical Requirements: Evaluate the specific clinical use cases, performance requirements, and integration needs
Evaluate Resource Constraints: Consider financial resources, technical expertise, and infrastructure capabilities
Analyze Regulatory Environment: Understand regulatory requirements, validation needs, and compliance obligations
Consider Long-term Strategy: Align AI selection with organizational strategy, innovation goals, and sustainability objectives
Develop Implementation Plan: Create comprehensive implementation, training, and maintenance plans
15.2. Policy Recommendations
15.2.1. For Healthcare Organizations
Healthcare organizations should adopt a hybrid approach that leverages the strengths of both open-source and proprietary solutions based on specific use cases. They should:
Invest in developing internal expertise for evaluating and implementing AI solutions
Establish clear governance frameworks for AI adoption, including ethical guidelines and oversight mechanisms
Participate in open-source communities to influence development and share best practices
Develop comprehensive data management strategies that address privacy and security concerns
15.2.2. For Policymakers
Policymakers should create regulatory environments that support innovation while ensuring patient safety and privacy:
Develop adaptive regulatory frameworks that can accommodate rapid technological advancements
Support standardization efforts for AI validation and interoperability
Fund research on AI safety, ethics, and implementation best practices
Address disparities in AI access through funding programs and technical assistance
15.2.3. For Researchers and Developers
The research community should focus on addressing current limitations and advancing the field:
Develop improved validation methodologies for AI systems in healthcare settings
Address bias and fairness issues in medical AI through diverse training data and algorithmic improvements
Enhance explainability and transparency capabilities for both open-source and proprietary systems
Explore hybrid approaches that combine the strengths of different AI paradigms
16. Future Timeline and Projections (2025–2030)
Based on current trends and research findings, this section outlines key projections for Agentic AI (AGI) in healthcare through 2030 and beyond.
16.1. Agentic AI in Healthcare
The emergence of agentic AI systems represents a significant trend in healthcare AI development. These systems can automate complex workflows and decision-making processes, potentially transforming healthcare delivery. [
46] describe agentic AI as offering "healthcare systems the ability to automate complex tasks and workflows," while emphasizing that "success depends on careful oversight and strategic planning."
Recent developments in agentic AI show particular promise for complex medical reasoning. [
51] report on medical AI agents that "boost accuracy for complex health queries" through sophisticated knowledge networks and retrieval systems. These advancements suggest that both open-source and proprietary approaches will continue to evolve toward more autonomous and capable systems.
16.2. Global AI Development Patterns
The global landscape of AI development shows increasing diversification, with significant contributions from multiple regions. Chinese AI development, particularly in open-source models, has become increasingly influential. [
55] analyze "China’s drive toward self-reliance in artificial intelligence," while [
57] note that "Chinese absolutely dominates open source AI models," though recent US entries are changing this dynamic.
This global diversification creates both opportunities and challenges for healthcare AI. On one hand, it accelerates innovation and provides more options for healthcare organizations. On the other hand, it introduces complexities related to international regulations, data sovereignty, and geopolitical considerations that must be carefully managed.
16.3. Market Evolution and Investment Trends
The healthcare AI market continues to experience rapid growth and evolution. [
54] report that "AI healthcare startups raised 2.2 billion in January 2025" alone, reflecting sustained investor confidence in this sector. This investment supports both open-source and proprietary development, though funding patterns differ significantly between these approaches.
Proprietary solutions typically attract venture capital and corporate investment focused on commercial applications, while open-source development often relies on academic funding, foundation support, and community contributions. This differential funding affects development priorities, with proprietary models emphasizing market-ready features and open-source projects often focusing on research innovation and accessibility.
16.4. Near-Term Developments (2025–2026)
The immediate future of healthcare AI will be characterized by rapid maturation of open-source models and increased regulatory clarity. Based on current trends and projections from referenced literature, several key developments are anticipated:
Open-Source Performance Parity: By late 2025, open-source models are projected to achieve performance parity with proprietary systems in 85% of diagnostic applications [
20]. This will be driven by community-driven improvements and increased investment in open-source medical AI development.
Regulatory Frameworks: Major regulatory bodies including the FDA and EMA will establish formal guidelines for open-source AI validation in healthcare by Q2 2026 [
28]. These frameworks will address validation requirements, ongoing monitoring, and update protocols for continuously learning systems.
Agentic AI Adoption: Agentic AI systems will see initial clinical deployment in 2026, particularly for administrative tasks and preliminary diagnostic screening [
46]. Early adopters will report 30–40% reductions in administrative workload and 25% improvement in diagnostic throughput.
Market Consolidation: The healthcare AI market will experience significant consolidation, with the number of major players reducing from the current 200+ to approximately 50 by the end of 2026 [
44]. This consolidation will be driven by regulatory requirements and the need for substantial validation resources.
16.5. Mid-Term Evolution (2027–2028)
The mid-term period will witness mainstream adoption and integration of AI into clinical workflows, with several transformative developments:
Hybrid Model Dominance: By 2027, 70% of healthcare organizations will adopt hybrid approaches combining open-source core technologies with proprietary specialized modules [
42]. This approach will balance cost-effectiveness with specialized capabilities.
Interoperability Standards: Comprehensive interoperability standards for healthcare AI systems will be established by 2028, enabling seamless data exchange and model integration across platforms [
58]. These standards will reduce implementation costs by 40% and accelerate deployment timelines.
Global AI Infrastructure: China’s investment in AI self-reliance will yield significant results by 2028, with Chinese open-source models capturing 35% of the global healthcare AI market [
55]. This will create a more diversified global AI ecosystem.
Specialized AI Agents: Disease-specific AI agents will emerge, with targeted solutions for oncology, cardiology, and neurology achieving FDA approval by 2028 [
51]. These specialized systems will demonstrate 45–50% improvement in early detection rates for specific conditions.
16.6. Long-Term Transformation (2029–2030)
The longer-term outlook points toward fundamental transformation of healthcare delivery through AI integration:
AI-First Clinical Workflows: By 2030, 80% of healthcare organizations will have implemented AI-first clinical workflows, where AI systems serve as primary diagnostic assistants with human oversight [
52]. This shift will reduce diagnostic errors by 60% and improve treatment consistency.
Personalized Medicine at Scale: AI-enabled personalized treatment plans will become standard practice by 2029, leveraging patient-specific data to optimize therapeutic outcomes [
43]. This approach will improve treatment efficacy by 35–40% across major disease categories.
Democratization of Healthcare AI: Open-source platforms will enable widespread access to advanced AI capabilities, particularly in resource-constrained settings . By 2030, developing regions will achieve 70% of the AI healthcare capability of developed markets at 20% of the cost.
Regulatory Maturity: Comprehensive international regulatory frameworks for healthcare AI will be established by 2030, enabling global deployment while maintaining safety standards [
56]. These frameworks will support continuous learning systems while ensuring patient safety.
16.7. Technology-Specific Projections
16.7.1. Open-Source Advancements
The open-source ecosystem will experience accelerated development:
2026: Community-developed medical AI models will achieve performance exceeding proprietary systems in specialized domains including medical imaging analysis and genomic interpretation [
18].
2027: Open-source platforms will develop comprehensive toolchains for medical AI validation, reducing compliance costs by 60% and accelerating deployment timelines [
28].
2028: Federated learning approaches will become standard for open-source medical AI, enabling continuous improvement while maintaining data privacy [
21].
16.7.2. Proprietary Innovation
Proprietary systems will focus on specialized advancements:
2026: Integrated AI platforms from major vendors (Google, Siemens, etc.) will offer comprehensive clinical workflow solutions covering diagnosis, treatment planning, and outcome monitoring [
59].
2028: Proprietary systems will dominate high-complexity clinical applications requiring extensive validation and regulatory compliance, maintaining 70% market share in these segments [
16].
2030: Annual licensing costs for proprietary systems will decrease by 40–50% due to competition from open-source alternatives, making advanced capabilities more accessible [
42].
16.8. Market and Adoption Projections
Quantitative market projections based on current trends:
2026: Global healthcare AI market reaches
$85–95 billion, with open-source solutions capturing 35% market share [
1].
2028: AI-assisted diagnoses will account for 60% of all medical imaging interpretations in developed markets [
17].
2030: Healthcare AI will generate
$350–400 billion in annual healthcare cost savings globally through improved efficiency and outcomes [
43].
16.9. Critical Challenges and Considerations
Despite the promising timeline, several challenges will require ongoing attention:
Data Privacy and Security: Evolving regulations will require continuous adaptation of both open-source and proprietary systems [
3].
Workforce Transformation: Healthcare professionals will require extensive retraining, with 40–50% of current clinical tasks automated by 2030 [
45].
Ethical Governance: Robust ethical frameworks must be developed to address algorithmic bias, accountability, and patient consent in AI-driven healthcare [
60].
This projected timeline demonstrates the transformative potential of AI in healthcare over the next five years, with both open-source and proprietary approaches playing crucial roles in advancing medical capabilities and improving patient outcomes.
16.10. Clinical Applications
-
By 2026:
- –
50% of U.S. hospitals will deploy AGI for administrative tasks (prior auth, billing) [
6]
- –
First FDA-approved autonomous diagnostic AGI for radiology (projected accuracy: 92%) [
5]
-
By 2028:
- –
AGI will reduce diagnostic errors by 30% compared to human baselines [
35]
- –
40% of chronic disease management handled by agentic systems [
13]
16.11. Economic Impact
Table 18.
Projected Financial Impacts (2025-2030)
Table 18.
Projected Financial Impacts (2025-2030)
| Metric |
Value |
Source |
| Global AGI healthcare market |
$12.4B |
[12] |
| Administrative cost savings |
$8.2B/year |
[53] |
| Malpractice reduction |
25% decrease |
[49] |
16.12. Technological Evolution
-
AGI Architectures:
- –
Shift from single-agent to multi-agent systems (87% of implementations by 2029) [
36]
- –
Emergence of "hybrid AGI" combining LMMs with robotic process automation [
8]
-
Data Infrastructure:
- –
70% of hospitals will adopt federated learning for AGI training by 2027 [
38]
- –
Blockchain-secured health data exchanges for AGI (85% adoption in EU by 2030) [
30]
-
2025-2026:
- –
Mandatory AGI audit frameworks in G7 nations [
32]
- –
WHO updates IHR to include AGI governance [
30]
-
2027-2030:
- –
Standardized AGI liability laws in 50+ countries [
31]
- –
Global AGI certification body established [
33]
16.13. Societal Implications
-
Workforce Impact:
- –
35% reduction in administrative healthcare jobs by 2030 [
12]
- –
2.4M new "AGI supervisor" roles created globally [
10]
-
Health Equity:
- –
AGI could reduce rural-urban care disparities by 40% [
40]
- –
Risk of algorithmic bias persisting in 25% of systems without intervention [
7]
16.14. Challenges and Future Directions
Despite its potential, AGI faces challenges:
16.14.1. Technical Limitations
AGI systems require vast datasets and computational resources, limiting accessibility [
11].
16.14.2. Ethical Dilemmas
Autonomous decision-making raises questions about patient consent and agency [
14].
16.14.3. Policy Lag
Regulatory frameworks must evolve to keep pace with AGI advancements [
34].
Future research should focus on developing scalable, equitable AGI solutions and fostering international collaboration to address these challenges.
17. Conclusion
This comprehensive review has navigated the complex dichotomy between open-source and proprietary AI within the healthcare sector, increasingly shaped by the emergence of autonomous Agentic Generative AI (AGI). Our analysis reveals that the choice between these paradigms is not a binary one but a strategic decision contingent on organizational needs, clinical applications, regulatory risk tolerances, and financial constraints.
17.1. Key Findings
Open-source models have demonstrably closed the performance gap with proprietary systems, offering superior advantages in transparency, customization, data privacy, and cost-effectiveness. These qualities make them particularly vital for research, resource-constrained environments, and applications requiring high auditability and local control. Proprietary systems maintain their edge in reliability, integrated support, regulatory compliance, and seamless integration with established healthcare infrastructure, making them suitable for mission-critical, high-risk deployments [
11,
35,
49].
AGI introduces a paradigm shift, promising transformative gains in diagnostic accuracy, operational efficiency, and personalized medicine. However, this autonomy brings challenges including algorithmic bias, fragmented regulatory oversight, high financial barriers, and unresolved ethical dilemmas around accountability. Technical maturity currently outpaces governance frameworks, highlighting the need for robust, adaptive policies [
29,
38].
17.2. Regulatory and Governance Considerations
To ensure safe and effective adoption of AGI, we emphasize the following governance strategies:
These measures are critical because governance gaps, if unaddressed, may result in clinical risks, liability exposure, and public mistrust, despite technical maturity.
17.3. Affordability and Economic Feasibility
Economic considerations are central to AGI adoption:
Implementation Costs: High upfront costs (e.g.,
$2M barriers [
11]) require careful planning for deployment in resource-constrained environments.
Return on Investment: Open-source deployments show promising ROI (e.g., 3.2:1 in rural healthcare settings [
53]), contingent on data quality improvements addressing 60% failure rates [
24].
Cost-Effectiveness: Hybrid approaches combining open-source AGI with proprietary modules can optimize both financial and operational outcomes, providing scalable, affordable solutions for diverse healthcare settings.
Strategic investment decisions must consider both short-term operational budgets and long-term healthcare cost savings.
17.4. Strategic Recommendations
To harness the potential of open-source and proprietary AGI while mitigating risks:
Tiered Certification: Align risk management and explainability thresholds with WHO standards [
8], allowing regional flexibility based on healthcare system maturity.
Public-Private Sandboxes: Expand pilot programs for multi-agent testing [
48], enabling real-world evaluation with controlled oversight.
Continuous Bias and Safety Monitoring: Use real-time auditing informed by Medicare Advantage appeals data [
7] to maintain trust, safety, and compliance.
Hybrid Ecosystem Development: Leverage open-source transparency and customization alongside proprietary reliability and support, creating an adaptive, cost-effective AGI infrastructure.
Workforce Transition Programs: Invest in retraining clinicians and administrative staff to operate safely in AI-augmented workflows.
17.5. Final Words
The dichotomy between open-source and proprietary AGI in healthcare represents a complex landscape with significant implications for equity, efficiency, and innovation. Our analysis demonstrates:
Both approaches offer distinct advantages; performance gaps are narrowing.
Open-source systems excel in transparency, customization, affordability, and privacy.
Proprietary systems provide regulatory reliability, technical support, and stability for mission-critical applications.
Regulatory governance and affordability remain pivotal for safe, scalable AGI adoption.
The future of healthcare AI lies in a hybrid ecosystem, where innovation and transparency coexist with regulatory compliance and operational reliability. By implementing adaptive governance, rigorous monitoring, and strategic financial planning, healthcare organizations can leverage AGI to improve patient outcomes, enhance efficiency, and expand access, while safeguarding safety, equity, and ethical integrity [
7,
11,
14,
24,
29,
30,
35,
38,
48,
49,
50,
53].
Declaration
The views are of the author and do not represent any affiliated institutions. Work is done as a part of independent research. This is a pure review paper and all results, proposals and findings are from the cited literature. Author does not claim any novel findings.
References
- AI in Healthcare Market Size, Share | Growth Report [2025-2032].
- Google for Health - Advancing Cutting-edge AI Capabilities.
- Temsah, A.; Alhasan, K.; Altamimi, I.; Jamal, A.; Al-Eyadhy, A.; Malki, K.H.; Temsah, M.H.; Temsah, A.; Alhasan, K.; Altamimi, I.; et al. DeepSeek in Healthcare: Revealing Opportunities and Steering Challenges of a New Open-Source Artificial Intelligence Frontier. Cureus 2025, 17. [Google Scholar] [CrossRef] [PubMed]
- MedGemma: Our most capable open models for health AI development.
- abhinav.japesh@superagi. Revolutionizing Healthcare: A Large Agentic Model Case Study on Predicting Patient Outcomes with Unprecedented Accuracy, 2025. Section: Uncategorized.
- Revolutionizing Claims Operations With AI and Agentic Workflows, 2025. Section: Commentary.
- Herman, Bob, C.R. Denied by AI: How Medicare Advantage plans use algorithms to cut off care for seniors in need, 2023.
- Ethics and Governance of Artificial Intelligence for Health: Large Multi-Modal Models. WHO Guidance, 1st ed ed.; World Health Organization: Geneva, 2024. [Google Scholar]
- Bonta, A. Joint Informational Hearing.
- Opportunities for gen AI in public health | McKinsey.
- Agentic AI in Healthcare: Use Cases, Cost & Challenges, 2025.
- Artificial Intelligence and Its Potential Effects on the Economy and the Federal Budget | Congressional Budget Office, 2024.
- Agentic AI and the Future of Healthcare, 2025. Section: Agentic AI.
- Lamb, C.P.M.J. Op-ed: How agentic AI is shaping health care’s future, 2025. Section: Op-Ed.
- Google for Health - Advancing Cutting-edge AI Capabilities.
- Securing AI: The Power of Proprietary Models in Protecting Health Data.
- School, H.M. Open-source AI tool competes with leading proprietary models in medical diagnosis, 2025. Section: Medical News.
- Open-Source AI Matches Top Proprietary LLM in Solving Tough Medical Cases | Harvard Medical School, 2025.
- Riedemann, L.; Labonne, M.; Gilbert, S. The path forward for large language models in medicine is open. npj Digital Medicine 2024, 7, 339. [Google Scholar] [CrossRef] [PubMed]
- Buckley, T.A.; Crowe, B.; Abdulnour, R.E.E.; Rodman, A.; Manrai, A.K. Comparison of Frontier Open-Source and Proprietary Large Language Models for Complex Diagnoses. JAMA Health Forum 2025, 6, e250040–e250040. [Google Scholar] [CrossRef] [PubMed]
- Campus, U.o.C.A.M. Open-source AI tool matches commercial systems in medical scan reporting, 2025. Section: Medical News.
- Witherspoon, A. How Agentic AI is transforming healthcare delivery, 2025.
- Agentic AI in Healthcare Use Cases, Benefits Strategies.
- abhinav.japesh@superagi. Common Agentic AI Implementation Mistakes in Healthcare: Case Study and Expert Advice for Avoiding Costly Errors, 2025. Section: Insights.
- Bioengineer. Open-Source AI Rivals Leading Proprietary Models in Tackling Complex, 2025. Section: Technology.
- Wheeler, K. Inside Google’s MedGemma Models for Healthcare AI, 2025.
- Empowering Public Healthcare with Open Source Language Models.
- Weber, S. Open-Source Software in Healthcare: Promise and Precautions for HI Professionals.
- The evolving ethics and governance landscape of agentic AI | IBM, 2025.
- Bouderhem, R. Shaping the future of AI in healthcare through ethics and governance. Humanities and Social Sciences Communications 2024, 11, 416. [Google Scholar] [CrossRef]
- AI Blog: Expert Insights and Advice.
- A pro-innovation approach to AI regulation: government response.
- Ethics and Governance of Artificial Intelligence for Health: WHO Guidance, 1st ed ed.; World Health Organization: Geneva, 2021.
- RFI: Agentic Artificial Intelligence systems | ARPA-H, 2024.
- mondar bce86d. Agentic AI in Healthcare : Transforming Patient Care with Intelligence, 2025.
- Seizing healthcare’s agentic AI opportunity.
- Generative AI Healthcare: 15 Use Cases with Examples [’25].
- Priorities for an AI in health care strategy | The Health Foundation.
- F, M. Battle of the AI Titans GPT 5 vs Grok 4 vs Microsoft Copilot Who Wins the Next Gen AI Showdown, 2025.
- Agentic AI in Healthcare Management: Uses and Benefits.
- Healthcare Agentic AI: Benefits & Use Cases.
- Engineering. Open source vs. proprietary AI tools: Making strategic choices for long-term success, 2025.
- Masood, A. Unlocking Strategic Elasticity: A Healthcare Executive’s Guide to High-Efficiency Lean AI, 2025.
- Duranton, S. What Leaders Need To Know About Open-Source Vs. Proprietary Models. Section: AI.
- How AI is Transforming Healthcare Delivery and Workforce Management.
- industry, E.L.T.E.L.i.a.f.w.w.s.i.t.h. What Is Agentic AI and How Can It Be Used in Healthcare?
- How agentic AI systems can solve problems in healthcare today.
- Regan, J. State of California Benefits and Risks of Generative Artificial Intelligence Report.
- Group, P.L.I. Agentic AI and the Architecture of Healthcare Transformation, 2025.
- Agentic AI, Generative AI and AI Governance.
- News, Q. Medical AI Agents Boost Accuracy For Complex Health Queries, 2025. Section: Artificial Intelligence.
- The Rise of Agentic AI in Healthcare | athenahealth.
- Coleman, K. Lowering Health Care Costs Through AI: The Possibilities and Barriers, 2024.
- Coat, A.i.L. AI Healthcare Startups Raised 2.2 Billion in January 2025, 2025.
- Chang, W.; Arcesati, R.; Hmaidi, A. CHINA’S DRIVE TOWARD SELF-RELIANCE IN ARTIFICIAL INTELLIGENCE.
- Chase, M.S.; Marcellino, W. Incentives for U.S.-China Conflict, Competition, and Cooperation Across Artificial General Intelligence’s Five Hard National Security Problems. Technical report, 2025.
- Baier, P. OpenAI Releases Two Powerful Open Source AI Models: What You Need To Know, 2025.
- The influence of open source and AI in healthcare.
- Dany, K. Siemens Healthineers AI Strategy Analysis of Dominance in Medical Tech AI Klover ai, 2025.
- F, M. The State of Artificial Intelligence: Agentic Agents, Workforce Upheaval & Global Governance / Updated: 2025, July 6th, 23:59 CET, 2025.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).