Introduction
Bacterial skin infections (BSIs) reveal when bacteria penetrate hair follicles or minor skin breaches, leading to the formation of blisters, abscesses, erythema, and tumefaction. The severity and extent of these infections can vary, affecting the entire body surface or subcutaneous tissues. Minor BSIs encompass conditions such as folliculitis, furuncles, impetigo, and skin abscesses. On the other hand, more severe infections include cellulitis, wound infections, and substantial skin abscesses. The most prevalent causes of bacterial infections are
Staphylococcus and
Streptococcus strains. Among these,
Staphylococcus aureus is particularly harmful due to its ability to cause bacteremia, infect heart valves, and cause endocarditis and osteomyelitis [
1].
Folliculitis is a serious bacterial, fungal, viral, or parasitic skin condition in which the hair follicle becomes infected or inflamed, forming a pustule or erythematous papule on the hair-covered skin that lies over the affected area. Its symptoms include mild pain, pruritus, or irritation and aetiology are often unclear, but factors such as perspiration, trauma, friction, and occlusion of the skin have been identified as potential risk factors for infection. Bacterial folliculitis is typically caused by
Staphylococcus aureus, though cases of
Pseudomonas aeruginosa (hot tub folliculitis) or other organisms have been documented on occasion. The majority of uncomplicated cases of folliculitis, characterized by a limited number of pustules, exhibit spontaneous resolution within several days. However, for more extensive manifestations, mupirocin and clindamycin have been proposed as topical antibiotic options and in treatment-resistant cases, oral cephalexin or dicloxacillin have proven to be an appropriate option. According to
Zha M, 2024, the preferred first-line nonantibiotic treatment is topical benzoyl peroxide [
2], which is often substituted with hydrogen peroxide in clinical practice due to its enhanced safety profile. Due to the restricted penetration capacity and the potentially dangerous adverse effects of topical antibiotics, there is an increasing emphasis on the development of novel, safe, and effective dermatological preparations to be routinely used in clinical practice for folliculitis. In this contest, it was recently developed Ialuxid
® gel (IgMD), registered as medical device and manufactured by BMG Pharma S.p.A., Milan, Italy. Even if the product was widely used for different skin infections and disorders and its optimal performance and safety have been confirmed by the daily practice of thousands of dermatologists, there were few clinical trials to support these activities and limited only to the treatment of acne [
3,
4]. The present study was designed to address this clinical gap (almost partially) by testing the product in folliculitis treatment over an 8-week course and to follow the European legislation on medical devices. In fact, Regulation (EU) 2017/745 requires manufacturers to demonstrate the performance and safety of existing medical devices on the market: this must be done by performing specific trials indicated as post-marketing clinical follow-up studies (PMCF) involving populations affected by the same severity of disease and treated with the same duration and administration reported in the instruction for use (IFU) of the product.
Materials and Methods
This was a non-comparative, open-label single-group assignment, interventional, pilot study involving an 8-week treatment with the IMD, which was administered to patients affected by folliculitis.
Originally, the idea was to conduct a comparative study to test IgMD in patients with folliculitis. In accordance with previous papers on folliculitis, we defined the study design (double-blind randomized vs. placebo), the duration of treatment (2 months) and the primary outcome (number of lesions). Unfortunately, no data were available on the effect size of IgMD in folliculitis, very few data were published for other therapies in this indication, and no useful information was found to calculate the sample size versus placebo [
5]. For this reason, we reported a minimally important difference of 2 ±3.0 points in the number of lesions using a comparison of two means, with a significance level of 5% and a power of 80% to calculate that approximately 60 evaluable participants will be required for each group. The figures regarding the prevalence and incidence of this disease are few and contradictory: no other study on folliculitis was ongoing in Romania at the time of starting our project. Therefore, it was quite impossible to estimate in advance the rate of enrolment of patients affected by folliculitis in a trial. In addition, the characteristic of the project recommended the use of a multicenter design, but the potential investigators never worked together in the past. These considerations suggested that we first carry out a pilot study to test the IgMD in a limited number of subjects, and secondly a randomized clinical trial (RCT) in a large population, but only if the results of the pilot study were favourable. In particular, our goal was to achieve a monthly enrolment rate of at least 0.8 patients/month/site in this pilot study to ensure that the planned sample size for the future comparative study could be enrolled in a reasonable amount of time. This conservative approach should also allow us to collect preliminary data on the performance of the IgMD before calculating the final sample size for the comparative study.
The Romanian Central Ethics Committee (National Commission for Bioethics of Medicines and Medical Devices - NCMMD) approved the study on October 6, 2022 (site SC Salvosan Ciobanca Srl in Zalău and site Darzas Aesthetic in Oradea) and May 11, 2023 (site Nova-Clin Medical Research Center in Timişoara). The trial was conducted in accordance with the Declaration of Helsinki and in adherence to the International Conference on Harmonisation (ICH), Good Clinical Practice (GCP), Medical Devices guidelines (MEDDEV), ISO 14155, and Romanian regulatory requirements. The study enrolled outpatients who had standard daily dermatological visits at three Romanian private clinics selected for their experience treating folliculitis. All participants completed a personal data treatment form in accordance with the General Data Protection Regulation (GDPR) 2016/679 and provided written informed consent for the study. In this form, it was specified that the images taken by the investigators at the different visits would only be used for a clinical trial. There was also an optional additional consent for the patients, allowing the use of their anonymized images for publication in a medical journal. No patient in the participating population agreed to sign this second consent. The study was identified as NCT05345093 on ClinicalTrials.gov. The study design and management followed the “CONSORT 2010 statement: extension to randomised pilot and feasibility trials” [
6].
Tested Product and Concomitant Treatment
IgMD is marketed as medical device in many European and extra EU countries. Its main component is Ialuvance
® Complex, a patented combination of hyaluronic acid (HA), hydrogen peroxide and glycine. HA is involved in many physiological processes, as essential part of the extracellular matrix, and it is essential in all stages of tissue repair thanks to its physicochemical and biological characteristics [
7,
8,
9]. Hydrogen peroxide, a mild antiseptic, favours tissue regeneration by increasing cytokine synthesis and gene expression related to inflammation [
10]. Recent publications confirm its activity in dermatology fields, especially regarding acne [
3,
4,
11]. Glycine can quicken the healing of the epidermal barrier [
12]. The components of IgMD have been observed to create a defensive barrier that maintains the skin’s moisture levels and inhibits bacterial growth by virtue of its film-forming properties. Therefore, the tested product, when applied as gel to the affected area, creates a barrier against outside elements, guards against bacterial infections, and is appropriate for Molluscum contagiosum, folliculitis, paronychia, and acne lesions. In the present pilot study, IgMD was administered directly by the patients at home for 8 weeks. The applications should be several per day and should completely cover the lesion area, as needed. These indications follow exactly what was reported in the IFU of IgMD, as specifically requested by the current legislation on PMCF studies.
During the course of the study, the administration of all drugs and therapies that were indicated as exclusion criteria was prohibited. On the contrary, the participants were permitted to continue receiving all previously prescribed therapeutic interventions for non-study-related clinical conditions.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: male and female adult patients (aged between 18 and 45 years), a diagnosis of folliculitis and consent to refrain from additional dermatological procedures or treatments for the duration of the trial. Patients were excluded if they had any dermal or systemic pathologies or infectious diseases that would compromise their capacity to participate in the trial. Subjects with a history of allergy or hypersensitivity to HA or any other ingredients of IgMD, who would require direct or indirect contact with quaternary ammonium salts during the study, and patients with any other medical condition that would compromise participation in the study were excluded. Furthermore, the following exclusion criteria were applied: the use of oral drugs or topical therapies for folliculitis within the previous month prior to enrolment; concomitant treatments or procedures aimed at improving the condition of the skin over the previous six months prior to the study; facial aesthetic surgery or facial rejuvenation surgery (including filler injections) within the previous three months prior the study; Botulinum toxin A injections in the face within the previous six months prior to enrolment.
Outcome Measures
In this pilot study, we evaluated the feasibility of the future comparative study according to the following parameters: monthly recruitment rate, duration of enrolment, choice of outcomes and difficulties in their measurement. In addition, we also defined the criterion to determine whether to proceed with the future main RCT. We defined that at the end of the pilot study, a monthly enrolment rate of at least 0.5 patient/month/site should be achieved. In fact, this minimum result should ensure that the planned sample size of approximately 120 subjects could be enrolled in two years and half using 8 centers in the future comparative study.
The Investigator collected preliminary data on performance by means of the following outcomes: the number of lesions present on the body area primarily affected by the disease; Total Severity Score (TSS), obtained by summing the intensity of each specific disease sign, graded on a 4-point scale from “absent” to “severe”; Treatment Satisfaction Questionnaires, assessed in accordance with a 4-point scale. A 5-point scale was used to assess patient satisfaction, with responses ranging from “very satisfied” to “not satisfied.” The Investigator Global Assessment of Performance (IGAP) was evaluated by photographs taken at each visit by the Investigators and assessed in blind by a different Investigator who was not involved in the patient visits. The blind Investigator evaluated the IGAP using a 4-point scale, ranging from 1 (very good) to 4 (poor performance). Dermatology Life Quality Index (DLQI) was evaluated based on the total score, with 0–1 indicating “no effect at all on the patient’s life,” and 2 indicating “mild,” 3 indicating “moderate,” 4 indicating “severe,” and 5 indicating “very severe.” A score of 5 indicates a “small effect on the patient’s life,” while a score of 6–10 denotes a “moderate effect,” a score of 11–20 signifies a “very large effect,” and a score of 21–30 represents an “extremely large effect.”
Safety has been evaluated from the Investigators by collecting all Adverse Events (AEs) and Serious Adverse Events (SAEs), comparing and evaluating them with the number of affected patients. The evaluation included related AEs, possibly related to or not evaluable. The incidence, type, and severity of AEs or SAEs were summarized in frequency tables using MedDRA (Medical Dictionary for Regulatory Activities) terms. Additional safety data were collected using the Investigator Global Assessment of Safety (IGAS) and the Patient Global Assessment of Safety (PGAS) assessments, which employed a 4-point scale, ranging from “very good safety” to “poor safety.”
Sample Size and Statistical Analysis
Due to the limited data available for folliculitis, the methodology proposed by
Billingham SA et al, in 2005 was applied [
13]. This implied that the inclusion of a sample size of 12 patients should have been enough to demonstrate the clinical performance of the IMD; in consideration of a potential dropout rate of 10% throughout the clinical trial, the total number of patients to be enrolled for participation in the clinical study has been determined in 13.
Statistical analyses were performed using the statistical software (version 4.1.0; R Foundation for Statistical Computing). The final analysis was conducted subsequent to the conclusion of the trial, the fulfilment of all queries, and the locking of the database (DBL). Media and standard deviation (SD) were used to describe normally distributed quantitative variables (i.e., demographic); median and range of interquartile were used for non-normally distributed variables. Comparative analyses were performed using the Student’s t-test for paired data and the Wilcoxon signed rank test according to the distribution of these variables. Frequencies and percentages were described using categorical variables; the χ2 test or Fisher’s exact test were used for comparative analyses. Secondary endpoints’ analyses were also performed according to AN(C)OVA for repeated measures and/or Linear Mixed Models. A Student’s t-test for paired data or a Wilcoxon signed rank test (if major deviations from the former’s test assumptions were recorded) were used for the analysis of the primary endpoint. ANOVA for repeated measures tests or ANCOVA for repeated measures tests (if considering covariates like age, sex, BMI, etc.), assuming completeness of data were used to analyse secondary endpoints, all continuous variables in nature.
Results
Recruitment Rate and Duration of Enrolment
Participants were recruited from November 11, 2022 to July 27. 2023. Therefore, the duration of enrolment was eight months, and the enrolment rate was 1.6 patients/month in total (0.54 patient/month/center). This value was in compliance with the total monthly recruitment rate planned for the main RCT (at least 0.5 patients/month/site) assuring for the future RCT the enrolment of approximately 120 patients involving 8 centers for 2.5 years. The pilot study ended on September 22nd 2023, date of the last patient’s visit.
Patients’ Characteristics
Four patients (30.8%) were males and 9 (69.2%) were females. Subjects were between 22 to 44 years old, with a mean age, height, weight, and BMI of 30.1±7.72 years, 170.30±7.47 cm, 74.50±18.60 kg, and 25.44±4.97, respectively and all subjects belonged to the Caucasian ethnic group. No statistically significant difference resulted between sex for age, height, and weight. (
Table 1). No relevant findings were evidenced either in the medical history or during physical examination and no concomitant medication was recorded during the screening evaluation and no major protocol deviations occurred concerning the study.
Choice of Outcomes
The outcomes used in the pilot study were the same that Investigators used in their routine visits, confirming that these outcomes are potentially available for future comparative studies.
Patients Disposition and Characteristics
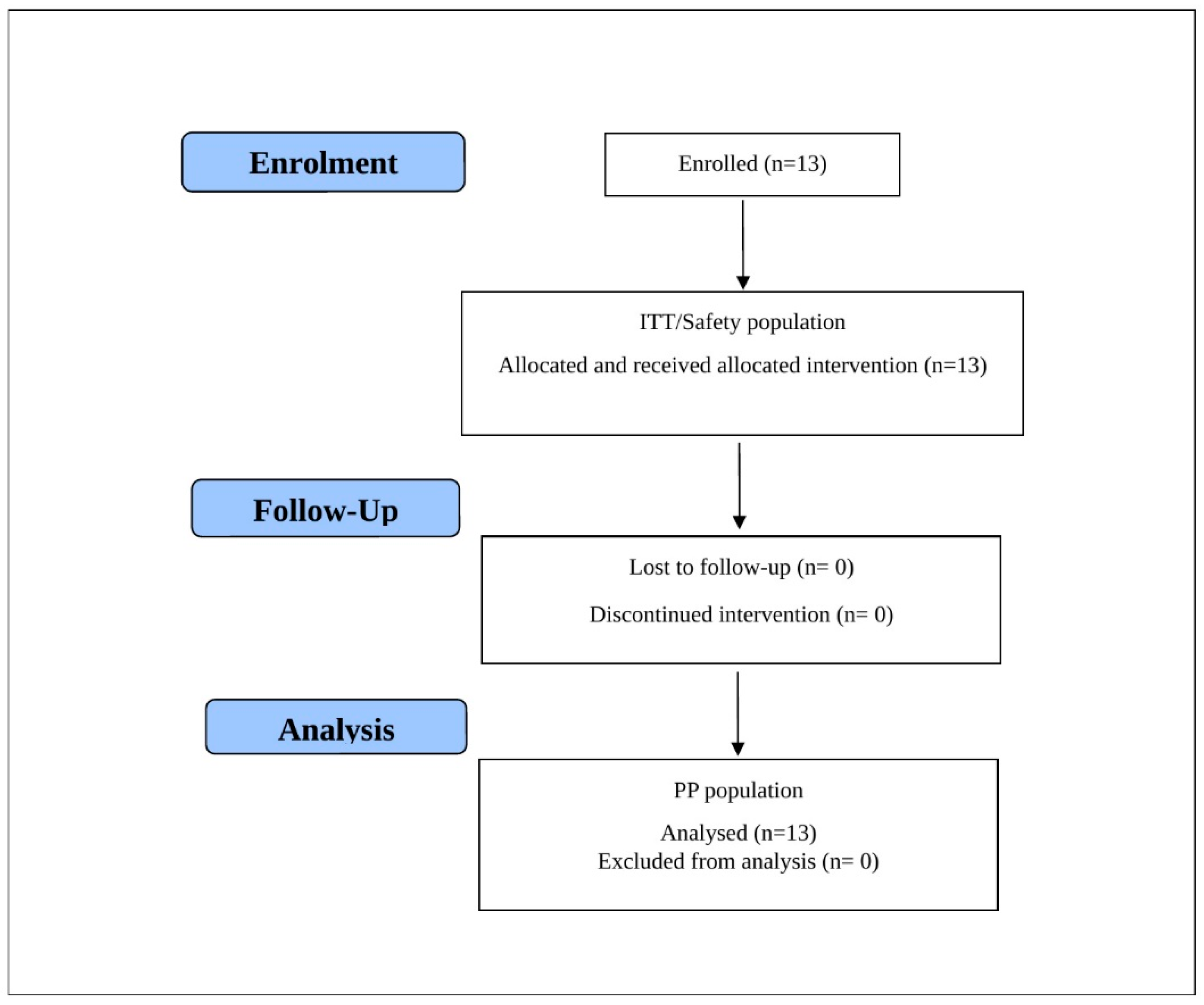
Thirteen patients affected by folliculitis were considered eligible, enrolled, and completed the trial. All patients were included in the Per-Protocol (PP), Intention-To-Treat (ITT), and safety populations, which coincided. The patient disposition is graphically displayed in
Figure 1 and the demographics characteristics at baseline are shown in
Table 1. Baseline characteristics were similar between sexes.
Total Number of Lesions
This parameter was the primary performance outcome and was evaluated on the body area mainly interested in the disease, according to the lesions count performed by the Investigator at 8 weeks after the initiation of treatment compared to Day 0 (
Table 2). A 3-fold decrease in the number of lesions between Day 0 and Day 56 visit was highly statistically significant (p = 0.005), performing the Wilcoxon test.
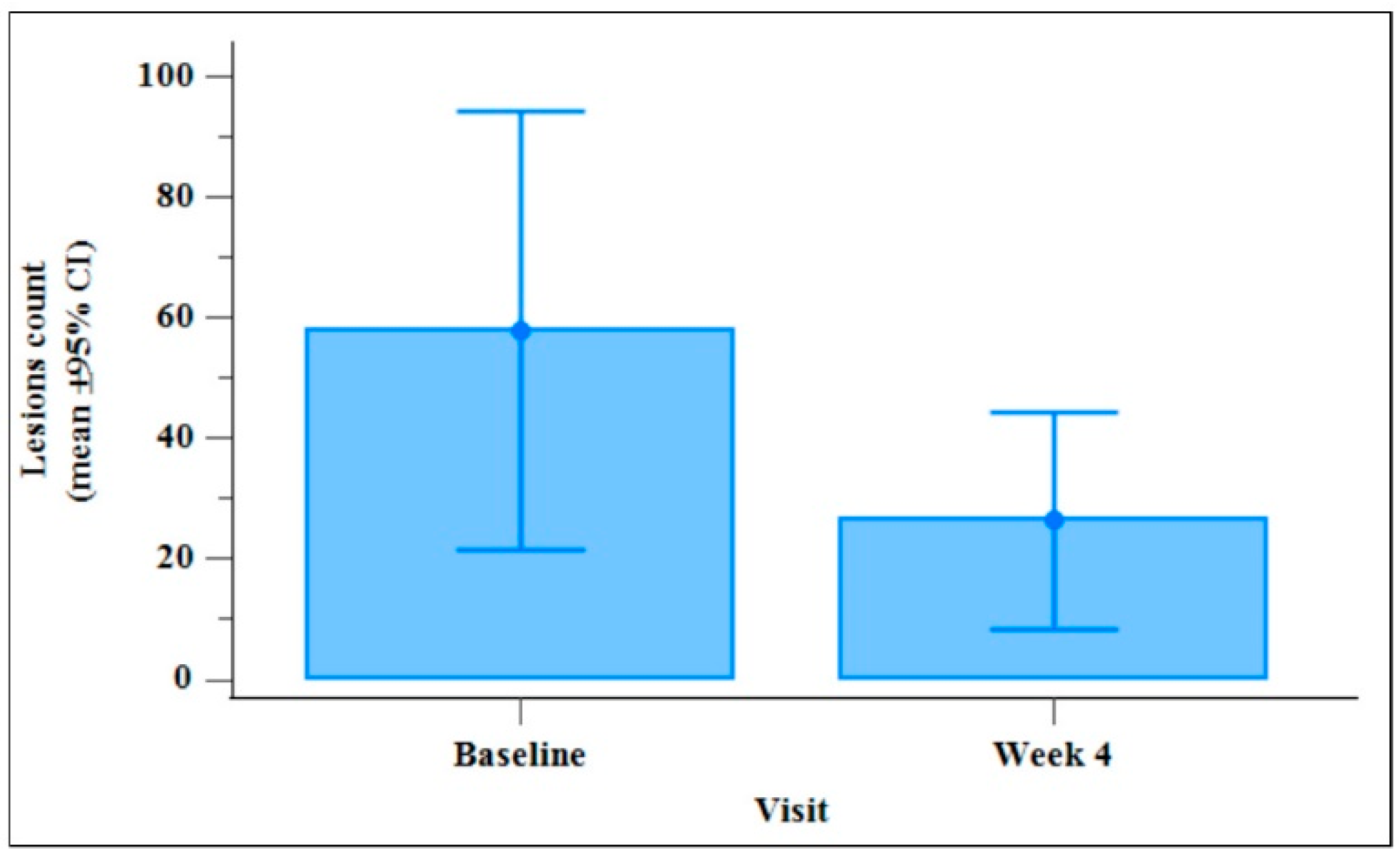
Figure 2 summarises the total number of lesions at baseline (Day 0) in comparison with the total number of lesions 4 weeks after treatment initiation. A highly statistically significant 2-fold decrease between Day 0 and Day 28 visits (p = 0.034) was shown performing a Wilcoxon test.
Total Severity Score
Table 3 summarises the change in TSS observed between the baseline, Day 28. and Day 56.
The statistical analysis revealed statistically significant reductions from baseline to both Day 28 (-33.3%, p = 0.003) and Day 56 (-66.6%, p = 0.002). Continued treatment after 4 weeks of IMD administration up to 8 weeks also improved the TSS (-50.0%, p=0.008).
Treatment Satisfaction Questionnaire
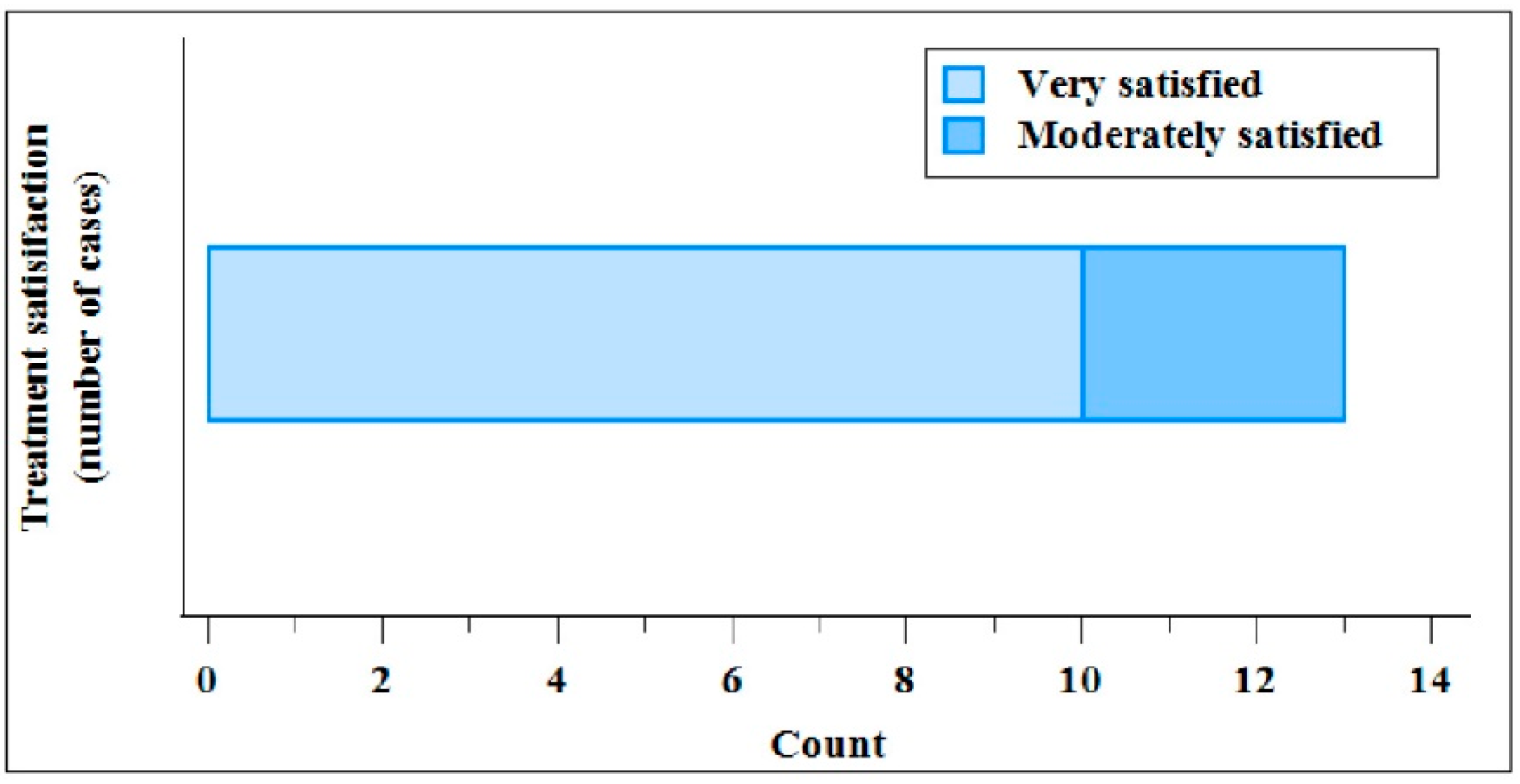
At the end of treatment period (Day 56), 10 patients (76.9%) declared to be very satisfied, and 3 (23.1%) were satisfied. No patient was moderately satisfied or not satisfied.
Figure 3 summarises, as counts, the patient’s satisfaction with the treatment at the Day 56.
Dermatology Life Quality Index
Table 4 summarises the change in the DLQI Total Score at 8 weeks after the initiation of treatment, compared to baseline.
Safety
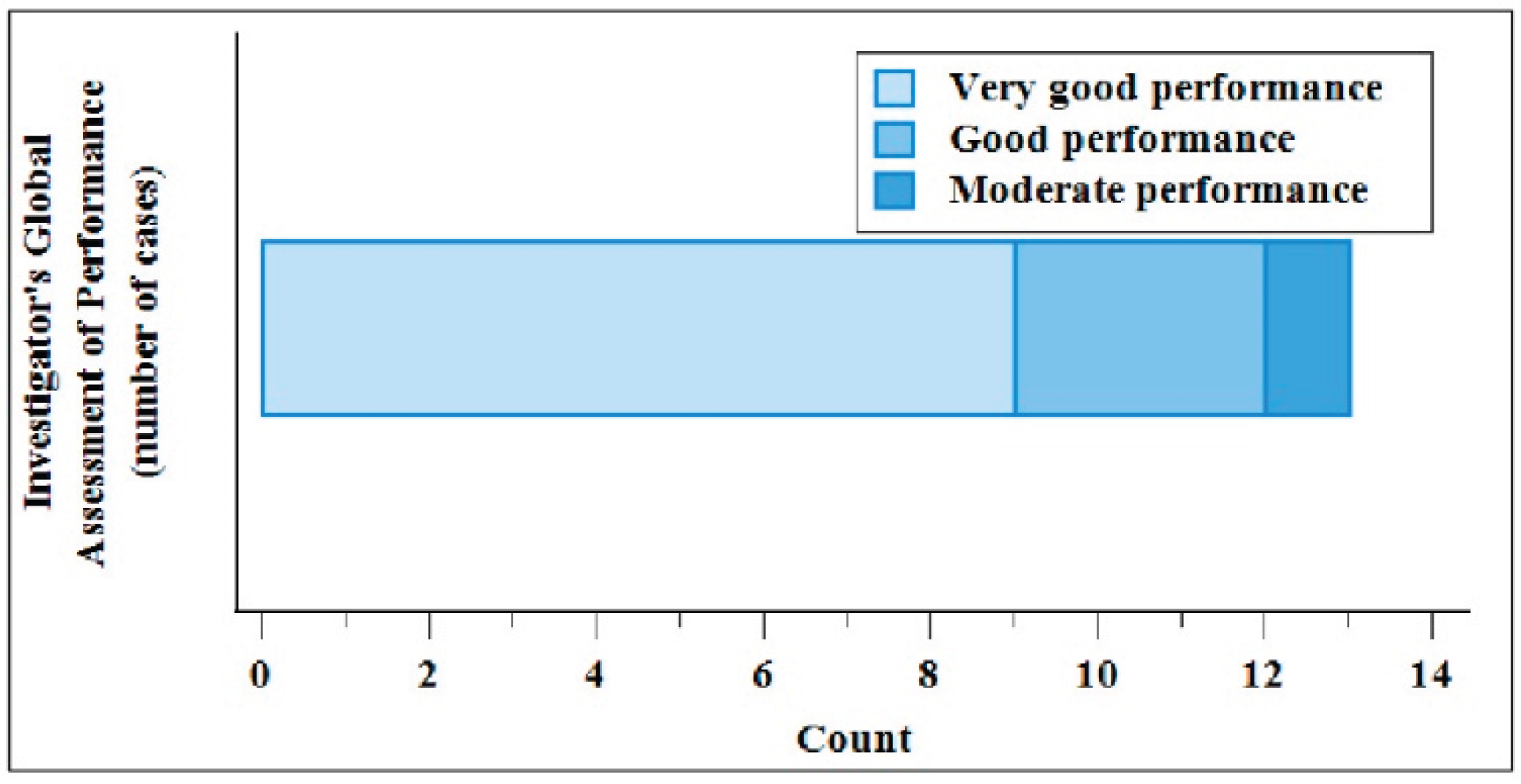
One patient reported two adverse events (AEs) during the study. Both AEs (application-site reactions, i.e., pruritus) were resolved by the end of the study; they were judged mild and related to the IMD. No Serious Adverse Events (SAEs), Adverse effects (ADEs) Device Deficiencies (DDs) were reported during the study period. In addition, the patient and the Investigator performed an overall safety evaluation at Day 56. For Investigator the judgment was “very good” and “good” in 92.3% and 7.7% of cases, respectively. Patients evaluated as “very good” and “good” in 84.6% and 15.4% of cases.
Discussion
The criterion that was established to determine whether to proceed with the future comparative study was satisfied. In fact, the recruitment rate at the conclusion of this pilot trial was 0.55 patients per month per site. This value was slightly above the 0.5 patients per month per site threshold. As the study was a PMCF, the collection of real-world data from a clinical setting of routine dermatologic visits was mandatory. Consequently, the investigators involved in the pilot study were satisfied with the outcomes and tests, which were the same as those used in routine practice. Data on performance and safety of the IgMD treatment were extremely satisfactory for planning the future study: patients showed substantial improvements in all parameters related to the severity of the disease, in particular with regards to the change in the total number of lesions. The product showed a statistically significant reduction of the total number of lesions at week 8 (from 57.8±60.3 to 17.7±29.5), an excellent safety (only two mild adverse events in one patient), and a positive changing in patient’s life (92.3% declared that folliculitis had no effect or small effect on their life after the IgMD treatment).
The limitations of this trial were due to its characteristic as a pilot study (with a limited sample size and the need to use outcomes and tests that could be used for future comparative studies) and to adhere to the PMCF requirements of the current legislation. These included the use of the same dosage regimen and duration of therapy as indicated in the IFU, the application of the same routine dermatology visit setting, and the avoidance of a randomized design.
Even if the trial included a small number of patients, its results provided fundamental confirmation of the safety and efficacy of the product and information on the feasibility of the future comparative study with IgMD.
Author Contributions
MES, DFB, and LB conceptualized the study. MES and DFB were involved in the methodology. MES, DFB, and LB were responsible for the formal and statistical analysis. The investigation during the study was performed by MES, IB, and CV. The original draft was written by MES, DFB, and LB, while IB, DFB and LB performed the review and editing of the manuscript. The visualization was created by LB and LS. The project was supervised by LS. DFB and LS were involved in the project administration. All authors have read and agreed to the published version of the manuscript.
Financial Support
A grant support was received from BMG PHARMA SpA, Milano - Italy (https://
https://bmgpharma.com) . BMG PHARMA SpA provided the medical devices needed for the studies to the clinics. The funder was not involved in the design of the trial, the collection, analysis, or interpretation of the data, the writing of the report, or the decision to submit this article for publication.
Ethical Statement
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The Romanian Central Ethics Committee (National Commission for Bioethics of Medicines and Medical Devices - NCMMD) approved the study on October 6, 2022 with number not reported (site SC Salvosan Ciobanca Srl in Zalău and site Darzas Aesthetic in Oradea) and May 11, 2023 with number not reported (site Nova-Clin Medical Research Center in Timişoara). The trial was conducted in accordance with the Declaration of Helsinki and in adherence to the International Conference on Harmonisation (ICH), Good Clinical Practice (GCP), Medical Devices guidelines (MEDDEV), ISO 14155, and Romanian regulatory requirements. All participants completed a personal data treatment form in accordance with the General Data Protection Regulation (GDPR) 2016/679 and provided written informed consent for the study.
Acknowledgements
We thank Marius Ardelean supporting us with the statistical analysis.
Conflicts of Interest
DFB is employed at Opera CRO, the contract research organization that managed the study. LB is a former internship at TIGERMED Italy, the company involved in data management and statistical analysis. LS is employed at BMG Pharma SpA, the Sponsor of the study. MES, IB, and CV declare no conflicts of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C.; et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef] [PubMed]
- Zha, M.; Usatine, R. Common Skin Conditions in Children and Adolescents: Bacterial Infections. FP Essent. 2024, 541, 14–19. [Google Scholar] [PubMed]
- Veraldi, S.; Micali, G.; Berardesca, E.; Dall'Oglio, F.; Sinagra, J.L.; Guanziroli, E. Results of a Multicenter, Randomized, Controlled Trial of a Hydrogen Peroxide-based Kit versus a Benzoyl Peroxide-based Kit in Mild-to-moderate Acne. . 2016, 9, 50–54. [Google Scholar] [PubMed]
- Stefancu, M.; Barattini, D.F.; Botnaru, I.; Vizman, C.; Stucchi, L.; Barattini, L. Performance and Safety of the Medical Device Ialuxid Gel in the Treatment of Mild–Moderate Acne Vulgaris: An Open-Label, Noncomparative Multicentre Interventional Clinical Trial. J. Cosmet. Dermatol. 2025, 24, e70084. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-S.; Lin, P.-T.; Tsai, Y.-S.; Wang, S.-H.; Chi, C.-C. ; Cochrane Skin Group Interventions for bacterial folliculitis and boils (furuncles and carbuncles). Cochrane Database Syst. Rev. 2021, 2021, CD013099. [Google Scholar] [CrossRef]
- Eldridge, S.M.; Chan, C.L.; Campbell, M.J.; Bond, C.M.; Hopewell, S.; Thabane, L.; Lancaster, G.A.; on behalf of the PAFS consensus group. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016, 2, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Sousa, D.A.; Cunha, A.; Andrade, R.; Espregueira-Mendes, J.; Oliveira, J.M.; et al. Hyaluronic Acid. Adv Exp Med Biol. 2018, 1059, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Salwowska, N.M.; Bebenek, K.A.; Żądło, D.A.; Wcisło-Dziadecka, D.L. Physiochemical properties and application of hyaluronic acid: a systematic review. J. Cosmet. Dermatol. 2016, 15, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Keen, M.A. Hyaluronic Acid in Dermatology. Skinmed 2017, 15, 441–448. [Google Scholar] [PubMed]
- Kapoor, P.; Kumar, S. Hydrogen peroxide in dermatology. Indian J. Dermatol. Venereol. Leprol. 2021, 89, 310–312. [Google Scholar] [CrossRef] [PubMed]
- Milani, M.; Bigardi, A.; Zavattarelli, M. Efficacy and safety of stabilised hydrogen peroxide cream (Crystacide) in mild-to-moderate acne vulgaris: a randomised, controlled trial versus benzoyl peroxide gel. Curr. Med Res. Opin. 2003, 19, 135–138. [Google Scholar] [CrossRef]
- Inoue, K.; Takei, K.; Denda, M. Functional glycine receptor in cultured human keratinocytes. Exp. Dermatol. 2015, 24, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Billingham, S.A.; Whitehead, A.L.; A Julious, S. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med Res. Methodol. 2013, 13, 104–104. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).