Submitted:
08 September 2025
Posted:
09 September 2025
You are already at the latest version
Abstract
Background: Cardiotoxicity remains a concern across cancer therapies. To date, there is a lack of extensive studies evaluating the potential impact of radioligand therapy (RLT) on myocardial injury in patients with neuroendocrine tumors (NETs), particularly in subgroups with increased susceptibility to such injury. This study aimed to assess the potential cardiotoxic effects and myocardial dysfunction associated with RLT using both [177Lu]Lu-DOTA-TATE and tandem therapy with [177Lu]Lu-DOTA-TATE/[90Y]Y-DOTA-TATE in patients with NETs, including specific high-risk subgroups such as patients with pre-existing heart failure, carcinoid heart disease or those previously treated with chemotherapy, by monitoring serum concentration of troponin I, CK-MB, and NT-proBNP before and after RLT. Methods: We retrospectively analyzed 60 consecutive NET patients who underwent 228 RLT courses. A comprehensive cardiac assessment, including detailed medical history, was performed. Additionally, serum troponin I, CKMB and NT-proBNP concentrations were measured prior to treatment and 48 hours post-therapy. 52 patients received [177Lu]Lu-DOTA-TATE monotherapy, while 8 patients were treated with tandem therapy. Results: No increase in cardiotoxicity markers was observed in the overall study population following RLT administration (DTroponin -0.2[-1.4-0.3]ng/L, p=0,007; DCKMB 0.0[-4.0-3.0]U/l, p=0,90; DNT-proBNP 4.0[-45.6-33.6]pg/mL) as well as in the subgroup receiving tandem therapy (DTroponin 0.7[-1.7-013]ng/L, p=0,68; DCKMB -0,5[-10.7-3.0]U/l, p=0,21; DNT-proBNP -21.6[-44.1-16.7]pg/mL). Furthermore, none of the predefined patient subgroups exhibited signs of cardiotoxicity or evidence of myocardial dysfunction. Conclusions: RLT is a safe anticancer treatment option for patients with NETs in terms of cardiotoxicity and cardiac dysfunction, including those at higher risk of cardiovascular complications.
Keywords:
1. Introduction
2. Materials and Methods
2.1. Laboratory Tests
2.2. Echocardiography and Blood Pressure Measurement
2.3. Statistical Analysis
3. Results
3.1. Patients Baseline Characteristics
3.2. Clinical Tolerability of RLT
3.3. Therapy-Related Renal, Hepatic, and Bone Marrow Toxicities
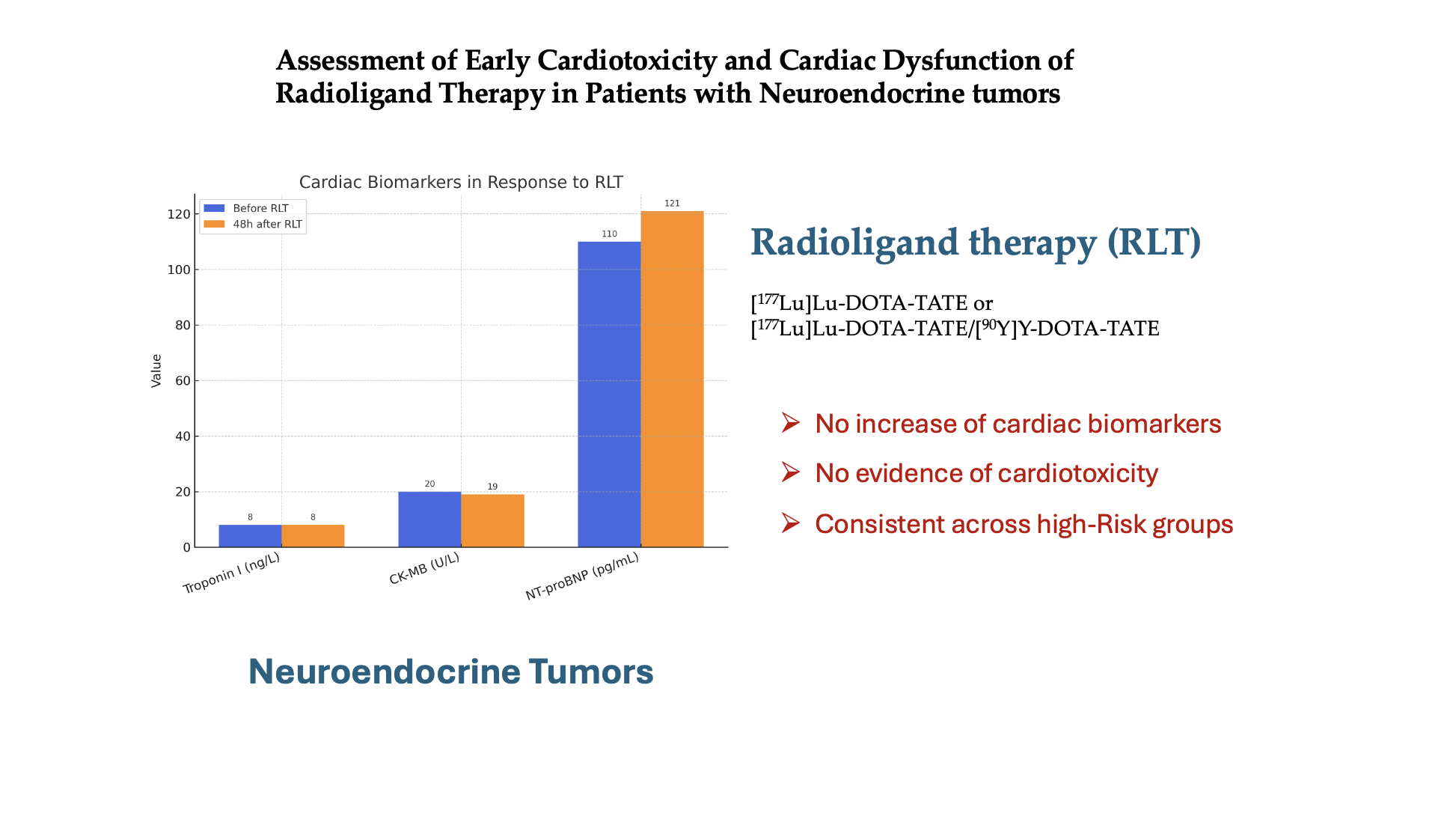
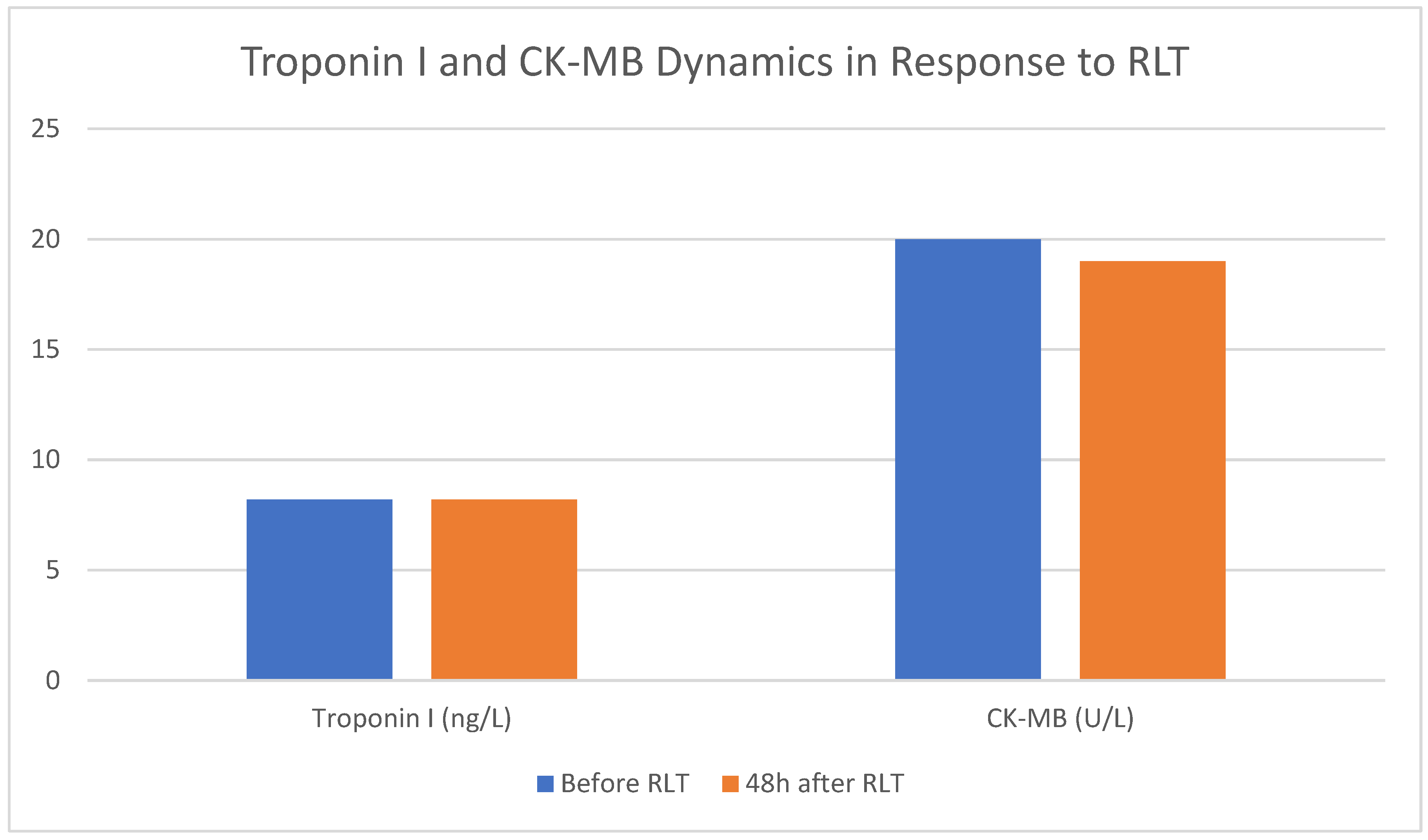
3.4. Investigation of the Potential Cardiotoxicity Associated with RLT
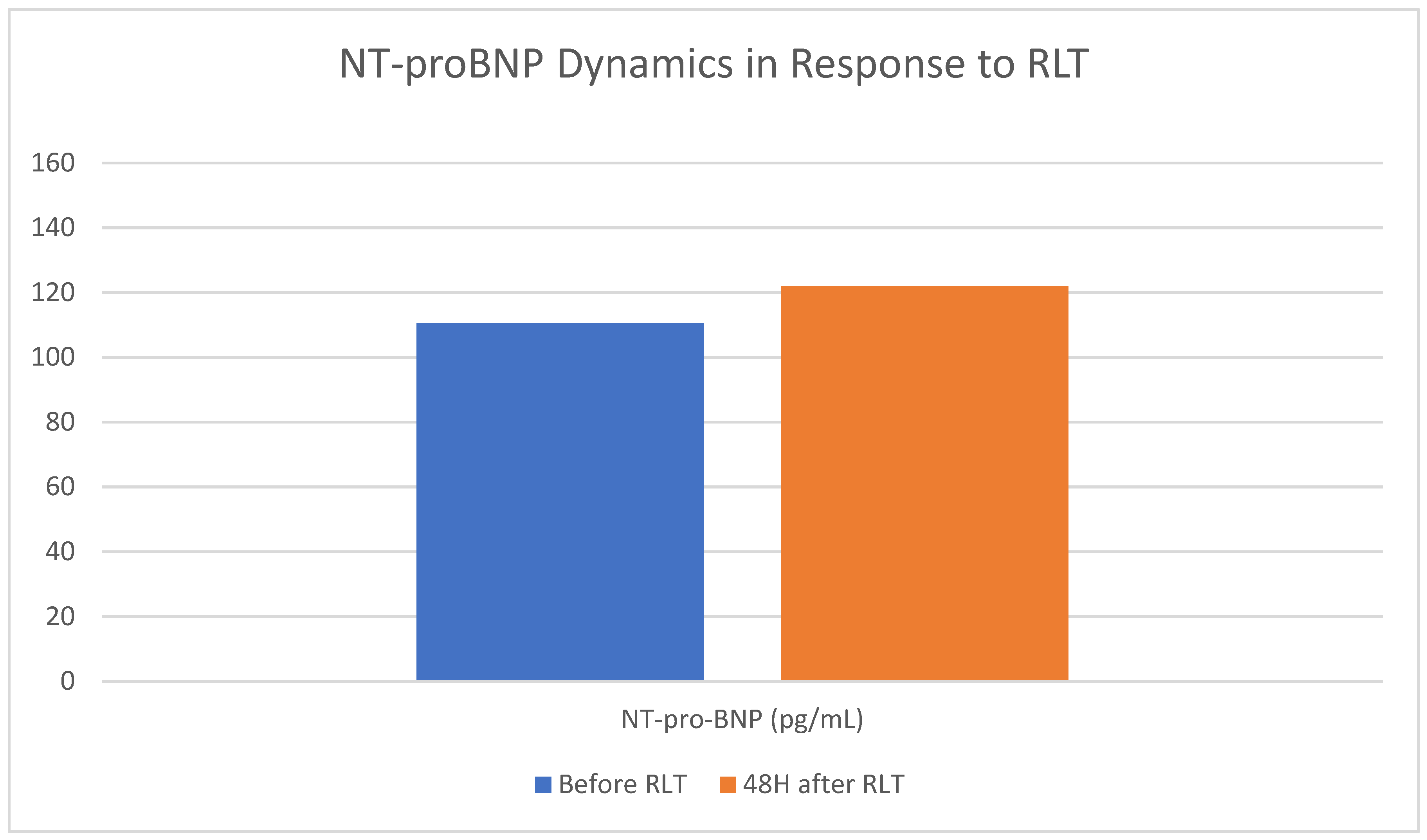
3.5. Effect of RLT on Heart Failure and Cardiac Overload
3.5. Impact of Clinical and Biochemical Parameters on RLT-Associated Cardiotoxicity
3.6. Clinically Overt Acute Cardiac Complications During Radioligand Therapy
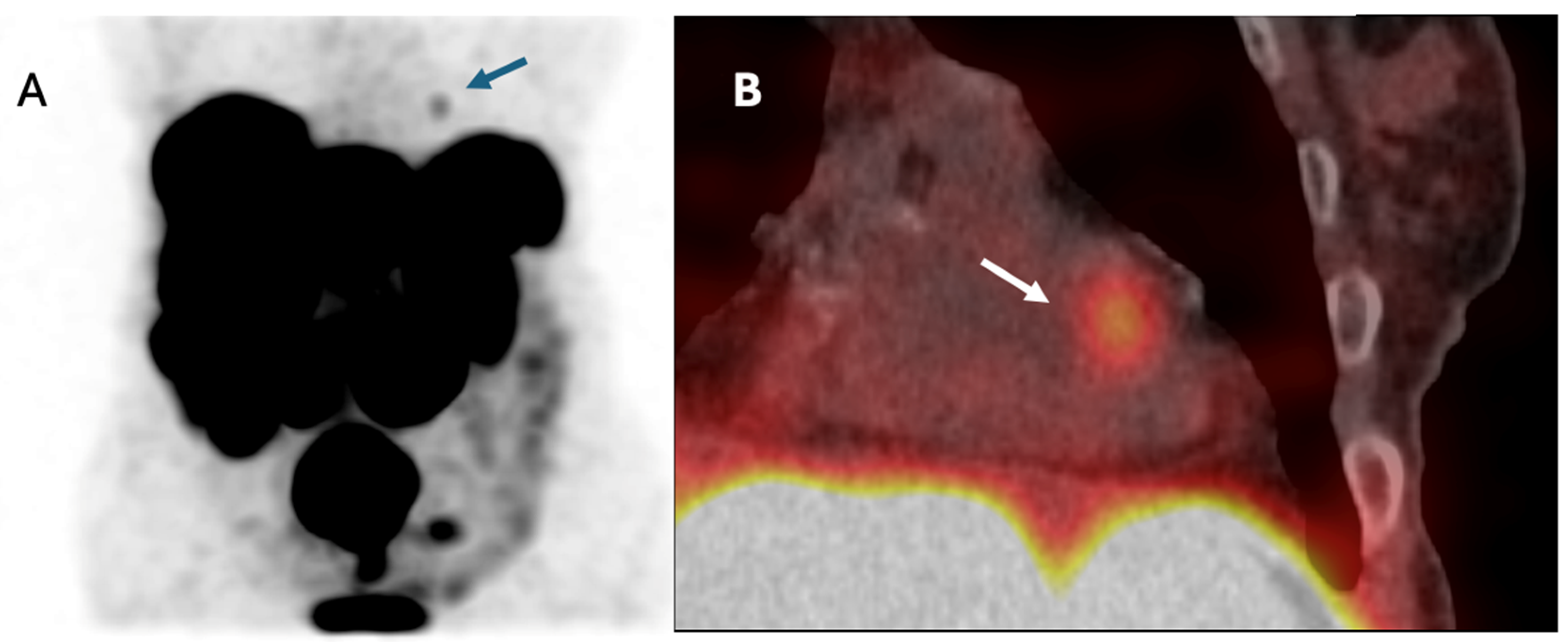
3.7. Patient with NET Metastasis to the Heart
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA AF AHA AST BMI BP CAPTEM CCS CHD CHT CgA CK-MB CS CV CVD DBP ELISA ESC GFR GLS HF ICIs IC-OS IQR LVEF M Med mTOR NET NT-proBNP NYHA PE RLT RVSP SBP SD STEMI |
Amino acid Atrial fibrillation American Heart Association Aspartate aminotransferase Body mass index Blood pressure Capecitabine/temozolomide Chronic coronary syndrome Carcinoid heart disease Chemotherapy Chromogranin A Creatine Kinase – MB isoenzyme Carcinoid syndrome Cardiovascular Cardiovascular disease Diastolic blood pressure Enzyme-linked immunosorbent assay European Society of Cardiology Glomerular filtration rate Global longitudinal strain Heart failure Immune checkpoint inhibitors International Cardio-Oncology Society Interquartile range Left ventricle ejection fraction Mean Median Mammalian Target of Rapamycin Inhibitors Neuroendocrine tumor N-terminal pro-B-type Natriuretic Peptide New York Heart Association Pulmonary embolism Radioligand therapy Right ventricular systolic pressure Systolic blood pressure Standard deviations ST-elevation myocardial infarction |
| TAPSE TKI |
Tricuspid annular plane systolic excursion Tyrosine kinase inhibitors |
| Troponin UPO |
Troponin I Unknown primary origin |
Appendix A
| Parameter | Unit | Reference range |
| Troponin | ng/L | 0.0–14.0 |
| CK-MB | U/L | 0.0 – 25.0 |
| NT-proBNP | pg/mL | 0.0 - 125.0 |
| Creatinine | mg/dL | 0.7 - 1.2 |
| GFR | mL/min/1.73 m² | >60 |
| AST | U/L | 0.0 - 50.0 |
| Leukocytes | ×10^9/L | 4.30 - 9.64 |
| Erythrocytes | ×1012/L | 4.36 - 5.78 |
| Blood platelets | ×10^9/L | 163 - 347 |
| Neutrophils | ×10^3/µL | 1.93 - 5.87 |
| Lymphocytes | ×10^3/µL | 1.23 - 3.42 |
| Chromogranin A | ng/mL | <100 |
| Parameter | Data | Unit | Reference range |
| LVEDD, median (IQR) | 51.7 (46.7–55.9) | mm | 39–52 |
| LVESD, median (IQR) | 34.8 (28.9–39.5) | mm | 25–40 |
| EF % (IQR) | 61.0 (56.0–65.0) | % | 55–70 |
| TAPSE, median (IQR) | 21.0 (19.0–22.0) | mm | >16 |
| IT, n (%) | 7 (11.7) | - | - |
| IP, n (%) | 3 (5.0) | - | - |
|
Troponin1 ng/L |
CK-MB1 U/L |
NT-proBNP 1 pg/mL |
|
| Before start of RLT | 10.0 [5.8–16.0] | 25.5 [17.2–35.7] | 158.0 [54.1–365.0] |
| Before each RLT administration | 8.2 [5.8–13.6] | 20.0 [16.0–32.7] | 110.5 [33.6–300.5] |
| Pts with CS, before each RLT administration | 8.3 [5.9–13.8] | 18.0 [15.0–27.0] | 191.0 [58.2–410.0] |
| Pts with CHD, before each RLT administration | 15.4 [5.8–19.1] | 58.0 [25.5–88.2] | 430 [224.0–3644.0] |
| Pts with HF, before each RLT administration | 13.1 [11.3–20.8] | 33.0 [19.0–45.0] | 580.0 [257.7–1228.2] |
| Pts with CCS, before each RLT administration | 13.0 [11.3–26.6] | 27.0 [18.0–45.0] | 214.0 [90.-672.0] |
| Before first RLT cycle | Before 4th RLT cycle | p | |
| Leukocytes, ×10^9/L | 6.4 [5.3–7.5] | 4.5 [3.9–6.4] | 0.004 |
| Erythrocytes, ×1012/L | 4.3 [3.9–4.6] | 3.9 [3.5–4.1] | 0.002 |
| Blood platelets, ×10^9/L | 223.5 [153.7–293.0] | 182.0 [146.5–254.0] | 0.190 |
| Neutrophils, ×10^3/µL | 4.0 [3.0–5.1] | 3.0 [2.1–4.3] | 0.039 |
| Lymphocytes, ×10^3/µL | 1.4 [1.1–2.0] | 0.8 [0.6–1.3] | <0,001 |
| Creatinine, mg/dL | 0.9 [0.8–1.0] | 0.9 [0.8–1.1] | 0.670 |
| GFR, mL/min/1.73m2 | 84.8 [58.4–109.7] | 73.7 [57.8–100.8] | 0.780 |
| AST, U/L | 29.5 [20.0–48.0] | 30.0 [26.8–37.0] | 0.260 |
| First RLT cycle | 4th RLT cycle | p | |
| Troponin, ng/L | 10.0 [5.8–16.0] | 7.9 [6.2–12.1] | 0.140 |
| CK-MB, U/L | 25.5 [17.2–35.7] | 7.9 [6.2–12.1] | 0.070 |
| NT-proBNP, pg/mL | 158.0 [54.1–365.0] | 106.0 [35.0–316.0] | 0.780 |
| ΔTroponin, ng/L | -0.7 [-1.8–0.2] | -0.1 [-1.7–0.8] | 0.810 |
| CK-MB, U/L | 0.0 [-4.7–6.7] | 0.0 [-3.0–2.5] | 0.600 |
| ΔNT-proBNP, pg/mL | -2.6 [-92.0–28.2] | -27.6 [-66.8- -0.7] | 0.850 |
| ΔTroponin | ΔCK-MB | ΔNT-proBNP | |
| Age | r = 0.13 p = 0.130 |
r = 0.02 p = 0.82 |
r = -0.103 p = 0.230 |
| BMI | r = -0.8 p = 0.37 |
r = 0.10 p = 0.22 |
r = 0.06 p = 0.480 |
| Troponin1 concentration before each RLT course | r = -0.24 p = 0.004 |
r = 0.02 p = 0.79 |
r = -0.10 p = 0.250 |
| CK-MB1 level before each RLT course | r = 0.02 p = 0.79 |
r = 0.51 p < 0.005 |
r = -0.04 p = 0.670 |
| NT-proBNP1 concentration before each RLT course | r = 0.05 p = 0.57 |
r = 0.12 p = 0.17 |
r = -0.41 p < 0.001 |
| Creatinine concentration before each RLT course | r = 0.005 p = 0.96 |
r = -0.09 p = 0.29 |
r = -0.32 p = 0.700 |
| GFR before each RLT course | r = -0.13 p = 0.13 |
r = 0.10 p = 0.26 |
r = 0.12 p = 0.140 |
| AST level before each RLT course | r = -0.10 p = 0.9 |
r = 0.51 p < 0.001 |
r = 0.04 p = 0.610 |
| Chromogranin A concentration before each RLT course | r = -0.08 p = 0.33 |
r = 0.39 p < 0.001 |
R = 0.03 p = 0.760 |
| LVEF before RLT | r = -0.13 p = 0.13 |
r = 0.004 p = 0.96 |
r = 0.09 p = 0.300 |
| TAPSE before RLT | r = -0.065 p = 0.44 |
r = 0.06 p = 0.45 |
r = 0.07 p = 0.440 |
|
Troponin1 (ng/L) |
Troponin2 (ng/L) | CK-MB1 (U/L) | CK-MB2 (U/L) | NT-proBNP1 (pg/mL) | NT-proBNP2 (pg/mL) | |
| I cycle of RLT 3500/1885 MBq [177Lu]Lu/[90Y]Y-DOTA-TATE |
18.7 | 26.1 | 29.0 | 19.0 | 364.0 | 293.0 |
| II cycle of RLT 3500/400 MBq [177Lu]Lu/[90Y]Y-DOTA-TATE |
17.0 | 20.1 | 38.0 | 19.0 | 273.0 | 301.0 |
| III cycle of RLT 3500/530 MBq [177Lu]Lu/[90Y]Y-DOTA-TATE |
17.2 | 31.8 | 21.0 | 26.0 | 224.0 | 354.0 |
| IV cycle of RLT 3500/600 MBq [177Lu]Lu/[90Y]Y-DOTA-TATE |
20.3 | 21.5 | 27.0 | 30.0 | 430.0 | 395.0 |
References
- Kennedy KR, Turner JH, MacDonald WBG, Claringbold PG, Boardman G, Ransom DT. Long-term survival and toxicity in patients with neuroendocrine tumors treated with 177 Lu-octreotate peptide radionuclide therapy. Cancer 2022, 128(11), 2182–2192. [CrossRef]
- Brabander, T.; van der Zwan, W.A.; Teunissen, J.J.M.; Kam, B.L.R.; Feelders, R.A.; de Herder, W.W.; van Eijck, C.H.J.; Franssen, G.J.H.; Krenning, E.p.; Kwekkeboom, D.J. Long-Term Efficacy, Survival, and Safety of [177Lu-DOTA0,Tyr3]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin Cancer Res 2017, 23, 4617–4624. doi: 10.1158/1078-0432.CCR-16-2743.
- Nilica B, Svirydenka A, Fritz J, Bayerschmidt S, Kroiss A, Gruber L, Virgolini IJ. Nephrotoxicity and hematotoxicity one year after four cycles of peptide receptor radionuclide therapy (PRRT) and its impact on future treatment planning - A retrospective analysis. Rev Esp Med Nucl Imagen Mol (Engl Ed) 2022, 41(3), 138–145. [CrossRef]
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan p, Tocchetti CG, van der Meer p, van der Pal HJH; ESC Scientific Document Group. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022, 43(41), 4229–4361. [CrossRef]
- Rao VU, Deswal A, Lenihan D, Dent S, Lopez-Fernandez T, Lyon AR, Barac A, Palaskas N, Chen MH, Villarraga HR, Sadler D, Campbell CM, Skurka K, Wagner MJ, Totzeck M, Ruddy KJ, Heidenreich p, Thomas R, Addison D, Ganatra S, Cheng R, Reeves D, Ghosh AK, Herrmann J. Quality-of-Care Measures for Cardio-Oncology: An IC-OS and ACC Cardio-Oncology Leadership Council Perspective. JACC CardioOncol 2025, 7(3), 191–202. [CrossRef]
- Alexandre J, Cautela J, Ederhy S, Damaj GL, Salem JE, Barlesi F, Farnault L, Charbonnier A, Mirabel M, Champiat S, Cohen-Solal A, Cohen A, Dolladille C, Thuny F. Cardiovascular Toxicity Related to Cancer Treatment: A Pragmatic Approach to the American and European Cardio-Oncology Guidelines. J Am Heart Assoc 2020, 9(18), e018403. [CrossRef]
- Lassmann M, Eberlein U, Verburg FA. Cardiovascular disease and radiopharmaceutical therapies- an underestimated risk? Eur J Nucl Med Mol Imaging 2025, 52(4), 1246–1248. [CrossRef]
- Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, Carver J, Dent S, Ky B, Lyon AR, López-Fernández T, Fradley MG, Ganatra S, Curigliano G, Mitchell JD, Minotti G, Lang NN, Liu JE, Neilan TG, Nohria A, O'Quinn R, Pusic I, Porter C, Reynolds KL, Ruddy KJ, Thavendiranathan p, Valent p. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J 2022, 43(4), 280–299. [CrossRef]
- Little MP, Azizova TV, Richardson DB, Tapio S, Bernier M-O, Kreuzer M, et al. Ionising radiation and cardiovascular disease: systematic review and meta-analysis. BMJ 2023, 380, e072924. doi: 10.1136/bmj-2022-072924.
- Jahng S, Cho KH, Kim KH, Kim N, Lee EJ, Oh Y, et al. Cardiovascular Toxicity of Radiotherapy: Pathophysiology, Diagnosis, and Management. Int J Mol Sci 2023, 24(5), 4379. [CrossRef]
- Kolbert KS, Pentlow KS, Pearson JR, Sheikh A, Finn RD, Humm JL, et al. Prediction of absorbed dose to normal organs in thyroid cancer patients treated with 131I by use of 124I PET and 3-dimensional internal dosimetry software. J Nucl Med 2007, 48, 143–9.
- Klein Hesselink EN, Klein Hesselink MS, de Bock GH, Gansevoort RT, Bakker SJ, Vredeveld EJ, et al. Long-term cardiovascular mortality in patients with differentiated thyroid carcinoma: an observational study. J Clin Oncol 2013, 31, 4046–4053. doi: 10.1200/JCO.2013.49.1043.
- Kao C-H, Chung C-H, Chien W-C, Shen DH-Y, Lin L-F, Chiu C-H, et al. Radioactive Iodine Treatment and the risk of LongTerm Cardiovascular morbidity and mortality in thyroid Cancer patients: a Nationwide Cohort Study. J Clin Med 2021, 10, 4032. doi: 10.3390/jcm10174032.
- Kim KJ, Song JE, Kim JY, Bae JH, Kim NH, Yoo HJ, et al. Effects of radioactive iodine treatment on cardiovascular disease in thyroid cancer patients: a nationwide cohort study. Annals Translational Med 2020, 8, 1235. doi: 10.21037/atm-20-5222.
- Bodei L, Cremonesi M, Ferrari M, Mittra ES, Kulkarni HR, Deroose CM, Srirajaskanthan R, Ramage J, Grana CM, Botta F, Weber MM, Miederer M, Reddy R, Chicco D, Mariani MF, Demange A, Erion JL, Gericke G, Krenning E. Dosimetry of [177Lu]Lu-DOTATATE in Patients with Advanced Midgut Neuroendocrine Tumors: Results from a Substudy of the Phase III NETTER-1 Trial. J Nucl Med. 2025, 66(3), 449–456. [CrossRef]
- Huot Daneault A, Desaulniers M, Beauregard JM, Beaulieu A, Arsenault F, April G, Turcotte É, Buteau FA. Highly Symptomatic Progressing Cardiac Paraganglioma With Intracardiac Extension Treated With 177Lu-DOTATATE: A Case Report. Front Endocrinol (Lausanne) 2021, 2, 705271. [CrossRef]
- Jafari E, Amini AL, Ahmadzadehfar H, Bagheri D, Assadi M. Cardiotoxicity and cardiac monitoring following the use of radiotheranostics agents including 177Lu-PSMA for prostate cancer and 177Lu-DOTATATE for neuroendocrine tumors. Nuklearmedizin 2021, 60(2), 99–105. [CrossRef]
- Afrouz Nayernama, Peter Waldron, Tracy Salaam, and Scott Proestel. Postmarketing safety review of everolimus and cardiac failure or left ventricular dysfunction. Journal of Clinical Oncology 2016, 34(15), suppl. [CrossRef]
- Altena R, Bajalica-Lagercrantz S, Papakonstantinou A. Pharmacogenomics for Prediction of Cardiovascular Toxicity: Landscape of Emerging Data in Breast Cancer Therapies. Cancers (Basel) 2022, 14(19), 4665. [CrossRef]
- Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan JA, Harris DM, Ismail NS, Chen JH, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T, Chen MH. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007, 370(9604), 2011–2019. [CrossRef]
- Dyhl-Polk A, Vaage-Nilsen M, Schou M, Vistisen KK, Lund CM, Kümler T, Appel JM, Nielsen DL. Incidence and risk markers of 5-fluorouracil and capecitabine cardiotoxicity in patients with colorectal cancer. Acta Onco 2020, 59(4), 475–483. [CrossRef]
- Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med 2017, 376(2), 125–135. [CrossRef]
- Strosberg JR, Caplin ME, Kunz PL, Ruszniewski p, Bodei L, Hendifar A, et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-Dotatate in the NETTER-1 trial. J Clin Oncol 2018, 36(8), 257–263. [CrossRef]
- Nicolini S, Imperiale A, Ruf J, Severi S, Mansi L, Chiti A, et al. Role of salvage peptide receptor radionuclide therapy in gastroenteropancreatic neuroendocrine tumors: a systematic review and meta-analysis. Cancers (Basel) 2021, 13(1), 107. [CrossRef]
- Durma AD, Saracyn M, Kołodziej M, Jóźwik-Plebanek K, Mróz A, Kapusta W, Dmochowska B, Kamiński G. Re-treatment with [177Lu]Lu-DOTA-TATE or [177Lu]Lu-DOTA-TATE and [90Y]Y-DOTA-TATE of patients with progressive neuroendocrine neoplasm. Nucl Med Rev Cent East Eur 2023, 26(0), 143–152. [CrossRef]
- Hendifar AE, Choi J, Modlin IM, Pisegna JR. Persistent cardiomyopathy associated with [^177Lu]Lu-DOTATATE therapy for metastatic neuroendocrine tumor. J Clin Oncol. 2018, 36(15_suppl), e16178. [CrossRef]
- Taïeb D, Jha A, Treglia G, Pacak K. Molecular imaging and radionuclide therapy of pheochromocytoma and paraganglioma in the era of genomic characterization of disease subgroups. Endocr Relat Cancer 2019, 26(11), R627-R652. [CrossRef]
- Brodowska-Kania DA, Saracyn M, Osial N, Kołodziej M, Kamiński G. Carcinoid crisis induced by radioligand therapy: a rare but life-threatening complication in patient with neuroendocrine tumor. Nucl Med Rev Cent East Eur 2024, 27(0), 36–38. [CrossRef]
- Michel L, Rassaf T, Totzeck M. Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. J Thorac Dis 2018, 10(Suppl 35), S4282-S4295. [CrossRef]
- Michel L, Mincu RI, Mahabadi AA, Settelmeier S, Al-Rashid F, Rassaf T, Totzeck M. Troponins and brain natriuretic peptides for the prediction of cardiotoxicity in cancer patients: a meta-analysis. Eur J Heart Fail 2020, 22(2), 350–361. [CrossRef]
- Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022, 43(41), 4229–4361. [CrossRef]
- Ky B, Vejpongsa p, Yeh ET, Force T, Moslehi JJ, Warnes CA, et al. Emerging paradigms in cardiotoxicity and cardiovascular monitoring in cancer therapeutics. Circulation 2014, 130(8), 719–31. [CrossRef]
- Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010, 55(22), 2135–41. [CrossRef]
- Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. J Am Coll Cardiol, 2012, 60(24), 2484–90. [CrossRef]
- Verburg FA, vern M, Mader U, Luster M, Reiners C, Hänscheid H. The absorbed dose to the blood is a better predictor of ablation success than the administered 131I activity in thyroid cancer patients. Eur J Nucl Med Mol Imaging 2011, 38, 673–80. doi: 10.1007/s00259-010-1689-5.
- Eberlein U, Nowak C, Bluemel C, Buck AK, Werner RA, Scherthan H, et al. DNA damage in blood lymphocytes in patients after 177Lu peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging 2015, 42, 1739–49. doi: 10.1007/s00259-015-3083-9.
- Schumann S, Scherthan H, Lapa C, Serfling S, Muhtadi R, Lassmann M, et al. DNA damage in blood leucocytes of prostate cancer patients during therapy with 177Lu-PSMA. Eur J Nucl Med Mol Imaging 2019, 46, 1723–32. [CrossRef]
- Huot Daneault A, Desaulniers M, Beauregard JM, Beaulieu A, Arsenault F, April G, Turcotte É, Buteau FA. Highly Symptomatic Progressing Cardiac Paraganglioma With Intracardiac Extension Treated With 177Lu-DOTATATE: A Case Report. Front Endocrinol (Lausanne) 2021, 12, 705271. [CrossRef]
- Stanciu, A. E., Stanciu, M., Gheorghe, D. C., & Zamfirescu, A. Cardiovascular effects of cumulative doses of radioiodine in differentiated thyroid cancer patients with type 2 diabetes mellitus. Cancers 2022, 14(9), 2287. [CrossRef]
- Fine RL, Gulati AP, Tsushima D, et al. Capecitabine and Temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: the Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol 2013, 71(3), 663–670. doi: 10.1007/s00280-012-2055-z.
- Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011, 117(2), 268–275. doi: 10.1002/cncr.25425.
- Polk A, Vaage-Nilsen M, Vistisen K, Nielsen DL. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev 2013, 39(8), 974–984. doi: 10.1016/j.ctrv.2013.03.005.
- Yeh ETH, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 2009, 53(24), 2231–2247. doi: 10.1016/j.jacc.2009.02.050.
- Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007, 370(9604), 2011–2019. doi: 10.1016/S0140-6736(07)61865-0.
- Telli mL, Witteles RM, Fisher GA, et al. Cardiotoxicity associated with the cancer therapeutic agent sunitinib malate. Ann Oncol 2008, 19(9), 1613–1618. doi: 10.1093/annonc/mdn168.
- Cremonesi M, Ferrari M, Bodei L, Tosi G, Paganelli G. Dosimetry in peptide radionuclide receptor therapy: a review. J Nucl Med 2006, 47(9), 1467–7145.
- Forrer F, Waldherr C, Maecke HR, Mueller-Brand J. Targeted radionuclide therapy with 90Y-DOTATOC in patients with neuroendocrine tumors. Anticancer Res 2006, 26(1B), 703–707. https://ar.iiarjournals.org/content/26/1B/703.
- Imhof A, Brunner p, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analog [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol 2011, 29(17), 2416–2423. doi: 10.1200/JCO.2010.33.7873.
- Del Prete M, Buteau FA, Arsenault F, et al. Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumors: initial results from the p-PRRT trial. Eur J Nucl Med Mol Imaging 2019, 46(3), 728–742. doi: 10.1007/s00259-018-4209-7.
- Kunikowska J, Pawlak D, Bańkowski T, et al. Long-term results and tolerability of tandem therapy with 90Y/177Lu-DOTATATE in neuroendocrine tumors: Results of a prospective study. Ann Oncol 2017, 28(12), 3103–3110. [CrossRef]
- Marinova M, Mücke M, Mahlknecht U, et al. Safety and efficacy of tandem peptide receptor radionuclide therapy using (177)Lu-DOTATOC and (90)Y-DOTATOC in advanced neuroendocrine tumors. Theranostics 2019, 9(3), 831–840. [CrossRef]
- Saracyn M, Durma AD, Bober B, Kołodziej M, Lubas A, Kapusta W, Niemczyk S, Kamiński G. Long-Term Complications of Radioligand Therapy with Lutetium-177 and Yttrium-90 in Patients with Neuroendocrine Neoplasms. Nutrients 2022,15(1), 185. doi: 10.3390/nu15010185.
- Curigliano G, Lenihan D, Fradley M, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020, 31(2), 171–190. doi: 10.1016/j.annonc.2019.10.023.
- Armenian SH, Lacchetti C, Barac A, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2017, 35(8), 893–911. doi: 10.1200/JCO.2016.70.5400.
- Zamorano JL, Lancellotti p, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur Heart J 2016, 37(36), 2768–2801. doi: 10.1093/eurheartj/ehw562.
- Bonsen LR, Aalbersberg EA, Tesselaar ME, Stokkel MP. Cardiac metastases in patients with neuroendocrine tumors: clinical relevance and imaging features. Neuroendocrinology 2019, 109(3), 256–263. [CrossRef]
- Hope TA, Bodei L, Chan JA, et al. NANETS/SNMMI Consensus Statement on Patient Selection and Appropriate Use of 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy. J Nucl Med 2023, 64(4), 444–454. [CrossRef]
| Parameter | Patients’ characteristics |
| Age, years median (IQR) | 66.5 (56.7–73.0) |
| Sex: Male, n (%) Female, n (%) |
33 (55.0) 27 (45.0) |
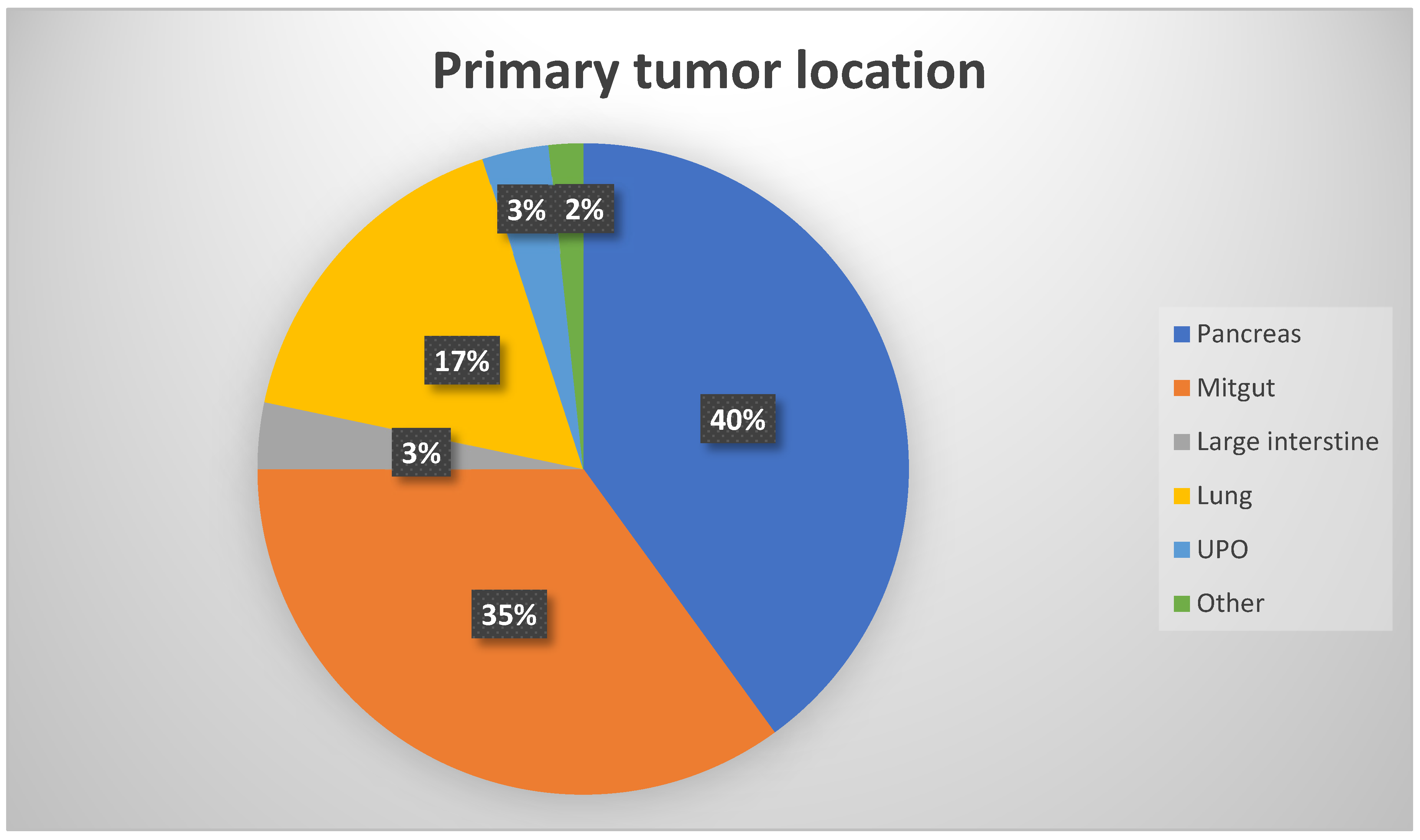
| Primary localisation of NET: Panceras, n (%) Mitgut, n (%) Large intestine, n (%) Lung, n (%) UPO, n (%) Other, n (%) |
24 (40) 21 (35) 2 (3.3) 10 (16.7) 2 (3.3) 1 (1.7) |
| Grade: G1, n (%) G2, n (%) G3, n (%) |
18 (30.0) 40 (66.7) 2 (3.3) |
| Ki-67, median (IQR) | 5 (2–10) |
| Prior RLT, n (%) | 9 (15.0) |
| Prior CHT, n (%) | 16 (26.7) |
| Prior treatment with TKI, n (%) | 4 (6.7) |
| BMI, kg/m2 , median (IQR) | 25.1 (22.6–27.2) |
| Obesity (BMI ≥30), n (%) | 8 (13.3) |
| Hypertension, n (%) | 32 (53.3) |
| Diabetes mellitus, n (%) | 19 (31.7) |
| hyperlipidemia, n (%) | 14 (23.3) |
| Smoking, n (%) | 10 (16.7) |
| Carcinoid syndrome, n (%) | 22 (36.7) |
| Carcinoid heart disease, n (%) | 4 (6.7) |
| Cardiac metastases, n (%) | 1 (1.7) |
| EF % (IQR) | 61 (56–65) |
| Heart failure, n (%): NYHA 1, n (%) NYHA 2, n (%) |
10 (16.7) 5 (8.3) 5 (8.3) |
| Other CVD, n (%): Chronic coronary syndrome, n (%) Atrial fibrillation, n (%) Chronic aortic aneurysm, n (%) History of pulmonary embolism, n (%) |
14 (23.3) 9 (15.0) 4 (6.7) 2 (3.3) 1 (1.7) |
| Parameter | Before each RLT course | 48h after RLT course | p |
| Leukocytes, ×10^9/L | 5.5 [4.3–6.7] | 5.2 [4.0–6.8] | 0,049 |
| Erythrocytes, ×1012/L | 4.0 [3.7–4.3] | 4.0 [3.7–4.5] | 0.18 |
| Blood platelets, ×10^9/L | 198.0 [162.0–258.0] | 185.0 [138.5–260.0] | <0.001 |
| Neutrophils, ×10^3/µL | 3.4 [2.6–4.6] | 3.2 [2.4–4.4] | 0.02 |
| Lymphocytes, ×10^3/µL | 1.1 [0.7–1.4] | 1.0 [0.6–1.4] | 0.11 |
| Creatinine, mg/dL | 0.9 [0.8–1.0] | 0.9 [0.8–1.0] | 0.37 |
| GFR, mL/min/1.73m2 | 79.3 [60.4–105.0] | 79.9 [60.4 – 107.1] | 0.56 |
| AST, U/L | 29.0 [24.0–39.0] | 27.0 [21.0–35.0] | 0.22 |
|
Troponin1 (ng/L) |
Troponin2 (ng/L) |
ΔTroponin (ng/L) |
p |
CKMB1 (U/L) |
CKMB2 (U/L) |
ΔCKMB (U/L) |
p | |
| All Pts | 8.2 [5.8–13.6] |
8.2 [6.0–13.6] |
-0.2 [-1.4–0.3] |
0.007 | 20.0 [16.0–32.7] |
19.0 [15.0–30.7] |
0.0 [-4.0–3.0] | 0.90 |
| Pts treated with [177Lu]Lu-DOTA-TATE |
8.3 [5.7–13.4] |
8.2 [5.9–13.7] |
-0.1 [-1.3–0.3] |
0.008 | 20.0 [16.0–32.7] |
19.0 [15.2–30.7] |
0.0 [-3.5–3.0] |
0.57 |
| Pts treated with tandem therapy | 11.3 [6.1–16.6] |
10.3 [6.3–15.3] |
-0.7 [-1.7–1.3] |
0.68 | 24.0 [15.2–51.0] |
19.0 [14.0–45.7] |
-0.5 [-10.7–3.0] |
0.21 |
| Pts with CS | 8.3 [5.9–13.8] |
8.6 [5.9–13.8] |
-0.2 [-1.3–0.3] |
0.10 | 18.0 [15.0–27.0] |
18.0 [14.0–26.0] |
0.0 [-0.4–3.0] |
0.59 |
| Pts with CHD | 15.4 [5.8–19.1] |
12.8 [5.9–22.6] |
0.2 [-2.2–4.1] |
0.68 | 58.0 [25.5–88.2] |
43.5 [19.0–78.2] |
-6.5 [-16.0–3.5] |
0.33 |
| All pts with HF | 13.5 [11.6–20.7] |
13.3 [10.4–21.7] |
-0.7 [-2.5–0.8] |
0.40 | 28.5 [17.7–45.0] |
30.5 [17.7–45.7] |
0.0 [-6.7–13.5] |
0.62 |
| HF NYHA I | 11.8 [10.7–18.9] |
12.8 [7.9–18.8] |
-0.7 [-3.0–0.65] |
0.21 | 21.0 [14.0–20.0] |
19.0 [17.0–43.0] |
1.0 [-4.0–19.5] |
0.67 |
| HF NYHA II | 16.7 [12.9–21.1] |
16.2 [10.8–25.6] |
-0.8 [-1.6–1.8] |
0.65 | 37.0 [21.7–56.0] |
33.5 [21.5–59.7] |
-0.5 [-9.7–4.7] |
0.89 |
| Pts with CCS | 13.8 [11.0–22.5] |
13.7 [9.9–24.0] |
-0.45 [-1.5–0.8] |
0.45 | 26.5 [17.0–37.2] |
26.5 [15.7–49.2] |
-0.5 [-7.2–3.5] |
0.90 |
| Pts with prior RLT | 9.0 [7.4–16.0] |
9.8 [6.1–15.6] |
-0.8 [-1.7–0.3] |
0.12 | 28.0 [21.0–75.5] |
22.0 [17.0–77.0] |
-1.0 [-8.0–34.0] |
0.88 |
| Pts with prior CHT | 5.7 [3.6–7.2] |
4.5 [3.1–8.1] |
-0.1 [-0.6–0.15] |
0.40 | 12.0 [11.0–16.0] |
15.0 [10.0–28.0] |
1.0 [0.0–5.2] |
0.08 |
| Pts with prior TKI | 7.1 [4.9–9.8] |
6.7 [4.9–7.8] |
-0.5 [-3.0–0.3] |
0.11 | 21.0 [18.0–34.5] |
19.5 [18.7–29.2] |
0.5 [-4.5–6.7] |
0.68 |
|
NT-proBNP1 (pg/mL) |
NT-proBNP2 (pg/mL) |
ΔNT-proBNP (pg/mL) |
p | |
| All Pts | 117.5 [40.5–319.7] |
121.5 [37.9–322.2] |
-4.0 [-45.6–33.6] |
0.32 |
| Pts treated with [177Lu]Lu-DOTA-TATE | 106.0 [28.1–316.0] |
127.0 [33.2–350.5] |
-4.0 [-46.0–33.6] |
0.53 |
| Pts treated with tandem therapy | 208.0 [103.0–358.5] |
185.0 [69.7–295.0] |
-21.6 [-44.1–16.7] |
0.08 |
| Pts with CS | 191.1 [58.2–410.0] |
187.0 [55.3–359.0] |
-0.5 [-46.0–34.7] |
0.49 |
| Pts with CHD | 430.0 [224.0–3644.0] |
301.0 [256.0–1925.0] |
-7.0 [-290.0–130.0] |
0.79 |
| All pts with HF | 531.5 [273.2–1166.7] |
659.0 [294.0–1255.0] |
-14.8 [-145.7–142.5] |
0.64 |
| Pts with HF NYHA I | 624.0 [166.0–1039.5] |
615.0 [107.2–906.5] |
-92.0 [-317.5–111.7] |
0.26 |
| Pts with HF NYHA II | 531.5 [296.0–2026.0] |
870.0 [297.0–1333.0] |
18.5 [-83.0–260.0] |
0.51 |
| Pts with CCS | 306.5 [115.5–609.0] |
274.0 [208.0–569.0] |
0.0 [-214.5–129.5] |
0.76 |
| Pts with prior RLT | 207.5 [15.8–303.7] |
283.0 [211.0–326.0] |
-4.4 [-37.3–116.7] |
0.33 |
| Pts with prior CHT | 47.4 [17.8–63.3] |
64.4 [30.0–118.0] |
9.9 [-3.6–52.7] |
0.18 |
| Pts with prior Other CVD | 79.9 [44.3–120.5] |
60.7 [31.7–316.7] |
-17.4 [-30.5–216.4] |
0.65 |
| ΔTroponin | ΔCKMB | ΔNT-proBNP | |
| Δcreatinine | r = 0.43 p < 0.001 |
r = 0.01 p = 0.90 |
r = 0.02 p = 0.83 |
| ΔGFR | r = 0.01 p = 0.95 |
r = 0.04 p = 0.63 |
r = -0.03 p = 0.73 |
| ΔTroponin | - | r = -0.01 p = 0.95 |
r = 0.13 p = 0.53 |
| ΔCKMB | r = -0.01 p = 0.95 |
- | r = -0.10 p = 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





