Submitted:
05 September 2025
Posted:
09 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Patient Cohorts and Exposure Definition
- Low LDH: LDH levels <200 Units per Liter (U/L).
- Moderate LDH: LDH levels = 201–280 U/L.
- High LDH: LDH levels ≥281 U/L.
2.3. Propensity Score Matching
- Demographics: Current age, age at NPDR diagnosis, sex, race, and Hispanic ethnicity.
- Metabolic and Vascular Comorbidities: HbA1c, body-mass index (BMI), hypertension, dyslipidemia, and proteinuria.
- Ocular Disease Stage: Granular NPDR severity codes, presence of macular edema, and open-angle glaucoma.
- Prior Ophthalmic Procedures: Codes for prior intravitreal injections and other ophthalmic procedures.
2.4. Outcomes
- Proliferative diabetic retinopathy (PDR)
- Tractional retinal detachment (TRD)
- Vitreous hemorrhage (VH)
3. Results
3.1. Comparison of Low vs. Moderate LDH Cohorts
3.2. Comparison of Low vs. High LDH Cohorts
4. Discussion
5. Conclusions
Acknowledgments
Declarations of interest
References
- Wilkinson, C.P., Ferris, F.L., Klein, R.E., Lee, P.P., Agardh, C.D., Davis, M., et al., 2003. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110:1677–1682. [CrossRef]
- Antonetti, D.A., Silva, P.S., Stitt, A.W., 2021. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat Rev Endocrinol 17:195–206. [CrossRef]
- Yang, P., Xu, W., Liu, L., Yang, G., 2024. Association of lactate dehydrogenase and diabetic retinopathy in US adults with diabetes mellitus. J Diabetes 16:e13476. [CrossRef]
- Lian, X., Zhu, M., 2024. Factors related to type 2 diabetic retinopathy and their clinical application value. Front Endocrinol (Lausanne) 15:1484197. [CrossRef]
- Li, Q., Gui, X., Zhang, H., Zhu, W., Zhang, R., Shen, W., et al., 2022. Role of glucose metabolism in ocular angiogenesis (Review). Mol Med Rep 26:363. [CrossRef]
- van Noorden, C.J.F., Yetkin-Arik, B., Serrano Martinez, P., Bakker, N., van Breest Smallenburg, M.E., Schlingemann, R.O., et al., 2024. New Insights in ATP Synthesis as Therapeutic Target in Cancer and Angiogenic Ocular Diseases. J Histochem Cytochem 72:329–352. [CrossRef]
- Wu, Y., Lu, C., Pan, N., Zhang, M., An, Y., Xu, M., et al., 2021. Serum lactate dehydrogenase activities as systems biomarkers for 48 types of human diseases. Sci Rep 11:12997. [CrossRef]
- Ghiam, B.K., Xu, L., Berry, J.L., 2019. Aqueous Humor Markers in Retinoblastoma, a Review. Transl Vis Sci Technol 8:13. [CrossRef]
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Characteristics |
Moderate LDH (n = 14,615) |
Low LDH (n = 13,514) |
Standardized Difference |
Moderate LDH (n = 11,708) |
Low LDH (n = 11,708) |
Standardized Difference |
| Age, mean ± SD | 63.5 ± 13.5 | 62.9 ± 13.7 | 0.0473 | 63.1 ± 13.5 | 63.2 ± 13.6 | 0.0034 |
| Gender, No. (%) | ||||||
| Male | 6,878 (49.92%) | 6,493 (52.94%) | 0.0603 | 6,121 (52.28%) | 6,106 (52.15%) | 0.0026 |
| Race, No. (%) | ||||||
| White | 7,350 (53.35%) | 7,120 (58.05%) | 0.0947 | 6,708 (57.29%) | 6,712 (57.33%) | 0.0007 |
| Black or African American | 3,688 (26.77%) | 2,764 (22.53%) | 0.0983 | 2,762 (23.59%) | 2,734 (23.35%) | 0.0056 |
| Asian | 554 (4.02%) | 512 (4.17%) | 0.0077 | 477 (4.07%) | 485 (4.14%) | 0.0034 |
| Native Hawaiian or Other Pacific Islander | 145 (1.05%) | 100 (0.82%) | 0.0247 | 99 (0.85%) | 97 (0.83%) | 0.0019 |
| American Indian or Alaska Native | 133 (0.97%) | 93 (0.76%) | 0.0224 | 91 (0.78%) | 93 (0.79%) | 0.0019 |
| Other Race | 631 (4.58%) | 568 (4.63%) | 0.0024 | 537 (4.59%) | 540 (4.61%) | 0.0012 |
| Unknown Race | 1,277 (9.27%) | 1,109 (9.04%) | 0.0079 | 1,034 (8.83%) | 1,047 (8.94%) | 0.0039 |
| Ethnicity, No. (%) | ||||||
| Hispanic/LatinX | 1,791 (13.00%) | 1,549 (12.63%) | 0.0111 | 1,482 (12.66%) | 1,497 (12.79%) | 0.0038 |
| Comorbidities, No. (%) | ||||||
| Essential (primary) hypertension | 11,983 (86.97%) | 10,369 (84.53%) | 0.0698 | 10,041 (85.76%) | 10,051 (85.85%) | 0.0024 |
| Hyperlipidemia | 11,076 (80.39%) | 9,563 (77.96%) | 0.0598 | 9,269 (79.17%) | 9,264 (79.13%) | 0.0011 |
| T2DM with mild NPDR without macular edema | 4,092 (29.70%) | 3,722 (30.34%) | 0.0141 | 3,535 (30.19%) | 3,502 (29.91%) | 0.0061 |
| T2DM with mild NPDR with macular edema | 787 (5.71%) | 656 (5.35%) | 0.0159 | 631 (5.39%) | 639 (5.46%) | 0.0030 |
| T2DM with moderate NPDR without macular edema | 1,423 (10.33%) | 1,141 (9.30%) | 0.0345 | 1,092 (9.33%) | 1,108 (9.46%) | 0.0047 |
| T2DM with moderate NPDR with macular edema | 764 (5.55%) | 607 (4.95%) | 0.0268 | 585 (5.00%) | 592 (5.06%) | 0.0027 |
| T2DM with severe NPDR without macular edema | 668 (4.85%) | 503 (4.10%) | 0.0362 | 474 (4.05%) | 489 (4.18%) | 0.0065 |
| T2DM with severe NPDR with macular edema | 464 (3.37%) | 353 (2.88%) | 0.0282 | 348 (2.97%) | 346 (2.96%) | 0.0010 |
| Primary open-angle glaucoma | 717 (5.20%) | 543 (4.43%) | 0.0363 | 531 (4.54%) | 533 (4.55%) | 0.0008 |
| Proteinuria | 3,442 (24.98%) | 2,599 (21.19%) | 0.0901 | 2,613 (22.32%) | 2,589 (22.11%) | 0.0049 |
| Alcohol dependence | 385 (2.79%) | 342 (2.79%) | 0.0004 | 320 (2.73%) | 327 (2.79%) | 0.0036 |
| Tobacco use | 783 (5.68%) | 679 (5.54%) | 0.0064 | 630 (5.38%) | 653 (5.58%) | 0.0086 |
| Central retinal artery occlusion | 57 (0.41%) | 45 (0.37%) | 0.0075 | 42 (0.36%) | 43 (0.37%) | 0.0014 |
| Retinal artery branch occlusion | 54 (0.39%) | 49 (0.40%) | 0.0012 | 49 (0.42%) | 47 (0.40%) | 0.0027 |
| Partial retinal artery occlusion | 43 (0.31%) | 41 (0.33%) | 0.0039 | 38 (0.33%) | 37 (0.32%) | 0.0015 |
| Central retinal vein occlusion | 199 (1.44%) | 160 (1.30%) | 0.0120 | 159 (1.36%) | 157 (1.34%) | 0.0015 |
| Tributary (branch) retinal vein occlusion | 193 (1.40%) | 150 (1.22%) | 0.0156 | 150 (1.28%) | 145 (1.24%) | 0.0038 |
| Low income | 68 (0.49%) | 41 (0.33%) | 0.0248 | 37 (0.32%) | 41 (0.35%) | 0.0059 |
| Intravitreal injection | 1,360 (9.87%) | 1,005 (8.19%) | 0.0585 | 998 (8.52%) | 993 (8.48%) | 0.0015 |
| Lab Values, mean ± SD | ||||||
| Hemoglobin | 10.5 ± 2.37 | 10.9 ± 2.42 | 0.1826 | 10.5 ± 2.39 | 10.9 ± 2.41 | 0.1474 |
| Hemoglobin A1c | 7.68 ± 1.97 | 7.64 ± 1.96 | 0.0246 | 7.69 ± 1.96 | 7.63 ± 1.96 | 0.0283 |
| Triglyceride | 148 ± 147 | 154 ± 136 | 0.0408 | 150 ± 153 | 154 ± 135 | 0.0301 |
| Cholesterol | 154 ± 53.7 | 152 ± 50.3 | 0.0511 | 154 ± 52.6 | 152 ± 50.3 | 0.0370 |
| Body Mass Index | 31.2 ± 7.88 | 30.8 ± 7.56 | 0.0461 | 31.3 ± 7.89 | 30.8 ± 7.54 | 0.0597 |
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| Characteristics |
High LDH (n = 21,915) |
Low LDH (n = 13,514) |
Standardized Difference |
High LDH (n = 12,154) |
Low LDH (n = 12,154) |
Standardized Difference |
| Age, mean ± SD | 62.6 ± 13.2 | 62.9 ± 13.7 | 0.0187 | 62.9 ± 13.3 | 62.9 ± 13.7 | 0.0009 |
| Gender, No. (%) | ||||||
| Male | 10,257 (50.05%) | 6,493 (52.94%) | 0.0577 | 6,468 (53.22%) | 6,417 (52.80%) | 0.0084 |
| Race, No. (%) | ||||||
| White | 10,202 (49.78%) | 7,120 (58.05%) | 0.1664 | 7,087 (58.31%) | 7,039 (57.92%) | 0.0080 |
| Black or African American | 6,169 (30.10%) | 2,764 (22.53%) | 0.1725 | 2,721 (22.39%) | 2,757 (22.68%) | 0.0071 |
| Asian | 909 (4.44%) | 512 (4.17%) | 0.0129 | 531 (4.37%) | 505 (4.16%) | 0.0106 |
| Native Hawaiian or Other Pacific Islander | 143 (0.70%) | 93 (0.76%) | 0.0071 | 96 (0.79%) | 93 (0.77%) | 0.0028 |
| American Indian or Alaska Native | 199 (0.97%) | 100 (0.82%) | 0.0166 | 91 (0.75%) | 99 (0.82%) | 0.0075 |
| Other Race | 862 (4.21%) | 568 (4.63%) | 0.0207 | 556 (4.58%) | 560 (4.61%) | 0.0016 |
| Unknown Race | 2,009 (9.80%) | 1,109 (9.04%) | 0.0261 | 1,072 (8.82%) | 1,101 (9.06%) | 0.0084 |
| Ethnicity, No. (%) | ||||||
| Hispanic/LatinX | 2,355 (11.49%) | 1,549 (12.63%) | 0.0349 | 1,504 (12.38%) | 1,527 (12.56%) | 0.0057 |
| Comorbidities, No. (%) | ||||||
| Essential (primary) hypertension | 18,027 (87.97%) | 10,369 (84.53%) | 0.0998 | 10,311 (84.84%) | 10,300 (84.75%) | 0.0025 |
| Hyperlipidemia | 16,602 (81.01%) | 9,563 (77.96%) | 0.0756 | 9,536 (78.46%) | 9,508 (78.23%) | 0.0056 |
| T2DM with mild NPDR without macular edema | 5,704 (27.83%) | 3,722 (30.34%) | 0.0553 | 3,703 (30.47%) | 3,673 (30.22%) | 0.0054 |
| T2DM with mild NPDR with macular edema | 980 (4.78%) | 656 (5.35%) | 0.0258 | 663 (5.46%) | 649 (5.34%) | 0.0051 |
| T2DM with moderate NPDR without macular edema | 1,958 (9.55%) | 1,141 (9.30%) | 0.0086 | 1,166 (9.59%) | 1,125 (9.26%) | 0.0115 |
| T2DM with moderate NPDR with macular edema | 928 (4.53%) | 607 (4.95%) | 0.0198 | 608 (5.00%) | 600 (4.94%) | 0.0030 |
| T2DM with severe NPDR without macular edema | 951 (4.64%) | 503 (4.10%) | 0.0264 | 501 (4.12%) | 501 (4.12%) | 0.0000 |
| T2DM with severe NPDR with macular edema | 589 (2.87%) | 353 (2.88%) | 0.0002 | 366 (3.01%) | 351 (2.89%) | 0.0073 |
| Primary open-angle glaucoma | 1,044 (5.09%) | 543 (4.43%) | 0.0314 | 516 (4.25%) | 542 (4.46%) | 0.0105 |
| Proteinuria | 5,033 (24.56%) | 2,599 (21.19%) | 0.0803 | 2,598 (21.38%) | 2,591 (21.32%) | 0.0014 |
| Alcohol dependence | 720 (3.51%) | 342 (2.79%) | 0.0415 | 323 (2.66%) | 340 (2.80%) | 0.0086 |
| Tobacco use | 1,155 (5.64%) | 679 (5.54%) | 0.0044 | 678 (5.58%) | 678 (5.58%) | 0.0000 |
| Central retinal artery occlusion | 86 (0.42%) | 45 (0.37%) | 0.0084 | 43 (0.35%) | 45 (0.37%) | 0.0027 |
| Retinal artery branch occlusion | 74 (0.36%) | 49 (0.40%) | 0.0062 | 46 (0.38%) | 49 (0.40%) | 0.0040 |
| Partial retinal artery occlusion | 65 (0.32%) | 41 (0.33%) | 0.0030 | 39 (0.32%) | 41 (0.34%) | 0.0029 |
| Central retinal vein occlusion | 243 (1.19%) | 160 (1.30%) | 0.0107 | 155 (1.28%) | 157 (1.29%) | 0.0015 |
| Tributary (branch) retinal vein occlusion | 252 (1.23%) | 150 (1.22%) | 0.0006 | 149 (1.23%) | 146 (1.20%) | 0.0023 |
| Low income | 86 (0.42%) | 41 (0.33%) | 0.0139 | 38 (0.31%) | 41 (0.34%) | 0.0043 |
| Intravitreal injection | 1,779 (8.68%) | 1,005 (8.19%) | 0.0175 | 991 (8.15%) | 1,000 (8.23%) | 0.0027 |
| Lab Values, mean ± SD | ||||||
| Hemoglobin | 10.1 ± 2.32 | 10.9 ± 2.42 | 0.3197 | 10.2 ± 2.34 | 10.9 ± 2.42 | 0.2917 |
| Hemoglobin A1c | 7.7 ± 2.01 | 7.64 ± 1.96 | 0.0340 | 7.69 ± 1.99 | 7.64 ± 1.96 | 0.0256 |
| Triglyceride | 155 ± 129 | 154 ± 136 | 0.0081 | 157 ± 131 | 154 ± 136 | 0.0202 |
| Cholesterol | 154 ± 54.2 | 152 ± 50.3 | 0.0466 | 152 ± 53.7 | 152 ± 50.4 | 0.0048 |
| Body Mass Index | 31 ± 7.79 | 30.8 ± 7.56 | 0.0169 | 30.9 ± 7.69 | 30.8 ± 7.55 | 0.0091 |
| Code System | Code | Description |
|---|---|---|
| ICD-10-CM | I10 | Essential (primary) hypertension |
| ICD-10-CM | E78 | Disorders of lipoprotein metabolism and other lipidemias |
| ICD-10-CM | E11.329 | Type 2 diabetes mellitus with mild NPDR without macular edema |
| ICD-10-CM | R80 | Proteinuria |
| ICD-10-CM | E11.339 | Type 2 diabetes mellitus with moderate NPDR without macular edema |
| ICD-10-CM | E11.321 | Type 2 diabetes mellitus with mild NPDR with macular edema |
| ICD-10-CM | Z72.0 | Tobacco use |
| ICD-10-CM | E11.331 | Type 2 diabetes mellitus with moderate NPDR with macular edema |
| ICD-10-CM | H40.11 | Primary open-angle glaucoma |
| ICD-10-CM | E11.349 | Type 2 diabetes mellitus with severe NPDR without macular edema |
| ICD-10-CM | E11.341 | Type 2 diabetes mellitus with severe NPDR with macular edema |
| ICD-10-CM | F10.2 | Alcohol dependence |
| ICD-10-CM | H34.81 | Central retinal vein occlusion |
| ICD-10-CM | H34.83 | Tributary (branch) retinal vein occlusion |
| ICD-10-CM | H34.23 | Retinal artery branch occlusion |
| ICD-10-CM | H34.1 | Central retinal artery occlusion |
| ICD-10-CM | H34.21 | Partial retinal artery occlusion |
| ICD-10-CM | Z59.6 | Low income |
| CPT | 67028 | Intravitreal injection of a pharmacologic agent (separate procedure) |
| TNX Curated | 9014 | Hemoglobin [Mass/volume] in Blood |
| TNX Curated | 9037 | Hemoglobin A1c/Hemoglobin.total in Blood |
| TNX Curated | 9004 | Triglyceride [Mass/volume] in Serum, Plasma or Blood |
| TNX Curated | 9083 | Body Mass Index (BMI) |
| TNX Curated | 9000 | Cholesterol [Mass/volume] in Serum or Plasma |
| ICD-10-CM | E11.33 | Type 2 diabetes mellitus with moderate NPDR |
| ICD-10-CM | E11.31 | Type 2 diabetes mellitus with unspecified diabetic retinopathy |
| ICD-10-CM | E11.32 | Type 2 diabetes mellitus with mild NPDR |
| ICD-10-CM | E11.34 | Type 2 diabetes mellitus with severe NPDR |
| TNX Curated | 9052 | Lactate dehydrogenase [Enzymatic activity/volume] in Serum or Plasma |
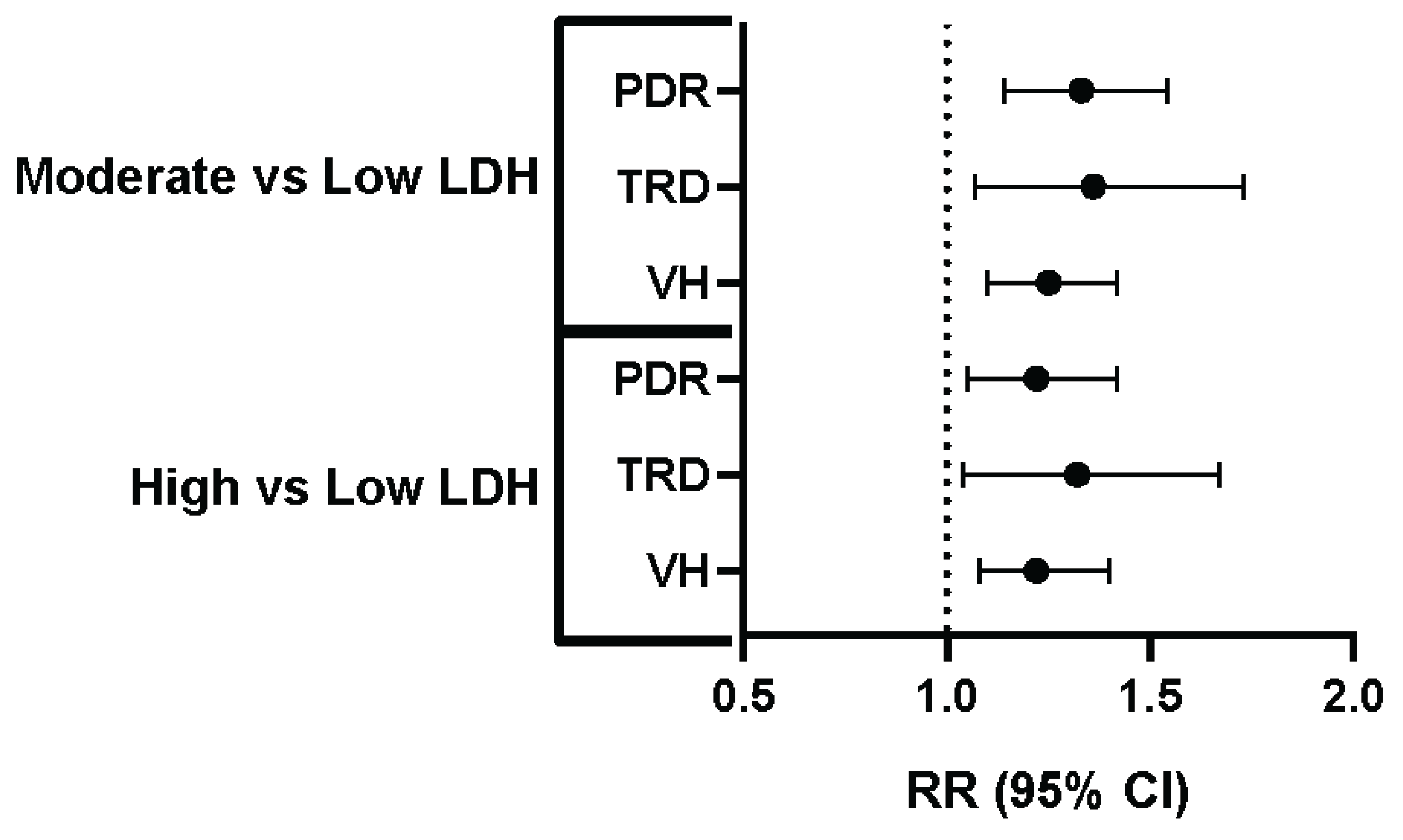
| Comparison | Outcome | Risk (%) - Low LDH | Risk (%) - Comparison Group | Relative Risk (95% CI) |
|---|---|---|---|---|
| Low vs. Moderate LDH | PDR | 2.96% | 3.93% | 1.33 (1.14-1.54) |
| Low vs. Moderate LDH | TRD | 0.99% | 1.35% | 1.36 (1.07-1.73) |
| Low vs. Moderate LDH | VH | 3.51% | 4.38% | 1.25 (1.10-1.42) |
| Low vs. High LDH | PDR | 3.00% | 3.66% | 1.22 (1.05-1.42) |
| Low vs. High LDH | TRD | 0.96% | 1.27% | 1.32 (1.04-1.67) |
| Low vs. High LDH | VH | 0.96% | 1.27% | 1.22 (1.08-1.40) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





