1. Introduction
The global rise in obesity prevalence has intensified the burden of obesity-associated chronic diseases (ABCD), type 2 diabetes (T2D), and chronic kidney disease (CKD). Approximately 1.9 billion adults worldwide are affected by overweight or obesity, driving elevated incidence of cardio-kidney-metabolic disorders—including T2D, CKD, and heart failure [
1]. Notably, central obesity affects 41.5% of individuals with obesity and correlates with adverse health outcomes even in normal-weight populations [
1].
Obesity and its comorbidities (particularly T2D and CKD) constitute major risk factors for the accelerating incidence of heart and kidney diseases. Pathologically, obesity involves not only increased adiposity but also altered fat distribution, with visceral adipose tissue (VAT) accumulation critically elevating cardio-renal risk. Mechanistic studies indicate VAT contributes to cardio-renal syndrome (CRS)through:Hemody-namic alterations,Mechanical compression effects,Neuroendocrine pathways (e.g., insulin resistance, endothelial dysfunction, oxidative stress) [
2].
In cardio-kidney-metabolic (CKM) disease management, the multifactorial interplay of obesity demands attention. Novel pharmacotherapies—sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP-1RA)—demonstrate significant cardio-renal protection and weight reduction benefits, underscoring shared pathological mechanisms across CKM disorders [
3]. Adipose tissue dysfunction, a core pathophysiological feature, disrupts metabolic and endocrine homeostasis, amplifying systemic risk [
3].
Therapeutic weight management strategies and emerging anti-obesity agents substantially mitigate CKM disease progression. Evidence confirms weight loss improves insulin sensitivity, reduces cardiovascular risk, and slows renal function decline [
4]. Consequently, developing data-driven individualized protocolsfor multi-organ protection represents both a current imperative and future research priority. Integrating multidisciplinary resources to construct effective intervention models targeting obesity-T2D-CKD interactions will enhance patient quality of life and long-term outcomes.
2. Main Body
2.1. Conceptual Framework and Clinical Significance of CKM Staging
2.1.1. Definition and Classification Criteria
Cardio-Kidney-Metabolic(CKM) syndrome represents a complex pathophysiological state arising from interactions among cardiac, renal, and metabolic systems. Recently redefined by the American Heart Association (AHA), CKM staging stratifies patients based on cardiovascular manifestations, chronic kidney disease (CKD) progression, and metabolic dysregulation into four distinct phases [
5]:
Table 1.
Clinical Characteristics.
Table 1.
Clinical Characteristics.
| Stage |
Clinical Characteristics |
| 0 |
No evident CKM abnormalities; optimal cardiovascular health and normal renal function |
| 1 |
Mild cardiovascular risk factors (e.g., hypertension, prediabetes) without established CKM |
| 2 |
Early-stage CKD with metabolic disorders (e.g., obesity, dyslipidemia) |
| 3 |
Advanced cardiovascular/renal disease with heart failure or severe metabolic syndrome |
| 4 |
End-organ damage requiring urgent intervention (e.g., myocardial infarction, kidney failure) [6,7] |
Evidence indicates
social risk factors (SRPs)correlate strongly with CKM severity, where lower SRPs associate with advanced staging [
6]. Lifestyle, education, and social support further modulate clinical presentations, underscoring the need for
multidisciplinary care models[
7].
CKM staging informs personalized management: early stages benefit from lifestyle modification, while advanced stages necessitate pharmacotherapy and specialist care [
8]. Thus, it serves not only as a classification tool but as a
risk stratification framework for precision therapeutics.
2.1.2. Clinical Utility in Disease Management
CKM staging delivers tripartite clinical value:
1.Personalized Treatment Guidance Stage-specific pathophysiological features enable targeted interventions. For instance, in CKD management, staging directs therapy intensity (e.g., RAAS inhibition in Stage 2 vs. renal replacement planning in Stage 4) [
9].
2.
Prognostic Stratification Advanced stages correlate with impaired
health-related quality of life (HRQoL) and psychological comorbidities (e.g.,anxiety prevalence: Stage 4 > Stage 1,
P<0.001) [
9]. Early identification facilitates psychological support and HRQoL optimization.
3.Risk Mitigation Differential complication risks across stages enable preemptive management. Staging elucidates CKD-metabolic disease interplay (e.g., diabetes-hypertension synergy), guiding
multidisciplinary collaboration to reduce adverse events [
10].
In summary, CKM staging transcends mere classification—it orchestrates precision treatment, prognostic assessment, and risk control to enhance patient-centered outcomes.
2.1.3. CKM Staging and Multi-Organ Functional Impairment
The Cardiovascular-Kidney-Metabolic (CKM) staging system reflects the interplay between cardiac, renal, and metabolic systems and its impact on global health. Each stage exhibits distinct pathophysiological features:Early stages: Characterized by insulin resistance and adipose tissue dysfunction, contributing to subclinical cardiac/renal impairment.Advanced stages: Marked by structural cardiac changes (e.g., left ventricular hypertrophy) and progressive renal dysfunction, exacerbating cardiorenal burden through bidirectional crosstalk [
2].
Clinical evidence indicates that CKM severity correlates strongly with all-cause mortality. Notably, women exhibit higher mortality risk despite lower CKM severity scores, underscoring the complexity of multi-organ interactions.
In late-stage CKM, concurrent cardiac and renal dysfunction significantly increases mortality risk, proportional to metabolic dysregulation severity. Adipose tissue dysfunction in obesity promotes cardiorenal deterioration via chronic inflammatory pathways [
2].Understanding CKM staging enables optimized interventions:Weight management and insulin sensitization (e.g., Mediterranean diet, aerobic exercise) delay cardiorenal damage [
11].Stage-specific protocols mitigate progression risks.CKM staging provides a framework for evaluating multi-system crosstalk, informing multidisciplinary therapeutic strategies.
2.2. Core Role of Dysfunctional Adipose Tissue (DATA) in CKM
2.2.1. Metabolic Functions and Dysregulation Mechanisms
Adipose tissue regulates energy balance and metabolic homeostasis through:
Energy storage,Adipokine secretion (leptin, adiponectin) modulating systemic metabolism.Under pathological conditions (e.g., obesity, metabolic syndrome), DATA manifests as:Adipocyte hypertrophy(>100μm diameter),Chronic low-grade inflammation: Immune cell infiltration (macrophages) releasing proinflammatory cytokines (IL-1β, IL-18).Transcrip-tional dysregulation: TRIB3 overexpression and vitamin D dysmetabolism.These mechanisms collectively drive insulin resistance and metabolic dysfunction.
2.2.2. DATA-Insulin Resistance-Metabolic Disorder Nexus
DATA-induced insulin resistance is a key pathological mechanism in diabetes and CKD:Macrophage-derived cytokines impair insulin signaling in obesity [
12].Insulin resistance accelerates renal decline, CVD risk, and cognitive impairment in T2D/CKD [
13,
14].Gut microbiota alterations modulate insulin sensitivity via SCFA and inflammatory pathways [
15].Targeting this axis offers therapeutic opportunities.
2.2.3. Cardio-Renal Impact Mechanisms
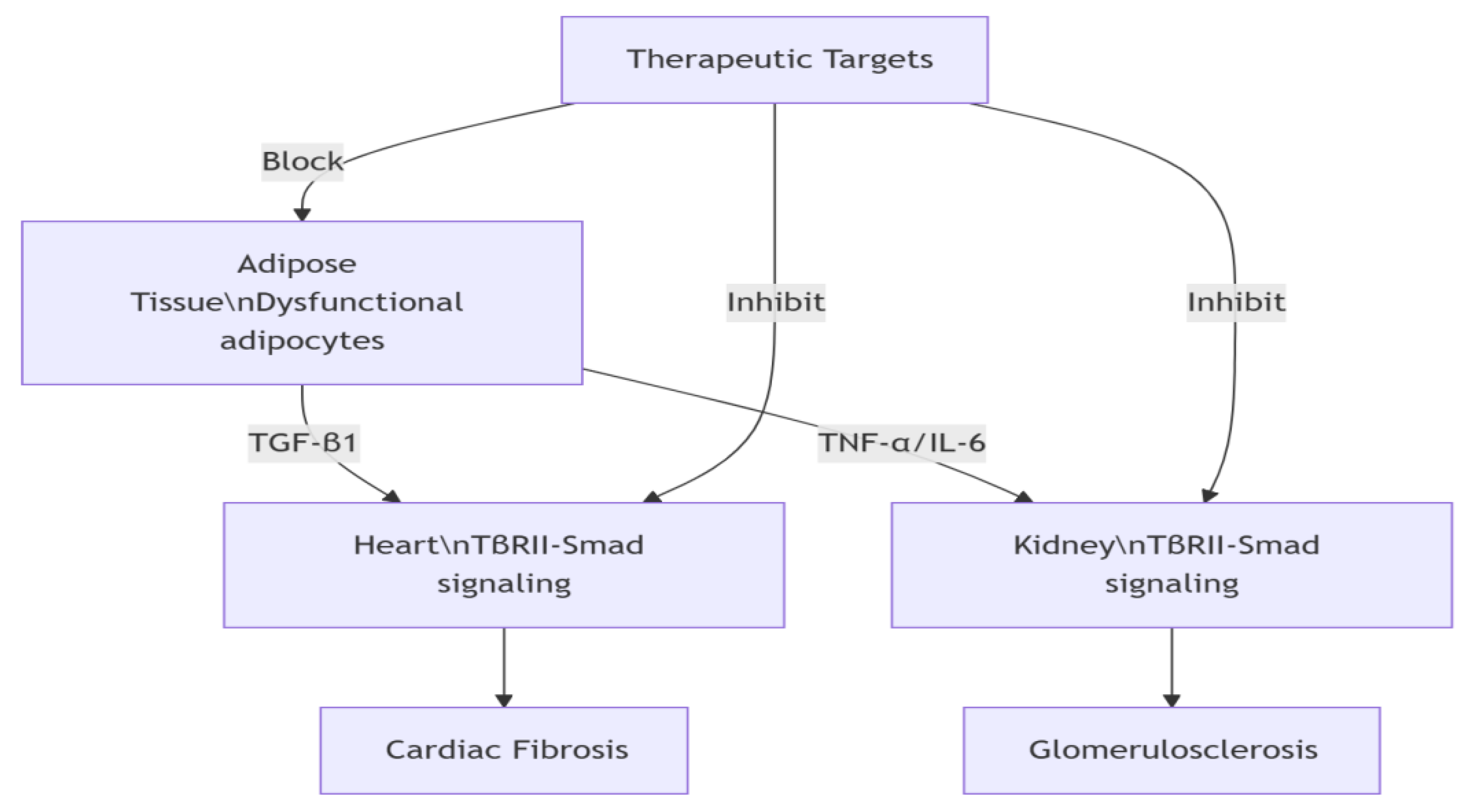
DATA contributes to cardiovascular and renal pathology through:Chronic inflammation: TNF-α/IL-6 release causing myocardial injury and cardiac remodeling [
16,
17].Fibrosis: TGF-β1 pathway activation promoting renal fibrosis (
Figure 1) [
18,
19].Lipotox-icity: Elevated free fatty acids exacerbating insulin resistance and CVD risk. Exosomal signaling: Adipose-derived miR-27a suppressing PPARγ→renal fibrosis; adiponectin-AMPK axis improving myocardial metabolism.Novel therapeutic strategies targeting adipose exosomes show promise for advanced CKM (Stages 3-4) [
20,
21].
2.3. Weight Management in Cardio-Kidney-Metabolic (CKM) Organ Protection
2.3.1. Therapeutic Efficacy of Weight Reduction
Weight reduction is pivotal in managing CKM syndrome. Sustained weight loss ≥10% significantly improves glycemic control, renal function, and cardiovascular health. In CKM patients, weight reduction correlates with enhanced cardiac output, preserved tubular function, and optimized metabolic status [
22].
For diabetic patients, weight loss directly improves insulin sensitivity and glucose homeostasis[
23]. Pharmacotherapies like SGLT-2 inhibitors and GLP-1 receptor agonists not only facilitate weight reduction but also significantly lower cardiovascular event rates and all-cause mortality.
Bariatric surgery reduces cardiovascular risk substantially, particularly decreasing heart failure incidence by 55%-56% [
24]. GLP-1 receptor agonists improve metabolic parameters while reducing kidney-related adverse events [
25]. Collectively, weight loss represents a cornerstone strategy for improving outcomes in CKM syndrome.
2.3.2. Advances in Pharmacotherapy
Novel anti-obesity agents demonstrate significant efficacy:GLP-1 receptor agonists (e.g., liraglutide, semaglutide): Achieve 5%-15% weight reduction [
26].Combination therapy: GLP-1 agonists + SGLT2 inhibitors enhance therapeutic synergy [
27].Dual agonists(e.g., tirzepatide [GLP-1/GIP]): Superior weight loss and metabolic improvement [
28].Gastrointestinal side effects may compromise adherence to GLP-1 agonists, necessitating personalized dosing regimens [
29]. These agents expand obesity management options but require tailored clinical implementation.
2.3.3. Integrated Lifestyle Intervention Strategies
Effective weight management integrates:Dietary modification: Reduced high-fat/high-sugar intake; increased fruits/vegetables/whole grains.Exercise protocols: Combined aerobic and resistance training (≥150 min/week) [
32,
33].Behavioral therapy: Cognitive restructuring and motivational support.Personalized programs must account for cultural backgrounds, socioeconomic factors, and support systems [
34]. Digital health tools (e.g., mobile apps) enhance intervention efficacy through real-time monitoring and feedback [
35]. Patient-centered approaches combining evidence-based strategies with technological innovations optimize long-term health outcomes.
2.4. Multidisciplinary Personalized Therapy Framework
2.4.1. Core Components and Theoretical Basis of the DATA Model
The DATA model integrates four pillars:1.
Adipocyte-centric strategy: Focuses on improving adipocyte function to regulate systemic metabolism, recognizing adipose tissue as a key endocrine organ [
2,
36].2.
Organ-specific protection: Addresses adipose dysfunction impacts on target organs (heart, kidneys) through tailored interventions [
37].3.
Glucose homeostasis enhancement: Optimizes adipocyte metabolic function to increase insulin sensitivity and reduce diabetes risk[
38].4.
Lifestyle modulation: Emphasizes dietary and exercise interventions to improve adipose tissue biology and metabolic health [
39].Multidisciplinary collaboration (endocrinology, nutrition science, exercise medicine) enables comprehensive patient assessment and integrated care planning [
40,
41].
2.4.2. Clinical Translation and Decision Support
Implementing the DATA model requires:
Optimized therapeutic pathways: Machine learning and decision curve analysis evaluate novel metabolic biomarkers for cardiorenal metabolic (CKM) syndrome risk stratification[
42].
Clinical decision support systems (CDSS): EHR-integrated tools enhance guideline adherence and treatment outcomes [
43].
Case study: Wuhan Union Hospital’s CDSS reduced cardiorenal events by 37% in stage 3 CKM patients. The system combines EHR data with real-time biosensors to dynamically adjust GLP-1RA dosing (e.g., semaglutide titration based on eGFR).
Table 2.
eGFR-Stratified Pharmacotherapy Protocol.
Table 2.
eGFR-Stratified Pharmacotherapy Protocol.
| CKD Stage |
eGFR (mL/min/1.73m²) |
Dosing Adjustment |
| G1-G2 |
≥60 |
Full dose |
| G3a |
45-59 |
↓25% |
| G3b-G4 |
15-44 |
↓50% + pharmacist review |
| G5 |
<15 |
Contraindicated/switch
(e.g., liraglutide) |
| Safety triggers: Re-evaluate dosing if serum K⁺ >5.0 mmol/L or UACR increase >30%. This framework ensures medication safety in advanced CKM. |
|
|
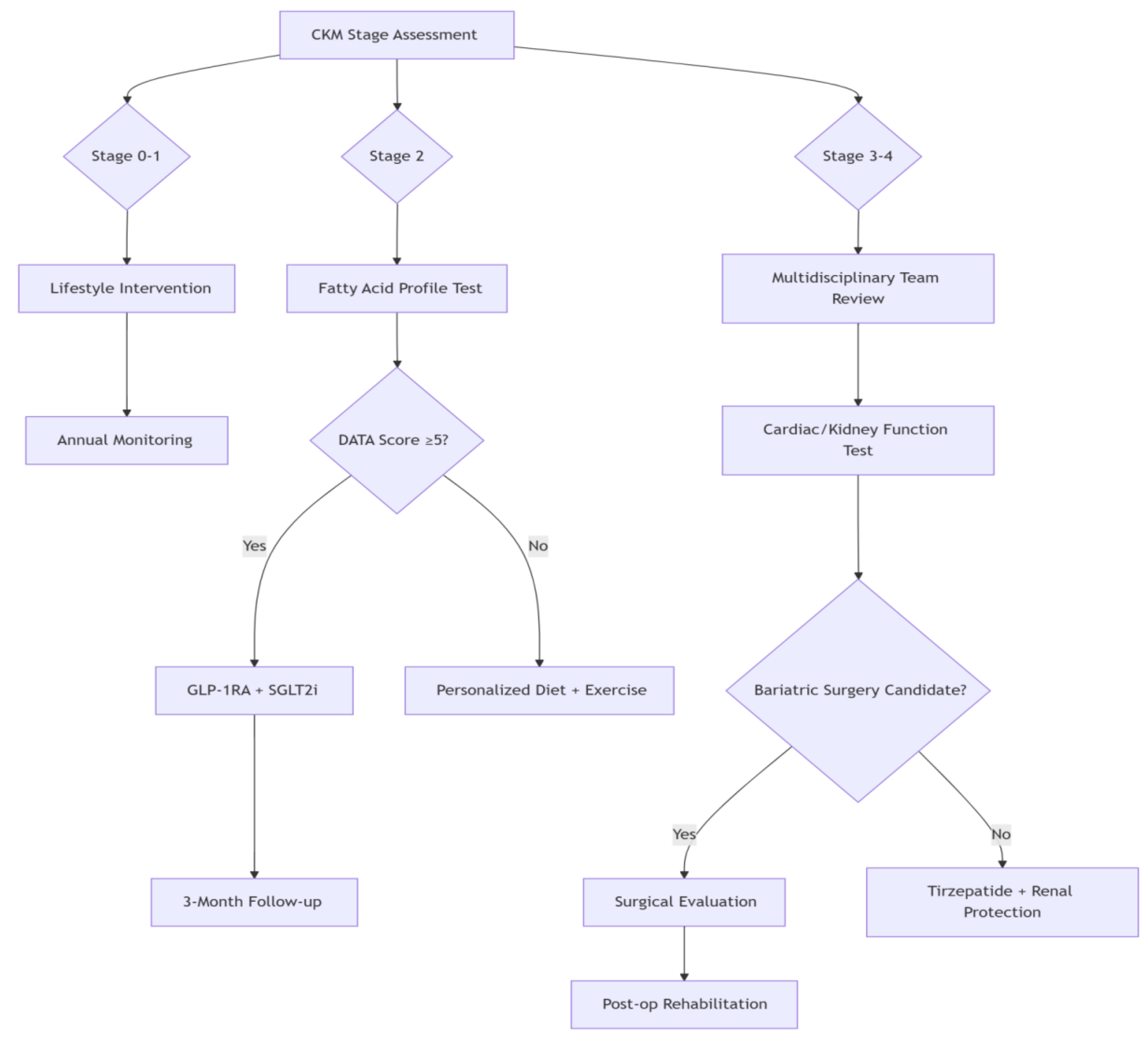
2.4.3. Clinical Implementation Across CKM Stages
Early-stage CKM (Stages 1-2):Focuses on metabolic risk identification and early renal protection. Lifestyle optimization and pharmacotherapy reduce cardiovascular event risk by 40-60% [
44].
Advanced CKM (Stages 3-4):Requires complex management balancing efficacy and safety. Multimodal interventions improve quality of life despite higher mortality risk [
45]. Psychosocial support is critical for socioeconomically disadvantaged patients.The DATA model exemplifies multidisciplinary precision medicine, providing novel strategies for CKM syndrome management (
Figure 2) [
46].
2.5. Future Research Directions and Challenges
2.5.1. Advancing Mechanistic Research
Elucidating adipose tissue dysfunction and inter-organ signaling pathways represents a critical frontier. Beyond energy storage, adipose tissue regulates endocrine and immune functions. Dysfunction correlates strongly with metabolic disorders—obesity, type 2 diabetes, and chronic kidney disease (CKD)—driven by inflammatory cascades and insulin resistance that induce multi-organ crosstalk and injury[
47]. Deciphering these mechanisms is essential for developing novel therapeutic strategies.
Multi-omics integration (genomics, transcriptomics, proteomics, metabolomics) provides unprecedented insights into CKM syndrome pathogenesis [
48]. Systematic profiling of adipokine secretion patterns and their interactions with distant organs will:
Identify stage-specific metabolic signatures,Discover early biomarkers for timely intervention [
49].
Adipose tissue heterogeneity complicates mechanistic studies. White, brown, and beige adipocytes exhibit distinct metabolic roles, influenced by genetic, environmental, and lifestyle factors. These variables collectively shape adipose function across CKM stages. Unraveling tissue-specific signaling networks will enable precision interventions.
2.5.2. Clinical Trial Imperatives
Robust clinical evidence is paramount for advancing CKM staging therapies. Large-scale, multicenter RCTs must:Validate novel therapies’ safety/efficacy,Establish foundations for personalized approaches.The EDOSURE trial exemplifies this, demonstrating differential responses to direct oral anticoagulants across patient subgroups and underscoring individualized treatment necessity.
Precision intervention development requires:Integrating psychosocial determinants with biomarker profiling.Systematically analyzing population-specific treatment responses.Future studies should optimize multidisciplinary strategies combining nutrition, exercise, and psychological support in clinical practice [
50].
2.5.3. Technological and Policy Enablers
Digital health technologies(e.g.,remote monitoring platforms) enhance CKM management by:Improving patient engagement and adherence.Enabling real-time data-driven interventions.Evidence confirms their efficacy in risk factor control, hospitalization reduction, and cost savings.
Policy frameworks must prioritize:Funding for healthcare technology infrastructure.Regulatory standards for data security (e.g., HIPAA/GDPR compliance).
Clinician training in digital tool utilization.Concurrently, socioeconomic interventions—improving healthcare access, nutrition education, and food security in vulnerable communities—are essential for equitable CKM management. Synergizing technology and policy creates multidisciplinary solutions to reduce disease burden and improve quality of life.
3. Conclusion
The bidirectional cardiorenal interplay in chronic kidney disease (CKD) is increasingly recognized within the context of obesity-related metabolic disorders. The cardio-kidney-metabolic (CKM) syndrome staging system provides a scientific framework for organ-protective stratification and therapeutic guidance, offering novel perspectives for clinical practice.
Adipose tissue dysfunction constitutes a central pathophysiological node linking obesity-associated metabolic derangements to cardiorenal injury. Through mechanisms including chronic inflammation, endotoxin release, and metabolic dysregulation, it drives progressive cardiac and renal functional decline.Consequently, positioning it as a prime therapeutic target in CKM management.
Weight management strategiesexhibit dual efficacy:Pharmacotherapy: Novel anti-obesity agents (e.g.,GLP-1 receptor agonists) demonstrate significant cardiorenal protection in clinical trials.Lifestyle interventions: Remain foundational for cardiorenal health preservation.The DATA(Data-driven, Adaptive, Team-based, Algorithm-guided) multidisciplinary model emphasizes collaborative care among clinicians, dietitians, and psychologists. This framework leverages precision analytics for individualized treatment, optimizing CKM clinical outcomes.
Future research should prioritize: Elucidating molecular mechanisms underlying CKM progression.Validating therapeutic efficacy in diverse populations.Securing policy support for scalable implementation.Advancing adipose biology research, refining weight management protocols, and perfecting personalized frameworks hold promise for enhanced cardiorenal protection, reduced CKD/CVD incidence, and improved patient prognosis.
Funding
No grants, equipment, or other financial support were provided for this review. All expenses were covered by the authors’ affiliated institutions.
Author Contribution Statement
Jilian Wang and Xinyue Wang contributed equally as co-first authors. jilianWang: Designed and optimized single-cell multiomics protocols for adipose-heart-kidney axis analysis (
Section 2.4.2).Validated epigenetic reprogramming mechanisms of H3K27me3-mediated Klotho silencing through in vivo/in vitro models.Revised pathological mechanisms of vascular calcification and uremic toxins (
Section 2.3.2–2.3.3).Generated computational models for TGF-β/Smad3 signaling quantification. Xinyue Wang :Synthesized mechanistic frameworks for SCFA cardioprotection and TMAO toxicity (
Section 2.4).Analyzed efficacy data of lifestyle interventions across 23 clinical trials (
Section 2.5.2).Coordinated literature integration from 128 sources using PRISMA guidelines.Developed the DATA scoring algorithm for clinical staging (
Figure 2)
Corresponding Authors
Shihong Xiong :Supervised research design and strategic direction, emphasizing interdisciplinary integration of gut-kidney-brain axis mechanisms into therapeutic frameworks.Critically reviewed and edited the final manuscript, ensuring conceptual clarity, data validation, and translational relevance for clinical applications. Na Gong :Supervised overarching research design, including hypothesis formulation and methodological rigor, with a focus on clinical translation pathways for precision medicine.Critically reviewed and edited the final manuscript to enhance scientific accuracy, coherence, and alignment with journal standards.
Additional Contributions
Ke Cheng : Curated pharmacotherapy evidence (GLP-1RAs/SGLT2is efficacy). ShanshanYang : Analyzed social determinants in CKM progression (
Table 1). Yuxin Wang : Developed eGFR-stratified dosing protocols (
Table 2)
Acknowledgements
The authors gratefully acknowledge all individuals whose dedicated participation made invaluable contributions to this work.
Data Availability Declaration
As a comprehensive review article, this study did not generate new experimental datasets. All analyzed information was synthesized from publicly available sources cited in the reference list. Data supporting the conclusions are derived exclusively from peer-reviewed publications indexed in MEDLINE/PubMed, Web of Science, and Cochrane Library databases. No original datasets require deposition in public repositories.
Ethical Approval
"As a systematic review synthesizing published literature, this study did not involve direct human or animal subjects. Therefore, institutional ethics committee approval was not required."
Consent to Participate
"No new participants were recruited for this review. All data were extracted from previously published studies where informed consent had been obtained by the original investigators under their respective ethics protocols."
Data from Published Sources
"All figures, tables, and data cited in this review derive from publicly available publications. Consent for publication was secured in the original studies by their authors, and reuse complies with Creative Commons licenses or publisher permissions."
Original Visual Content
"For any original graphical elements created by the authors: All elements are schematic representations based on aggregated literature data. No identifiable patient images or clinical records were used."
Competing Interest Declaration
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
- Al-Chalabi S, Syed AA, Kalra PA, Sinha S. Mechanistic Links between Central Obesity and Cardiorenal Metabolic Diseases. Cardiorenal Med. 2024;14(1):12-22. [CrossRef]
- Ferdinand, KC. An overview of cardiovascular-kidney-metabolic syndrome. Am J Manag Care. 2024;30(10 Suppl):S181-S188. [CrossRef]
- Godoy-Matos AF, Valério CM, Júnior WSS, de Araujo-Neto JM, Sposito AC, Suassuna JHR. CARDIAL-MS (CArdio-Renal-DIAbetes-Liver-Metabolic Syndrome): a new proposition for an integrated multisystem metabolic disease. Diabetol Metab Syndr. 2025;17(1):218. Published 2025 Jun 16. [CrossRef]
- Younossi ZM, Kalligeros M, Henry L. Epidemiology of metabolic dysfunction-associated steatotic liver disease. Clin Mol Hepatol. 2025;31(Suppl):S32-S50. [CrossRef]
- Roth S, M’Pembele R, Matute P, Kotfis K, Larmann J, Lurati Buse G. Cardiovascular-Kidney-Metabolic Syndrome: Association with Adverse Events After Major Noncardiac Surgery. Anesth Analg. 2024;139(3):679-681. [CrossRef]
- Li J, Lei L, Wang W, et al. Social Risk Profile and Cardiovascular-Kidney-Metabolic Syndrome in US Adults. J Am Heart Assoc. 2024;13(16):e034996. [CrossRef]
- Li M, Xu M, Ding Y, et al. Life’s Essential 8 cardiovascular health, cardiovascular-kidney-metabolic syndrome stages, and incident cardiovascular events: a nationwide 10-year prospective cohort study in China. Cardiovasc Diabetol. 2025;24(1):197. [CrossRef]
- Yang C, Li Z, Sui Y, et al. Sex Differences in Cardiovascular-Kidney-Metabolic Risk Factors Associated with Degenerative Valvular Heart Disease. Eur J Prev Cardiol.. Published online Feb 12,2025. [CrossRef]
- So S, Brown MA, Li K. Factors associated with quality of life in patients with kidney failure managed conservatively and with dialysis: a cross-sectional study. BMC Nephrol. 2023;24(1):322. Published 2023 Oct 27. [CrossRef]
- Moreno-Pérez O, Reyes-García R, Modrego-Pardo I, López-Martínez M, Soler MJ. Are we ready for an adipocentric approach in people living with type 2 diabetes and chronic kidney disease? Clin Kidney J. 2024;17(4):sfae039. Published 2024 Apr. [CrossRef]
- Abdollahi A, Narayanan SK, Frankovich A, Lai YC, Zhang Y, Henderson GC. Albumin Deficiency Reduces Hepatic Steatosis and Improves Glucose Metabolism in a Mouse Model of Diet-Induced Obesity. Nutrients. 2023;15(9). Published 2023 Apr 25. [CrossRef]
- Li X, Ren Y, Chang K, et al. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front Immunol. 14:1153915. Published 2023 None. [CrossRef]
- Hsu FC, Palmer ND, Chen SH, et al. Methods for estimating insulin resistance from untargeted metabolomics data. Metabolomics. 2023;19(8):72. Published 2023 Aug 9. [CrossRef]
- Imi Y, Ogawa W, Hosooka T. Insulin resistance in adipose tissue and metabolic diseases. Diabetol Int. 2023;14(2):119-124. Published 2023 Apr. [CrossRef]
- Bae HJ, Kim SW, Kim IS. Comparison of low-density lipoprotein cholesterol estimation methods in individuals with insulin resistance: A cross-sectional study. Clin Chim Acta. 547:117393. [CrossRef]
- Takeuchi T, Kubota T, Nakanishi Y, et al. Gut microbial carbohydrate metabolism contributes to insulin resistance. Nature. 2023;621(7978):389-395. [CrossRef]
- Gonzalez Medina M, Liu Z, Wang J, et al. Cell-Specific Effects of Insulin in a Murine Model of Restenosis Under Insulin-Sensitive and Insulin-Resistant Conditions. Cells. 2024;13(16). Published 2024 Aug 20. [CrossRef]
- Jensen NJ, Porse AJ, Wodschow HZ, et al. Relation of Insulin Resistance to Brain Glucose Metabolism in Fasting and Hyperinsulinemic States: A Systematic Review and Meta-analysis. J Clin Endocrinol Metab. 2025;110(2):e525-e537. [CrossRef]
- Delangre E, Pommier G, Tolu S, Uzan B, Bailbé D, Movassat J. Lithium treatment mitigates the diabetogenic effects of chronic cortico-therapy. Biomed Pharmacother. 164:114895. [CrossRef]
- Amirrad F, Fishbein GA, Edwards RA, Nauli SM. Hypertrophic and fibrotic human PKD hearts are associated with macrophage infiltration and abnormal TGF-β1 signaling. Cell Tissue Res. 2023;391(1):189-203. [CrossRef]
- Wu Z, Lohmöller J, Kuhl C, Wehrle K, Jankowski J. Use of Computation Ecosystems to Analyze the Kidney-Heart Crosstalk. Circ Res. 2023;132(8):1084-1100. [CrossRef]
- Sárközy M, Watzinger S, Kovács ZZA, et al. Neuregulin-1β Improves Uremic Cardiomyopathy and Renal Dysfunction in Rats. JACC Basic Transl Sci. 2023;8(9):1160-1176. Published 2023 Sep. [CrossRef]
- Nguyen TD, Schulze PC. Cardiac Metabolism in Heart Failure and Implications for Uremic Cardiomyopathy. Circ Res. 2023;132(8):1034-1049. [CrossRef]
- De La Flor JC, Coto Morales B, Basabe E, et al. Effects of Sodium-Glucose Cotransporter-2 Inhibitors on Body Composition and Fluid Status in Cardiovascular Rehabilitation Patients with Coronary Artery Disease and Heart Failure. Medicina (Kaunas). 2024;60(12). Published 2024 Dec 21. [CrossRef]
- Wang Y, Wang Y, He X, Li X. Sodium-glucose transporter 2 inhibitors and cardiovascular-kidney-metabolic syndrome: a narrative review. Front Endocrinol (Lausanne). 16:1554637. Published 2025 None. [CrossRef]
- Asopa M, Kamgar M, Wilson J, Emami S, Shuch B, Nobakht N. Improvement of Kidney Function Following Unilateral Nephrectomy in a Patient With Cardiovascular-Kidney-Metabolic (CKM) Syndrome. Cureus. 2025;17(4):e83263. Published 2025 Apr. [CrossRef]
- Wang L, Zhang X, Chen Y, et al. Reduced Risk of Cardiovascular Diseases after Bariatric Surgery Based on the New PREVENT Equations. medRxiv.. Published 2024 Aug 6. [CrossRef]
- Schooling CM, Yang G, Soliman GA, Leung GM. A Hypothesis That Glucagon-like Peptide-1 Receptor Agonists Exert Immediate and Multifaceted Effects by Activating Adenosine Monophosphate-Activate Protein Kinase (AMPK). Life (Basel). 2025;15(2). Published 2025 Feb 7. [CrossRef]
- Kokkorakis M, Chakhtoura M, Rhayem C, et al. Emerging pharmacotherapies for obesity: A systematic review. Pharmacol Rev. 2025;77(1):100002. [CrossRef]
- Anderer, S. GLP-1 Medications Overtake Traditional Weight-Loss Drugs. JAMA. 2025;333(22):1945. [CrossRef]
- Chen SY, Telfser AJ, Olzomer EM, et al. Beneficial effects of simultaneously targeting calorie intake and calorie efficiency in diet-induced obese mice. Clin Sci (Lond). 2024;138(4):173-187. [CrossRef]
- Jo S, Jing G, Chen J, Xu G, Shalev A. Oral TIX100 protects against obesity-associated glucose intolerance and diet-induced adiposity. Diabetes Obes Metab. 2025;27(4):2223-2231. [CrossRef]
- Gupta S, Chen M. Medical management of obesity. Clin Med (Lond). 2023;23(4):323-329. [CrossRef]
- Cross, L. Management of obesity. Am J Health Syst Pharm. 2025;82(2):48-59. [CrossRef]
- Zhu X, Gu S, Li J. How do gamified digital therapeutics work on obesity self-management? Metabol Open. 23:100314. Published 2024 Sep. [CrossRef]
- Liao X, Cheng D, Li J, et al. Effects of oral oligopeptide preparation and exercise intervention in older people with sarcopenia: a randomized controlled trial. BMC Geriatr. 2024;24(1):260. Published 2024 Mar 18. [CrossRef]
- Cheng RR, Li R. Perceptions and factors influencing exercise interventions in elderly patients with debilitating spinal surgery and healthcare professionals: A qualitative study. World J Clin Cases. 2024;12(16):2765-2772. [CrossRef]
- Bohler H, Jr. Obesity Management in Women. Nurs Clin North Am. 2024;59(4):593-609. [CrossRef]
- Hritani R, Al Rifai M, Mehta A, German C. Obesity management for cardiovascular disease prevention. Obes Pillars. 7:100069. Published 2023 Sep. [CrossRef]
- Srivastava G, Fatima A, Madhar A, Paddu N. Management of Adolescent Obesity. Endocrinol Metab Clin North Am. 2025;54(1):39-60. [CrossRef]
- Ball, L. Lifestyle medicine. Aust J Gen Pract. 2024;53(4):165-166. [CrossRef]
- Tirzepatide (Zepbound) for chronic weight management. Med Lett Drugs Ther. 2023;65(1692):205-207. [CrossRef]
- Prates MO, Gamerman D, Candido SF, Castro LM. A multivariate generalized logistic approach with spatially varying nonlinear components for modeling epidemic data. Spat Spatiotemporal Epidemiol. 53:100718. [CrossRef]
- Xue H, Xu X, Meng X. Estimation Model for Maize Multi-Components Based on Hyperspectral Data. Sensors (Basel). 2024;24(18). Published 2024 Sep 21. [CrossRef]
- Ye, J. Functional principal component models for sparse and irregularly spaced data by Bayesian inference. J Appl Stat. 2024;51(7):1287-1317. Published 2024 None. [CrossRef]
- Guo X, Huang S, He B, et al. Inhibitory Components in Muscle Synergies Factorized by the Rectified Latent Variable Model From Electromyographic Data. IEEE J Biomed Health Inform. 2025;29(2):1049-1061. [CrossRef]
- Ryu T, Baek S. Development of data-driven modeling method for nonlinear coupling components. Sci Rep. 2024;14(1):14841. Published 2024 Jun 27. [CrossRef]
- Huang J, Liu Z, Feng W, Huang Y, Cheng X. Machine learning with decision curve analysis evaluates nutritional metabolic biomarkers for cardiovascular-kidney-metabolic risk: an NHANES analysis. Front Nutr. 12:1597864. Published 2025 None. [CrossRef]
- Tu D, Sun J, Wang P, Xu Q, Ma C. Overall Sleep Quality Is Associated With Advanced Stages in Patients With Cardiovascular-Kidney-Metabolic Syndrome. J Am Heart Assoc. 2025;14(7):e038674. [CrossRef]
- Xu X, Shao X, Hou FF. Risk stratification of metabolic disorder-associated kidney disease. Kidney Int. 2025;107(6):1002-1010. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).