Submitted:
01 September 2025
Posted:
02 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Omentin: Biochemistry and General Functions
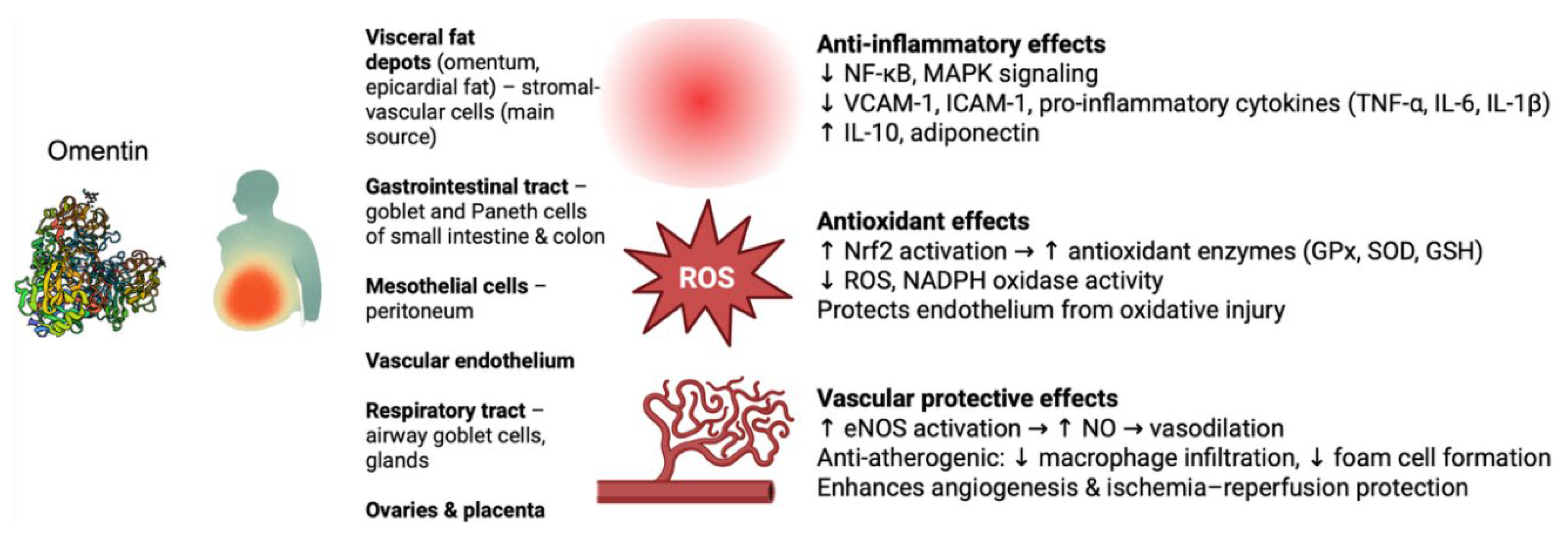
2.1. Anti-Inflammatory and Immune Regulatory Roles
2.2. Antioxidant Effects and Oxidative Stress Modulation
2.3. Vascular Biology and Vascular Protective Functions
3. The Role of Omentin in Metabolic Disorders
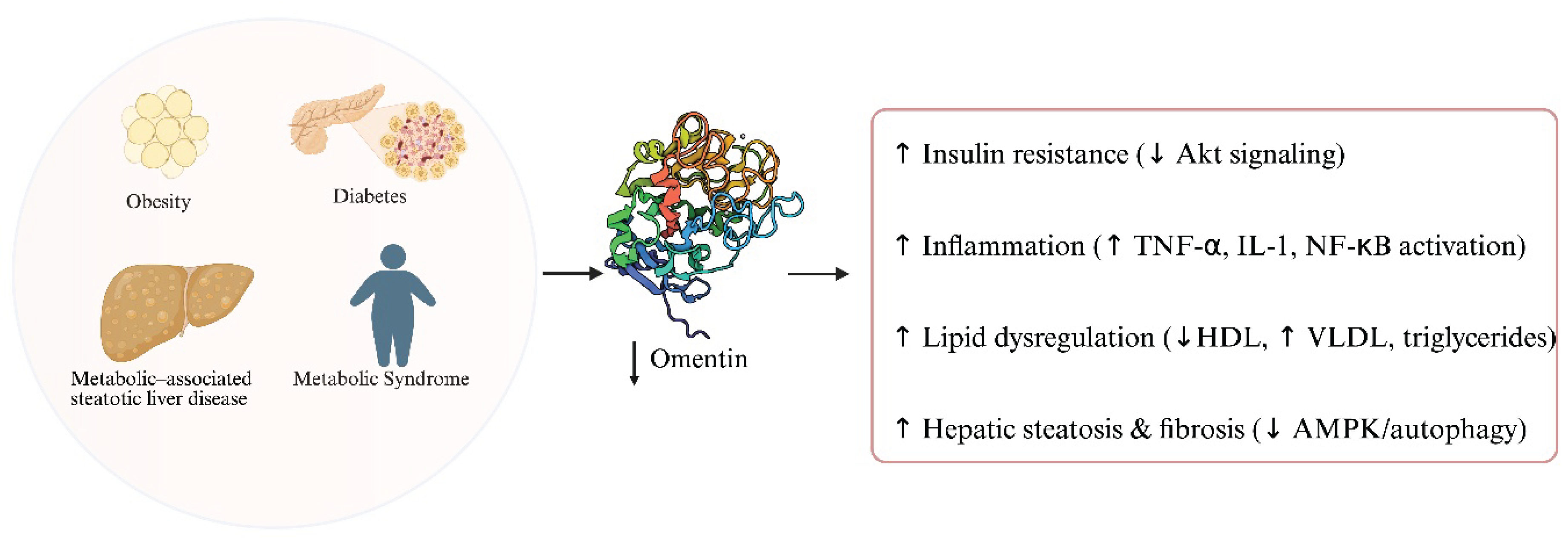
3.1. Omentin and Obesity
3.2. Omentin, Insulin Resistance and Diabetes
3.3. Omentin and Metabolic Syndrome
3.4. Omentin and Metabolic–Associated Steatotic Liver Disease
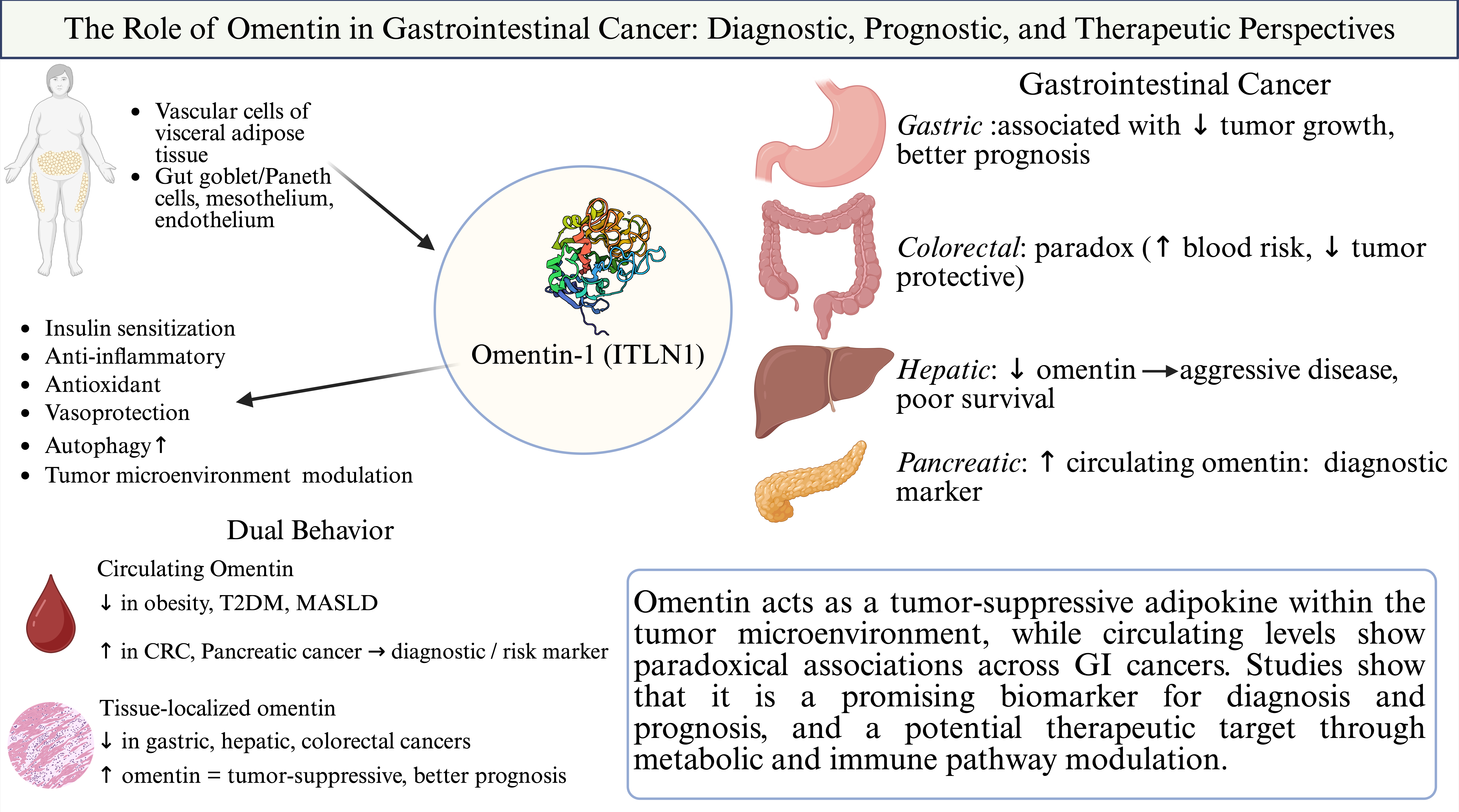
4. The Role of Omentin in Gastrointestinal Cancers
4.1. Upper Gastrointestinal Cancer
4.1.1. Esophageal Cancer
4.1.2. Gastric Cancer
4.2. Hepato-Pancreato-Biliary (HPB) Cancer
4.2.1. Hepatocellular Cancer
4.2.2. Pancreatic Cancer
4.3. Colorectal Cancer
5. Directions and Research Needs
5.1. What Is Missing About Omentin’s Role in GI Cancers?
5.2. Proposals for Future Research Focusing on Translational and Clinical Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| AUC | Area under the curve |
| BE | Barrett’s esophagus |
| CA19-9 | Carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| CDX2 | Caudal-type homeobox 2 |
| CP | Chronic pancreatitis |
| CRC | Colorectal cancer |
| CSC | Cancer stem cell |
| CXCL2 | C-X-C motif chemokine ligand 2 |
| EAC | Esophageal adenocarcinoma |
| EPC | Endothelial progenitor cell |
| ERK | Extracellular signal–regulated kinase |
| ESCC | Esophageal squamous cell carcinoma |
| HCC | Hepatocellular carcinoma |
| HNF4α | Hepatocyte nuclear factor 4 alpha |
| HOMA-IR | Homeostatic model assessment of insulin resistance |
| HR | Hazard ratio |
| IHC | Immunohistochemistry |
| ITLN1 | Intelectin-1 (gene encoding omentin-1) |
| JNK | c-Jun N-terminal kinase |
| LF | Lactoferrin |
| MAPK | Mitogen-activated protein kinase |
| MASLD | Metabolic-associated steatotic liver disease |
| MASH | Metabolic-associated steatohepatitis |
| MDSC | Myeloid-derived suppressor cell |
| miRNA | MicroRNA |
| NF-κB | Nuclear factor kappa B |
| NK cell | Natural killer cell |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| OR | Odds ratio |
| PA | Pancreatic adenocarcinoma |
| PDAC | Pancreatic ductal adenocarcinoma |
| PI3K | Phosphoinositide 3-kinase |
| PPAR-δ | Peroxisome proliferator-activated receptor delta |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RFS | Recurrence-free survival |
| ROC | Receiver operating characteristic |
| ROS | Reactive oxygen species |
| SIRT1 | Sirtuin 1 |
| SNP | Single-nucleotide polymorphism |
| TCGA | The Cancer Genome Atlas |
| TMEM207 | Transmembrane protein 207 |
| TNF-α | Tumor necrosis factor alpha |
| TNM | Tumor–node–metastasis (staging) |
| TOP/FOP | TCF reporter constructs for Wnt/β-catenin signaling |
| VAT | Visceral adipose tissue |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| VEGF | Vascular endothelial growth factor |
| VLDL | Very low–density lipoprotein |
References
- Lu, L.; Mullins, C.S.; Schafmayer, C.; Zeißig, S.; Linnebacher, M. A Global Assessment of Recent Trends in Gastrointestinal Cancer and Lifestyle-Associated Risk Factors. Cancer Communications 2021, 41, 1137–1151. [Google Scholar] [CrossRef]
- Tan, B.K.; Adya, R.; Randeva, H.S. Omentin: A Novel Link Between Inflammation, Diabesity, and Cardiovascular Disease. Trends in Cardiovascular Medicine 2010, 20, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-Y.; Chan, L.; Zhou, S.-W. Omentin: Linking Metabolic Syndrome and Cardiovascular Disease. Curr Vasc Pharmacol 2014, 12, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhu, L.; Zheng, M.; Fan, C.; Li, Y.; Zhang, D.; He, Y.; Yang, H. Changes of Serum Omentin-1 Levels in Normal Subjects, Type 2 Diabetes and Type 2 Diabetes with Overweight and Obesity in Chinese Adults. Ann Endocrinol (Paris) 2014, 75, 171–175. [Google Scholar] [CrossRef] [PubMed]
- de Souza Batista, C.M.; Yang, R.-Z.; Lee, M.-J.; Glynn, N.M.; Yu, D.-Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; et al. Omentin Plasma Levels and Gene Expression Are Decreased in Obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef]
- Rothermel, J.; Lass, N.; Barth, A.; Reinehr, T. Link between Omentin-1, Obesity and Insulin Resistance in Children: Findings from a Longitudinal Intervention Study. Pediatr Obes 2020, 15, e12605. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Catalán, V.; Ortega, F.; Gómez-Ambrosi, J.; Ricart, W.; Frühbeck, G.; Fernández-Real, J.M. Circulating Omentin Concentration Increases after Weight Loss. Nutr Metab (Lond) 2010, 7, 27. [Google Scholar] [CrossRef]
- Saremi, A.; Asghari, M.; Ghorbani, A. Effects of Aerobic Training on Serum Omentin-1 and Cardiometabolic Risk Factors in Overweight and Obese Men. J Sports Sci 2010, 28, 993–998. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef]
- Paval, D.R.; Di Virgilio, T.G.; Skipworth, R.J.E.; Gallagher, I.J. The Emerging Role of Intelectin-1 in Cancer. Front Oncol 2022, 12, 767859. [Google Scholar] [CrossRef]
- Yang, R.-Z.; Lee, M.-J.; Hu, H.; Pray, J.; Wu, H.-B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.-W. Identification of Omentin as a Novel Depot-Specific Adipokine in Human Adipose Tissue: Possible Role in Modulating Insulin Action. Am J Physiol Endocrinol Metab 2006, 290, E1253–1261. [Google Scholar] [CrossRef]
- Dec, P.; Poniewierska-Baran, A.; Modrzejewski, A.; Pawlik, A. The Role of Omentin-1 in Cancers Development and Progression. Cancers 2023, 15, 3797. [Google Scholar] [CrossRef]
- Fain, J.N.; Sacks, H.S.; Buehrer, B.; Bahouth, S.W.; Garrett, E.; Wolf, R.Y.; Carter, R.A.; Tichansky, D.S.; Madan, A.K. Identification of Omentin mRNA in Human Epicardial Adipose Tissue: Comparison to Omentin in Subcutaneous, Internal Mammary Artery Periadventitial and Visceral Abdominal Depots. Int J Obes (Lond) 2008, 32, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Sena, C.M. Omentin: A Key Player in Glucose Homeostasis, Atheroprotection, and Anti-Inflammatory Potential for Cardiovascular Health in Obesity and Diabetes. Biomedicines 2024, 12, 284. [Google Scholar] [CrossRef]
- Washimi, K.; Yokose, T.; Yamashita, M.; Kageyama, T.; Suzuki, K.; Yoshihara, M.; Miyagi, Y.; Hayashi, H.; Tsuji, S. Specific Expression of Human Intelectin-1 in Malignant Pleural Mesothelioma and Gastrointestinal Goblet Cells. PLoS One 2012, 7, e39889. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, B.; Hao, C.; Huang, X.; Li, X.; Huang, Y.; Luo, Z. Omentin-A Novel Adipokine in Respiratory Diseases. International Journal of Molecular Sciences 2018, 19, 73. [Google Scholar] [CrossRef] [PubMed]
- Czechowski, P.; Hagemann, T.; Ghosh, A.; Sun, W.; Dong, H.; Noé, F.; Niersmann, C.; Reinisch, I.; Wolfrum, C.; Herder, C.; et al. Expression of Intelectin-1, Also Known as Omentin-1, Is Related to Clinical Phenotypes Such as Overweight, Obesity, Insulin Resistance, and Changes after Bariatric Surgery. Sci Rep 2024, 14, 22286. [Google Scholar] [CrossRef] [PubMed]
- Peña-Cano, M.I.; Valencia-Ortega, J.; Morales-Ávila, E.; Díaz-Velázquez, M.F.; Gómez-Díaz, R.; Saucedo, R. Omentin-1 and Its Relationship with Inflammatory Factors in Maternal Plasma and Visceral Adipose Tissue of Women with Gestational Diabetes Mellitus. J Endocrinol Invest 2022, 45, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Nonnecke, E.B.; Castillo, P.A.; Dugan, A.E.; Almalki, F.; Underwood, M.A.; De La Motte, C.A.; Yuan, W.; Lu, W.; Shen, B.; Johansson, M.E.V.; et al. Human Intelectin-1 (ITLN1) Genetic Variation and Intestinal Expression. Sci Rep 2021, 11, 12889. [Google Scholar] [CrossRef]
- Andresen, S.; Fantone, K.; Chapla, D.; Rada, B.; Moremen, K.W.; Pierce, M.; Szymanski, C.M. Human Intelectin-1 Promotes Cellular Attachment and Neutrophil Killing of Streptococcus Pneumoniae in a Serotype-Dependent Manner. Infect Immun 90, e00682-21. [CrossRef]
- Yamawaki, H.; Kuramoto, J.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. Omentin, a Novel Adipocytokine Inhibits TNF-Induced Vascular Inflammation in Human Endothelial Cells. Biochem Biophys Res Commun 2011, 408, 339–343. [Google Scholar] [CrossRef]
- Watanabe, T.; Watanabe-Kominato, K.; Takahashi, Y.; Kojima, M.; Watanabe, R. Adipose Tissue-Derived Omentin-1 Function and Regulation. In Comprehensive Physiology; Terjung, R., Ed.; Wiley, 2017; pp. 765–781 ISBN 978-0-470-65071-4.
- Hiramatsu-Ito, M.; Shibata, R.; Ohashi, K.; Uemura, Y.; Kanemura, N.; Kambara, T.; Enomoto, T.; Yuasa, D.; Matsuo, K.; Ito, M.; et al. Omentin Attenuates Atherosclerotic Lesion Formation in Apolipoprotein E-Deficient Mice. Cardiovasc Res 2016, 110, 107–117. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Qian, G.; Zhou, J. Omentin-1 Attenuates Adipose Tissue Inflammation via Restoration of TXNIP/NLRP3 Signaling in High-Fat Diet-Induced Obese Mice. Fundam Clin Pharmacol 2020, 34, 721–735. [Google Scholar] [CrossRef]
- Sigal, M.; Reinés, M. del M.; Müllerke, S.; Fischer, C.; Kapalczynska, M.; Berger, H.; Bakker, E.R.M.; Mollenkopf, H.-J.; Rothenberg, M.E.; Wiedenmann, B.; et al. R-Spondin-3 Induces Secretory, Antimicrobial Lgr5+ Cells in the Stomach. Nat Cell Biol 2019, 21, 812–823. [Google Scholar] [CrossRef]
- Kazama, K.; Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Omentin Plays an Anti-Inflammatory Role through Inhibition of TNF-α-Induced Superoxide Production in Vascular Smooth Muscle Cells. Eur J Pharmacol 2012, 686, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Queiroz, M.; Azul, L.; Seiça, R.; Sena, C.M. Omentin: A Novel Therapeutic Approach for the Treatment of Endothelial Dysfunction in Type 2 Diabetes. Free Radic Biol Med 2021, 162, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Fang, S.; Liu, X.; Li, J.; Wang, X.; Cui, J.; Chen, T.; Li, Z.; Yang, F.; Tian, J.; et al. Omentin-1 Protects against High Glucose-Induced Endothelial Dysfunction via the AMPK/PPARδ Signaling Pathway. Biochem Pharmacol 2020, 174, 113830. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Yan, W.; Chen, C.; Tang, M.; Zhao, X.; Feng, Q.; Fei, X.; Fu, Y. Omentin-1 Ameliorates Experimental Inflammatory Bowel Disease via Nrf2 Activation and Redox Regulation. Life Sci 2023, 328, 121847. [Google Scholar] [CrossRef] [PubMed]
- Binti Kamaruddin, N.A.; Fong, L.Y.; Tan, J.J.; Abdullah, M.N.H.; Singh Cheema, M.; Bin Yakop, F.; Yong, Y.K. Cytoprotective Role of Omentin Against Oxidative Stress-Induced Vascular Endothelial Cells Injury. Molecules 2020, 25, 2534. [Google Scholar] [CrossRef]
- Ohashi, K.; Shibata, R.; Murohara, T.; Ouchi, N. Role of Anti-Inflammatory Adipokines in Obesity-Related Diseases. Trends Endocrinol Metab 2014, 25, 348–355. [Google Scholar] [CrossRef]
- Zhong, X.; Li, X.; Liu, F.; Tan, H.; Shang, D. Omentin Inhibits TNF-α-Induced Expression of Adhesion Molecules in Endothelial Cells via ERK/NF-κB Pathway. Biochem Biophys Res Commun 2012, 425, 401–406. [Google Scholar] [CrossRef]
- Watanabe, K.; Watanabe, R.; Konii, H.; Shirai, R.; Sato, K.; Matsuyama, T.-A.; Ishibashi-Ueda, H.; Koba, S.; Kobayashi, Y.; Hirano, T.; et al. Counteractive Effects of Omentin-1 against Atherogenesis†. Cardiovasc Res 2016, 110, 118–128. [Google Scholar] [CrossRef]
- Shibata, R.; Ouchi, N.; Takahashi, R.; Terakura, Y.; Ohashi, K.; Ikeda, N.; Higuchi, A.; Terasaki, H.; Kihara, S.; Murohara, T. Omentin as a Novel Biomarker of Metabolic Risk Factors. Diabetol Metab Syndr 2012, 4, 37. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, S.; Ke, Y.; Li, Q.; Shen, C.; Ruan, Y.; Wu, K.; Hu, J.; Liu, S. Association of Circulating Omentin Level and Metabolic-Associated Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2023, 14. [Google Scholar] [CrossRef]
- Salvoza, N.; Giraudi, P.; Gazzin, S.; Bonazza, D.; Palmisano, S.; de Manzini, N.; Zanconati, F.; Raseni, A.; Sirianni, F.; Tiribelli, C.; et al. The Potential Role of Omentin-1 in Obesity-Related Metabolic Dysfunction-Associated Steatotic Liver Disease: Evidence from Translational Studies. Journal of Translational Medicine 2023, 21, 906. [Google Scholar] [CrossRef] [PubMed]
- As Habi, A.; Sadeghi, M.; Arab, A.; Hajianfar, H. The Association between Omentin and Diabetes: A Systematic Review and Meta-Analysis of Observational Studies. Diabetes Metab Syndr Obes 2019, 12, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, X.; Bu, P. Omentin-1 Is Associated with Carotid Atherosclerosis in Patients with Metabolic Syndrome. Diabetes Res Clin Pract 2011, 93, 21–25. [Google Scholar] [CrossRef]
- Cetin Sanlialp, S.; Nar, G.; Nar, R. Relationship between Circulating Serum Omentin-1 Levels and Nascent Metabolic Syndrome in Patients with Hypertension. J Investig Med 2022, 70, 780–785. [Google Scholar] [CrossRef]
- Varona, J.F.; Ortiz-Regalón, R.; Sánchez-Vera, I.; López-Melgar, B.; García-Durango, C.; Castellano Vázquez, J.M.; Solís, J.; Fernández-Friera, L.; Vidal-Vanaclocha, F. Soluble ICAM 1 and VCAM 1 Blood Levels Alert on Subclinical Atherosclerosis in Non Smokers with Asymptomatic Metabolic Syndrome. Arch Med Res 2019, 50, 20–28. [Google Scholar] [CrossRef]
- Huang, Z.; Luo, L.; Xiao, Z.; Xiong, M.; Wen, Z. Omentin-1 Mitigates Non-Alcoholic Fatty Liver Disease by Preserving Autophagy through AMPKα/mTOR Signaling Pathway. Sci Rep 2024, 14, 31464. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Dreczkowski, G.; Ramage, M.I.; Wigmore, S.J.; Gallagher, I.J.; Skipworth, R.J.E. Adipose Depot Gene Expression and Intelectin-1 in the Metabolic Response to Cancer and Cachexia. J Cachexia Sarcopenia Muscle 2020, 11, 1141–1153. [Google Scholar] [CrossRef]
- Huang, F.-L.; Yu, S.-J. Esophageal Cancer: Risk Factors, Genetic Association, and Treatment. Asian Journal of Surgery 2018, 41, 210–215. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, Y.; Zhang, Z.; Zhang, Z.; Zhang, G.; Wang, F.; Che, Y.; Fang, L.; Zhang, Y.; Sun, N.; et al. Identification of a Prognostic Immune Signature for Esophageal Squamous Cell Carcinoma to Predict Survival and Inflammatory Landscapes. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Zhu, L.; Liang, F.; Han, X.; Ye, B.; Xue, L. Machine Learning-Based Glycolipid Metabolism Gene Signature Predicts Prognosis and Immune Landscape in Oesophageal Squamous Cell Carcinoma. J Cell Mol Med 2025, 29, e70434. [Google Scholar] [CrossRef] [PubMed]
- Busslinger, G.A.; de Barbanson, B.; Oka, R.; Weusten, B.L.A.; de Maat, M.; van Hillegersberg, R.; Brosens, L.A.A.; van Boxtel, R.; van Oudenaarden, A.; Clevers, H. Molecular Characterization of Barrett’s Esophagus at Single-Cell Resolution. Proceedings of the National Academy of Sciences 2021, 118, e2113061118. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, S.; Jonas, C.; Wenzel, M.C.; Velazquez Camacho, O.; Arolt, C.; Zhao, Y.; Büttner, R.; Quaas, A.; Plum, P.S.; Hillmer, A.M. Genomic and Transcriptomic Characteristics of Esophageal Adenocarcinoma. Cancers (Basel) 2021, 13, 4300. [Google Scholar] [CrossRef]
- Zheng, L.; Weng, M.; Qi, M.; Qi, T.; Tong, L.; Hou, X.; Tong, Q. Aberrant Expression of Intelectin-1 in Gastric Cancer: Its Relationship with Clinicopathological Features and Prognosis. J Cancer Res Clin Oncol 2012, 138, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, X.; Xiao, Y.; Mei, H.; Pu, J.; Xiang, X.; Jiao, W.; Song, H.; Qu, H.; Huang, K.; et al. Intelectin 1 Suppresses Tumor Progression and Is Associated with Improved Survival in Gastric Cancer. Oncotarget 2015, 6, 16168–16182. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Zhou, L.-M. Omentin-1, a New Adipokine, Promotes Apoptosis through Regulating Sirt1-Dependent P53 Deacetylation in Hepatocellular Carcinoma Cells. European Journal of Pharmacology 2013, 698, 137–144. [Google Scholar] [CrossRef]
- Li, J.; Tao, H.-S.; Yuan, T.; Huang, Z.-Y.; Zhang, E.-L. Intelectin-1 Is a Novel Prognostic Biomarker for Hepatocellular Carcinoma. Medicine (Baltimore) 2023, 102, e36474. [Google Scholar] [CrossRef] [PubMed]
- Cidem, A.; Chang, G.R.-L.; Yen, C.-C.; Chen, M.-S.; Yang, S.-H.; Chen, C.-M. Lactoferrin Targeting INTL1 Receptor Inhibits Hepatocellular Carcinoma Progression via Apoptosis and Cell Cycle Signaling Pathways. Sci Rep 2024, 14, 31210. [Google Scholar] [CrossRef]
- Karabulut, S.; Afsar, C.U.; Karabulut, M.; Alis, H.; Bozkurt, M.A.; Aydogan, F.; Serilmez, M.; Tas, F. Clinical Significance of Serum Omentin-1 Levels in Patients with Pancreatic Adenocarcinoma. BBA Clin 2016, 6, 138–142. [Google Scholar] [CrossRef]
- Kiczmer, P.; Szydło, B.; Seńkowska, A.P.; Jopek, J.; Wiewiora, M.; Piecuch, J.; Ostrowska, Z.; Świętochowska, E. Serum Omentin-1 and Chemerin Concentrations in Pancreatic Cancer and Chronic Pancreatitis. Folia medica Cracoviensia 2018. [Google Scholar] [CrossRef]
- Aleksandrova, K.; di Giuseppe, R.; Isermann, B.; Biemann, R.; Schulze, M.; Wittenbecher, C.; Fritsche, A.; Lehmann, R.; Menzel, J.; Weikert, C.; et al. Circulating Omentin as a Novel Biomarker for Colorectal Cancer Risk: Data from the EPIC–Potsdam Cohort Study. Cancer Research 2016, 76, 3862–3871. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Y.; Deng, L.; Wang, Y.; Li, Y.; Chen, M. The Association between Chinese Patients’ Elevated Omentin-1 Levels, Their Clinicopathological Features, and the Risk of Colorectal Cancer. Int J Clin Exp Pathol 2019, 12, 2264–2274. [Google Scholar]
- Feng, Z.; Sun, H.; Liu, P.; Shi, W.; Han, W.; Ma, L. Analysis of the Expression of Plasma Omentin-1 Level in Colorectal Cancer and Its Correlation with Prognosis. Transl Cancer Res 2020, 9, 6479–6486. [Google Scholar] [CrossRef]
- Maeda, K.; Saigo, C.; Kito, Y.; Sakuratani, T.; Yoshida, K.; Takeuchi, T. Expression of TMEM207 in Colorectal Cancer: Relation between TMEM207 and Intelectin-1. J Cancer 2016, 7, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, K.; Maeda, K.; Saigo, C.; Kito, Y.; Yoshida, K.; Takeuchi, T. Adiponectin and Intelectin-1: Important Adipokine Players in Obesity-Related Colorectal Carcinogenesis. International Journal of Molecular Sciences 2017, 18, 866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, X.; Chen, M. Autocrine Action of Adipokine Omentin-1 in the SW480 Colon Cancer Cell Line. Oncol Lett 2020, 19, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Wan, L.; Zhang, Q.; Chen, M.; Zhao, X. The Effect of Omentin-1 on the Proliferation and Apoptosis of Colon Cancer Stem Cells and the Potential Mechanism. J BUON 2019, 24, 91–98. [Google Scholar]
- Uyeturk, U.; Alcelik, A.; Aktas, G.; Tekce, B.K. Post-Treatment Plasma Omentin Levels in Patients with Stage III Colon Carcinoma. J BUON 2014, 19, 681–685. [Google Scholar]
- Zhang, Y.; Zhao, X.; Li, Y.; Wang, Y.; Chen, M. Association between the Omentin-1 Gene Rs2274907 A>T Polymorphism and Colorectal Cancer in the Chinese Han Population: A Case-Control Study. J Int Med Res 2021, 49, 03000605211006522. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, M.S.; Dashti, H.; Akbarzadeh, S.; Assadi, M.; Aminian, A.; Keramati, M.R.; Nabipour, I. Circulating Levels of Novel Adipocytokines in Patients with Colorectal Cancer. Cytokine 2013, 62, 81–85. [Google Scholar] [CrossRef]
- Chen, L.; Jin, X.-H.; Luo, J.; Duan, J.-L.; Cai, M.-Y.; Chen, J.-W.; Feng, Z.-H.; Guo, A.M.; Wang, F.-W.; Xie, D. ITLN1 Inhibits Tumor Neovascularization and Myeloid Derived Suppressor Cells Accumulation in Colorectal Carcinoma. Oncogene 2021, 40, 5925–5937. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kang, U.-B.; Lee, H.; Jung, J.-H.; Lee, S.-T.; Yu, M.-H.; Kim, H.; Lee, C. Profiling of Differentially Expressed Proteins in Stage IV Colorectal Cancers with Good and Poor Outcomes. Journal of Proteomics 2012, 75, 2983–2997. [Google Scholar] [CrossRef] [PubMed]
| Authors and Year | Cancer Type | Model | Findings | Role | Clinical Implication |
| Zhang et al., 2020 | ESCC | ESCC (microarray + qRT-PCR) | ↑ ITLN1 linked to better OS/RFS; protective gene in 6-gene immune signature | Protective, prognostic | Favorable immune profile and improved survival |
| Liang et al., 2021 | ESCC | ESCC tumor and adjacent normal tissue | ↓ ITLN1 in tumors; associated with better immune contexture and lower immune risk score | Immune-modulating, prognostic | Immune biomarker; favorable prognosis |
| Busslinger et al., 2021 | EAC | Barrett’s esophagus and dysplasia/EAC tissue | ITLN1 expressed in BE epithelium; unchanged in progression to dysplasia or adenocarcinoma | Marker of metaplasia | Diagnostic utility in Barrett’s; no role in malignant transformation |
| Hoppe et al., 2021 | EAC | Review of Barrett’s esophagus and EAC data | ITLN1 marks goblet-like BE cells; no expression change in EAC | Marker of cell identity | Limited diagnostic/prognostic value in EAC |
| Zheng et al., 2012 | Gastric | qPCR, IHC, survival analysis on tumors and normal tissue | ITLN1 mRNA 8× higher in tumors; associated with CDX2 expression, favorable histology, lower stage, improved survival (HR = 2.25 for ITLN1−) | Tumor suppressor downstream of CDX2 | Prognostic marker and therapeutic target for intestinal-type GC |
| Li et al., 2015 | Gastric | In vitro (SGC-7901, AGS), in vivo (xenografts) | ITLN1 inhibits PI3K/NF-κB, restores HNF4α, suppresses β-catenin; reduces invasion/metastasis; positively correlates with OS (R = 0.82) | Tumor-suppressive adipokine | Dual biomarker with HNF4α; therapeutic target |
| Zhang et al., 2013 | HCC | HepG2, HuH-7 cell lines | Omentin-1 stabilizes p53 via SIRT1 inhibition; activates caspase-mediated apoptosis via JNK | Tumor suppressor | Potential therapeutic via p53 reactivation |
| Li et al., 2023 | hCC | 149 HCC patients (tissue analysis) | ↓ ITLN1 in 78% of tumors; associated with aggressive features and worse survival (HR ≈ 0.5) | Prognostic biomarker | Risk stratification tool post-resection |
| Cidem et al., 2024 | HCC | HepG2, Hep3B, SK-Hep1 + orthotopic mice | LF requires omentin-1 for uptake; triggers apoptosis, cell-cycle arrest, inhibits angiogenesis | Therapeutic co-factor | Predictive biomarker and therapeutic enhancer for LF-based treatment |
| Karabulut et al., 2016 | Pancreatic | 33 PA patients vs 30 controls (ELISA) | ↑ Omentin-1 (~9.6 vs ~1.6 ng/mL); correlates with tumor size, not OS or chemo response | Diagnostic marker | Potential biomarker for detection, not for prognosis |
| Kiczmer et al., 2018 | Pancreatic | 25 PDAC, 10 CP, 36 controls (serum) | ↑ Omentin-1 in PDAC vs controls; AUC 0.73; no correlation with TNM stage or BMI | Diagnostic adjunct | Support diagnosis; limited prognostic use |
| Aleksandrova et al., 2016 | CRC | Prospective cohort (n=2,295 subcohort + 251 CRC cases) | High baseline omentin-1 associated with increased CRC risk, especially in non-obese | Risk biomarker | Stratify risk, particularly in lean individuals |
| Zhao et al., 2019 | CRC | Case-control (358 CRC vs 286 controls) | ↑ Omentin-1 in CRC; associated with advanced stage (pT3/4, TNM III–IV); AUC 0.88 | Diagnostic and staging marker | Non-invasive diagnostic tool with good sensitivity |
| Feng et al., 2020 | CRC | Prospective cohort (319 CRC vs 300 controls) | Elevated pre-op omentin-1 predicts recurrence (HR 3.3) and mortality (HR 2.1) | Prognostic biomarker | Post-operative monitoring and treatment |
| Kawashima et al., 2016 | CRC | Review of mechanistic and IHC data | TMEM207 stabilizes omentin-1; loss linked to aggressive, non-mucinous CRC | Facilitator of tumor suppression | TMEM207–omentin axis: prognostic and therapeutic value |
| Maeda et al., 2016 | CRC | IHC + in vitro (siRNA) | TMEM207 loss reduces omentin secretion; correlates with nodal metastasis | Post-translational regulator | Potential target for therapy and prognosis |
| Zhang et al., 2020 | CRC | CRC tissue + SW480 cells | Tumors overexpress omentin-1; secreted in autocrine/paracrine fashion | Context-dependent role | Targeting tumor-derived omentin alters CRC progression |
| Ji et al., 2019 | CRC | CD133⁺ SW480 cells | Omentin-1 inhibits PI3K/Akt; reduces stemness and promotes apoptosis | Cancer stem cell suppressor | Adjuvant therapy targeting cancer stem cells |
| Uyeturk et al., 2014 | CRC | Stage III post-FOLFOX patients (n=45) | ↑ Omentin-1 after chemo; not correlated with metabolic parameters | Reactive or compensatory marker | Host response to therapy |
| Zhang et al., 2021 | CRC | Genetic association study (SNP) | Variant not associated with CRC overall; modifies risk in obesity | Gene–environment interaction | Stratify risk in obese patients based on genotype |
| Fazeli et al., 2013 | CRC | 39 CRC vs 30 controls | ≈20× higher plasma omentin-1 in CRC; independent of BMI or stage | Carcinogenesis biomarker | Early detection |
| Chen et al., 2021 | CRC | TCGA + IHC (n=229) | Low ITLN1 linked to worse OS; re-expression reduces EPCs and MDSCs | Microenvironment modulator | Therapeutic restoration enhances anti-tumor immunity |
| Kim et al., 2012 | CRC | Stage IV CRC proteomics | ITLN1 41× higher in long-term survivors; validated by WB/IHC | Prognostic biomarker | Survival indicator; guides treatment intensity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





