Submitted:
30 August 2025
Posted:
03 September 2025
You are already at the latest version
Abstract
Keywords:
1. Background
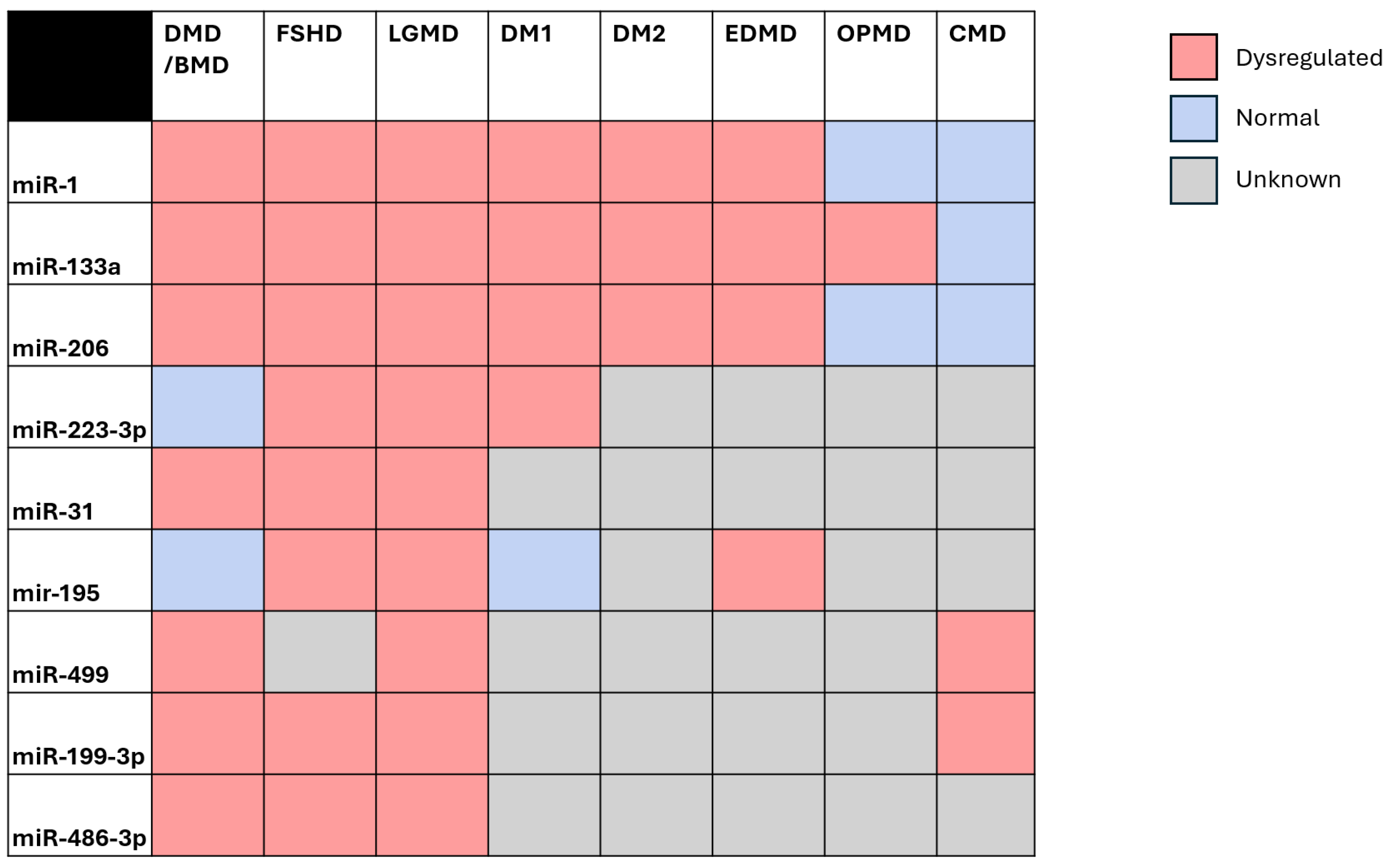
2. Shared Non-Coding Regulation in the Muscular Dystrophies
3. Non-Coding Regulatory Elements Local to Muscular Dystrophy Loci
3.1. Duchenne/Becker Muscular Dystrophy
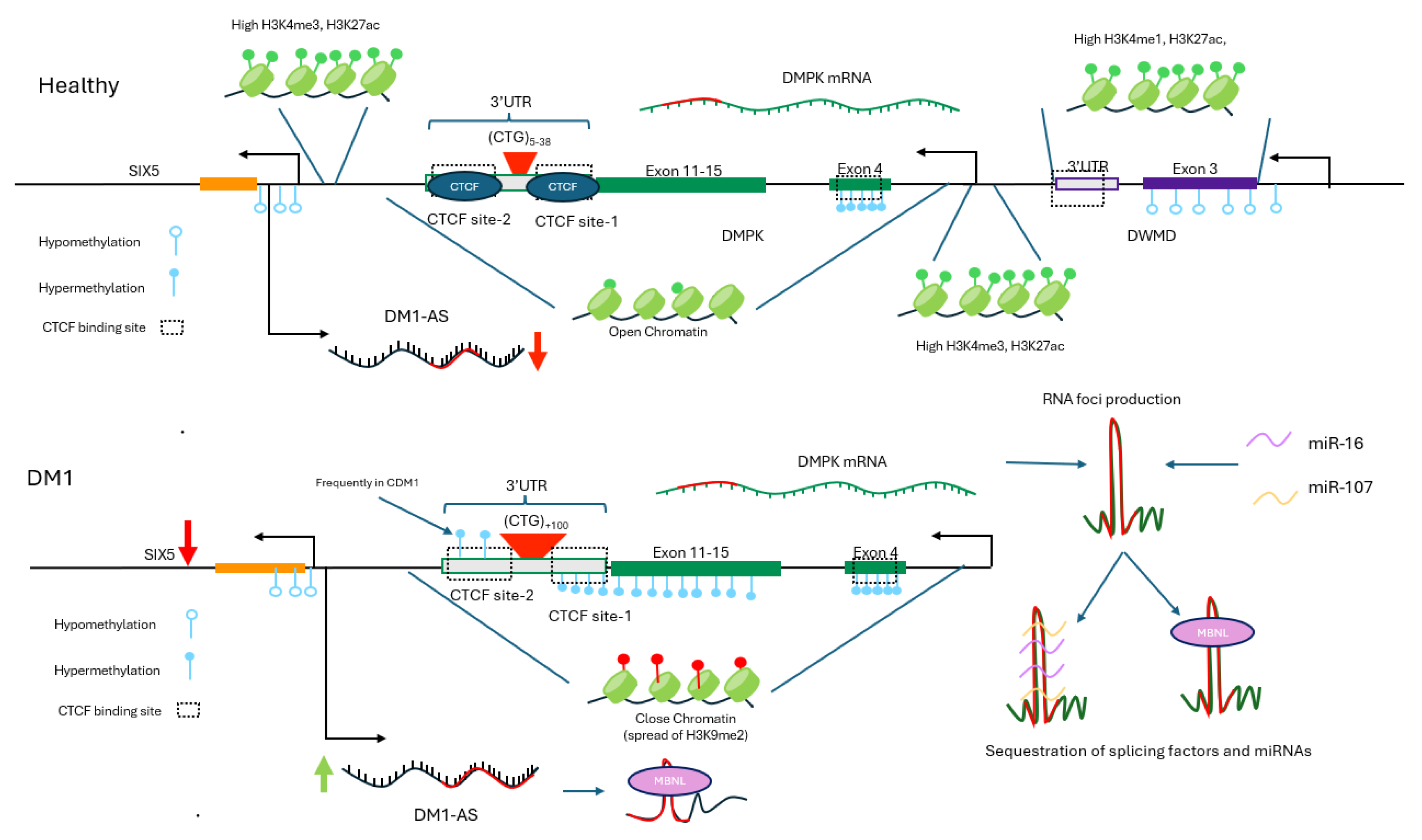
3.2. Myotonic Muscular Dystrophy
3.3. Facioscapulohumeral Dystrophy
3.4. Limb-Girdle Muscular Dystrophy
3.5. Emery-Dreifuss Muscular Dystrophy
3.6. Oculopharyngeal Muscular Dystrophy
3.7. Congenital Muscular Dystrophies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wicklund, M.P. The Muscular Dystrophies. Continuum : Lifelong Learning in Neurology 2013, 19, 1535. [Google Scholar] [CrossRef] [PubMed]
- Mousa, N.O.; Abdellatif, A.; Fahmy, N.; El-Fawal, H.; Osman, A. MicroRNAs as a Tool for Differential Diagnosis of Neuromuscular Disorders. Neuromolecular Med 2023, 25, 603. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Moon, J.; Yu, J.; Kho, C. MicroRNAs in Dystrophinopathy. Int J Mol Sci 2022, 23, 7785. [Google Scholar] [CrossRef] [PubMed]
- Hoang, T.; Dowdy, R.A.E. A Review of Muscular Dystrophies. Anesth Prog 2024, 71, 44. [Google Scholar] [CrossRef]
- Neguembor, M. V.; Jothi, M.; Gabellini, D. Long Noncoding RNAs, Emerging Players in Muscle Differentiation and Disease. Skeletal Muscle 2014 4:1 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Falcone, G.; Perfetti, A.; Cardinali, B.; Martelli, F. Noncoding RNAs: Emerging Players in Muscular Dystrophies. Biomed Res Int 2014, 2014, 503634. [Google Scholar] [CrossRef]
- Brusa, R.; Magri, F.; Bresolin, N.; Comi, G. Pietro; Corti, S. Noncoding RNAs in Duchenne and Becker Muscular Dystrophies: Role in Pathogenesis and Future Prognostic and Therapeutic Perspectives. Cellular and Molecular Life Sciences 2020, 77, 4299–4313. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nature Reviews Molecular Cell Biology 2023 24:6 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Mattick, J.S.; Makunin, I. V. Non-Coding RNA. Hum Mol Genet 2006, 15 Spec No 1. [Google Scholar] [CrossRef]
- Kaikkonen, M.U.; Lam, M.T.Y.; Glass, C.K. Non-Coding RNAs as Regulators of Gene Expression and Epigenetics. Cardiovasc Res 2011, 90, 430. [Google Scholar] [CrossRef]
- Patil, V.S.; Zhou, R.; Rana, T.M. Gene Regulation by Noncoding RNAs. Crit Rev Biochem Mol Biol 2013, 49, 16. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne) 2018, 9, 402. [Google Scholar] [CrossRef]
- Filipowicz, W.; Bhattacharyya, S.N.; Sonenberg, N. Mechanisms of Post-Transcriptional Regulation by MicroRNAs: Are the Answers in Sight? Nature Reviews Genetics 2008 9:2 2008, 9, 102–114. [Google Scholar] [CrossRef]
- Jo, M.H.; Shin, S.; Jung, S.R.; Kim, E.; Song, J.J.; Hohng, S. Human Argonaute 2 Has Diverse Reaction Pathways on Target RNAs. Mol Cell 2015, 59, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Aronin, N. Target Selectivity in MRNA Silencing. Gene Ther 2006, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Ui-Tei, K.; Naito, Y.; Takahashi, F.; Haraguchi, T.; Ohki-Hamazaki, H.; Juni, A.; Ueda, R.; Saigo, K. Guidelines for the Selection of Highly Effective SiRNA Sequences for Mammalian and Chick RNA Interference. Nucleic Acids Res 2004, 32, 936. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular Mechanisms and Biological Functions of SiRNA. Int J Biomed Sci 2017, 13, 48. [Google Scholar] [CrossRef]
- Thomson, T.; Lin, H. The Biogenesis and Function of PIWI Proteins and PiRNAs: Progress and Prospect. Annu Rev Cell Dev Biol 2009, 25, 355–376. [Google Scholar] [CrossRef]
- Iwasaki, Y.W.; Siomi, M.C.; Siomi, H. PIWI-Interacting RNA: Its Biogenesis and Functions. Annu Rev Biochem 2015, 84, 405–433. [Google Scholar] [CrossRef]
- Höck, J.; Meister, G. The Argonaute Protein Family. Genome Biol 2008, 9, 1–8. [Google Scholar] [CrossRef]
- Cook, M.S.; Blelloch, R. Small RNAs in Germline Development. Curr Top Dev Biol 2013, 102, 159–205. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, P.J. Mammalian PiRNAs: Biogenesis, Function, and Mysteries. Spermatogenesis 2014, 4, e27889. [Google Scholar] [CrossRef]
- Sienski, G.; Dönertas, D.; Brennecke, J. Transcriptional Silencing of Transposons by Piwi and Maelstrom and Its Impact on Chromatin State and Gene Expression. Cell 2012, 151, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Klenov, M.S.; Sokolova, O.A.; Yakushev, E.Y.; Stolyarenko, A.D.; Mikhaleva, E.A.; Lavrov, S.A.; Gvozdev, V.A. Separation of Stem Cell Maintenance and Transposon Silencing Functions of Piwi Protein. Proc Natl Acad Sci U S A 2011, 108, 18760–18765. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nature Reviews Molecular Cell Biology 2020 22:2 2020, 22, 96–118. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat Rev Mol Cell Biol 2023, 24, 430. [Google Scholar] [CrossRef]
- Deveson, I.W.; Hardwick, S.A.; Mercer, T.R.; Mattick, J.S. The Dimensions, Dynamics, and Relevance of the Mammalian Noncoding Transcriptome. Trends in Genetics 2017, 33, 464–478. [Google Scholar] [CrossRef]
- Aliperti, V.; Skonieczna, J.; Cerase, A. Long Non-Coding Rna (Lncrna) Roles in Cell Biology, Neurodevelopment and Neurological Disorders. Noncoding RNA 2021, 7. [Google Scholar] [CrossRef]
- Robinson, E.K.; Covarrubias, S.; Carpenter, S. The How and Why of LncRNA Function: An Innate Immune Perspective. Biochim Biophys Acta Gene Regul Mech 2019, 1863, 194419. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, L.R.; Blom, S.; Henriques, R.; Bachem, C.W.B.; Immink, R.G.H. LncRNAs: The Art of Being Influential without Protein. Trends Plant Sci 2024, 29, 770–785. [Google Scholar] [CrossRef]
- Li, Y.; Syed, J.; Sugiyama, H. RNA-DNA Triplex Formation by Long Noncoding RNAs. Cell Chem Biol 2016, 23, 1325–1333. [Google Scholar] [CrossRef]
- Aránega, A.E.; Lozano-velasco, E.; Rodriguez-outeiriño, L.; Ramírez de Acuña, F.; Franco, D.; Hernández-torres, F. MiRNAs and Muscle Regeneration: Therapeutic Targets in Duchenne Muscular Dystrophy. Int J Mol Sci 2021, 22, 4236. [Google Scholar] [CrossRef]
- Koutsoulidou, A.; Koutalianos, D.; Georgiou, K.; Kakouri, A.C.; Oulas, A.; Tomazou, M.; Kyriakides, T.C.; Roos, A.; Papadimas, G.K.; Papadopoulos, C.; et al. Serum MiRNAs as Biomarkers for the Rare Types of Muscular Dystrophy. Neuromuscular Disorders 2022, 32, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.E.G.; Almeida-Becerril, T.; Rodríguez-Cruz, M. Advances in MicroRNAs in Pathophysiology of Duchenne Muscular Dystrophy. Muscle Nerve 2025, 0, 1–15. [Google Scholar] [CrossRef]
- Neguembor, M. V.; Jothi, M.; Gabellini, D. Long Noncoding RNAs, Emerging Players in Muscle Differentiation and Disease. Skeletal Muscle 2014, 4, 1–12. [Google Scholar] [CrossRef]
- Pegoraro, V.; Cudia, P.; Baba, A.; Angelini, C. MyomiRNAs and Myostatin as Physical Rehabilitation Biomarkers for Myotonic Dystrophy. Neurological Sciences 2020, 41, 2953–2960. [Google Scholar] [CrossRef]
- Pivonello, C.; Patalano, R.; Simeoli, C.; Montò, T.; Negri, M.; Amatrudo, F.; Di Paola, N.; Larocca, A.; Crescenzo, E.M.; Pirchio, R.; et al. Circulating MyomiRNAs as Biomarkers in Patients with Cushing’s Syndrome. J Endocrinol Invest 2024, 47, 655–669. [Google Scholar] [CrossRef]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-Specific MicroRNAs in Skeletal Muscle Development. Dev Biol 2016, 410, 1–13. [Google Scholar] [CrossRef]
- Safa, A.; Bahroudi, Z.; Shoorei, H.; Majidpoor, J.; Abak, A.; Taheri, M.; Ghafouri-Fard, S. MiR-1: A Comprehensive Review of Its Role in Normal Development and Diverse Disorders. Biomedicine & Pharmacotherapy 2020, 132, 110903. [Google Scholar] [CrossRef] [PubMed]
- Benzoni, P.; Nava, L.; Giannetti, F.; Guerini, G.; Gualdoni, A.; Bazzini, C.; Milanesi, R.; Bucchi, A.; Baruscotti, M.; Barbuti, A. Dual Role of MiR-1 in the Development and Function of Sinoatrial Cells. J Mol Cell Cardiol 2021, 157, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Ishibashi, J.; Scimè, A.; Rudnicki, M.A. Pax7 Is Necessary and Sufficient for the Myogenic Specification of CD45+:Sca1+ Stem Cells from Injured Muscle. PLoS Biol 2004, 2, e130. [Google Scholar] [CrossRef]
- Renzini, A.; Marroncelli, N.; Noviello, C.; Moresi, V.; Adamo, S. HDAC4 Regulates Skeletal Muscle Regeneration via Soluble Factors. Front Physiol 2018, 9, 1387. [Google Scholar] [CrossRef]
- Chen, J.F.; Mandel, E.M.; Thomson, J.M.; Wu, Q.; Callis, T.E.; Hammond, S.M.; Conlon, F.L.; Wang, D.Z. The Role of MicroRNA-1 and MicroRNA-133 in Skeletal Muscle Proliferation and Differentiation. Nat Genet 2005, 38, 228. [Google Scholar] [CrossRef]
- Brusa, R.; Magri, F.; Bresolin, N.; Comi, G. Pietro; Corti, S. Noncoding RNAs in Duchenne and Becker Muscular Dystrophies: Role in Pathogenesis and Future Prognostic and Therapeutic Perspectives. Cellular and Molecular Life Sciences 2020, 77, 4299–4313. [Google Scholar] [CrossRef] [PubMed]
- Cacchiarelli, D.; Legnini, I.; Martone, J.; Cazzella, V.; D’Amico, A.; Bertini, E.; Bozzoni, I. MiRNAs as Serum Biomarkers for Duchenne Muscular Dystrophy. EMBO Mol Med 2011, 3, 258. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Moon, J.; Yu, J.; Kho, C. MicroRNAs in Dystrophinopathy. Int J Mol Sci 2022, 23, 7785. [Google Scholar] [CrossRef] [PubMed]
- Cacchiarelli, D.; Legnini, I.; Martone, J.; Cazzella, V.; D’Amico, A.; Bertini, E.; Bozzoni, I. MiRNAs as Serum Biomarkers for Duchenne Muscular Dystrophy. EMBO Mol Med 2011, 3, 258. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Kishi, S.; Aoki, Y.; Komaki, H.; Oya, Y.; Takeda, S. ichi; Hashido, K. Three Novel Serum Biomarkers, MiR-1, MiR-133a, and MiR-206 for Limb-Girdle Muscular Dystrophy, Facioscapulohumeral Muscular Dystrophy, and Becker Muscular Dystrophy. Environ Health Prev Med 2014, 19, 452. [Google Scholar] [CrossRef]
- Koutsoulidou, A.; Kyriakides, T.C.; Papadimas, G.K.; Christou, Y.; Kararizou, E.; Papanicolaou, E.Z.; Phylactou, L.A. Elevated Muscle-Specific MiRNAs in Serum of Myotonic Dystrophy Patients Relate to Muscle Disease Progress. PLoS One 2015, 10, e0125341. [Google Scholar] [CrossRef]
- Koutsoulidou, A.; Koutalianos, D.; Georgiou, K.; Kakouri, A.C.; Oulas, A.; Tomazou, M.; Kyriakides, T.C.; Roos, A.; Papadimas, G.K.; Papadopoulos, C.; et al. Serum MiRNAs as Biomarkers for the Rare Types of Muscular Dystrophy. Neuromuscular Disorders 2022, 32, 332–346. [Google Scholar] [CrossRef]
- Bonanno, S.; Malacarne, C.; Tarasco, M.; Farinazzo, G.; Mattioli, E.; Schena, E.; Saraceno, F.; Fiorillo, C.; Andreetta, F.; Schirmer, E.; et al. 593P Emerging Role of Muscle and Fibrosis-Related MicroRNAs as Biomarkers and Therapeutic Targets in Emery-Dreifuss Muscular Dystrophies. Neuromuscular Disorders 2024, 43, 104441.348. [Google Scholar] [CrossRef]
- Raz, V.; Kroon, R.H.M.J.M.; Mei, H.; Riaz, M.; Buermans, H.; Lassche, S.; Horlings, C.; De Swart, B.; Kalf, J.; Harish, P.; et al. Age-Associated Salivary MicroRNA Biomarkers for Oculopharyngeal Muscular Dystrophy. Int J Mol Sci 2020, 21, 6059. [Google Scholar] [CrossRef]
- Aksu-Menges, E.; Akkaya-Ulum, Y.Z.; Dayangac-Erden, D.; Balci-Peynircioglu, B.; Yuzbasioglu, A.; Topaloglu, H.; Talim, B.; Balci-Hayta, B. The Common MiRNA Signatures Associated with Mitochondrial Dysfunction in Different Muscular Dystrophies. American Journal of Pathology 2020, 190, 2136–2145. [Google Scholar] [CrossRef]
- Boštjančič, E.; Zidar, N.; Štajer, D.; Glavač, D. MicroRNAs MiR-1, MiR-133a, MiR-133b and MiR-208 Are Dysregulated in Human Myocardial Infarction. Cardiology 2010, 115, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lu, Y.; Li, Z.; Wang, Q. MicroRNA-133: Expression, Function and Therapeutic Potential in Muscle Diseases and Cancer. Curr Drug Targets 2014, 15, 817–828. [Google Scholar] [CrossRef]
- Dmitriev, P.; Stankevicins, L.; Ansseau, E.; Petrov, A.; Barat, A.; Dessen, P.; Robert, T.; Turki, A.; Lazar, V.; Labourer, E.; et al. Defective Regulation of MicroRNA Target Genes in Myoblasts from Facioscapulohumeral Dystrophy Patients. Journal of Biological Chemistry 2013, 288, 34989–35002. [Google Scholar] [CrossRef]
- Li, S.; Czubryt, M.P.; McAnally, J.; Bassel-Duby, R.; Richardson, J.A.; Wiebel, F.F.; Nordheim, A.; Olson, E.N. Requirement for Serum Response Factor for Skeletal Muscle Growth and Maturation Revealed by Tissue-Specific Gene Deletion in Mice. Proc Natl Acad Sci U S A 2005, 102, 1082. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Qiu, H. Recent Advances in Serum Response Factor Posttranslational Modifications and Their Therapeutic Potential in Cardiovascular and Neurological Diseases. Vascul Pharmacol 2024, 156, 107421. [Google Scholar] [CrossRef]
- Salant, G.M.; Tat, K.L.; Goodrich, J.A.; Kugel, J.F. MiR-206 Knockout Shows It Is Critical for Myogenesis and Directly Regulates Newly Identified Target MRNAs. RNA Biol 2020, 17, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Boettger, T.; Wüst, S.; Nolte, H.; Braun, T. The MiR-206/133b Cluster Is Dispensable for Development, Survival and Regeneration of Skeletal Muscle. Skelet Muscle 2014, 4, 1–13. [Google Scholar] [CrossRef]
- McCarthy, J.J. MicroRNA-206: The Skeletal Muscle-Specific MyomiR. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 2008, 1779, 682–691. [Google Scholar] [CrossRef]
- Ma, G.; Wang, Y.; Li, Y.; Cui, L.; Zhao, Y.; Zhao, B.; Li, K. MiR-206, a Key Modulator of Skeletal Muscle Development and Disease. Int J Biol Sci 2015, 11, 345. [Google Scholar] [CrossRef]
- García-Giménez, J.L.; García-Trevijano, E.R.; Avilés-Alía, A.I.; Ibañez-Cabellos, J.S.; Bovea-Marco, M.; Bas, T.; Pallardó, F. V.; Viña, J.R.; Zaragozá, R. Identification of Circulating MiRNAs Differentially Expressed in Patients with Limb-Girdle, Duchenne or Facioscapulohumeral Muscular Dystrophies. Orphanet J Rare Dis 2022, 17, 1–14. [Google Scholar] [CrossRef]
- Samani, A.; Hightower, R.M.; Reid, A.L.; English, K.G.; Lopez, M.A.; Scott Doyle, J.; Conklin, M.J.; Schneider, D.A.; Bamman, M.M.; Widrick, J.J.; et al. MiR-486 Is Essential for Muscle Function and Suppresses a Dystrophic Transcriptome. Life Sci Alliance 2022, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yue, B.; Lan, X.; Wang, Y.; Fang, X.; Ma, Y.; Bai, Y.; Qi, X.; Zhang, C.; Chen, H. MiR-499 Regulates Myoblast Proliferation and Differentiation by Targeting Transforming Growth Factor β Receptor 1. J Cell Physiol 2019, 234, 2523–2536. [Google Scholar] [CrossRef]
- Alexander, M.S.; Casar, J.C.; Motohashi, N.; Myers, J.A.; Eisenberg, I.; Gonzalez, R.T.; Estrella, E.A.; Kang, P.B.; Kawahara, G.; Kunkel, L.M. Regulation of DMD Pathology by an Ankyrin-Encoded MiRNA. Skelet Muscle 2011, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Heier, C.R.; Zhang, A.; Nguyen, N.Y.; Tully, C.B.; Panigrahi, A.; Gordish-Dressman, H.; Pandey, S.N.; Guglieri, M.; Ryan, M.M.; Clemens, P.R.; et al. Multi-Omics Identifies Circulating MiRNA and Protein Biomarkers for Facioscapulohumeral Dystrophy. J Pers Med 2020, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Jeanson-Leh, L.; Lameth, J.; Krimi, S.; Buisset, J.; Amor, F.; Le Guiner, C.; Barthélémy, I.; Servais, L.; Blot, S.; Voit, T.; et al. Serum Profiling Identifies Novel Muscle MiRNA and Cardiomyopathy-Related MiRNA Biomarkers in Golden Retriever Muscular Dystrophy Dogs and Duchenne Muscular Dystrophy Patients. American Journal of Pathology 2014, 184, 2885–2898. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, W.H.; Chen, Q.; Li, Q.G.; Qiu, Z.M. Downregulation of MiR-199a-3p Mediated by the CtBP2-HDAC1-FOXP3 Transcriptional Complex Contributes to Acute Lung Injury by Targeting NLRP1. Int J Biol Sci 2019, 15, 2627. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Chai, B.; Wu, Z.; Gu, Z.; Zou, H.; Zhang, H.; Li, Y.; Sun, Q.; Fang, W.; et al. MiR-199a-3p/5p Regulate Tumorgenesis via Targeting Rheb in Non-Small Cell Lung Cancer. Int J Biol Sci 2022, 18, 4187. [Google Scholar] [CrossRef]
- Yu, W.; Liang, X.; Li, X.; Zhang, Y.; Sun, Z.; Liu, Y.; Wang, J. MicroRNA-195: A Review of Its Role in Cancers. Onco Targets Ther 2018, 11, 7109. [Google Scholar] [CrossRef]
- Lin, X.; Wu, W.; Ying, Y.; Luo, J.; Xu, X.; Zheng, L.; Wu, W.; Yang, S.; Zhao, S. MicroRNA-31: A Pivotal Oncogenic Factor in Oral Squamous Cell Carcinoma. Cell Death Discov 2022, 8, 1–9. [Google Scholar] [CrossRef]
- Yu, T.; Ma, P.; Wu, D.; Shu, Y.; Gao, W. Functions and Mechanisms of MicroRNA-31 in Human Cancers. Biomedicine & Pharmacotherapy 2018, 108, 1162–1169. [Google Scholar] [CrossRef]
- Alexander, M.S.; Kawahara, G.; Motohashi, N.; Casar, J.C.; Eisenberg, I.; Myers, J.A.; Gasperini, M.J.; Estrella, E.A.; Kho, A.T.; Mitsuhashi, S.; et al. MicroRNA-199a Is Induced in Dystrophic Muscle and Affects WNT Signaling, Cell Proliferation, and Myogenic Differentiation. Cell Death Differ 2013, 20, 1194–1208. [Google Scholar] [CrossRef]
- Eisenberg, I.; Eran, A.; Nishino, I.; Moggio, M.; Lamperti, C.; Amato, A.A.; Lidov, H.G.; Kang, P.B.; North, K.N.; Mitrani-Rosenbaum, S.; et al. Distinctive Patterns of MicroRNA Expression in Primary Muscular Disorders. Proc Natl Acad Sci U S A 2007, 104, 17016–17021. [Google Scholar] [CrossRef]
- Israeli, D.; Poupiot, J.; Amor, F.; Charton, K.; Lostal, W.; Jeanson-Leh, L.; Richard, I. Circulating MiRNAs Are Generic and Versatile Therapeutic Monitoring Biomarkers in Muscular Dystrophies. Sci Rep 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Nunes, A.M.; Ramirez, M.; Jones, T.I.; Jones, P.L. Identification of Candidate MiRNA Biomarkers for Facioscapulohumeral Muscular Dystrophy Using DUX4-Based Mouse Models. DMM Disease Models and Mechanisms 2021, 14. [Google Scholar] [CrossRef]
- Cacchiarelli, D.; Incitti, T.; Martone, J.; Cesana, M.; Cazzella, V.; Santini, T.; Sthandier, O.; Bozzoni, I. MiR-31 Modulates Dystrophin Expression: New Implications for Duchenne Muscular Dystrophy Therapy. EMBO Rep 2011, 12, 136. [Google Scholar] [CrossRef]
- Jiao, P.; Wang, X.P.; Luoreng, Z.M.; Yang, J.; Jia, L.; Ma, Y.; Wei, D.W. MiR-223: An Effective Regulator of Immune Cell Differentiation and Inflammation. Int J Biol Sci 2021, 17, 2308. [Google Scholar] [CrossRef]
- Koutalianos, D.; Koutsoulidou, A.; Mytidou, C.; Kakouri, A.C.; Oulas, A.; Tomazou, M.; Kyriakides, T.C.; Prokopi, M.; Kapnisis, K.; Nikolenko, N.; et al. MiR-223-3p and MiR-24-3p as Novel Serum-Based Biomarkers for Myotonic Dystrophy Type 1. Mol Ther Methods Clin Dev 2021, 23, 169–183. [Google Scholar] [CrossRef]
- Dmitriev, P.; Stankevicins, L.; Ansseau, E.; Petrov, A.; Barat, A.; Dessen, P.; Robert, T.; Turki, A.; Lazar, V.; Labourer, E.; et al. Defective Regulation of MicroRNA Target Genes in Myoblasts from Facioscapulohumeral Dystrophy Patients. Journal of Biological Chemistry 2013, 288, 34989–35002. [Google Scholar] [CrossRef]
- Alexander, M.S.; Casar, J.C.; Motohashi, N.; Vieira, N.M.; Eisenberg, I.; Marshall, J.L.; Gasperini, M.J.; Lek, A.; Myers, J.A.; Estrella, E.A.; et al. MicroRNA-486–Dependent Modulation of DOCK3/PTEN/AKT Signaling Pathways Improves Muscular Dystrophy–Associated Symptoms. J Clin Invest 2014, 124, 2651–2667. [Google Scholar] [CrossRef]
- Wang, R.; Kumar, B.; Doud, E.H.; Mosley, A.L.; Alexander, M.S.; Kunkel, L.M.; Nakshatri, H. Skeletal Muscle-Specific Overexpression of MiR-486 Limits Mammary Tumor-Induced Skeletal Muscle Functional Limitations. Mol Ther Nucleic Acids 2022, 28, 231. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Chen, B.; Zhu, Z.; Du, C.; Gao, S.; Zhao, G.; Zhao, P.; Wang, Y.; Wang, A.; Schwartz, Z.; et al. Long Noncoding RNA (LncRNA) H19: An Essential Developmental Regulator with Expanding Roles in Cancer, Stem Cell Differentiation, and Metabolic Diseases. Genes Dis 2023, 10, 1351. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Pei, T.; Zhao, J.; Wang, Z.; Shen, Y.; Yang, Y.; Liang, J. Long Noncoding RNA H19: Functions and Mechanisms in Regulating Programmed Cell Death in Cancer. Cell Death Discovery 2024 10:1 2024, 10, 1–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Hu, Q.; Xi, Y.; Xing, Z.; Zhang, Z.; Huang, L.; Wu, J.; Liang, K.; Nguyen, T.K.; et al. LncRNA H19 Alleviates Muscular Dystrophy Through Stabilizing Dystrophin. Nat Cell Biol 2020, 22, 1332. [Google Scholar] [CrossRef]
- Saad, N.Y.; Al-Kharsan, M.; Garwick-Coppens, S.E.; Chermahini, G.A.; Harper, M.A.; Palo, A.; Boudreau, R.L.; Harper, S.Q. Human MiRNA MiR-675 Inhibits DUX4 Expression and May Be Exploited as a Potential Treatment for Facioscapulohumeral Muscular Dystrophy. Nature Communications 2021 12:1 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic Modifications: Basic Mechanisms and Role in Cardiovascular Disease. Circulation 2011, 123, 2145. [Google Scholar] [CrossRef]
- Aboud, N.M. Al; Tupper, C.; Jialal, I. Genetics, Epigenetic Mechanism; StatPearls, 2023. [Google Scholar]
- Rugowska, A.; Starosta, A.; Konieczny, P. Epigenetic Modifications in Muscle Regeneration and Progression of Duchenne Muscular Dystrophy. Clinical Epigenetics 2021, 13, 1–25. [Google Scholar] [CrossRef]
- Thomas, E.A. Histone Posttranslational Modifications in Schizophrenia. Adv Exp Med Biol 2017, 978, 237–254. [Google Scholar] [CrossRef]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne Muscular Dystrophy. Nature Reviews Disease Primers 2021, 7, 1–19. [Google Scholar] [CrossRef]
- Romitti, P.A.; Zhu, Y.; Puzhankara, S.; James, K.A.; Nabukera, S.K.; Zamba, G.K.D.; Ciafaloni, E.; Cunniff, C.; Druschel, C.M.; Mathews, K.D.; et al. Prevalence of Duchenne and Becker Muscular Dystrophies in the United States. Pediatrics 2015, 135, 513–521. [Google Scholar] [CrossRef]
- Adorisio, R.; Mencarelli, E.; Cantarutti, N.; Calvieri, C.; Amato, L.; Cicenia, M.; Silvetti, M.; D’Amico, A.; Grandinetti, M.; Drago, F.; et al. Duchenne Dilated Cardiomyopathy: Cardiac Management from Prevention to Advanced Cardiovascular Therapies. J Clin Med 2020, 9, 1–18. [Google Scholar] [CrossRef]
- Shirokova, N.; Niggli, E. Cardiac Phenotype of Duchenne Muscular Dystrophy: Insights from Cellular Studies. J Mol Cell Cardiol 2012, 58, 217. [Google Scholar] [CrossRef]
- KRQ, L.; Q, N.; T, Y. Genotype-Phenotype Correlations in Duchenne and Becker Muscular Dystrophy Patients from the Canadian Neuromuscular Disease Registry. J Pers Med 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Wilson, K.; Faelan, C.; Patterson-Kane, J.C.; Rudmann, D.G.; Moore, S.A.; Frank, D.; Charleston, J.; Tinsley, J.; Young, G.D.; Milici, A.J. Duchenne and Becker Muscular Dystrophies: A Review of Animal Models, Clinical Endpoints, and Biomarker Quantification. Toxicol Pathol 2017, 45, 961. [Google Scholar] [CrossRef]
- Tennyson, C.N.; Klamut, H.J.; Worton, R.G. The Human Dystrophin Gene Requires 16 Hours to Be Transcribed and Is Cotranscriptionally Spliced. Nat Genet 1995, 9, 184–190. [Google Scholar] [CrossRef]
- Loperfido, M.; Jarmin, S.; Dastidar, S.; Di Matteo, M.; Perini, I.; Moore, M.; Nair, N.; Samara-Kuko, E.; Athanasopoulos, T.; Tedesco, F.S.; et al. PiggyBac Transposons Expressing Full-Length Human Dystrophin Enable Genetic Correction of Dystrophic Mesoangioblasts. Nucleic Acids Res 2016, 44, 744–760. [Google Scholar] [CrossRef]
- Bovolenta, M.; Erriquez, D.; Valli, E.; Brioschi, S.; Scotton, C.; Neri, M.; Falzarano, M.S.; Gherardi, S.; Fabris, M.; Rimessi, P.; et al. The DMD Locus Harbours Multiple Long Non-Coding RNAs Which Orchestrate and Control Transcription of Muscle Dystrophin MRNA Isoforms. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Gherardi, S.; Bovolenta, M.; Passarelli, C.; Falzarano, M.S.; Pigini, P.; Scotton, C.; Neri, M.; Armaroli, A.; Osman, H.; Selvatici, R.; et al. Transcriptional and Epigenetic Analyses of the DMD Locus Reveal Novel Cis-acting DNA Elements That Govern Muscle Dystrophin Expression. Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 2017, 1860, 1138–1147. [Google Scholar] [CrossRef]
- García-Rodríguez, R.; Hiller, M.; Jiménez-Gracia, L.; van der Pal, Z.; Balog, J.; Adamzek, K.; Aartsma-Rus, A.; Spitali, P. Premature Termination Codons in the DMD Gene Cause Reduced Local MRNA Synthesis. Proc Natl Acad Sci U S A 2020, 117, 15664–16464. [Google Scholar] [CrossRef]
- Liao, Q.; Zhang, Y.; He, J.; Huang, K. Global Prevalence of Myotonic Dystrophy: An Updated Systematic Review and Meta-Analysis. Neuroepidemiology 2022, 56, 163–173. [Google Scholar] [CrossRef]
- Martorell, L.; Monckton, D.G.; Sanchez, A.; Lopez de Munain, A.; Baiget, M. Frequency and Stability of the Myotonic Dystrophy Type 1 Premutation. Neurology 2001, 56, 328–335. [Google Scholar] [CrossRef]
- Damen, M.J.; Muilwijk, O.G.; Olde Dubbelink, T.B.; van Engelen, B.G.; Voermans, N.C.; Tieleman, A.A. Life Expectancy and Causes of Death in Patients with Myotonic Dystrophy Type 2. J Neuromuscul Dis 2024, 11, 1221–1228. [Google Scholar] [CrossRef]
- Wenninger, S.; Montagnese, F.; Schoser, B. Core Clinical Phenotypes in Myotonic Dystrophies. Front Neurol 2018, 9. [Google Scholar] [CrossRef]
- Malik, I.; Kelley, C.P.; Wang, E.T.; Todd, P.K. Molecular Mechanisms Underlying Nucleotide Repeat Expansion Disorders. Nat Rev Mol Cell Biol 2021, 22, 589–607. [Google Scholar] [CrossRef]
- Kamsteeg, E.J.; Kress, W.; Catalli, C.; Hertz, J.M.; Witsch-Baumgartner, M.; Buckley, M.F.; Van Engelen, B.G.M.; Schwartz, M.; Scheffer, H. Best Practice Guidelines and Recommendations on the Molecular Diagnosis of Myotonic Dystrophy Types 1 and 2. European Journal of Human Genetics 2012, 20, 1203–1208. [Google Scholar] [CrossRef]
- Visconti, V.V.; Centofanti, F.; Fittipaldi, S.; Macrì, E.; Novelli, G.; Botta, A. Epigenetics of Myotonic Dystrophies: A Minireview. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Lanzuolo, C. Epigenetic Alterations in Muscular Disorders. Comp Funct Genomics 2012, 2012, 256892. [Google Scholar] [CrossRef]
- Morales, F.; Corrales, E.; Zhang, B.; Vásquez, M.; Santamaría-Ulloa, C.; Quesada, H.; Sirito, M.; Estecio, M.R.; Monckton, D.G.; Krahe, R. Myotonic Dystrophy Type 1 (DM1) Clinical Subtypes and CTCF Site Methylation Status Flanking the CTG Expansion Are Mutant Allele Length-Dependent. Hum Mol Genet 2022, 31, 262–274. [Google Scholar] [CrossRef]
- Steinbach, P.; Gläser, D.; Vogel, W.; Wolf, M.; Schwemmle, S. The DMPK Gene of Severely Affected Myotonic Dystrophy Patients Is Hypermethylated Proximal to the Largely Expanded CTG Repeat. Am J Hum Genet 1998, 62, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Filippova, G.N.; Thienes, C.P.; Penn, B.H.; Cho, D.H.; Hu, Y.J.; Moore, J.M.; Klesert, T.R.; Lobanenkov, V. V.; Tapscott, S.J. CTCF-Binding Sites Flank CTG/CAG Repeats and Form a Methylation-Sensitive Insulator at the DM1 Locus. Nat Genet 2001, 28, 335–343. [Google Scholar] [CrossRef]
- Shin, K.-J.; Kang, J.; Kim, A. CTCF-Binding Sites Demarcate Chromatin Domains Enriched with H3K9me2 or H3K9me3 and Restrict the Spreading of These Histone Modifications in Human Cells. bioRxiv 2025. [Google Scholar] [CrossRef]
- Yanovsky-Dagan, S.; Avitzour, M.; Altarescu, G.; Renbaum, P.; Eldar-Geva, T.; Schonberger, O.; Mitrani-Rosenbaum, S.; Levy-Lahad, E.; Birnbaum, R.Y.; Gepstein, L.; et al. Uncovering the Role of Hypermethylation by CTG Expansion in Myotonic Dystrophy Type 1 Using Mutant Human Embryonic Stem Cells. Stem Cell Reports 2015, 5, 221–231. [Google Scholar] [CrossRef]
- Cho, D.H.; Thienes, C.P.; Mahoney, S.E.; Analau, E.; Filippova, G.N.; Tapscott, S.J. Antisense Transcription and Heterochromatin at the DM1 CTG Repeats Are Constrained by CTCF. Mol Cell 2005, 20, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Castel, A.L.; Nakamori, M.; Tomé, S.; Chitayat, D.; Gourdon, G.; Thornton, C.A.; Pearson, C.E. Expanded CTG Repeat Demarcates a Boundary for Abnormal CpG Methylation in Myotonic Dystrophy Patient Tissues. Hum Mol Genet 2010, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Barbé, L.; Lanni, S.; López-Castel, A.; Franck, S.; Spits, C.; Keymolen, K.; Seneca, S.; Tomé, S.; Miron, I.; Letourneau, J.; et al. CpG Methylation, a Parent-of-Origin Effect for Maternal-Biased Transmission of Congenital Myotonic Dystrophy. Am J Hum Genet 2017, 100, 488–505. [Google Scholar] [CrossRef]
- Brouwer, J.R.; Huguet, A.; Nicole, A.; Munnich, A.; Gourdon, G. Transcriptionally Repressive Chromatin Remodelling and CpG Methylation in the Presence of Expanded CTG-Repeats at the DM1 Locus. J Nucleic Acids 2013, 2013. [Google Scholar] [CrossRef]
- Buendía, G.A.R.; Leleu, M.; Marzetta, F.; Vanzan, L.; Tan, J.Y.; Ythier, V.; Randal, E.L.; Marque, A.C.; Baubec, T.; Murr, R.; et al. Three-Dimensional Chromatin Interactions Remain Stable upon CAG/CTG Repeat Expansion. Sci Adv 2020, 6. [Google Scholar] [CrossRef]
- Buckley, L.; Lacey, M.; Ehrlich, M. Epigenetics of the Myotonic Dystrophy-Associated DMPK Gene Neighborhood. Epigenomics 2016, 8, 13–31. [Google Scholar] [CrossRef]
- Franck, S.; De Deckersberg, E.C.; Bubenik, J.L.; Markouli, C.; Barbé, L.; Allemeersch, J.; Hilven, P.; Duqué, G.; Swanson, M.S.; Gheldof, A.; et al. Myotonic Dystrophy Type 1 Embryonic Stem Cells Show Decreased Myogenic Potential, Increased CpG Methylation at the DMPK Locus and RNA Mis-Splicing. Biol Open 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Manchón, J.; Capó, J.; Martínez-Piñeiro, A.; Juanola, E.; Pesovic, J.; Mosqueira-Martín, L.; González-Imaz, K.; Maestre-Mora, P.; Odria, R.; Savic-Pavicevic, D.; et al. Immortalized Human Myotonic Dystrophy Type 1 Muscle Cell Lines to Address Patient Heterogeneity. iScience 2024, 27. [Google Scholar] [CrossRef]
- Handal, T.; Juster, S.; Abu Diab, M.; Yanovsky-Dagan, S.; Zahdeh, F.; Aviel, U.; Sarel-Gallily, R.; Michael, S.; Bnaya, E.; Sebban, S.; et al. Differentiation Shifts from a Reversible to an Irreversible Heterochromatin State at the DM1 Locus. Nature Communications 2024 15:1 2024, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Fontana, L.; Maiorca, F.; Centofanti, F.; Massa, R.; Silvestri, G.; Novelli, G.; Botta, A. Expanded [CCTG]n Repetitions Are Not Associated with Abnormal Methylation at the CNBP Locus in Myotonic Dystrophy Type 2 (DM2) Patients. Biochim Biophys Acta Mol Basis Dis 2018, 1864, 917–924. [Google Scholar] [CrossRef]
- Malatesta, M.; Giagnacovo, M.; Renna, L. V.; Cardani, R.; Meola, G.; Pellicciari, C. Cultured Myoblasts from Patients Affected by Myotonic Dystrophy Type 2 Exhibit Senescence-Related Features: Ultrastructural Evidence. Eur J Histochem 2011, 55. [Google Scholar] [CrossRef]
- Falcone, G.; Perfetti, A.; Cardinali, B.; Martelli, F. Noncoding RNAs: Emerging Players in Muscular Dystrophies. Biomed Res Int 2014, 2014, 503634. [Google Scholar] [CrossRef]
- Tawil, R.; Van Der Maarel, S.M. Facioscapulohumeral Muscular Dystrophy. Muscle Nerve 2006, 34, 1–15. [Google Scholar] [CrossRef]
- Fecek, C.; Emmady, P.D. Facioscapulohumeral Muscular Dystrophy; StatPearls, 2023. [Google Scholar]
- Statland, J.M.; Tawil, R. Facioscapulohumeral Muscular Dystrophy. Continuum : Lifelong Learning in Neurology 2016, 22, 1916. [Google Scholar] [CrossRef]
- Cabianca, D.S.; Casa, V.; Bodega, B.; Xynos, A.; Ginelli, E.; Tanaka, Y.; Gabellini, D. A Long NcRNA Links Copy Number Variation to a Polycomb/Trithorax Epigenetic Switch in FSHD Muscular Dystrophy. Cell 2012, 149, 819. [Google Scholar] [CrossRef]
- Snider, L.; Asawachaicharn, A.; Tyler, A.E.; Geng, L.N.; Petek, L.M.; Maves, L.; Miller, D.G.; Lemmers, R.J.L.F.; Winokur, S.T.; Tawil, R.; et al. RNA Transcripts, MiRNA-Sized Fragments and Proteins Produced from D4Z4 Units: New Candidates for the Pathophysiology of Facioscapulohumeral Dystrophy. Hum Mol Genet 2009, 18, 2414–2430. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.W.; Snider, L.; Yao, Z.; Tawil, R.; Van Der Maarel, S.M.; Rigo, F.; Bennett, C.F.; Filippova, G.N.; Tapscott, S.J. DICER/AGO-Dependent Epigenetic Silencing of D4Z4 Repeats Enhanced by Exogenous SiRNA Suggests Mechanisms and Therapies for FSHD. Hum Mol Genet 2015, 24, 4817–4828. [Google Scholar] [CrossRef] [PubMed]
- Block, G.J.; Petek, L.M.; Narayanan, D.; Amell, A.M.; Moore, J.M.; Rabaia, N.A.; Tyler, A.; van der Maarel, S.M.; Tawil, R.; Filippova, G.N.; et al. Asymmetric Bidirectional Transcription from the FSHD-Causing D4Z4 Array Modulates DUX4 Production. PLoS One 2012, 7, e35532. [Google Scholar] [CrossRef]
- Koscianska, E.; Witkos, T.M.; Kozlowska, E.; Wojciechowska, M.; Krzyzosiak, W.J. Cooperation Meets Competition in MicroRNA-Mediated DMPK Transcript Regulation. Nucleic Acids Res 2015, 43, 9500. [Google Scholar] [CrossRef] [PubMed]
- Moreno, N.; Sabater-Arcis, M.; Espinosa-Espinosa, J.; Mulet-Rivero, L.; García-España, E.; González-García, J.; Seoane-Miraz, D.; Wood, M.J.A.; Varela, M.A.; Ohana, J.; et al. MiR-107 Represses DMPK and Is Sequestered by CUG Repeats Triggering the MSI2/MiR-7 Pathogenesis Axis in Myotonic Dystrophy. Mol Ther Nucleic Acids 2025, 36. [Google Scholar] [CrossRef]
- Gudde, A.E.E.G.; van Heeringen, S.J.; de Oude, A.I.; van Kessel, I.D.G.; Estabrook, J.; Wang, E.T.; Wieringa, B.; Wansink, D.G. Antisense Transcription of the Myotonic Dystrophy Locus Yields Low-Abundant RNAs with and without (CAG)n Repeat. RNA Biol 2017, 14, 1374–1388. [Google Scholar] [CrossRef]
- Gallardo, E.; Ankala, A.; Núñez-Álvarez, Y.; Hegde, M.; Diaz-Manera, J.; Luna, N. De; Pastoret, A.; Suelves, M.; Illa, I. Genetic and Epigenetic Determinants of Low Dysferlin Expression in Monocytes. Hum Mutat 2014, 35, 990–997. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Xiang, Y.; Wang, C.; Liang, B.; Li, B.; Qi, D.; Deng, Q.; Yu, H.; Lu, Z.; et al. DYSF Promotes Monocyte Activation in Atherosclerotic Cardiovascular Disease as a DNA Methylation-Driven Gene. Translational Research 2022, 247, 19–38. [Google Scholar] [CrossRef]
- Santini, G.T.; Shah, P.P.; Karnay, A.; Jain, R. Aberrant Chromatin Organization at the Nexus of Laminopathy Disease Pathways. Nucleus 2022, 13, 300–312. [Google Scholar] [CrossRef]
- Morival, J.L.P.; Widyastuti, H.P.; Nguyen, C.H.H.; Zaragoza, M. V.; Downing, T.L. DNA Methylation Analysis Reveals Epimutation Hotspots in Patients with Dilated Cardiomyopathy-Associated Laminopathies. Clin Epigenetics 2021, 13, 1–20. [Google Scholar] [CrossRef]
- Shah, P.P.; Lv, W.; Rhoades, J.H.; Poleshko, A.; Abbey, D.; Caporizzo, M.A.; Linares-Saldana, R.; Heffler, J.G.; Sayed, N.; Thomas, D.; et al. Pathogenic LMNA Variants Disrupt Cardiac Lamina-Chromatin Interactions and de-Repress Alternative Fate Genes. Cell Stem Cell 2021, 28, 938–954.e9. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Bolin, E.H.; Qasim, A.; Orloff, M.S.; Lupo, P.J.; Nembhard, W.N. DNA Methylation of the Lamin A/C Gene Is Associated with Congenital Heart Disease. Birth Defects Res 2024, 116. [Google Scholar] [CrossRef]
- Lammerding, J.; Fong, L.G.; Ji, J.Y.; Reue, K.; Stewart, C.L.; Young, S.G.; Lee, R.T. Lamins a and C but Not Lamin B1 Regulate Nuclear Mechanics. Journal of Biological Chemistry 2006, 281, 25768–25780. [Google Scholar] [CrossRef]
- Norwood, F.L.M.; Harling, C.; Chinnery, P.F.; Eagle, M.; Bushby, K.; Straub, V. Prevalence of Genetic Muscle Disease in Northern England: In-Depth Analysis of a Muscle Clinic Population. Brain 2009, 132, 3175–3186. [Google Scholar] [CrossRef]
- Bonne, G.; Leturcq, F.; Yaou, R. Ben Emery-Dreifuss Muscular Dystrophy. Current Clinical Neurology 2019, Part F2297, 159–174. [Google Scholar] [CrossRef]
- Heller, S.A.; Shih, R.; Kalra, R.; Kang, P.B. Emery-Dreifuss Muscular Dystrophy. Muscle Nerve 2019, 61, 436. [Google Scholar] [CrossRef] [PubMed]
- Madej-Pilarczyk, A.; Kochański, A. Emery-Dreifuss Muscular Dystrophy: The Most Recognizable Laminopathy. Folia Neuropathol 2016, 54, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, R.; Yang, W.; Qi, B. SP1-SYNE1-AS1-MiR-525-5p Feedback Loop Regulates Ang-II-Induced Cardiac Hypertrophy. J Cell Physiol 2019, 234, 14319–14329. [Google Scholar] [CrossRef]
- Abu-Baker, A.; Rouleau, G.A. Oculopharyngeal Muscular Dystrophy: Recent Advances in the Understanding of the Molecular Pathogenic Mechanisms and Treatment Strategies. Biochim Biophys Acta Mol Basis Dis 2007, 1772, 173–185. [Google Scholar] [CrossRef]
- Yamashita, S. Recent Progress in Oculopharyngeal Muscular Dystrophy. J Clin Med 2021, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Trollet, C.; Boulinguiez, A.; Roth, F.; Stojkovic, T.; Butler-Browne, G.; Evangelista, T.; Guily, J.L.S.; Richard, P. Oculopharyngeal Muscular Dystrophy. Current Clinical Neurology 2020, Part F2297, 123–130. [Google Scholar] [CrossRef]
- Abdelmohsen, K.; Panda, A.C.; Munk, R.; Grammatikakis, I.; Dudekula, D.B.; De, S.; Kim, J.; Noh, J.H.; Kim, K.M.; Martindale, J.L.; et al. Identification of HuR Target Circular RNAs Uncovers Suppression of PABPN1 Translation by CircPABPN1. RNA Biol 2017, 14, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Shademan, M.; Mei, H.; van Engelen, B.; Ariyurek, Y.; Kloet, S.; Raz, V. PABPN1 Loss-of-Function Causes APA-Shift in Oculopharyngeal Muscular Dystrophy. Human Genetics and Genomics Advances 2024, 5, 100269. [Google Scholar] [CrossRef] [PubMed]
- Bertini, E.; D’Amico, A.; Gualandi, F.; Petrini, S. Congenital Muscular Dystrophies: A Brief Review. Semin Pediatr Neurol 2011, 18, 277. [Google Scholar] [CrossRef]
- Bönnemann, C.G.; Wang, C.H.; Quijano-Roy, S.; Deconinck, N.; Bertini, E.; Ferreiro, A.; Muntoni, F.; Sewry, C.; Béroud, C.; Mathews, K.D.; et al. Diagnostic Approach to the Congenital Muscular Dystrophies. Neuromuscul Disord 2014, 24, 289. [Google Scholar] [CrossRef]
- Zambon, A.A.; Muntoni, F. Congenital Muscular Dystrophies: What Is New? Neuromuscular Disorders 2021, 31, 931–942. [Google Scholar] [CrossRef]
- Li, S.; Hu, J.; Li, G.; Mai, H.; Gao, Y.; Liang, B.; Wu, H.; Guo, J.; Duan, Y. Epigenetic Regulation of LINC01270 in Breast Cancer Progression by Mediating LAMA2 Promoter Methylation and MAPK Signaling Pathway. Cell Biol Toxicol 2023, 39, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




