Submitted:
20 August 2025
Posted:
22 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Overview of Air Pollutants: Definitions and Classifications
3. Sources of HAPs in PR
4. Key Pollutants and Carcinogenic Classifications
5. Mechanisms of Respiratory-Related Carcinogenesis by Air Pollutants
6. Other Respiratory Conditions Induced by Air Pollutants
7. Epidemiological and Toxicological Evidence in PR
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- (HEI), H.E.I. State of Global Air 2024: A Special Report on Global Exposure to Air Pollution and Its Health Impacts, With a Focus on Children’s Health. Available online: https://www.stateofglobalair.org/resources/report/state-global-air-report-2024 (accessed on 05/28/2025).
- (WHO), W.H.O. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 4 October 2025).
- Cancer, I.A.f.R.o.; Outdoor air pollution a leading environmental cause of cancer deaths. Press Release No 221. Available online: https://www.iarc.who.int/wp-content/uploads/2018/07/pr221_E.pdf (accessed on 04/10/2025).
- Turner, M.C.; Andersen, Z.J.; Baccarelli, A.; Diver, W.R.; Gapstur, S.M.; Pope, C.A., 3rd; Prada, D.; Samet, J.; Thurston, G.; Cohen, A. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J Clin 2020. [Google Scholar] [CrossRef]
- Leitch, C. Air Pollution Found to Drive Lung Cancer in Non Smokers. Available online: https://www.labroots.com/trending/genetics-and-genomics/29299/air-pollution-found-drive-lung-cancer-smokers (accessed on 07/20/2025).
- Díaz-Gay, M.; Zhang, T.; Hoang, P.H.; Leduc, C.; Baine, M.K.; Travis, W.D.; Sholl, L.M.; Joubert, P.; Khandekar, A.; Zhao, W.; et al. The mutagenic forces shaping the genomes of lung cancer in never smokers. Nature 2025. [Google Scholar] [CrossRef]
- Subramanian, R.; Ellis, A.; Torres-Delgado, E.; Tanzer, R.; Malings, C.; Rivera, F.; Morales, M.; Baumgardner, D.; Presto, A.A.; Mayol-Bracero, O.L. Air Quality in Puerto Rico in the Aftermath of Hurricane Maria: A Case Study on the Use of Lower Cost Air Quality Monitors. ACS Earth and Space Chemistry 2018, 2, 1179–1186. [Google Scholar] [CrossRef]
- Jirau-Colón, H.; Cosme, A.; Marcial-Vega, V.; Jiménez-Vélez, B. Toxic Metals Depuration Profiles from a Population Adjacent to a Military Target Range (Vieques) and Main Island Puerto Rico. International journal of environmental research and public health 2020, 17, 264. [Google Scholar] [CrossRef]
- Toro-Heredia, J.; Jirau-Colón, H.; Jiménez-Vélez, B.D. Linking PM2.5 organic constituents, relative toxicity and health effects in Puerto Rico. Environmental Challenges 2021, 5, 100350. [Google Scholar] [CrossRef]
- Jirau-Colón, H.; Toro-Heredia, J.; Layuno, J.; Calderon, E.D.; Gioda, A.; Jiménez-Vélez, B.D. Distribution of toxic metals and relative toxicity of airborne PM2.5 in Puerto Rico. Environ Sci Pollut R 2021, 28, 16504–16516. [Google Scholar] [CrossRef]
- Massol-Deyá, A.; Pérez, D.; Pérez, E.; Berrios, M.; Diaz, E. Trace elements analysis in forage samples from a US Navy bombing range (Vieques, Puerto Rico). International journal of environmental research and public health 2005, 2, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, H.; Patrik, F.; Steven, S.R.; Jesper, C.; Per, L.; and Becker, T. Civilian exposure to munitions-specific carcinogens and resulting cancer risks for civilians on the Puerto Rican island of Vieques following military exercises from 1947 to 1998. Global Security: Health, Science and Policy 2017, 2, 40–61. [Google Scholar] [CrossRef]
- Ortiz-Roque, C.; López-Rivera, Y. Mercury contamination in reproductive age women in a Caribbean island: Vieques. Journal of Epidemiology and Community Health 2004, 58, 756–757. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, B.; Lu, Y.; Cao, Z.; Yu, S.; Fan, F.; Zhu, M. Malignant transformation of human bronchial epithelial cell (BEAS-2B) induced by depleted uranium. Ai Zheng 2002, 21, 944–948. [Google Scholar]
- Briner, W. The toxicity of depleted uranium. International journal of environmental research and public health 2010, 7, 303–313. [Google Scholar] [CrossRef]
- Singh, P.; Tiwari, D.; Mishra, M.; Kumar, D. Molecular Mechanisms of Heavy Metal Toxicity in Cancer Progression. In Networking of Mutagens in Environmental Toxicology, Kesari, K.K., Ed.; Springer International Publishing: Cham, 2019; pp. 49–79. [Google Scholar]
- Rodríguez-Cotto, R.I.; Ortiz-Martínez, M.G.; Rivera-Ramírez, E.; Méndez, L.B.; Dávila, J.C.; Jiménez-Vélez, B.D. African Dust Storms Reaching Puerto Rican Coast Stimulate the Secretion of IL-6 and IL-8 and Cause Cytotoxicity to Human Bronchial Epithelial Cells (BEAS-2B). Health (Irvine Calif) 2013, 5, 14–28. [Google Scholar] [CrossRef]
- Ferlay, J.E.M.; Lam, F.; et al. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/630-puerto-rico-fact-sheet.pdf (accessed on 08/10/2025).
- Tirado-Delgado, J.M. The spatial and temporal distribution of particulate matter pollution on the island of Puerto Rico. Howard University, 2009.
- Agency, U.S.E.P. Hazardous Air Pollutants (HAPs). Available online: https://www.epa.gov/haps (accessed on 06/04/2025).
- Thurston, G.D. Outdoor Air Pollution: Sources, Atmospheric Transport and Human Health Effects; Academic Press: 2025; pp. 184–197.
- (NHDES), N.H.D.o.E.S. Criteria Pollutants. Available online: https://www.des.nh.gov/air/state-implementation-plans/criteria-pollutants (accessed on 04/25/2025).
- Kim, B.-J.; Kwon, J.-W.; Seo, J.-H.; Kim, H.-B.; Lee, S.-Y.; Park, K.-S.; Yu, J.; Kim, H.-C.; Leem, J.-H.; Sakong, J.; et al. Association of ozone exposure with asthma, allergic rhinitis, and allergic sensitization. Annals of Allergy, Asthma & Immunology 2011, 107, 214–219.e211. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-M.; Kuschner, W.G.; Gokhale, J.; Shofer, S. Outdoor Air Pollution: Nitrogen Dioxide, Sulfur Dioxide, and Carbon Monoxide Health Effects. The American Journal of the Medical Sciences 2007, 333, 249–256. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Frontiers in Public Health 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Ortega, D.; González Esquivel, D.F.; Blanco Ayala, T.; Pineda, B.; Gómez Manzo, S.; Marcial Quino, J.; Carrillo Mora, P.; Pérez de la Cruz, V. Cognitive Impairment Induced by Lead Exposure during Lifespan: Mechanisms of Lead Neurotoxicity. Toxics 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Agency, U.S.E.P. News Release: EPA proposes to find that two areas in Puerto Rico do not meet AQS for sulfur dioxide and proposes to approve elements of Puerto Rico’s plan to improve air quality. Available online: https://www.epa.gov/newsreleases/epa-proposes-find-two-areas-puerto-rico-do-not-meet-air-quality-standards-sulfur-0 (accessed on 06/04/2025).
- Agency, U.S.E.P. Initial List of Hazardous Air Pollutants with Modifications. Available online: https://www.epa.gov/haps/initial-list-hazardous-air-pollutants-modifications#mods (accessed on 06/04/2025).
- Agency, U.S.E.P. Health and Environmental Effects of Hazardous Air Pollutants. Available online: https://www.epa.gov/haps/health-and-environmental-effects-hazardous-air-pollutants (accessed on 06/04/2025).
- Agency, U.S.E.P. Air Data: Air Quality Data Collected at Outdoor Monitors Across the US. Available online: https://www.epa.gov/outdoor-air-quality-data (accessed on 06/04/2025).
- (UCS), U.o.C.S. Ethylene Oxide in Puerto Rico. Available online: https://www.ucs.org/resources/puerto-rico (accessed on 04/25/2025).
- Earthjustice. Toxic Coal Ash in Puerto Rico: The Hazardous Legacy of the AES-PR Coal Plant. Available online: https://earthjustice.org/feature/coal-ash-states/puerto-rico (accessed on 04/25/2025).
- Lafarga Previdi, I.; Vélez Vega, C.M. Health Disparities Research Framework Adaptation to Reflect Puerto Rico’s Socio-Cultural Context. International journal of environmental research and public health 2020, 17. [Google Scholar] [CrossRef]
- Loyo-Berríos, N.I.; Irizarry, R.; Hennessey, J.G.; Tao, X.G.; Matanoski, G. Air Pollution Sources and Childhood Asthma Attacks in Cataño, Puerto Rico. American Journal of Epidemiology 2007, 165, 927–935. [Google Scholar] [CrossRef]
- Agency, U.S.E.P. The Battery Recycling Company, Superfund Site Profile, Superfund Site Information. Available online: https://cumulis.epa.gov/supercpad/SiteProfiles/index.cfm?fuseaction=second.cleanup&id=0206263 (accessed on 06/11/2025).
- PBS. PBS NewsHour: Coal ash raising concerns over health risks in Puerto Rico. Available online: https://www.pbs.org/newshour/show/coal-ash-raising-concerns-over-health-risks-in-puerto-rico (accessed on 04/25/2025).
- Rosado Lebrón, J.A. EPA Took Six Years to Regulate Toxic Emission in Puerto Rico That Could Cause Cancer. Centro de Periodismo Investigativo (CPI), 2022. [Google Scholar]
- Agency, U.S.E.P. EPA gives public picture of Puerto Rico’s environmental releases: Many new industry groups, including utilities, report toxic releases for first time; U.S. Environmental Protection Agency: 2000/05/11 2000.
- Madrigal, J.M.; Flory, A.; Fisher, J.A.; Sharp, E.; Graubard, B.I.; Ward, M.H.; Jones, R.R. Sociodemographic inequities in the burden of carcinogenic industrial air emissions in the United States. J Natl Cancer Inst 2024, 116, 737–744. [Google Scholar] [CrossRef]
- Padilla Dalmau, C.A.; Ocasio Torres, M.E. Disaster debris is pushing Puerto Rico’s landfills to the brink. Available online: https://economichardship.org/2022/10/disaster-debris-is-pushing-puerto-ricos-landfills-to-the-brink/ (accessed on 07/27/2025).
- Padilla Dalmau, C.A.; Ocasio Torres, M.E. Disaster debris is pushing Puerto Rico’s landfills to the brink. Available online: https://grist.org/extreme-weather/disaster-debris-is-pushing-puerto-ricos-landfills-to-the-brink/ (accessed on 07/27/2025).
- Cancer, I.A.f.R.o. Diesel and Gasoline Engine Exhausts and Some Nitroarenes. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: 2014; Volume 105, pp. 9–699.
- Silverman, D.T. Diesel Exhaust and Lung Cancer-Aftermath of Becoming an IARC Group 1 Carcinogen. Am J Epidemiol 2018, 187, 1149–1152. [Google Scholar] [CrossRef]
- Agency, U.S.E.P. Toxics Release Inventory (TRI) Program: 2019 TRI National Analysis. Available online: https://www.epa.gov/trinationalanalysis (accessed on 05/20/2025).
- Reid, J.S. Analysis of measurements of Saharan dust by airborne and ground-based remote sensing methods during the Puerto Rico Dust Experiment (PRIDE). J. Geophys. Res. 2003, 108, 8586. [Google Scholar] [CrossRef]
- Ortiz-Martínez, M.G.; Rodríguez-Cotto, R.I.; Ortiz-Rivera, M.A.; Pluguez-Turull, C.W.; Jiménez-Vélez, B.D. Linking Endotoxins, African Dust PM10 and Asthma in an Urban and Rural Environment of Puerto Rico. Mediators Inflammation 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Ortiz-Martínez, M.G.; Rodríguez-Cotto, R.I.; Ortiz-Rivera, M.A.; Pluguez-Turull, C.W.; Jiménez-Vélez, B.D. Linking Endotoxins, African Dust PM10 and Asthma in an Urban and Rural Environment of Puerto Rico. Mediators of Inflammation 2015, 2015, 784212. [Google Scholar] [CrossRef]
- Morales-Medina, M.; Ortíz-Martínez, A.P.; Pérez-Cardona, C.M.; Rueda-Roa, D.; Otis, D.; Pérez-Matías, E.; Muller-Karger, F.; Mayol-Bracero, O.; Méndez-Lázaro, P. Who Is Affected by Saharan Dust in the Caribbean? A Spatial Analysis and Citizen’s Perspective from Puerto Rico during the Godzilla Dust Event in June 2020. Weather, Climate, and Society 2024, 16, 205–219. [Google Scholar] [CrossRef]
- Sabia, S.; Morris, K.R. NASA Helps Puerto Rico Prepare for Saharan Dust Impacts, 2020. Available online: https://www.nasa.gov/missions/aqua/nasa-helps-puerto-rico-prepare-for-saharan-dust-impacts (accessed on 04/25/2025).
- Horwell, C.J.; Baxter, P.J. The respiratory health hazards of volcanic ash: a review for volcanic risk mitigation. Bulletin of Volcanology 2006, 69, 1–24. [Google Scholar] [CrossRef]
- Kim, B.M.; Seo, J.; Kim, J.Y.; Lee, J.Y.; Kim, Y. Transported vs. local contributions from secondary and biomass burning sources to PM2.5. Atmospheric Environment 2016, 144, 24–36. [Google Scholar] [CrossRef]
- Pathak, G.; Nichter, M.; Hardon, A.; Moyer, E. The Open Burning of Plastic Wastes is an Urgent Global Health Issue. Ann Glob Health 2024, 90, 3. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C. Army Corps engineers begin burning hurricane debris in Puerto Rico. Available online: https://www.sierraclub.org/press-releases/2018/02/army-corps-engineers-begin-burning-hurricane-debris-puerto-rico (accessed on 07/27/2025).
- Zúñiga-Venegas Liliana, A.; Hyland, C.; Muñoz-Quezada María, T.; Quirós-Alcalá, L.; Butinof, M.; Buralli, R.; Cardenas, A.; Fernandez Ricardo, A.; Foerster, C.; Gouveia, N.; et al. Health Effects of Pesticide Exposure in Latin American and the Caribbean Populations: A Scoping Review. Environ Health Persp 2022, 130. [Google Scholar] [CrossRef]
- Santiago, X.B.; Rivera, D.; Pabon, A.; Garcia, A. An Examination of the Use of Pesticides in Puerto Rican Agriculture. RURALS: Review of Undergraduate Research in Agricultural and Life Sciences 2016, 10, Article–1. [Google Scholar]
- Agency, U.S.E.P. Paraquat Dichloride: Tier II Epidemiology Report. Available online: https://19january2021snapshot.epa.gov/sites/static/files/2019-08/documents/paraquat_epi_review.pdf (accessed on 06/04/2025).
- Bryant, D.J.; Nelson, B.S.; Swift, S.J.; Budisulistiorini, S.H.; Drysdale, W.S.; Vaughan, A.R.; Newland, M.J.; Hopkins, J.R.; Cash, J.M.; Langford, B.; et al. Biogenic and anthropogenic sources of isoprene and monoterpenes and their secondary organic aerosol in Delhi, India. Atmos. Chem. Phys. 2023, 23, 61–83. [Google Scholar] [CrossRef]
- Hantson, S.; Knorr, W.; Schurgers, G.; Pugh, T.A.M.; Arneth, A. Global isoprene and monoterpene emissions under changing climate, vegetation, CO2 and land use. Atmospheric Environment 2017, 155, 35–45. [Google Scholar] [CrossRef]
- McFiggans, G.; Mentel, T.F.; Wildt, J.; Pullinen, I.; Kang, S.; Kleist, E.; Schmitt, S.; Springer, M.; Tillmann, R.; Wu, C.; et al. Secondary organic aerosol reduced by mixture of atmospheric vapours. Nature 2019, 565, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Guo, H.; Boyd, C.M.; Klein, M.; Bougiatioti, A.; Cerully, K.M.; Hite, J.R.; Isaacman-VanWertz, G.; Kreisberg, N.M.; Knote, C.; et al. Effects of anthropogenic emissions on aerosol formation from isoprene and monoterpenes in the southeastern United States. Proceedings of the National Academy of Sciences 2015, 112, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.; Lin, J.; Martin, R.; Millet, D.B.; Jaeglé, L.; Ridley, D.; Keller, C.; Li, C.; Du, M.; Meng, J. Global high-resolution emissions of soil NOx, sea salt aerosols, and biogenic volatile organic compounds. Scientific Data 2020, 7, 148. [Google Scholar] [CrossRef]
- Kelly, J.M.; Doherty, R.M.; O'Connor, F.M.; Mann, G.W. The impact of biogenic, anthropogenic, and biomass burning volatile organic compound emissions on regional and seasonal variations in secondary organic aerosol. Atmos. Chem. Phys. 2018, 18, 7393–7422. [Google Scholar] [CrossRef]
- Agency, U.S.E.P. IRIS Advanced Search. Available online: https://cfpub.epa.gov/ncea/iris/search/index.cfm (accessed on 06/04/2025).
- Agency, U.S.E.P. Integrated Risk Information System (IRIS). Available online: https://iris.epa.gov (accessed on 06/04/2025).
- Cancer, I.A.f.R.o. Chemical Agents and Related Occupations; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100F. [Google Scholar]
- Agency, U.S.E.P. 2014 National Air Toxics Assessment (NATA) Summary of Results. Available online: https://www.epa.gov/sites/default/files/2020-07/documents/nata_2014_summary_of_results.pdf (accessed on 06/04/2025).
- Cancer, I.A.f.R.o. List of classifications by cancer sites with sufficient or limited evidence in humans (IARC Monographs Volumes 1–138a). Available online: https://monographs.iarc.who.int/wp-content/uploads/2019/07/Classifications_by_cancer_site.pdf (accessed on 05/22/2025).
- Cancer, I.A.f.R.o. Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans (accessed on 05/22/2025).
- (NTP), N.T.P. Completed RoC Evaluations. Available online: https://ntp.niehs.nih.gov/research/assessments/cancer/completed/roc (accessed on 05/25/2025).
- Agency, U.S.E.P. AP-42 Section 1.3: Fuel Oil Combustion. Available online: https://www.epa.gov/sites/default/files/2020-09/documents/1.3_fuel_oil_combustion.pdf (accessed on 06/04/2025).
- Joshi, D.R.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. Journal of Pharmaceutical Research International 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Davidson, C.J.; Hannigan, J.H.; Bowen, S.E. Effects of inhaled combined Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX): Toward an environmental exposure model. Environmental toxicology and pharmacology 2021, 81, 103518. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.H.; Obot, I.B. Corrosion challenges in petroleum refinery operations: Sources, mechanisms, mitigation, and future outlook. Journal of Saudi Chemical Society 2021, 25, 101370. [Google Scholar] [CrossRef]
- Agency, U.S.E.P. Toxicological Review of Benzene (Noncancer Effects). Available online: https://iris.epa.gov/static/pdfs/0276tr.pdf (accessed on 06/04/2025).
- Corbin, J.C.; Mensah, A.A.; Pieber, S.M.; Orasche, J.; Michalke, B.; Zanatta, M.; Czech, H.; Massabò, D.; Buatier de Mongeot, F.; Mennucci, C.; et al. Trace Metals in Soot and PM(2.5) from Heavy-Fuel-Oil Combustion in a Marine Engine. Environ Sci Technol 2018, 52, 6714–6722. [Google Scholar] [CrossRef]
- Chin, J.Y.; Batterman, S.A.; Northrop, W.F.; Bohac, S.V.; Assanis, D.N. Gaseous and Particulate Emissions from Diesel Engines at Idle and under Load: Comparison of Biodiesel Blend and Ultralow Sulfur Diesel Fuels. Energy Fuels 2012, 26, 6737–6748. [Google Scholar] [CrossRef]
- Bendtsen, K.M.; Bengtsen, E.; Saber, A.T.; Vogel, U. A review of health effects associated with exposure to jet engine emissions in and around airports. Environmental health : a global access science source 2021, 20, 10. [Google Scholar] [CrossRef]
- Chatterjee, S.; Deb, U.; Datta, S.; Walther, C.; Gupta, D.K. Common explosives (TNT, RDX, HMX) and their fate in the environment: Emphasizing bioremediation. Chemosphere 2017, 184, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Matei, E.; Râpă, M.; Mateș, I.M.; Popescu, A.-F.; Bădiceanu, A.; Balint, A.I.; Covaliu-Mierlă, C.I. Heavy Metals in Particulate Matter—Trends and Impacts on Environment. Molecules (Basel, Switzerland) 2025, 30, 1455. [Google Scholar] [CrossRef] [PubMed]
- Golden, D.M. Interaction of combustion with the atmosphere. Proceedings of the Combustion Institute 2000, 28, 2383–2392. [Google Scholar] [CrossRef]
- Ünlü Endirlik, B.; Wincent, E.; Dreij, K. Non-additive mixture effects of benzo[a]pyrene and pesticides in vitro and in vivo: Role of AhR signaling. Environ Pollut 2023, 316, 120510. [Google Scholar] [CrossRef]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. Journal of environmental management 2022, 323, 116285. [Google Scholar] [CrossRef] [PubMed]
- Shaked, Y.; Yang, J.; Monaghan, M.; van Gerwen, M. The Association between Metals and Thyroid Cancer in Puerto Rico—A National Health and Nutrition Examination Survey Analysis and Ecological Study. Toxics 2024, 12, 632. [Google Scholar] [CrossRef]
- Lydersen, K. In Puerto Rico, residents wait for accountability, cleanup of toxic coal ash “caminos blancos” – Sierra Nevada Ally. Available online: https://sierranevadaally.org/2023/10/15/in-puerto-rico-residents-wait-for-accountability-cleanup-of-toxic-coal-ash-caminos-blancos/ (accessed on 06/20/2025).
- (CDPI), C.d.P.I. Damage by coal ash to the southern aquifer cannot be undone. Available online: https://periodismoinvestigativo.com/2019/03/damage-by-coal-ash-to-the-southern-aquifer-cannot-be-undone/ (accessed on 06/20/2025).
- Cancer, I.A.f.R.o. Agents Classified by the IARC Monographs, Volumes 1–138. Available online: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed on 6/20/2025).
- Evans, L. Ash in Lungs: How Breathing Coal Ash is Hazardous to Your Health. Available online: https://earthjustice.org/article/ash-in-lungs-how-breathing-coal-ash-is-hazardous-to-your-health (accessed on 06/20/2025).
- (CDPI), C.d.P.I. Centro de Periodismo Investigativo (CDPI): Gobierno aprueba ley contra las cenizas, pero se enreda en su aplicación. Available online: https://periodismoinvestigativo.com/2017/07/gobierno-aprueba-ley-contra-las-cenizas-pero-se-enreda-en-su-aplicacion/ (accessed on 06/20/2025).
- Agency, U.S.E.P. EPA Settlement with AES Requires More Monitoring and Payment of Penalty for Clean Air Act Violations in Guayama, Puerto Rico. Available online: https://www.epa.gov/newsreleases/epa-settlement-aes-requires-more-monitoring-and-payment-penalty-clean-air-act (accessed on 06/04/2025).
- Kantrow, M. EPA slaps $3.1M fine on AES Puerto Rico, requires more monitoring. Available online: https://newsismybusiness.com/epa-slaps-3-1m-fine-on-aes-puerto-rico-requires-more-monitoring/ (accessed on 06/20/2025).
- Aponte, J. Corporations Are Poisoning People in Puerto Rico With Coal Ash. Available online: https://truthout.org/articles/corporations-are-poisoning-people-in-puerto-rico-with-coal-ash/ (accessed on 06/20/2025).
- Dávila-Ruhaak, S. Making the Case for a Right to a Healthy Environment for the Protection of Vulnerable Communities: A Case of Coal-Ash Disaster in Puerto Rico. Michigan Journal of Environmental & Administrative Law 2020, 9. [Google Scholar]
- (IRIS), I.R.I.S. A to Z List. Available online: https://iris.epa.gov/AtoZ/?list_type=alpha (accessed on 06/20/2025).
- Yu, X.J.; Yang, M.J.; Zhou, B.; Wang, G.Z.; Huang, Y.C.; Wu, L.C.; Cheng, X.; Wen, Z.S.; Huang, J.Y.; Zhang, Y.D.; et al. Characterization of Somatic Mutations in Air Pollution-Related Lung Cancer. EBioMedicine 2015, 2, 583–590. [Google Scholar] [CrossRef]
- Mudipalli, A. Airborne Carcinogens: Mechanisms of Cancer. In Air Pollution and Health Effects, Nadadur, S.S., Hollingsworth, J.W., Eds.; Springer London: London, 2015; pp. 151–184. [Google Scholar]
- Wang, L.H.; Wu, C.F.; Rajasekaran, N.; Shin, Y.K. Loss of Tumor Suppressor Gene Function in Human Cancer: An Overview. Cell Physiol Biochem 2018, 51, 2647–2693. [Google Scholar] [CrossRef]
- Cho, A.K.; Sioutas, C.; Miguel, A.H.; Kumagai, Y.; Schmitz, D.A.; Singh, M.; Eiguren-Fernandez, A.; Froines, J.R. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ Res 2005, 99, 40–47. [Google Scholar] [CrossRef]
- Lee, C.W.; Vo, T.T.T.; Wu, C.Z.; Chi, M.C.; Lin, C.M.; Fang, M.L.; Lee, I.T. The Inducible Role of Ambient Particulate Matter in Cancer Progression via Oxidative Stress-Mediated Reactive Oxygen Species Pathways: A Recent Perception. Cancers (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Li, T.; Hu, R.; Chen, Z.; Li, Q.; Huang, S.; Zhu, Z.; Zhou, L.F. Fine particulate matter (PM(2.5)): The culprit for chronic lung diseases in China. Chronic Dis Transl Med 2018, 4, 176–186. [Google Scholar] [CrossRef]
- Guo, C.; Hoek, G.; Chang, L.Y.; Bo, Y.; Lin, C.; Huang, B.; Chan, T.C.; Tam, T.; Lau, A.K.H.; Lao, X.Q. Long-Term Exposure to Ambient Fine Particulate Matter (PM2.5) and Lung Function in Children, Adolescents, and Young Adults: A Longitudinal Cohort Study. Environ Health Perspect 2019, 127, 127008. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Yoon, S.J.; Choi, S.; Jung, J.; Park, J.Y.; Park, Y.H.; Seo, J.; Lee, J.; Lee, M.S.; Lee, S.J.; et al. Particulate matter promotes cancer metastasis through increased HBEGF expression in macrophages. Exp Mol Med 2022, 54, 1901–1912. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ J 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Baccarelli, A.; Wright, R.O.; Bollati, V.; Tarantini, L.; Litonjua, A.A.; Suh, H.H.; Zanobetti, A.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Rapid DNA Methylation Changes after Exposure to Traffic Particles. Am J Respir Crit Care Med 2009, 179, 572–578. [Google Scholar] [CrossRef]
- Mostafavi, N.; Vermeulen, R.; Ghantous, A.; Hoek, G.; Probst-Hensch, N.; Herceg, Z.; Tarallo, S.; Naccarati, A.; Kleinjans, J.C.S.; Imboden, M.; et al. Acute changes in DNA methylation in relation to 24 h personal air pollution exposure measurements: A panel study in four European countries. Environment international 2018, 120, 11–21. [Google Scholar] [CrossRef]
- Peng, D.; Liu, X.-Y.; Sheng, Y.-H.; Li, S.-Q.; Zhang, D.; Chen, B.; Yu, P.; Li, Z.-Y.; Li, S.; Xu, R.-B. Ambient air pollution and the risk of cancer: Evidence from global cohort studies and epigenetic-related causal inference. J Hazard Mater 2025, 489, 137619. [Google Scholar] [CrossRef]
- Wu, H.; Eckhardt, C.M.; Baccarelli, A.A. Molecular mechanisms of environmental exposures and human disease. Nature Reviews Genetics 2023, 24, 332–344. [Google Scholar] [CrossRef]
- Szende, B.; Tyihák, E. Effect of formaldehyde on cell proliferation and death. Cell Biol Int 2010, 34, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Vogeley, C.; Sondermann, N.C.; Woeste, S.; Momin, A.A.; Gilardino, V.; Hartung, F.; Heinen, M.; Maaß, S.K.; Mescher, M.; Pollet, M.; et al. Unraveling the differential impact of PAHs and dioxin-like compounds on AKR1C3 reveals the EGFR extracellular domain as a critical determinant of the AHR response. Environment international 2022, 158, 106989. [Google Scholar] [CrossRef] [PubMed]
- Kelly-Reif, K.; Bertke, S.J.; Stayner, L.; Steenland, K. Exposure to Ethylene Oxide and Relative Rates of Female Breast Cancer Mortality: 62 Years of Follow-Up in a Large US Occupational Cohort. Environ Health Persp 2025, 133, 057013. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.N.; Falk, R.T.; Schairer, C.; Moore, S.C.; Fuhrman, B.J.; Dallal, C.M.; Bauer, D.C.; Dorgan, J.F.; Shu, X.-O.; Zheng, W.; et al. Association of Estrogen Metabolism with Breast Cancer Risk in Different Cohorts of Postmenopausal Women. Cancer Research 2017, 77, 918–925. [Google Scholar] [CrossRef]
- Chen, R.-J.; Siao, S.-H.; Hsu, C.-H.; Chang, C.-Y.; Chang, L.W.; Wu, C.-H.; Lin, P.; Wang, Y.-J. TCDD Promotes Lung Tumors via Attenuation of Apoptosis through Activation of the Akt and ERK1/2 Signaling Pathways. Plos One 2014, 9, e99586. [Google Scholar] [CrossRef]
- Buñay, J.; Kossai, M.; Damon-Soubeyrant, C.; De Haze, A.; Saru, J.P.; Trousson, A.; de Joussineau, C.; Bouchareb, E.; Kocer, A.; Vialat, M.; et al. Persistent organic pollutants promote aggressiveness in prostate cancer. Oncogene 2023, 42, 2854–2867. [Google Scholar] [CrossRef]
- Beyersmann, D.; Hartwig, A. Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Archives of Toxicology 2008, 82, 493–512. [Google Scholar] [CrossRef]
- Wu, X.; Ciminieri, C.; Bos, I.S.T.; Woest, M.E.; D'Ambrosi, A.; Wardenaar, R.; Spierings, D.C.J.; Königshoff, M.; Schmidt, M.W.I.; Kistemaker, L.E.M.; et al. Diesel exhaust particles distort lung epithelial progenitors and their fibroblast niche. Environ Pollut 2022, 305, 119292. [Google Scholar] [CrossRef]
- Risom, L.; Møller, P.; Loft, S. Oxidative stress-induced DNA damage by particulate air pollution. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 2005, 592, 119–137. [Google Scholar] [CrossRef]
- Potera, C. Making Sense of Carcinogens: A New Method for Navigating Mechanistic Data. Environ Health Persp 2016, 124, A113–A113. [Google Scholar] [CrossRef]
- DeMarini, D.M.; Gwinn, W.; Watkins, E.; Reisfeld, B.; Chiu, W.A.; Zeise, L.; Barupal, D.; Bhatti, P.; Cross, K.; Dogliotti, E.; et al. IARC Workshop on the Key Characteristics of Carcinogens: Assessment of End Points for Evaluating Mechanistic Evidence of Carcinogenic Hazards. Environ Health Perspect 2025, 133, 25001. [Google Scholar] [CrossRef]
- Brender, J.D.; Maantay, J.A.; Chakraborty, J. Residential Proximity to Environmental Hazards and Adverse Health Outcomes. American Journal of Public Health 2011, 101, S37–S52. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, E. , Medicine (NASEM). Using 21st Century Science to Improve Risk-Related Evaluations; The National Academies Press: Washington, DC, 2017; p. 200. [Google Scholar]
- Prüss-Üstün, A.; Wolf, J.; Corvalán, C.; Bos, R.; Neira, D.M. Preventing Disease through Healthy Environments; World Health Organization: 2016.
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. The Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- (WHO), W.H.O. Executive summary: Guidelines for Indoor Air Quality: Selected Pollutants; WHO: Geneva, 2010. [Google Scholar]
- Rai, R.; El-Zaemey, S.; Dorji, N.; Fritschi, L. Occupational exposures to hazardous chemicals and agents among healthcare workers in Bhutan. Am J Ind Med 2020, 63, 1109–1115. [Google Scholar] [CrossRef]
- Bello, A.; Xue, Y.; Gore, R.; Woskie, S.; Bello, D. Assessment and control of exposures to polymeric methylene diphenyl diisocyanate (pMDI) in spray polyurethane foam applicators. Int J Hyg Environ Health 2019, 222, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Aslam, R.; Sharif, F.; Baqar, M.; Nizami, A.S.; Ashraf, U. Role of ambient air pollution in asthma spread among various population groups of Lahore City: a case study. Environmental science and pollution research international 2023, 30, 8682–8697. [Google Scholar] [CrossRef]
- Kamis, A.; Cao, R.; He, Y.; Tian, Y.; Wu, C. Predicting Lung Cancer in the United States: A Multiple Model Examination of Public Health Factors. International journal of environmental research and public health 2021, 18, 6127. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, J.; Sun, J.; Xin, L. PM2.5 induces inflammatory responses via oxidative stress-mediated mitophagy in human bronchial epithelial cells. Toxicology Research 2022, 11, 195–205. [Google Scholar] [CrossRef]
- Loomis, D.; Grosse, Y.; Lauby-Secretan, B.; Ghissassi, F.E.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Baan, R.; Mattock, H.; Straif, K. The carcinogenicity of outdoor air pollution. The Lancet Oncology 2013, 14, 1262–1263. [Google Scholar] [CrossRef]
- (WHO), W.H.O. Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide; World Health Organization: Geneva, 2021. [Google Scholar]
- Ho, G.Y.; Figueroa-Vallés, N.R.; De La Torre-Feliciano, T.; Tucker, K.L.; Tortolero-Luna, G.; Rivera, W.T.; Jiménez-Velázquez, I.Z.; Ortiz-Martínez, A.P.; Rohan, T.E. Cancer disparities between mainland and island Puerto Ricans. Rev Panam Salud Publica 2009, 25, 394–400. [Google Scholar] [CrossRef]
- Figueroa-Vallés, N.R.; Ortiz-Ortiz, K.J.; Pérez-Ríos, N.; Villanueva-Rosa, E.; Traverso-Ortiz, M.; Torres-Cintrón, C.R.; Suárez-Ramos, T. Cancer in Puerto Rico, 2004–2009; Puerto Rico Central Cancer Registry: San Juan, PR, 2012. [Google Scholar]
- Fernández Porto, J. Vieques: Environmental and Ecological Damage. Diálogo 2000, 4, 7. [Google Scholar]
- Remigio, R.V.; Andreotti, G.; Sandler, D.P.; Erickson, P.A.; Koutros, S.; Albert, P.S.; Hurwitz, L.M.; Parks, C.G.; Lubin, J.H.; Hofmann, J.N.; et al. An Updated Evaluation of Atrazine-Cancer Incidence Associations among Pesticide Applicators in the Agricultural Health Study Cohort. Environ Health Perspect 2024, 132, 27010. [Google Scholar] [CrossRef]
- (NCBI):, N.C.f.B.I.; (NIH), N.I.o.H. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 05/22/2025).
- Agency, U.S.E.P. Technical Fact Sheet - 2,4,6-Trinitrotoluene (TNT); U.S. Environmental Protection Agency: 2014.
- Agency, U.S.E.P. NAAQS Table. Available online: https://www.epa.gov/criteria-air-pollutants/naaqs-table#4 (accessed on 05/22/2025).
- Office of Environmental Health Hazard Assessment (OEHHA), C.E.P.A. Chronic Toxicity Summary: Silica (Crystalline, Respirable), 2: of Environmental Health Hazard Assessment (OEHHA), California Environmental Protection Agency, 2005.
- Energy, U.S.D.o. Puerto Rico Grid Resilience and Transitions to 100% Renewable Energy Study (PR100). Available online: https://www.energy.gov/gdo/puerto-rico-grid-resilience-and-transitions-100-renewable-energy-study-pr100 (accessed on 06/04/2025).
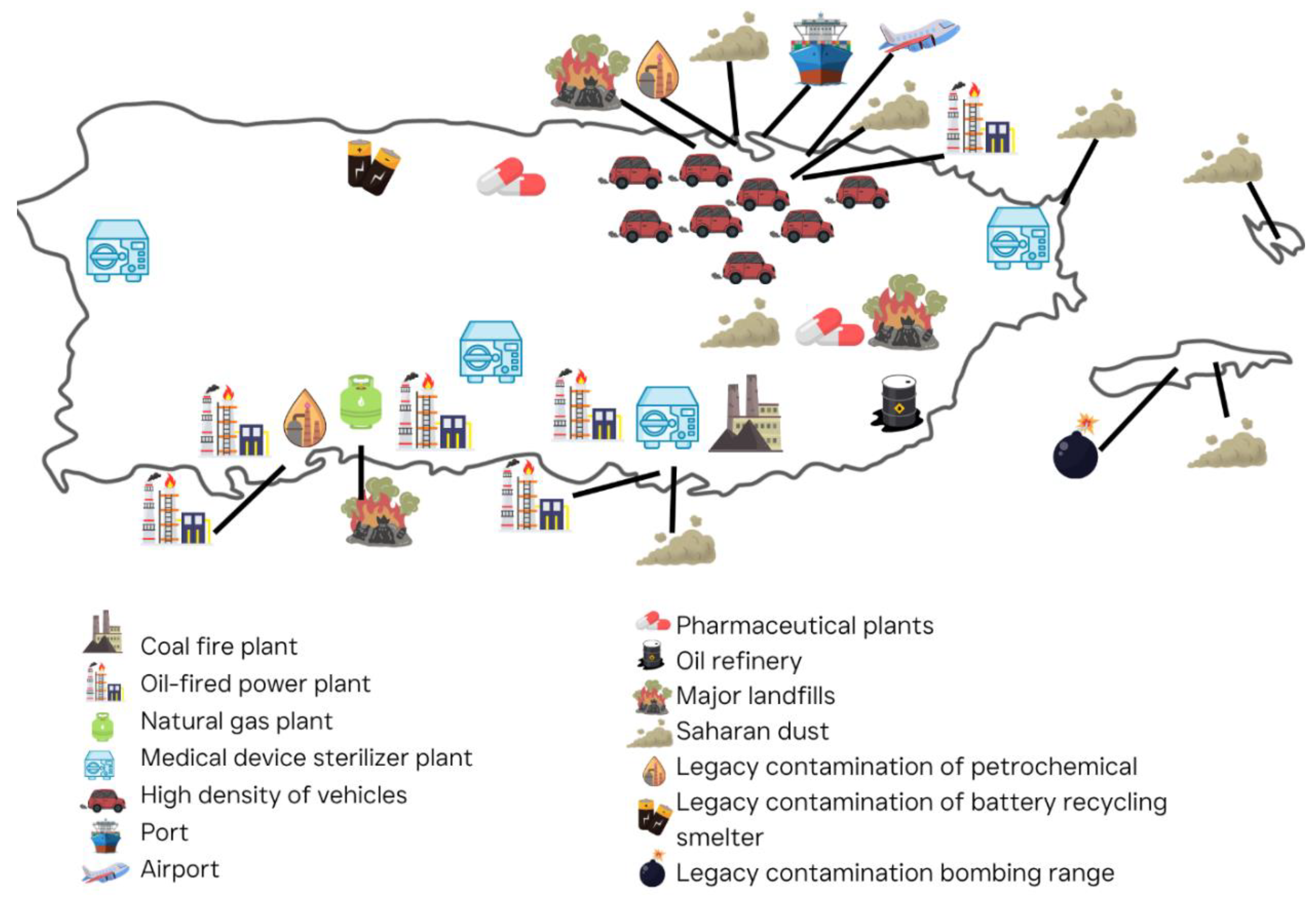
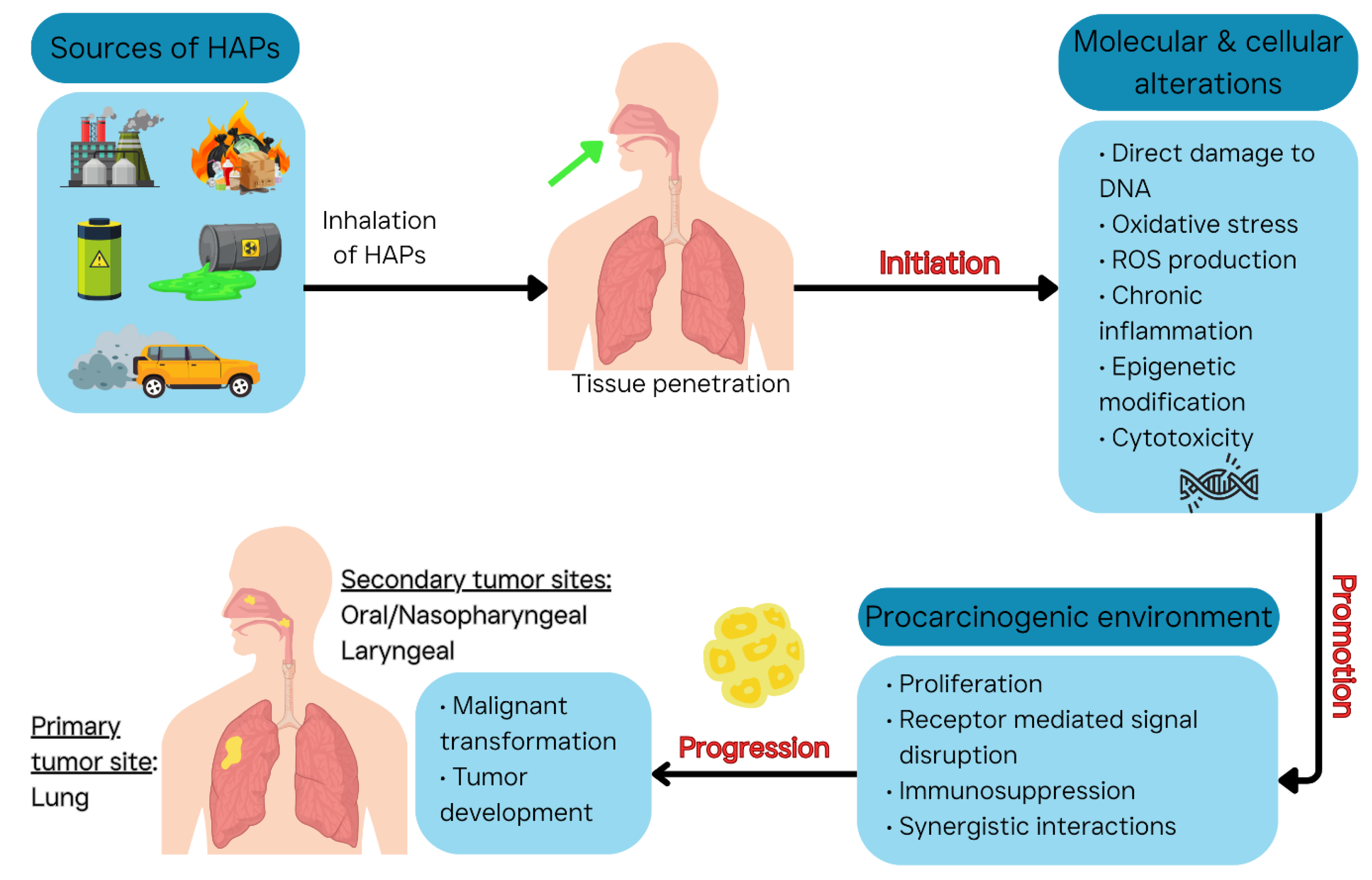
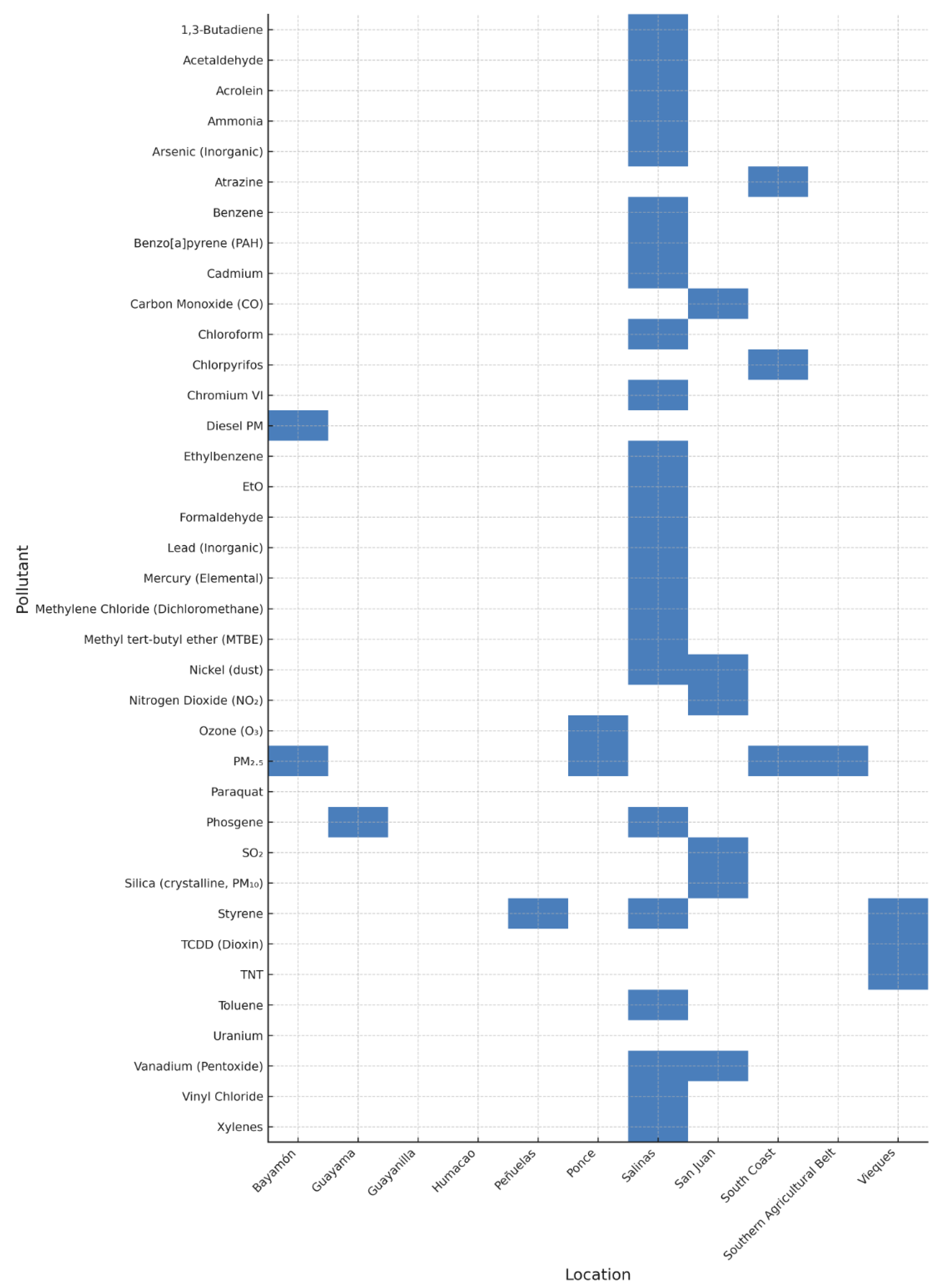
| Pollutant | Major Sources | Key Health Effects |
|---|---|---|
| Particulate Matter (PM2.5, PM10) | Combustion (vehicles, power plants, fires); construction dust; Saharan dust (natural) | Lung irritation, asthma exacerbation, reduced lung function; heart attack, arrhythmia, stroke; lung cancer (PM2.5 classified as Group 1 carcinogen). |
| Ozone (O3) | Not directly emitted; formed from NOx + VOCs in sunlight (photochemical smog) | Coughing, throat irritation; asthma attack; impaired lung development in children; oxidative stress on respiratory tract. |
| Nitrogen Dioxide (NO2) | Vehicle exhaust; power plants (burning fuel at high temps) | Airway inflammation; aggravates asthma and increases susceptibility to infections; contributes to smog and secondary PM formation. |
| Sulfur Dioxide (SO2) | Coal and oil combustion (power plants, ships); refining ore | Bronchoconstriction especially in asthmatics; wheezing, shortness of breath; precursor to sulfate particles and acid rain |
| Carbon Monoxide (CO) | Incomplete combustion (vehicles, generators, fires) | Reduces oxygen delivery (forms carboxyhemoglobin); at moderate levels causes headaches, dizziness; at high levels can be fatal (affects heart and brain). |
| Lead (Pb) | Historically, leaded gasoline (now phased out); metal smelters; battery recycling; old paint dust | Neurotoxin: cognitive impairment, behavioral problems in children; hypertension, kidney damage in adults; a probable carcinogen. |
| Source Category | Potential Sources in PR | Notable Hazardous Pollutants Emitted | IARC Classification (Group) [67,68] | EPA Classification (IRIS/NTP) [63,69] |
|---|---|---|---|---|
| Coal-Fired Power Plant (Combustion) | AES-PR Guayama coal plant (since 2002) | Arsenic, Chromium VI, Nickel, Lead, Mercury (in fly ash), PAHs, fine PM [32]. | Group 1 (Arsenic, Chromium VI, Nickel, PAHs), Group 2B (Lead) | Carcinogenic (Arsenic, Chromium VI, Nickel), Probable Carcinogen (PAHs) |
| Oil-Fired Power Plants | PREPA plants (Palo Seco, San Juan, Aguirre, Costa Sur) | SO2 (→ sulfate PM), Nickel, Vanadium; formaldehyde and benzene, NOx (→O3 formation) [70]. | Group 1 (Benzene, Formaldehyde), Group 2B (Vanadium) | Carcinogenic (Benzene, Formaldehyde); Nickel - Probable |
| Medical Device Sterilizers | Steri-Tech (Salinas), Edwards Lifesciences (Anasco), Customed (Fajardo), Medtronic (Villalba), etc. | EtO, ethylene chlorohydrin, trace amounts of other VOCs [31]. | Group 1 (EtO) | Carcinogenic (EtO) |
| Pharmaceutical & Chemical Manufacturing | Pharma plants in Barceloneta, Gurabo, etc. | Solvents (methylene chloride, chloroform, toluene), alcohols and ethers, ethylene dichloride, dichlorobenzene, acid gases, ammonia, phosgene [71]. | Group 2A (Methylene chloride), Group 2B (Chloroform) | Probable Carcinogen (Methylene chloride) |
| Petrochemical Storage & Refineries | Penuelas and Yabucoa oil terminals; former CORCO | Benzene, Toluene, Ethylbenzene, Xylenes (BTEX) [72], hydrogen sulfide [73], PAHs, vinyl chloride, styrene. | Group 1 (Benzene, Vinyl chloride), Group 2B (Styrene) | Carcinogenic (Benzene, Vinyl chloride); Styrene - Reasonably Anticipated |
| Waste Disposal | Landfills (Juncos, Penuelas, Toa Baja), open burning, proposed Arecibo incinerator (not built) | Dioxins/Furans (if waste burned), PCBs (if old electrical waste burned), mercury and lead fumes, methanol, benzene, methane with VOC mix, airborne arsenic, molybdenum, radium in dust [32]. | Group 1 (Dioxins, PCBs); Mercury compounds - Group 2B | Carcinogenic (Dioxins, PCBs); Mercury - Probable Carcinogen |
| Transportation – Road (engines) | ~3 million vehicles (San Juan metro, PR-52, PR-22, etc.) | Benzene and 1,3-butadiene, formaldehyde, acetaldehyde (from fuel combustion), diesel PM (soot with adsorbed PAHs like benzo[a]pyrene and nitropyrenes), acrolein (a reactive irritant), MTBE (from fuel) traces [4,74]. | Group 1 (Benzene, 1,3-butadiene, Diesel exhaust), Group 2B (Acrolein, MTBE) | Carcinogenic (Benzene, 1,3-butadiene, Diesel exhaust particles) |
| Transportation – Marine/Air | Port of San Juan (ships, ferries), airports (SJU, BQN, PSE) | SO2, PM, vanadium, nickel [75], benzene, PAHs [76], formaldehyde [77]. | Group 1 (Benzene, PAHs); Vanadium compounds Group 2B | Carcinogenic (Benzene, PAHs); Formaldehyde - Carcinogenic |
| Natural Dust Events | Saharan dust episodes | Silica particles, iron, aluminum, endotoxins and pollen (not classified as HAP, but relevant to particulate toxicity), pesticide residues [49]. | PM Group 1 (Silica crystalline); Endotoxins not classified | Silica (Crystalline) - Known Human Carcinogen; Dust/PM - Respiratory hazard |
| Military/ Ordnance | Vieques bombing range (historic), training sites | RDX, TNT [78], lead, mercury, uranium [79], NO2 and CO from blasts [80]. | Group 2A (TNT), Group 2B (Lead inorganic, Uranium compounds) | TNT - Reasonably anticipated carcinogen; Lead/Uranium - Probable Carcinogens |
| Agricultural Emissions | Sugar cane field burning; pest control fumigation | Benzo[a]pyrene, atrazine, paraquat, chlorpyrifos [81], ammonia [82]. | Group 1 (Benzo[a]pyrene); Group 2B (Atrazine, Paraquat) | Benzo[a]pyrene - Known carcinogen; Atrazine, Paraquat - Possible carcinogens |
| Mechanism | Description | Example HAPs [4,6,93] |
|---|---|---|
| Direct DNA Damage (Genotoxicity) | Chemical binds to or chemically alters DNA, causing mutations if not repaired. | PAHs (e.g., benzo[a]pyrene) form DNA adducts; EtO alkylates DNA bases; Benzene metabolites cause chromosomal breaks. |
| Oxidative Stress & ROS | Overproduction of reactive oxygen species leading to DNA strand breaks, base damage; also, lipid peroxidation. | Diesel PM and ultrafine particles (generate ROS in lungs); Arsenic (impairs antioxidants); O3 (an oxidant gas) causes oxidative DNA damage indirectly. |
| Chronic Inflammation | Persistent activation of immune/inflammatory cells, releasing cytokines and ROS, promoting cell proliferation and DNA damage. | PM (coal ash dust, silica from Saharan dust) causing lung inflammation; Wood smoke and endotoxin also contribute. |
| Epigenetic Modification | Changes in gene expression without DNA mutation: DNA methylation, histone modification, microRNAs. | Traffic-related air pollution associated with DNA hypermethylation of p16, p53 genes; Nickel and arsenic cause DNA methylation changes (silencing genes); Diesel exhaust can alter microRNA profiles in respiratory cells. |
| Cytotoxicity & Proliferation | Cell injury or death followed by regenerative proliferation increases risk of cancerous growth. | Formaldehyde (cytotoxic to nasal cells, causing compensatory hyperplasia); Acrolein (toxic to lung cells); strong acids or alkalis in aerosols can injure airway lining. |
| Receptor-Mediated Pathways | Activation of cellular receptors that drive proliferation or inhibit apoptosis. | Dioxins/PAHs activating AhR (leads to altered expression of growth-related genes); Endocrine disruptors (some pesticides, bisphenol A in dust) activating estrogen receptors potentially promoting hormone-sensitive tumors. |
| Immunosuppression | Impaired immune surveillance of tumors. | Dioxins (TCDD) and PCBs – diminish T-cell function; Polycyclic aromatic hydrocarbons – some evidence of immune modulation; high lead exposure – affects immune responses. |
| Interaction of Mixed Exposures | Synergistic or additive effects of multiple pollutants. | Tobacco smoke + asbestos (synergistic lung cancer risk, relevant as smokers in polluted areas have higher risk than additive); Diesel PM + viruses (inflammation can potentiate other carcinogens). |
| Air Pollutants | Concentration (µg/m³) | Years | Location | Midpoint (µg/m³) | IUR per (µg/m³)a ,e [7,63,133,134,135,136,137] |
RfC (mg/m³)b,e [7,63,133,134,135,136,137] |
LCRc | CRLd,e | HQd,e |
|---|---|---|---|---|---|---|---|---|---|
| 1,3-Butadiene | 0.1–0.3 | 2015–2016 | Salinas | 0.2 | 3.00 × 10⁻⁵ | 2.00 × 10⁻³ | 6.00 × 10⁻⁶ | Moderate | 1.00 × 10⁻¹ |
| Acetaldehyde | 1.0–2.0 | 2015–2016 | Salinas | 1.5 | 2.20 × 10⁻⁶ | 9.00 × 10⁻³ | 3.30 × 10⁻⁶ | Moderate | 1.67 × 10⁻¹ |
| Acrolein | 0.02–0.05 | 2015–2016 | Salinas | 0.04 | 2.00 × 10⁻⁵ | 2.00 × 10⁰ | |||
| Ammonia | 1.0–3.0 | 2015–2016 | Salinas | 2 | 5.00 × 10⁻¹ | 4.00 × 10⁻³ | |||
| Arsenic (Inorganic) | 0.0005–0.0023 | 2015–2016 | Salinas | 0.0014 | 4.30 × 10⁻³ | 1.50 × 10⁻⁵ | 6.02 × 10⁻⁶ | Moderate | 9.33 × 10⁻² |
| Atrazine | 0.3–0.7 | 2022 | South Coast | 0.5 | 1.00 × 10⁻⁵ | 5.00 × 10⁻⁶ | Moderate | ||
| Benzene | 0.5–1.5 | 2015–2016 | Salinas | 1 | 7.80 × 10⁻⁶ | 3.00 × 10⁻² | 7.80 × 10⁻⁶ | Moderate | 3.33 × 10⁻² |
| Benzo[a]pyrene (PAH) | 0.0001–0.0005 | 2015–2016 | Salinas | 0.0003 | 6.00 × 10⁻⁴ | 2.00 × 10⁻⁶ | 1.80 × 10⁻⁷ | Low | 1.50 × 10⁻¹ |
| Cadmium | 0.003–0.007 | 2015–2016 | Salinas | 0.005 | 1.80 × 10⁻³ | 1.00 × 10⁻⁵ | 9.00 × 10⁻⁶ | Moderate | 5.00 × 10⁻¹ |
| Carbon Monoxide (CO) | 600–10,000 | 2017 | San Juan | 5,300 | 2.30 × 10¹ | 2.30 × 10⁻¹ | |||
| Chloroform | 0.1–0.3 | 2015–2016 | Salinas | 0.2 | 2.30 × 10⁻⁵ | 1.95 × 10⁻³ | 4.60 × 10⁻⁶ | Moderate | 1.03 × 10⁻¹ |
| Chlorpyrifos | 0.2–0.4 | 2022 | South Coast | 0.3 | |||||
| Chromium VI | 0.0001–0.0005 | 2015–2016 | Salinas | 0.0003 | 1.80 × 10⁻² | 3.00 × 10⁻⁵ | 5.40 × 10⁻⁶ | Moderate | 1.00 × 10⁻² |
| Diesel PM | 0.3–1.2 | 2017–2018 | San Juan, Bayamón | 0.8 | 3.00 × 10⁻⁴ | 2.40 × 10⁻⁴ | High | ||
| Ethylbenzene | 0.6–1.5 | 2015–2016 | Salinas | 1.05 | 2.50 × 10⁻⁶ | 1.00 × 10⁰ | 2.63 × 10⁻⁶ | Moderate | 1.05 × 10⁻³ |
| EtO | 0.3–121 | 2023 | Salinas | 60 | 3.00 × 10⁻³ | 3.00 × 10⁻² | 1.80 × 10⁻¹ | High | 2.00 × 10⁰ |
| Formaldehyde | 1.0–3.0 | 2015–2016 | Salinas | 2 | 1.10 × 10⁻⁵ | 7.00 × 10⁻³ | 2.20 × 10⁻⁵ | Elevated | 2.86 × 10⁻¹ |
| Lead (Inorganic) | 0.05–0.2 | 2015–2016 | Salinas | 0.125 | 1.20 × 10⁻⁵ | 1.50 × 10⁻⁶ | Moderate | ||
| Mercury (Elemental) | 0.0005–0.0015 | 2015–2016 | Salinas | 0.001 | 3.00 × 10⁻⁴ | 3.00 × 10⁻⁴ | 3.00 × 10⁻⁷ | Low | 3.33 × 10⁻³ |
| Methylene Chloride (Dichloromethane) | 0.1–0.5 | 2015–2016 | Salinas | 0.3 | 1.70 × 10⁻⁸ | 6.00 × 10⁻¹ | 5.10 × 10⁻⁹ | Low | 5.00 × 10⁻⁴ |
| Methyl tert-butyl ether (MTBE) | 0.5–1.0 | 2015–2016 | Salinas | 0.8 | 2.60 × 10⁻⁷ | 3.00 × 10⁰ | 2.08 × 10⁻⁷ | Low | 2.67 × 10⁻⁴ |
| Nickel (dust) | 0.0012–0.0034 | 2015–2016 | Salinas | 0.0023 | 2.60 × 10⁻⁴ | 1.00 × 10⁻⁵ | 5.98 × 10⁻⁷ | Low | 2.30 × 10⁻¹ |
| Nitrogen Dioxide (NO₂) | 10–45 | 2015–2017 | San Juan | 27.5 | 4.70 × 10⁻¹ | 5.85 × 10⁻² | |||
| Ozone (O3) | 50–100 | 2015–2017 | San Juan Ponce |
75 | 1.80 × 10⁻¹ | 4.17 × 10⁻¹ | |||
| Paraquat | 0.3–0.5 | 2022 | Southern Agricultural Belt | 0.4 | |||||
| Phosgene | 0.1–0.3 | 2015–2016 | Salinas | 0.2 | 3.00 × 10⁻⁴ | 6.67 × 10⁻¹ | |||
| PM2.5 | 4.33–5.82 | 2015–2016 | Ponce, Bayamón, Guayama, Guayanilla, Humacao | 5.2 | |||||
| Silica (crystalline, PM10) | 0.3–0.6 | 2015–2016 | San Juan (Saharan dust events) | 0.5 | 3.00 × 10⁻³ | 1.67 × 10⁻¹ | |||
| SO₂ | 10–80 | 2017 | San Juan, Guayama | 45 | 2.62 × 10⁻² | 1.72 × 10⁰ | |||
| Styrene | 0.5–1.5 | 2015–2016 | Salinas | 1 | 1.00 × 10⁰ | 1.00 × 10⁻³ | |||
| TCDD (Dioxin, 2,3,7,8-Tetrachlorodibenzo-p-dioxin) | 0.000005–0.00002 | Legacy/Modeled Penuelas (burning), Vieques (legacy) | 0.00001 | 3.80 × 10¹ | 4.00 × 10⁻⁸ | 3.80 × 10⁻⁴ | High | 2.50 × 10⁻¹ | |
| 2,4,6-Trinitrotoluene (TNT)e | 0.2–0.4 | Historical (2000s) | Vieques | 0.3 | 5.00 × 10⁻¹ | 6.00 × 10⁻⁴ | |||
| Toluene | 0.5–1.5 | 2015–2016 | Salinas | 1 | 5.00 × 10⁰ | 2.00 × 10⁻⁴ | |||
| Uranium | 0.1–0.3 | 2003–2005 | Vieques | 0.2 | 4.00 × 10⁻⁵ | 5.00 × 10⁰ | |||
| Vanadium (Pentoxide) | 0.0005–0.0015 | 2015–2016 | San Juan, Salinas | 0.001 | 8.30 × 10⁻³ | 7.00 × 10⁻⁶ | 8.30 × 10⁻⁶ | Moderate | 1.43 × 10⁻¹ |
| Vinyl Chloride | 0.1–0.5 | 2015–2016 | Salinas | 0.3 | 4.40 × 10⁻⁶ | 1.00 × 10⁻¹ | 1.32 × 10⁻⁶ | Moderate | 3.00 × 10⁻³ |
| Xylenes | 1.0–1.5 | 2015–2016 | Salinas | 1.2 | 1.00 × 10⁻¹ | 1.20 × 10⁻² |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





