Submitted:
18 August 2025
Posted:
20 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Tumour Microenvironment
3. Exosomes
4. Cancer Associated Fibroblasts
5. Tumour Associated Macrophages (TAMs)
6. Cytotoxic CD8+ T-Cell
7. Natural Killer Cells
8. Dissemination of Tumour Cells to Distant Tissues
9. Subclassification of MRD
10. The Effect of Treatment on the TME and MRD
11. The Latency Period of MRD
12. The Detection of Minimal Residual Disease in Breast Cancer, What It Means and How Can It Be Used to Direct Treatment Options in Non-Metastatic Breast Cancer Patients
13. The Effect of Neoadjuvant, Surgical Excision, Adjuvant Treatment on the Immune System
14. Immunosuppression in Breast Cancer
15. Immunomodulation and Immunotherapy in Non-Metastatic Breast Cancer Patients
16. Immunotherapy Using Monoclonal Antibodies
17. Tyrosine Kinase Inhibitors of HER2
18. Antibody Drug Conjugates
19. Check-Point Inhibitors in Breast Cancer
20. Cyclin-Dependent Kinase (CDK) 4/6 Inhibitors
21. Cancer Vaccines
22. Minimal Residual Disease, Clinical Utility, and a Guide to Treatment Options
23. Tumour Associated Macrophages
24. Myeloid Derived Suppressor Cells
25. Poly (ADP-Ribose) Polymerase Inhibitors (PARP)
26. Chimeric Antigen Receptor (CAR) T-Cell Therapy
27. Inhibition of MMP-2
28. The Use of Bisphosphonates in Breast Cancer
29. Minimal Residual Disease, What It Means and How Do We Treat It
30. Treating the Tumour Microenvironment
31. Conclusions
References
- Siegel, R.; Miller, K.D.; Fuchs, H.E.; Jamal, A. Cancer statistics, 2022. CA A Cancer J Clin 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Giuquinto, N.; Colla, S.; Morandi, F.; Rizzoli, V. Breast cancer statistics, 2022. A Cancer J Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Makki, J. Diversity of breast carcinoma: Histological subtypes and clinical relevance. Pathology. 2015, 8, 23-3. [Google Scholar] [CrossRef]
- Anderson, W.F.; Chatterjee, N.; Ershler, T.; Brawley, O.W. Oestrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology and End Results database. Breast Cancer Res Treat. 2002, 76, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, J.S.; Korlimarla ADesai k Alexander, A.; Raghacvan, R.; Anupama, C.; Dendukuri, N.A.; Manjunath, S.; Correa, M.; Raman, N.; et al. majority of low (1-10%) ER positive breast cancer behave like hormone receptor negative tumors. J Cancer 2014, 5, 156–165. [Google Scholar] [CrossRef]
- Gloyeske, N.C.; Dabbs, D.J.; Bhargava, R. Low ER + breast cancer: Is this a distinct group? Am J Clin Pathol 2014, 141, 697–701. [Google Scholar] [CrossRef]
- Bouchard-Fortier, A.; Provencher, L.; Blanchette, C.; Diorio, C. Prognostic, and predictive value of low estrogen receptor expression in breast cancer. Curr Oncol. 2017, 24, e106–e114. [Google Scholar] [CrossRef]
- Sleightholm, R.; Nielson, B.K.; Elkhatib, S.; Flores, L.; Dukkipati, S.; Zhao, R.; Choudbury, S.; Gardner, B.; Carmichael, J.; Smith, L.; Bennion, N.; Wahl, A.; Baine, M. Percentage of hormone receptor positivity in breast cancer provides prognostic value: A single-institute study. J Clin Med Res 2021, 13, 9–19. [Google Scholar] [CrossRef]
- Marchio, C.; Annaratone, L.; Marques, A.; Casorzo, L.; Berrino, E.; Sapino, A. Evolving concepts in HER2 evaluation in breast cancer: Heterogeneity, HER2 low carcinomas and beyond. Semin Cancer Bio 2021, 72, 123–135. [Google Scholar] [CrossRef]
- Wahl, G.M.; Spike, B.T. Cell state plasticity, stem cells, EMT, and the generation of intra-tumoral heterogeneity. NPJ Breast Cancer 2017, 3, 14. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, S.; Caldas, C. The implications of clonal genome evolution for cancer medicine. N Eng J Med 2013, 368, 842–851. [Google Scholar] [CrossRef]
- Marusky, A.; Almendro, V.; Polyak, K. Intra-tumoral heterogeneity: a looking glass for cancer? Nat Rev Cancer 2012, 12, 323–334. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Clonal heterogeneity, and tumor evolution: past, present and future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef]
- Liu, T.; Liu, C.; Yan, M.; Zhang, L.; Zhang, J.; Xiao, M.; Li, Z.; Wei, X.; Zhang. Single cell profiling of primary and paired metastatic lymph node tumors in breast cancer patients. Nat Commun 2022, 13, 6823. [Google Scholar] [CrossRef]
- Mao, X.; Zhou, D.; Lin, K.; Zhang, B.; Gao, J.; Ling, F.; Zhu, L.; Yu, S.; Chen, P.; Zhang, C.; et al. Single cell and spatial transcriptome analysis analyses reveal cell heterogeneity and immune environment alterations in metastatic axillary lymph nodes in breast cancer. Cancer Immunol Immunother 2023, 72, 679–695. [Google Scholar] [CrossRef]
- Gulati, G.S.; Sikander, S.S.; Wesche, D.; Manjunath, A.; Bharadwaj, A.; Berger, M.J.; Ilagan, F.; Kuo, A.H.; Hsieh, R.W.; Cai, S.; et al. Single cell transcriptional diversity is a hallmark of development potential. Science 2020, 367, 405–411. [Google Scholar] [CrossRef]
- Van de Sande, B.; Flerin, C.; Davie, K.; De Weageneer, M.; Hulselmans, G.; Aibar, S.; Seurinck, R.; Saalens, W.; Cannoodt, R.; Rouchon, Q.; et al. A scalable SCENIC workflow for single cell gene regulatory network analysis. Nat Proc 2020, 15, 2247–2276. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, R.; Xie, H.; Hu, L.; Wang, C.; Xu, J.; Zhu, C.; Liu, Y.; Gao, F.; Li, X.; et al. Single-cell RNA sequencing reveals cell heterogeneity and transcriptome profile of breast cancer lymph node metastasis. Oncogenesis 2021, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Fidler, I.J.; Poste, G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008, 9, 8. [Google Scholar] [CrossRef]
- Luo, W. Nasopharyngeal carcinoma ecology theory: Cancer as a multidimension spatiotemporal “unity of ecology and evolution” pathological ecosystem. Theranostics 2023, 13, 1607–1631. [Google Scholar] [CrossRef]
- Chen, X.; Song, E. The theory of tumour ecosystem. Cancer Commun. 2022, 42, 587–608. [Google Scholar] [CrossRef]
- Murray, N.P.; Miranda, R.; Ruiz, A.; Droguett, E. Diagnostic yield of primary circulating tumour cells in women suspected of breast cancer: the BEST (Breast Early Screening Test) Study. Asian Pac J Cancer Prev. 2015, 16, 1929–1934. [Google Scholar] [CrossRef]
- Sanger, N.; Effenberger, K.E.; Reithdorf, S.; Van Haasteren, V.; Gauwerky, J.; Wiegratz, I.; Strebhardt, K.; Kaufmann, M.; Pantel, K. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int J Cancer 2011, 129, 2522–2526. [Google Scholar] [CrossRef]
- Rodriguez-Bejerano, O.H.; Parra-Lopez, C.; Patarroyo, M.A. A review concerning the breast cancer-related tumour microenvironment. Crit Rev Oncol Hematol 2024, 199, 104389. [Google Scholar] [CrossRef] [PubMed]
- Agahozo, M.C.; van Boskstal, M.R.; Groenendijk, F.H.; van den Bosch, T.P.P.; Westenend, P.J.; van Deurzen, C.H.M. Ductal carcinoma in situ of the breast: immune cell composition according to subtype. Mod Pathol. 2020, 33, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, H. Prognostic values of tumoral MMP2 and MMP9 overexpression in breast cancer: a systematic review and meta-analysis. BMC Cancer 2012, 21, 149. [Google Scholar] [CrossRef]
- Yang, C.; Yu, H.; Chen, R.; Tao, K.; Jian, L.; Peng, M.; Li, X.; Liu, M.; Liu, S. CXCL1 stimulates migration and invasion in ER-negative breast cancer cells via activation of the ERK/MMP2/9 signalling pathway. Int J Oncol 2019, 55, 684–696. [Google Scholar] [PubMed]
- Kargozaran, H.; Yuan, S.Y.; Breslin, J.W.; Watson, K.D.; Gaudreault, N.; Breen, A.; Wu, M.H. A role for endothelial derived matrix metalloproteinase-2 in breast cancer cell transmigration across the endothelial-basement membrane barrier. Clin Exp Metastasis 2007, 24, 495–502. [Google Scholar] [CrossRef]
- Ross, J.S.; Kaur, P.; Sheehan, C.E.; Fisher, H.A.; Kaufman RAJr Kallakury, B.V. Prognostic significance of metalloproteinase 2 and tissue inhibitor of metalloproteinase 2 expression in prostate cancer. Mod. Pathol. 2003, 16, 198–205. [Google Scholar] [CrossRef]
- Trudel, D.; Fradet, Y.; Meyer, F.; Harel, F. Tetu, B. Significance of MMP-2 expression in prostate cancer: An immunohistochemical study. Cancer Res. 2003, 63, 8511–8515. [Google Scholar]
- Nissinen, L.; Kahari, V.M. MMPs in inflammation. Biochem. Biophys. Acta 2014, 1840, 2571–2580. [Google Scholar] [CrossRef]
- Lee, B.K.; Kim, M.J.; Jang, H.S.; Lee, H.R.; Ahn, K.M.; Lee, J.H.; Choung, P.H.; Kim, M.J. High concentrations of MMP-2 and MMP-9 reduce NK mediated cytotoxicity against oral squamous cell carcinoma line. In Vivo 2008, 22, 593–598. [Google Scholar]
- Li, J.; Yu, S.; Rao, M.; Cheng, B. Tumor-derived extracellular vesicles: key drivers of immunomodulation in breast cancer. Front Immunol 2025, 16, 1543585. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.; Gazinska, P.; Zhang, B.; Khiabany, A.; Sinha, S.; Alaguthuri, T.; Flores-Borja, F.; Vicencio, J.; Beuron, F.; Roxanis, I.; et al. Serum derived extracellular vesicles from breast cancer patients contribute to differential regulation of T-cell mediated immune-escape mechanisms in breast cancer subtypes. Front Immunol 2023, 14, 1204224. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Mark, M.T.; Molina, H.; Kohsaka, S.; Di Giannatalo, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Syn, N.; Wang, L.; Sethi, G.; Thiery, J.P.; Goh, B.C. Exosome-mediated metastasis: From epithelial-mesenchymal transition to escape from immunosurveillance. Trends Pharmacol. Sci. 2016, 37, 606–617. [Google Scholar]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O´Connor, S.T.F.; Chin, A.R.; et al. Cancer secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef]
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G.; et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 2021, 11, 1429–1445. [Google Scholar] [CrossRef]
- Korbecki, J.; Kojder, K.; Siminska, D.; Bohatyrewicz, R.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. CC chemokines in a tumor. A review of pro-cancer and anti-cancer properties of the ligands of receptors od CCR1, CCR2, CCR3. And CCR4. Int J Mol Sci. 2020, 21, 8412. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Poliakov, A.; Liu, C.; Liu, Y.; Deng, Z.B.; Wang, J.; Cheng, Z.; Shah, S.V.; Wang, G.J.; Zhang, L.; et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int. J. Cancer 2009, 124, 2621–2633. [Google Scholar] [CrossRef]
- Morrisey, S.M.; Zhang, F.; Ding, C.; Montoya-Durango, D.E.; Hu, X.; Yang, C.; Wang, Z.; Yuan, F.; Fox, M.; Zhang, H.G.; et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021, 33, 2020–2058. [Google Scholar] [CrossRef]
- Wieckowski, E.U.; Visus, C.; Szajnik, M.; Szczepanski, M.J.; Storkus, W.J. Whiteside. T.L. Tumor-derived micro-vesicles promote regulatory T-cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol. 2009, 183, 3720–3730. [Google Scholar] [CrossRef] [PubMed]
- Figueiro, F.; Muller, L.; Funk, S.; Jackson, E.K.; Battastini, A.M.; Whiteside, T.L. Phenotypic and functional characteristics of CD39 high human regulatory B-cells (Breg). Oncoimmunology 2016, 5, e1082703. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer associated fibroblasts. Nat Rev Cancer 2020, 20, 174–86. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Cheng, P.; Pan, J.; Wang, S.; Ding, Q.; Jiang, Z.; Cheng, L.; Shao, X.; Huang, L.; Huang, J.; et al. An IL6-adenosine positive feedback loop between CD73 (+) Tregs and CAFs promotes tumor progression in human breast cancer. Cancer Immunol Res 2020, 8, 1273–1286. [Google Scholar] [CrossRef] [PubMed]
- Kennel, K.B.; Bozlar, M.; De Valk, A.F.; Greten, F.R. Cancer associated fibroblasts in inflammation and antitumor immunity. Clin Cancer Res 2023, 29, 1009–1016. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Dis 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Waaijer, S.J.H.; Zwager, M.C.; de Vries, E.G.E.; van der Vegt, B.; Schroder, C.; et al. Tumor-associated macrophages in breast cancer: innocent bystanders or important player? Cancer Treat Rev 2018, 70, 178–189. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci 2019, 26, 78. [Google Scholar] [CrossRef]
- Salmaninejad, A.; Valilou, S.F.; Soltani, A.; Ahmadi, S.; Abarghan, Y.J.; Rosengren, R.J.; Sahebkar, A. Tumor associated macrophages: role in cancer development and therapeutic implictions. Cell Oncol (Dordr) 2019, 42, 591–608. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8 (+) T-cell lymphocytes on cancer immunotherapy: a review. J Cell Physiol 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Hossain, M.A.; Liu, G.; Dai, B.; Si, Y.; Yang, Q.; Wazir, J.; Bimbauer, L.; Yang, Y. Reinvigorating exhausted CD8 (+) cytotoxic T-lymphocytes in the tumor microenvironment and current strategies in cancer immunotherapy. Med Res Rev 2021, 41, 156–201. [Google Scholar] [CrossRef]
- Ugel, S.; de Sanctis, F.; Mandruzzato. Tumor-induced myeloid deviation: when myeloide derived suppressor cells meet tumor associated macrophages. J Clin Invest 2015, 125, 3365–3376. [Google Scholar] [CrossRef]
- Huang, X.; Cao, J.; Zu, X. Tumor-associated macrophages: An important player in breast cancer progression. Thorac Cancer 2022, 13, 269–276. [Google Scholar] [CrossRef]
- Qin, R.; Ren, W.; Ya, G.; Wang, B.; He, J.; Ren, S.; Jiang, L.; Zhou, S. Role of chemokines in the crosstalk between tumor cells and tumor-associated macrophages. Clin Exp Med 2023, 23, 1359–1373. [Google Scholar] [CrossRef] [PubMed]
- Marme, F. Antibody conjugates for breast cancer. Oncol Res Treat 2012, 45, 26.36. [Google Scholar] [CrossRef]
- Moura, T.; Caramelo, O.; Silva, I.; Silva, S.; Goncalo, M.; Portilha, M.A.; Moreira, J.N.; Gil, A.M.; Laranjiera, P. Early-stage Luminal B-like breast cancer exhibits a more tumor suppressive microenvironment than Luminal A type breast cancer. Biomolecules 2025, 15, 78. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.P. The role of matrix metalloproteinase-2 in the metastatic cascade: a review. Oncologie 2024, 26, 27–40. [Google Scholar] [CrossRef]
- Morgunova, E.; Tuuttila, A.; Bergmann, M.I.; Isupov, M.; Lindquist, Y.; Schneider, G.; Tryggvason, K. Structure of human pro-matrix metalloproteinase 2: activation mechanism revealed. Science 1999, 284, 1667–1670. [Google Scholar] [CrossRef]
- Mayhew, V.; Omokehinde, T.; Johnson, R.W. Tumor dormancy in bone. Cancer Rep 2020, 3, e1156. [Google Scholar] [CrossRef] [PubMed]
- Sandiford, O.A.; Donnelly, R.J.; El-Far, M.H.; Burgmeyer, L.M.; Sinha, G.; Pamarthi, S.H.; Sherman, L.S.; Ferrer, A.I.; de Vore, D.E.; Patel, S.A.; et al. Mesenchymal stem cell-secreted extracellular vesicles instruct stepwise dedifferentiation of breast cancer cells into dormancy at the bone marrow perivascular region. Cancer Res 2021, 81, 1567–1582. [Google Scholar] [CrossRef] [PubMed]
- Tamamouna V, Pavlou E, Neophytou CM, Papageorgis P, Costeas P- Regulation of metastatic tumor dormancy and emerging opportunities for therapeutic intervention. Int J Mol Sci 2022, 23, 13931. [CrossRef] [PubMed]
- Byme, N.M.; Summers, M.A.; McDonald, M.M. Tumor cell dormancy and reactivation in bone: Skeletal biology and therapeutic opportunities. JBMR Plus 2019, 3, e10125. [Google Scholar]
- Muller, V.; Alix-Panabieres, C.; Pantel, K. Insights into minimal residual disease in cancer patients: implications for anti-cancer therapies. Cancer 2010, 46, 1189–1197. [Google Scholar] [CrossRef]
- Aurilio, G.; Mondardini, L.; Rizzo, S.; Sciandivasci, A.; Preda, L.; Bagnardi, V.; Disalvatore, D.; Pruneri, G.; Munzone, E.; Vigna, P.D.; et al. Discordant hormone receptor and human epidermal growth factor receptor 2 status in bone metastases compared to primary breast cancer. Acta Oncol 2013, 52, 1649–1656. [Google Scholar] [CrossRef]
- Aurilio, G.; Dislavatore, D.; Pruneri, G.; Bagnardi, V.; Viale, G.; Curigliano, G.; Adamoli, L.; Munzone, E.; Scianduivasci, A.; De Vita, F.; et al. A meta-analysis of oestroegen receptor, progesterone receptor and human epidermal growth factor receptor discordance between primary breast cancer and metastasis. Eur J Cancer 2014, 50, 277–289. [Google Scholar] [CrossRef]
- Konig, T.; Dogan, S.; Hohn, A.K.; Weydandt, L.; Aktas, B.; Nel, I. Multi-parameter analisis of disseminated tumor cells (DTCs) in early breast cancer patients with hormone receptor positive tumors. Cancer 2023, 15, 568. [Google Scholar] [CrossRef]
- 69Ditsch, N.; Meyer, B.; Rolle, M.; Untch, M.; Schildberg, F.W.; Funke, I. Estrogen receptor expression profile of disseminated epithelial tumor cells in bone marrow of breast cancer patients. Recent Results cancer Res. 2003, 162, 141–147. [Google Scholar]
- Jager, B.A.S.; Finkenzeller, C.; Bock, C.; Majunke, L.; Jueckstock, J.K.; Andergassen, U.; Neugebauer, J.K.; Pestka, A.; Friedl, T.W.P.; Jeschke, U.; et al. Estrogen receptor and HER-2 status on disseminated tumor cells and primary tumor in patients with early breast cancer. Transl Oncol 2015, 8, 509–516. [Google Scholar] [CrossRef]
- Murray, N.P.; Aedo, S.; Villalon, R.; Albarran, V.; Orrego, S.; Guzman, E. Subtypes of minimal residual disease and outcome for stage II colon cancer treated by surgery alone. Ecancermedicine 2020, 14, 1119. [Google Scholar]
- Murray, N.P.; Aedo, S.; Villalon, R.; Lopez, M.A.; Minzer, S.; Munoz, L.; Orrego, S.; Contreras, L.; Arzeno, L.; Guzman, E. Effect of FOLFOX on minimal residual disease in stage III colon cancer and risk of relapse. Ecancermedicine 2019, 13, 935. [Google Scholar] [CrossRef]
- Murray, N.P.; Aedo, S.; Fuentealba, C.; Reyes, E.; Salazar, A.; Lopez, M.A.; Minzer, S.; Orrego, S.; Guzman, E. Subtypes of minimal residual disease, association with Gleason score, risk and time to biochemical failure in pT2 prostate cancer treated with radical prostatectomy. Ecancermedicine 2019, 13, 934. [Google Scholar] [CrossRef]
- Magbanua, M.J.M.; Yau, C.; Wolf, D.M.; Lee, J.S.; Chattopadhyay, A.; Scott, J.H.; Bowlby-Yoder, E.; Hwang, E.S.; Alvarado, N.; Ewing, AL.; et al. Synchronous detection in blood and disseminated tumor cells in breast cancer predicts adverse outcome in early breast cancer. Clin Cancer Res 2019, 25, 5388–5397. [Google Scholar] [CrossRef]
- Bilani, N.; Elson, L.; Liang, H.; Elimimian, E.B.; Arteta-Bulos, R.; Nahleh, Z. Prognostic and predictive value of circulating and disseminated tumor cells in breast cancer: A National Cancer Database (NCDB) analysis. Technol Cancer Res Treat 2020, 19, 1533033820980107. [Google Scholar] [CrossRef]
- Pantel, K.; Alix-Panabieres, C. Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. Bonekey Rep 2014, 3, 584. [Google Scholar] [CrossRef]
- Sai, B.; Xiang, J. Disseminated tumour cells in bone marrow are the source of cancer relapse after therapy. J Cell Mol Med 2018, 22, 5776–5786. [Google Scholar] [CrossRef] [PubMed]
- NCCN clinical practice guidelines in oncology. Breast cancer version 4.2025. www.nccn.org/professional/physician_gls/pdf/breastpdf accessed June 2025.
- Kabak, E.C.; Foo, S.L.; Rafaeva, M.; Martin, I.; Bentires-Alj, M. Microenvironment regulation of dormancy in breast cancer metastasis: “An ally that changes allegiances”. Adv Exp Med Biol 2025, 1464, 373–395. [Google Scholar]
- Lennart, N.A.; Rao, S.S. Cell-cell interactions mediating primary and metastatic breast cancer dormancy. Cancer Metastasis Rev 2024, 44, 6. [Google Scholar] [CrossRef] [PubMed]
- Sandiford, O.A.; Donnelly, R.J.; El-Far, M.K.; Burgmeyer, L.M.; Sinha, G.; Pamarthi, S.H.; Sherman, L.S.; Ferrer, A.I.; DeVore, D.E.; Patel, SA.; et al. Mesenchymal stem-cell secreted extracellular vesicles instruct stepwise dedifferentiation of breast cancer cells into dormancy at the bone marrow perivascular region. Cancer Res 2021, 81, 1567–1582. [Google Scholar] [CrossRef]
- Khadge, S.; Coler, K.; Talmadge, J.E. Myeloid derived suppressor cells and the release of micro-metastasis from dormancy. Clin Exp Metastasis 2021, 38, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Huang, X.; Fu, X.; Zhnag, X.; Lin, R.; Zhang, W.; Zhang, J.; Lu, Y. Myeloid-like tumor hybrid cells in bone marrow promote progression of prostate cancer bone metastasis. J Haematol Oncol 2023, 16, 46. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Liu, M.; Xiang, X.; Xi, Z.; Xu, H. Osteoblasts and osteoclasts: an important switch of tumour cell dormancy during bone metastasis. J Exp Clin Cancer Res 2023, 41, 316. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.P.; Reyes, E.; Tapia, P.; Badinez, L.; Orellana, N.; Fuentealba, C.; Olivares, R.; Porcell, J.; Duenas, R. Redefining micrometastasis in prostate cáncer, bone marrow disseminated tumour cells and micrometastasis: Implications in determining local or systemic treatment for biochemical failure after radical prostatectomy. Int J Mol Med 2012, 30, 896–904. [Google Scholar] [CrossRef]
- Bain, B. Bone marrow biopsy morbidity: review of 2003. J Clin Pathol 2005, 58, 406–40. [Google Scholar] [CrossRef]
- Galvis, M.M.; Ranschez-Romero, C.; Oliveira-Bueno, T.; Teng, Y. Toward a new era for the management of circulating tumour cells. Adv Exp Med Biol 2021, 1286, 125–134. [Google Scholar]
- Papadaki, M.A.; Koutsopoulos, A.V.; Tsoufas, P.G.; Lagoudaki, E.; Aggouraki, D.; Monastirrioti, A.; Koutoulaki, C.; Apostolopoulou, C.A.; Merodoulaki, A.C.; Papadaki, C. Clinical revelance of immune checkpoints on circulating tumor cells in breast cancer. Cancers 2020, 12, 376. [Google Scholar] [CrossRef]
- Kim, J.W.; Lim, A.R.; You, J.Y.; Lee, L.H.; Song, S.E.; Lee, N.K.; Jung, S.P.; Cho, K.R.; Kim, C.Y.; Park, H. PIK3CA mutation is associated with a poor response to HER-2 targeted therapy in breast cancer patients. Cancer Res Treat 2023, 55, 531–541. [Google Scholar] [CrossRef]
- Nakai, M.; Yamada, T.; Sekiya, K.; Sato, A.; Hankyo, M.; Kuriyama, S.; Takahashi, G.; Kurita, T.; Yanagihara, K.; Yoshida, H.; et al. PIK3CA detected by liquid biopsy in patients with metastatic breast cancer. J Nippon Med Sch 2021, 89, 66–71. [Google Scholar] [CrossRef]
- Urso, L.; Vernaci, G.; Carlet, J.; Lo Mele, M.; Fassan, M.; Zulato, E.; Faggioni, G.; Menichetti, A.; Di Liso, E.; Griguolo, G. ESR1 gene mutation in hormone receptor positive HER2 negative metastatic breast cancer patients. Concordance between tumour tissue and circulating tumor DNA analysis. Front Oncol 2021, 11, 625636. [Google Scholar] [CrossRef]
- Ashworth, T.R. A case of cancer in which cells similar to those in tumors were seen in the blood after death. Australian Med 1869, 14, 146–147. [Google Scholar]
- Cristofanilli, M.; Hayes, D.F.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Rueben, J.M.; Doyle, G.V.; Matera, J.; Allard, W.J.; Miller, MC.; et al. Circulating tumor cells: a novel prognostic factor for newly diagnostic metastatic breast cancer. J Clin Oncol. 2005, 23, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Bidard, F.C.; Peeters, D.J.; Fehm, T.; Nole, F.; Gisbert-Criado, R. Mavroudis D, Grisanti S, Generali D., Garcia.Saenz R., Stebbing J et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient dsts. Lancet Oncol 2014, 15, 406–414. [Google Scholar] [CrossRef]
- Janni, W.; Friedl, T.W.P.; Yab, T.C.; Bidard, F.C.; Cristofanilli, M.; Hayes, D.F.; Ignatiadis, M.; Regan, M.M.; Alix-Panabieres, C.; Barlow, WE.; et al. Clinical validity of repeated circulating tumor cell enumeration as an early treatment monitoring tool for metastatic breast cancer in the PREDICT global pooled analysis. Clin Cancer Res 2025, 31, 2196–2029. [Google Scholar] [CrossRef]
- Dvir, K.; Gordiano, S.; Leone, J.P. Immunotherapy in breast cancer. Int J Mol Sci 2024, 25, 7517. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Pierga, J.Y.; Rueban, J.; Rademaker, A.; Davis, A.A.; Peeters, D.J.; Fehm, T.; Nole, F.; Gisbert-Criado, R.; Mavroudia, D.; et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): international expert consensus paper. Crit Rev Oncol Hematol 2019, 134, 39–45. [Google Scholar] [CrossRef]
- Bidard, F.C.; Kiavue, N.; Jacot, W.; Bachelot, T.; Dureau, S.; Bourgeois, H. Gonclaves A, Brain E, Ladoire S, Dalenc F et al. Overall survival with circulating tumor cell count-driven choice of therapy in advanced breast cancers: a randomized trial. J Clin Oncol 2024, 42, 383–389. [Google Scholar] [CrossRef]
- Cabal, L.; Berger, F.; Cottu, P.; Loirat, D.; Rampanou, A.; Brain, E.; Cyrille, S.; Bourgeois, H.; Klavue, N.; DeLuche, E.; et al. Clinical utility of circulating tumour cell-based monitoring of late-line chemotherapy for metastatic breast cancer: the randomized CirCe01 trial. Br J Cancer 2021, 124, 1207–1213. [Google Scholar] [CrossRef]
- Fehm, T.; Mueller, V.; Banys-Paluchowski, M.; Fasching, P.A.; Fried, T.W.P.; Hartkopf, A.; Huober, J.; Loehberg CRack, B.; Riethdorf, A.; et al. Efficacy of lapatinib in patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells: the DETECT III clinical trial. Clin Chem 2024, 70, 307–318. [Google Scholar] [CrossRef]
- Grigoryeva, E.S.; Tashireva, L.A.; Alifanov, V.V.; Savelieva, O.E.; Vtorsuhin, S.V.; Zavyalova, M.V.; Bragina, O.D.; Garbukov, E.Y.; Cherdyntyseva, N.V.; Choinzonov, E.L.; Perelmuter, V.M. Molecular subtype conversion in CTCs as indicator of treatment adequacy associated with metastasis-free survival in breast cancer. Sci Rep 2022, 12, 20949. [Google Scholar] [CrossRef]
- Ho, H.Y.; Chung, K.S.; Kan, C.M.; Wong, S.C. Liquid biopsy in the clinical management of cancer. Int J Med Sci 2024, 25, 8594. [Google Scholar] [CrossRef]
- de Miranda, F.S.; Barauna, V.G.; dos Santos, L.; Costa, G.; Vassallo, P.F.; Campos, L.C.G. Properties and application of cell free DNA as a clinical biomarker. Int J Mol Sci 2021, 22, 9110. [Google Scholar] [CrossRef] [PubMed]
- Kolenda, T.; Guglas, K.; Baranowski, D.; Sobocinska, J.; Kopcznska, M.; Teresiak, A.; Blizniak, R.; Ramperska, K. cfRNAs as biomarkers in oncology-still experimental or applied tool for personalized medicine already? Rep Pract Oncol Radiother 2020, 25, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, C.; Santini, D.; Corradini, A.G.; Zamagni, C.; Trere, D.; Montanaro, L.; Taffurelli, M. Liquid biopsy in the management of breast cancer patients: Where are we now and where are we going? Diagnostics 2023, 13, 1241. [Google Scholar] [CrossRef]
- Shegekar T, Vodithala S, Juganavar A, The emerging role of liquid biopsies in revolutionizing cancer diagnosis and therapy. Cureus 2023, 15, e43650.
- Nel, I.; Herzog, H.; Atkas, B. Combined analysis of disseminated tumor cells (DTCs) and circulating tumour DNA (ctDNA) in a patient suffering from triple negative breast cancer revealed elevated risk. Front Biosci 2022, 27, 208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Nguyen, E.T.; Pagano, I.; Fukui, J.A. Genomic landscape of circulating tumor DNA in a diverse cohort of metastatic breast cancer patients. Oncol Res Treat 2023, 46, 26–32. [Google Scholar] [CrossRef]
- 109 Tang, Y.; Li, J.; Lu, B.; Ran, J.; Hu, Z.Y.; Ouyang, Q. Circulating tumor DNA profile and its clinical significance in patients with hormone receptor positive and HER2 negative MBC. Front Endocrinol (Lausanne) 2022, 13, 1075830. [Google Scholar] [CrossRef]
- Wimmer, K.; Sachet, M.; Ramos, C.; Frantal, S.; Birnleitner, H.; Brostjan, C.; Exner, R.; Filipits, M.; Bago-Horvath, Z.; Rudas, M.; et al. Differential immunomodulatory effects of epirubicin/cyclophosphamide and docetaxel in breast cancer patients. J Exp Clin Cancer Res 2023, 42, 300. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, X.; Wang, Z.; Wu, P.; Huang, J. Anthracyclines potentiate anti-tumor immunity: A new opportunity for chemoimmunotherapy. Cancer Lett 2015, 369, 331–335. [Google Scholar] [CrossRef]
- Wennerberg, E.; Sarhan, D.; Carlsten MKaminskyy, V.O.; DÁrcy, P.; Zhivotovsky, B.; Childs, R.; Lundqvist, A. Doxorubicin sensitizes human tumor cells to NK-cell and T-cell mediating killing by augmented TRAIL receptor signalling. Int J Cancer 2013, 133, 1643–1652. [Google Scholar] [CrossRef]
- Sawasdee, N.; Wattanapanitch, M.; Thongsin, M.; Phanthaphol, N.; Chiawpanit, C.; Thuwajit, C.; Yenchitsomanus, P.T.; Panya, A. Doxorubicin sensitizes breast cancer cells to natural killer cells in connection with increased Fas receptors. Int J Mol Med 2022, 49. [Google Scholar] [CrossRef]
- Shenasa, E.; He, Y.; Wang, Z.; Dongsheng, T.; Gao, D.; Kos, Z.; Thornton, S.; O´Nielsen, T.O. Digital profiling of immune biomarkers in breast cancer: Relation to anthracycline benefit. Mod Pathol 2025, 38, 100718. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, W.; Dong, B.; Xin, Z.; Ji, Y.; Su, R.; Shen, K.; Pan, J.; Wang, Q.; Wie, W. Docetaxol remodels prostate cancer immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. Theranostics 2022, 12, 4965–4979. [Google Scholar] [CrossRef] [PubMed]
- Wang P, Wang H, Huang Q, Peng C, Yao L, Chen H, Qiu Z, Wu Y, Wang L, Chen W, Exosomes from M1-polarized macrophages enhance paclitaxel antitumor activity by activating macrophages-mediated inflammation. Theranostics 2019, 9, 1714–1727. [CrossRef] [PubMed]
- Maldondo, M.T.; Quinn, M.; Plebanski, M. Low dose cyclophosphamide: Mechanisms of T cell modulation. Cancer Treat Rev 2016, 42, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Che, Y.Q.; Zhang, Y.; Wang, D.; Liu, H.Y.; Shen, D.; Luo, Y. Baseline lymphopenia: A predictor of poor outcomes in HER2 positive metastatic breast cancer treated with trastuzumab. Drug Des Devel Ther. 2019, 13, 3727–3734. [Google Scholar] [CrossRef]
- Roghanian, A.; Hu, G.; Fraser, C.; Singh, M.; Foxall, R.B.; Meyer, M.J.; Lees, E.; Huet, H.; Glennie, M.J.; Beers, R.B.; et al. Cyclophosphamide enhances cancer antibody immunotherapy in the resistant bone marrow niche by modulating macrophage FcR expression. Cancer Immunol Res 2019, 7, 1876–1890. [Google Scholar] [CrossRef]
- Tang, F.; Zhong, Q.; Yang, Z.; Li, H.; Pan, C.; Huang, L.; Ni, T.; Deng, R.; Wang, Z.; Tan, S.; et al. Low-dose cyclophosphamide combined with IL-2 inhibits tumor growth by decreasing regulatory TY cells and increasing CD8 + T cells and natural killer cells in mice. Immunobiol 2022, 227, 152212. [Google Scholar] [CrossRef]
- Zhong, H.; Lai, Y.; Zhang, R.; Daoud, A.; Feng, Q.; Zhou, J.; Shang, J. Low dose cyclophosphamide modulates tumor microenvironment by TGF-ß signalling pathway. Int J Mol Sci 2020, 21, 957. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Lv, S.; Liu, X.; Li, W.; Song, Y.; Rong, D.; Zheng, P.; Huang, H.; Zheng, H. Combined oral low-dose cyclophosphamide endocrine therapy may improve clinical response among patients with metastatic breast cancer via Tregs in TLSs. Sci Rep 2024, 14, 13432. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.M.; O´Donovan, N.; McGowan, P.M.; O´Sullivan, F.; Duffy, M.J.; Crown, J. Trastuzumab induces antibody-dependent-cell-mediated cytotoxicity (ADCC) in HER2-non-amplified breast cancer cell lines. Ann Oncol 2012, 23, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Lower, E.E.; Glass, E.; Blau, R.; Harman, S. HER-2/neu expression in primary and metastatic breast cancer. Breast cancer Res Treat 2009, 113, 301–306. [Google Scholar] [CrossRef]
- Cai, M.; Ming, L.; Lv, H.; Zhou, S.; Xu, X.; Shui, R.; Yang, W. HER2-low breast cancer: evolution of HER2 expression from primary tumour to distant metastasis. BMC Cancer 2023, 23, 656. [Google Scholar] [CrossRef]
- Geukens, T.; de Schepper, M.; Richard, F.; Maetens, M.; Van Baelen, K.; Mahdami, A.; Nguyen, H.L.; Isnaldi, E.; Leduc, S.; Pabba, A.; et al. Intra-patient and inter-metastatic heterogeneity of HER-2 low status in metastatic breast cancer. Eur J Cancer 2023, 188, 152–160. [Google Scholar] [CrossRef]
- Lin, M.; Luo, T.; Jin, Y.; Zhong, X.; Zheng, D.; Zeng, C.; Guo, Q.; Wu, J.; Shao, Z.M.; Hu, X.; et al. Her-2 low heterogeneity between primary and paired recurrent/metastatic breast cancer: Implications in treatment and prognosis. Cancer 2024, 130, 851–862. [Google Scholar] [CrossRef]
- Krawczyk, N.; Banys, M.; Neubauer, H.; Solomayer, E.F.; Gall, C.; Hahn, M.; Becker, S.; Bachmann, R.; Wallwiener, D.; Fehm, T. HER2 status on persistent disseminated tumor cells after adjuvant therapy may differ from initial status on primary tumor. Anticancer Res 2009, 29, 4019–4024. [Google Scholar]
- Volmer, L.L.; Dannehl, D.; Matovina, S.; Taran, F.A.; Walter, C.B.; Wallwiener, M.; Brucker, S.Y.; Hartkopf, A.D.; Engler, T. Comparing the HER2 status of the primary tumor to that of disseminated tumor cells in early breast cancer. Int J Mol Sci 2024, 25, 5910. [Google Scholar] [CrossRef]
- Rack, B.; Juckstock, J.; Gunther-Biller, M.; Andergassen, U.; Neugebauer, J.; Hepp, P.; Schoberth, A.; Mayr, D.; Zwingers, T.; Schindlbeck, C.; et al. Trastuzumab clears HER2/neu-positive isolated tumor cells from bone marrow in primary breast cancer. Arch Gynecol Obstet 2012, 285, 485–492. [Google Scholar] [CrossRef]
- Barok, M.; Balazas, M.; Nagy, P.; Rokosy, Z.; Treszl, A.; Toth, E.; Juhasz, I.; Park, J.W.; Isola, J.; Vereb, G.; et al. Trastuzumab decreases the number of circulating tumor cells despite trastuzumab resistance of the primary tumor. Cancer Lett 2008, 260, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Collins, D.M.; O´Donovan, N.; McGowan, P.M.; O´Sullivan, F.; Duffy, M.J.; Crown, J. Trastuzumab induces antibody-dependent-cell-mediated cytotoxicity (ADCC) in HER2-non-amplified breast cancer cell lines. Ann Oncol 2012, 23, 1788–1795. [Google Scholar] [CrossRef]
- Munzone, E.; Nole, F.; Goldhirsch, A.; Botteri, E.; Espirito, A.; Zorzino, L.; Curiliano, G.; Minchella, I.; Adamoli, L.; Cassatella, M.C.; et al. Changes in HER2 status in circulating tumor cells compared with the primary tumor during treatment for advanced breast cancer. Clin Breast Cancer 2010, 10, 392–397. [Google Scholar] [CrossRef]
- Jordan, N.V.; Bardia, A.; Wittner, B.S.; Benes, C.; Ligorio, M.; Zheng, Y.; Yu, M.; Sundaresan, T.K.; Licausi, J.A.; Desai, R.; et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 2016, 537, 102–106. [Google Scholar] [CrossRef]
- Li, Z.; Lai, X.; Fu, S.; Ren, L.; Cai, H.; Zhang, H.; Gu, Z.; Ma, X.; Luo, K. Immunogenic cell death activates the tumor immune microenvironment to boost the immunotherapy effcieincy. Adv Sci (Weinh) 2022, 9, e2201734. [Google Scholar] [CrossRef]
- Meng, X.; Song, S.; Jiang, Z.; Sun, B.; Wang, T.; Zhang, S.; Wu, S. Receptor conversion in metastatic breast cancer: a prognosticator of survival. Oncotarget 2016, 7, 71887–17903. [Google Scholar] [CrossRef] [PubMed]
- Hodge, J.W.; Kwilas, A.R.; Ardiani, A.; Gameiro, S.R. Attacking malignant cells that survive therapy: exploiting immunogenic modulation. Oncoimmunology 2013, 2, e26937. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, B.; Padget, M.R.; Schlom, J.; Hodge, J.W. Exploiting off-target effects of estrogen deprivation to sensitize estrogen negative breast cancer to immune killing. J Immunother Cancer 2021, 9, e002258. [Google Scholar] [CrossRef]
- Huhn, D.; Marti-Rodrigo, P.; Mouron, S.; Hansel, C.; Tschapalda, K.; Porebski, B.; Haggblad, M.; Lidemalm, L.; Quintela-Fandino, M.; Carreras-Puigvert, J.; et al. Prolonged estrogen deprivation triggers a broad immunosuppressive phenotype in breast cancer cells. Mol Oncol 2022, 16, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liao, J.; Tan, J.T.; Hu, H. Comparison of minimal access and open breast surgery: a propensity score-matched study on postoperative immune function in breast cancer. W J Surg Oncol 2024, 22, 183. [Google Scholar] [CrossRef]
- Michaels, E.; Chen, N.; Nanda, R. The role of immunotherapy in triple negative breast cancer (TNBC). Clin Breast cancer 2024, 24, 263–270. [Google Scholar] [CrossRef]
- Mathiesen, R.R.; Borgen, E.; Renolen, A.; Lokkevik, E.; Nesland, J.M.; Anker, G.; Ostenstad, B.; Lundgren, S.; Risberg, T.; Mjaaland, I.; et al. Persistence of disseminated tumor cells of neoadjuvent treatment for locally advanced breast cancer predicts poor survival. Breast Cancer Res 2012, 14, R117. [Google Scholar] [CrossRef]
- Hall, C.; Krishnamurthy, S.; Lodhi, A.; Bhattachaeryva, A.; Anderson, A.; Kuerer, H.; Bedrosian, I.; Singh, B.; Lucci, A. Disseminated tumor cells predict survival after neoadjuvant therapy in primary breast cancer. Cancer 2012, 118, 342–348. [Google Scholar] [CrossRef]
- Ven Becker Solomayer, E.; Becker-Pergola, G.; Wallwiener, D.; Fehm, T. Primary systemic therapy does not eradicate disseminated tumor cells in breast cancer patients. Breast Cancer Res Treat 2007, 106, 239–243. [Google Scholar]
- Braun, S.; Kentenich, C.; Janni, W.; Hepp, F.; de Waal, J.; Willgeroth, F.; Sommer, H.; Pantel, K. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high risk breast cancer patients. J Clin Oncol 2000, 18, 80–86. [Google Scholar] [CrossRef]
- Fehm, T.; Krawczyk, N.; Solomayer, E.F.; Becker-Pergola, G.; Durr-Storzer, S.; Neubauer, H.; Seeger, H.; Staebler, A.; Wallwiener, D. ER alpha status of disseminated tumour cells in bone marrow of primary breast cancer patients. Breast Cancer Res 2008, 10, R76. [Google Scholar] [CrossRef]
- Lower, E.E.; Glass, E.; Blau, R.; Harman, S. HER-2/neu expression in primary and metastatic breast cancer. Breast cancer Res Treat 2009, 113, 301–306. [Google Scholar] [CrossRef]
- Cai, M.; Ming, L.; Lv, H.; Zhou, S.; Xu, X.; Shui, R.; Yang, W. HER2-low breast cancer: evolution of HER2 expression from primary tumour to distant metastasis. BMC Cancer 2023, 23, 656. [Google Scholar] [CrossRef]
- Geukens, T.; de Schepper, M.; Richard, F.; Maetens, M.; Van Baelen, K.; Mahdami, A.; Nguyen, H.L.; Isnaldi, E.; Leduc, S.; Pabba, A.; et al. Intra-patient and inter-metastatic heterogeneity of HER-2 low status in metastatic breast cancer. Eur J Cancer 2023, 188, 152–160. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, I.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab and docetaxel in HER2 positive metastatic breast cancer. N Eng J Med 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Aroino, G.; de la Haba-Rodriguez, J.; Ferrero, J.M.; De Placido, S.; Osborne, C.K.; Klingbiel, D.; Revelant, V.; Wohlfarth, C.; Poppe, R.; Rimawi, M.; et al. Pertuzumab, trastuzumab and an aromatase inhibitor for HER2 positive and hormone receptor positive metastatic/locally advanced breast cancer: PERTAIN final analysis. Clin Cancer Res 2023, CCR-22-1092. [Google Scholar]
- Rugo, H.; Im, S.A.; Cardoso, F.; Cortes, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Bachelot, T.; Wright, G.S.; Saura, C.; et al. Margetuximab versus trastuzumab in patients with previously treated HER2 positive advanced breast cancer (SOPHIA): final overall survival results from a randomized phase 3 trial. J Clin Oncol 2023, 41, 198–205. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Cecchini, R.S.; Geyer Jr, C.E.; Rastogi, P.; Costantino, J.P.; Atkins, J.N.; Crown, J.P.; Polikoff, J.; Boileau, J.F.; Provencer, L.; et al. NSABP B-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol 2020, 38, 444–453. [Google Scholar] [CrossRef]
- de Nonneville, A.; Houvenaeghel, G.; Cohen, M.; Sabiani, L.; Bannier, M.; Viret, F.; Goncalves, A.; Bertucci, F. Pathological complete response rate and disease-free survival after neoadjuvant chemotherapy in patients with HER2-low and HER2-0 breast cancers. Eur J Cancer 2022, 176, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, X.; Wang, Y.; Zhang, X.; Ye, Y.; Xu, S.; Zhou, L.; Lin, Y.; Lu, J.; Yin, W. Stable or at least once HER2-low status during neoadjuvant chemotherapy confers survival benefit in patients with breast cancer. Ann Med 2024, 56, 2409343. [Google Scholar] [CrossRef]
- Geyer, C.E.; Forster, L.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus capecitabine for HER2 positive advanced breast cancer. N Eng J Med 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, K.L.; Burstein, H.J.; Storniolo, A.M.; Rugo, H.S.; Sledge, G.; Atkan, G.; Ellis, C.; Florance, A.; Vukelija, S.; Bischoff, J.; et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2 positive, trastuzimab refractory metastatic breast cancer. J Clin Cancer 2010, 28, 1124–1130. [Google Scholar]
- Johnson, S.; Pippen Jr, J.; Pivot, X.; Lichinitser, M.; Sadeghi, S.; Dieras, V.; Gomez, H.L.; Romieu, G.; Manikhas, A.; Kennedy, MJ.; et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor positive metastatic breast cancer. J Clin Oncol 2009, 27, 5538–5546. [Google Scholar] [CrossRef]
- Johnston, S.; Hegg, R.; Im, S.A.; Park, I.H.; Burdaeva, O.; Kurteva, G.; Press, M.F.; Tjulandin, S.; Iwata, H.; Simon, SD.; et al. Phase III randomized study of dual human epidermal growth factor receptor 2 (HER2) blockage with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2 positive hormone receptor positive metastatic breast cancer: updated results of alternative treatment options. J Clin Oncol 2021, 39, 79–89. [Google Scholar]
- Stanowicka-Grada, M.; Senkus, E. Anti-HER2 drugs for the treatment of advanced HER2 positive breast cancer. Curr Treat Options Oncol 2023, 24, 1633–1650. [Google Scholar] [CrossRef]
- Li, L.; Zhang, D.; Liu, B.; Lv, D.; Zhai, J.; Guan, X.; Ji, Z.; Ma, F. Antibody-drug conjugates in HER2 positive breast cancer. Chin Med J 2021, 135, 261–262. [Google Scholar] [CrossRef]
- Goldenberg, D.M.; Stein, R.; Sharkey, R.M. The emergence of trophoblast cell surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget 2018, 9, 28989–29006. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau MBaselga, J.; Pegream, M.; Oh, D.Y.; Dieras, V.; Guardino, E.; et al. Trastuzumab-emtansine for HER2 positive advanced breast cancer. N Eng J Med 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Huang, C.S. Mano MS, Loibl S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A, Redondo A et al. Trastuzumab emansine for residual invasive HER2 positive breast cancer. N Eng J Med 2019, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Redman, J.; Hill, E.; Al Degaither, D.; Weiner, L. Mechanisms of action of therapeutic antibodies for cancer. Mol Immunol 2015, 67, 28–45. [Google Scholar] [CrossRef]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor-2 antibody-drug conjugate, in tumors with human epidermal factor receptor 2 heterogeneity. Cancer Sci 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in previously treated HER2 positive breast cancer. N Eng J Med 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Cortes, J.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.M.; Petry, V.; Chung, C.F.; et al. Trastuzumab Deruxtecan versus trastuzumab emtansine for breast cancer. N Eng J Med 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- FDA FDA approves Fam-trastuzumab-Deruxtecan-Nxki for HER2 low breast cancer. FDA: Silver Spring, MD, USA 2022.
- Bardia, A.; Mayer, L.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond JRO´Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Goviectan-hziy in refractory metastatic triple negative breast cancer. N Eng J Med 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Bardia, A.; Marme, F.; Cortes, J.; Schmid, P.; Loirat, D.; Tredan, O.; Ciruelos, E.M.; Dalenc, F.; Gomez- Pardo, PG.; et al. Overall survival with Sacituzumab govitecan in hormone receptor positive and human epidermal growth factor negative metastatic breast cancer. (TROPiCS-02): A randomized open-label multicentre phase3 trail. Lancet 2023, 402, 1423–1433. [Google Scholar]
- Rugo, H.S.; Bardia, A.; Marme, F.; Cortes, J.; Schmid, P.; Loirat, D.; Tredan, O.; Ciruelos, E.; Delenc FPardo, PG.; et al. Overall survival (OS) with sacituzunab govitecan in hormone receptor positive and human epidermal growth factor receptor-2 negative metastatic breast cancer (TROPiCS-02) A randomized open-label multicentre phase 3 trial. Lancet 2023, 402, 1423–1433. [Google Scholar] [CrossRef]
- Trerotola; Cantanelli, P.; Guera, E.; Tripaldi, R.; Aloisi, A.L.; Bonasera, V.; Lattanzio, R.; de Lange, R.; Weidle, U.H.; Piantelli, M.; et al. Upregulation of Trop-2 quantitativly stimulates human cancer growth. Oncogene 2013, 32, 222–233. [Google Scholar] [CrossRef]
- Ambrogi, F.; Fornilli, M.; Boracchi, P.; Trerotola, M.; Relli, V.; Simone, P.; La Sorda, R.; Lattanzio, R.; Querzoli, P.; Pedriali, M.; et al. Trop-2 is a determinant of breast cancer survival. PLos One 2014, 9, e110606. [Google Scholar] [CrossRef] [PubMed]
- Vidula N, Yan C, Rugo H, Trophoblast cell surface antigen gene (TACSTD2) expression in primary breast cancer. Breast Cancer ResTreat 2022, 194, 569–571. [CrossRef]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop2 is a novel target for solid cancer therapy with Sacituzumab-govitcan (IMMU-132) an antibody drug conjugate (ADC). Oncotarget 2015, 6, 22496–22512. [Google Scholar] [CrossRef]
- Okajima, D.; Yasuda, S.; Maejima, T.; Karibe, T.; Sakurai, K.; Aida, T.; Toki, T.; Yamaguchi, J.; Kitamura, M.; Kamai, R.; et al. Datopotamab deruxtecan a novel TROP2 directed antibody drug conjugate, demonstrates potential antitumor activity by efficient drug delivery to tumor cells. Mol Cancer Therapeut 2021, 20, 2329–2340. [Google Scholar] [CrossRef]
- Krop, I.E.; Masuda, N.; Mukohara, T.; Takahashi, S.; Nakayama, T.; Inoue, K.; Iwata, H.; Toyoma, T.; Yamamoto, Y.; Hansra, DM.; et al. Results from the phase I/II study of patritumab deruxtecan, a HER3-directed antibody drug conjugate (ADC) in patients with HER3 expressing metastatic breast cancer (MBC). J Clin Oncol 2022, 40, 1002. [Google Scholar] [CrossRef]
- Hamilton, E.P.; Dosunmu, O.; Sjastry, M.; Finney, L.; Sellami, D.B.; Sternberg, D.W.; Wright-Browne, V.; Toppmeyer, D.; Gwin, W.R.; Thaddeus, JT.; et al. A phase 2 study of HER3-DXd in patients (pts) with metastatic breast cancer (MBC). J Clin Oncol 2023, 41, 1004. [Google Scholar] [CrossRef]
- Plitas, G.Y.; Rudensky, A.Y. Regulatory T cells in cancer. Ann Rev Cancer Biol 2020, 4, 459–477. [Google Scholar] [CrossRef]
- Zhang, H.; Mi, J.; Xin, Q.; Cao, W.; Song, C.; Zhang, N.; Yuan, C. Recent research and clinical progress of CTLA-4 based immunotherapy for breast cancer. Front Oncol 2023, 13, 1256360. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Shi, Y.; Kim, B.C.; Jo, M.H.; Cruz, L.O.; Gou, Z.; Ha, T.; Lu, L.F.; Reich, D.H.; Chen, Y. Force dependent trans-endocytosis by breast cancer cells depletes costimulatory CD80 and attenuates T-cell activation. Biosensors bioelectronics 2020, 165, 112389. [Google Scholar] [CrossRef]
- Jama, M.; Tabana, Y.; Barakat, K.H. Targeting cytotoxic lymphocyte antigen 4 (CTLA-4) in breast cancer. Eur J Med Res 2024, 29, 353. [Google Scholar] [CrossRef]
- Krishnamuethy N, Nishizaki D, Lippman SM, Miyahita H, Nesline MK, Pabla S, Conroy JM, DePietro P, Kato S, Kuurock. High CLTA-4 transcription expression correlates with high expression of other checkpoints and immunotherapy outcome. Ther Adv Med Oncol 2024, 16, 1758835923122050.
- Paucek, R.D.; Baltimore, D.; Li, G. Cancer immunotherapy using checkpoint inhibition. Science 2018, 359, 1350–1355. [Google Scholar]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O´Bryne, K.; Kulasinghe, A. Immune checkpoint inhibitors in cancer therapy. Curr Oncol 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Rad, H.S.; Monkman, J.; Warkiani, M.E.; Ladwa, R.; O´Bryne, K.; Rezaei, N.; Kulasinghe, A. Understanding the tumor microenvironment for effective immunotherapy. Med Res Rev 2021, 41, 1474–1498. [Google Scholar]
- O´Meara, T.A.; Tolaney, S.M. Tumor mutational burden as a predictor of immunotherapy response in breast cancer. Oncotarget 2021, 12, 394–400. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kummel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. VPT-2021: KEYNOTE-522. Phase III study of neoadjuvant pembrolizumab + chemotherapy vs placebo + chemotherapy followed by adjuvant pembrolizumab vs placebo in early stage TNBC. Annal Oncol 2021, 32, 1198–1200. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Asare, S.; Hylton, N.; Leer, L.V.; Perlmutter, J.; Wallace, A.M.; Chien, A.J.; Forero-Torreds, A.; et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): http://ascopubs.org/doi/abs/10.1200/JCO.20127.35.15_suppl.506 (accessed 23 June 2024).
- Andresen, N.K.; Rossevold, A.H.; Borgen, E.; Schirmer, C.B.; Gilje, B.; Garred, O.; Lomo, J.; Stensland, M.; Nordgard, O.; Falk, RS.; et al. Circulating tumour cells in metastatic breast cancer patients treated with immune checkpoint inhibitors- a biomarker analysis of the ALICE and ICON trials. Mol Oncol 2024, 8. [Google Scholar]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Van Bianchi, G.; Esteva, F.J. Martin M et al. Updated overall survival (OS) results from the phase III MONALESSA-3 trial of postmenopausal patients with HR+/HER2- advanced breast cancer (ABC) treated with fulvestrant (FUL) +/- ribociclib (RIB). J Clin Oncol suppl 2021, 39, 1001. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall survival with ribociclib plus letrozole in advanced breast cancer. N Eng J Med 2022, 386, 942–950. [Google Scholar] [CrossRef]
- Lu, Y.S.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Cardoso, F.; Harbeck, N.; Hurvitz, S.; Chow, L.; Sohn, J.; et al. Updated overall survival of ribociclib plus endocrine therapy versus endocrine therapy alone in pre- and perimenopausal patients with HR+/HER” – advanced breast cancer in MONALESSA-7. A phase III trial. J Am Assoc Cancer Res 2022, 28, 851–859. [Google Scholar]
- Cai, Z.; Wang, J.; Li, Y.; Shi, Q.; Jin, L.; Li, S.; Zhu, M.; Wang, Q.; Wong, L.L.; Wang, W.; et al. Overexpression of cyclin D1 and CDK proteins are responsible for the resistance to CDK 4/6 inhibitor in breast cancer that can be reversed by PI3K7mTOR inhibitors. Sci China Life Sci 2023, 66, 94–109. [Google Scholar] [CrossRef]
- Kappel, C.; Elliott, M.J.; Kumar, V.; Nadler, M.B.; Desnoyers, A.; Amir, E. Comparative overall survival of CDK 4/6 inhibitors in combination with endocrine therapy in advanced breast cancer. SCI rep 2024, 14, 3129. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Lacko, A.; Sohn, J.; Cruz, F.; Borrego, M.R.; Manikhas, A.; Park, Y.H.; Stroyakovskiy, D.; Yardley, D.A.; Huang, C.S.; et al. A phase III trial of adjuvant ribociclib plus endocrine therapy versus endocrine therapy alone in patients with HR-positive/HER2 negative early breast cancer: final invasive disease-free survival results from the NATALEE trial. Ann Oncol 2025, 36, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Pallerla, S.; Abdul, A.R.M.; Comeau, J.; Jolis, S. Cancer vaccines, treatment of the future: with emphasis on HER2-positive breast cancer. Int J Med Sci 2021, 22, 779. [Google Scholar] [CrossRef] [PubMed]
- Garnett, C.T.; Schlom, J.; Hodge, J.W. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res 2008, 14, 3536–3544. [Google Scholar] [CrossRef]
- Corti, C.; Giachetti, P.P.M.B.; Eggermont, A.M.M.; Delaloge, S.; Curigliano, G. Therapeutic vaccines for breast cancer: Has the time finally come? Eur J Cancer 2022, 160, 150–174. [Google Scholar] [CrossRef]
- Davodabdia, F.; Sarhadi, M.; Arabour, J.; Sargazi, S.; Rahdar, A.; Diez-Pascual, A.M. Breast cancer vaccines: New insights into immunomodulatory and bano-therapeitic approaches. J Control Release 2022, 349, 844–875. [Google Scholar] [CrossRef]
- Zhoa, Y.; Song, D.; Wang, Z.; Huang, Q.; Huang, F.; Ye, Z.; Wich, D.; Chen, M.; Khirallah, J.; Goa, S.; et al. Antitumour vaccination vias targeted proteolysis of antigens isolated from tumour lysates. Nat Biomed Eng 2025, 9, 234–248. [Google Scholar] [CrossRef]
- Volmer, L.; Koch, A.; Matovina, S.; Dannehl, D.; Weiss, M.; Welker, G.; Hahn, M.; Engler, T.; Wallweiner, M.; Walter, CB.; et al. Neoadjuvant chemotherapy of patients with early breast cancer is associated with increased detection of disseminated tumor cells in the bone marrow. Cancers (Basel) 2022, 14, 635. [Google Scholar] [CrossRef]
- D´Alerio, S.; Scala, S.; Sozzi, G.; Roz, L.; Bertolini, G. Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion. Semin Cancer Biol 2020, 60, 351–361. [Google Scholar]
- Perkins, D.W.; Steiner, I.; Halder, S.; Robertson, D.; Buus, R.; O´Leary, L.; Isacke, C.M. Therapy induced normal tissue damage promotes breast cancer metastasis. iScience 2023, 27, 108503. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Condeelis, J.S.; Oktay, M.H. Chemotherapy-induced metastasis, mechanisms and translational opportunities. Clin Exp Metastasis 2018, 35, 269–284. [Google Scholar] [CrossRef]
- Garcia, A.J.; Rediti, M.; Venet, D.; Majjaj, S.; Kammler, R.; Munzone, E.; Gianni, L.; Thurlmann, B.; Laang, I.; Colleoni, M.; et al. Differential benefit of metronomic chemotherapy among triple negative breast cancer subtypes treated in the IBCSG trial 22-00. Clin Cancer Res 2023, 29, 4908–4919. [Google Scholar] [CrossRef]
- Alkan, F.K.; Caglayan, A.B.; Alkan, H.K.; Benson, E.; Gunduz, Y.E.; Sensoy, O.; Durdagi, S.; Zarbaliyev, E.; Dyson, G.; Assad, H.; et al. Dual activity of minnelide chemosensitize basal/triple negative breast cancer stem cells and reprograms immunosuppressive tumor microenvironment. Sci Rep 2024, 28, 22487. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.; Tan, J.; Huang, J.; Zhang, X.; Guo, Y.; Huang, Y.; Yang, J. The dynamic change of neutrophil to lymphocyte ratio is predictive of pathological complete response after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer 2020, 27, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Alshamsan, B.; Elshenawy, M.A.; Aseafan, M.; Fahmy, N.; Badran, A.; Elhassan, T.; Alsayed, A.; Suleman, K.; Al-Tweigeri, T. Prognostic significance of the neutrophil to lymphocyte ratio in locally advanced breast cancer. Oncel Lett 2024, 28, 429. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Li, H.; Liu, X.; Song, G.; Jiang, H.; Yan, Y.; Zhang, R.; Ran, R.; Zhang, J.; Liu, Y.; et al. The prognostic value of neutrophil to lymphocyte ratio in de novo stage IV breast cancer: a retrospective cohort study. Ann Transl Med 2023, 31, 45. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Miller, G.T.; Jesson, M.I.; Watanabe, T.; Jones, B.; Wallner, B.P. PT-100, a small molecule dipeptidyl peptidase inhibitor, has potent antitumour effects and augments anti-body mediated cytotoxicity via a novel immune mechanism. Cancer Res 2004, 64, 5471–5480. [Google Scholar] [CrossRef]
- Narra, K.; Mullins, S.R.; Lee, H.O.; Strzemkowski-Brun, B.; Magalong, K.; Christiansen, V.J.; McKee, P.A.; Egleston, B.; Cohen, S.J.; Weiner, LM.; et al. Phase II trial of single agent Val.boroPro (Talabostat) inhibiting fibroblast activation patients in patients with metastatic colorectal cancer. Cancer Biol Ther 2007, 6, 1691–1699. [Google Scholar] [CrossRef]
- Hofheinz, R.D.; al-Batran, S.E.; Hartmann, F.; Hartung, G.; Jager, D.; Renner, C.; Tanswell, P.; Kunz, U.; Amelsberg, A.; Kuthan, H.; et al. Stromal antigen targeting by a humanised monoclonal antibody: An early phase II of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie 2003, 26, 44–48. [Google Scholar] [CrossRef]
- Huang, S.; Fang, R.; Xu, J.; Qui, S.; Zhang, H.; Du, J.; Cai, S. Evaluation of the tumor targeting of a FAP alpha based doxorubicin prodrug. J Drug Target 2011, 19, 487–496. [Google Scholar] [CrossRef]
- Brennen, W.N.; Isaacs, J.T.; Denmeade, S.R. Rational behind targeting fibroblast activation protein-expressing carcinoma associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther 2012, 11, 257–266. [Google Scholar] [CrossRef]
- Fang, J.; Xiao, L.; Joo, K.I.; Liu, Y.; Zhang, C.; Lui, S.; Conti, P.S.; Li, Z.; Wang, P.A. A potent immunotoxin targeting fibroblast activating protein for treatment of breast cancer in mice. Int J Cancer 2016, 138, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, Z.; Sun, J.; Song, Q.; He, B.; Zhang, H.; Wang, X.; Dai, W.; Zhang, Q. A tenascin C targeted nanoliposome with navitoclax for specifically eradicating of cancer associated fibroblasts. Nanomedicine 2016, 12, 131–141. [Google Scholar] [CrossRef]
- Truffi, M.; Mazzucchelli, S.; Bonizzi, A.; Sorentino, L.; Allevi, R.; Vanna, R.; Morasso, C.; Corsi, F. Nano-strategies to target breast cancer fibroblasts: Rearranging the tumor microenvironment to achieve antitumor efficacy. Int J Mol Sci 2019, 20, 1263. [Google Scholar] [CrossRef]
- Lee, J.; Fassnacht, M.; Nair, S.; Boczkowski, D.; Gilboa, E. Tumour immunotherapy targeting fibroblast activating protein, a product expressed in tumor-associated fibroblasts. Cancer Res 2005, 65, 11156–11163. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.D.; Duffy, M.R. Lei-Rossmann J, Muntzer A, Scott EM, Hagel J, Campo L, Bryant RJ, Verril C, Lambert A et al. An oncolytic virus expressing a T-cell engager simultaneously targets cancer and immunosuppressive stromal cells. Cancer Res 2018, 78, 6852–6865. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Chinnasamy, D.; Yu, Z.; Morgan, R.A.; Lee, C.C.; Restifo, N.P.; Rosenberg, S.A. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med 2013, 210, 1125–1135. [Google Scholar] [CrossRef]
- Rastegar-Pouyani, N.; Abdolvahab, M.H.; Farzin, M.A.; Zare, H.; Kesharwani, P.; Sahebkar, A. Targeting cancer-associated fibroblasts with pirfenidone: A novel approach for cancer therapy. Tissue cancer 2024, 91, 102624. [Google Scholar] [CrossRef]
- Glabman, R.A.; Choyke, P.L.; Sato, N. Cancer-associated fibroblasts: Tumorigenicity and targeting for cancer therapy. Cancers 2022, 14, 3906. [Google Scholar] [CrossRef]
- Sun, R.; Luo, H.; Su, J.; Di, S.; Zhou, M.; Shi, B.; Sun, Y.; Du, G.; Zhang, H.; Jiang, H.; Li, J. Olaparib supresses MDSC recruitment via SDF1alpha/CXCR4 axis to improve the anti-tumor efficacy of CAR-T cells on breast cancer in mice. Mol Ther 2021, 29, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Johnson, K.C.C.; Gatti-Mays, M.E.; Li, Z. Emerging strategies in targeting tumor resident myeloid cells for cancer immunotherapy. J Haematol Oncol 2022, 15, 118. [Google Scholar] [CrossRef]
- Beatson, R.; Graham, R.; Grundland Freile, F.; Kannambath, S.; Pfiefer EWoodman, N.; Owen, J.; Nuamah, R.; Mandel, U.; Pinder, S.; et al. Cancer-associated hypersialylated MUC1 drives the differentiation of human monocytes into macrophages with a pathogenic phenotype. Commun Biol 2020, 3, 644. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur J Pharmacol 2020, 877, 173090. [Google Scholar] [CrossRef]
- Goswami, K.K.; Ghosh, T.; Ghosh, S.; Sarkar, M.; Bose, A.; Baral, R. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol 2017, 316. [Google Scholar] [CrossRef]
- Giannone G, Ghisoni E, Genta S, Scotto G, Tuninetti V, Turinetto M, Valabrega. Immuno-metabolism and microenvironment in cancer. Int J Mol Sci 2020, 21, 4414.
- Vaupel, P.; Multhoff, G. Revisting the Warberg effect: historical dogma versus current understanding. J Physiol 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Chen, P.; Zuo, H.; Xiong, H.; Kolar, M.J.; Chu, Q.; Saghatelian, A.; Siegwart, D.J.; Wan, Y. Gpr132 sensing of lactate mediates tumour-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci USA 2017, 114, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Bohn T, Rapp S, Luther N, Bruehl TJ, Kojima N, Lopez PA, Hahlbrock J, Muth S, Endo S, Pektor S et al Tumor immunoevasion via acidosis- dependent induction of regulatory tumor-associated macrophages. Nat Immunol 2018, 19, 1319–1329. [CrossRef]
- Jiang, H.; Wei, H.; Wang, H.; Wang, Z.; Li, J.; Ou, Y.; et al. Zeb1 induced metabolic reprogramming of glycolysis is essential for macrophage polarization in breast cancer. Cell Death Dis 2022, 13, 206. [Google Scholar] [CrossRef]
- Farabegoli, F.; Vettraino MManberba, M.; Fiume, I.; Roberti, M.; Din Stefano, G. Galloflavin, a new lactate dehydrogenase inhibitor induces death of human breast cancer cells with different glycolytic attitude by affecting distinct signalling pathways. Eur. J. Pharm. Sci. 2012, 47, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Kim, E.Y.; Han, C.W.; Park, S.Y.; Jeong, M.S.; Yoon, D.; Choi, H.J.; Jin, L.; Park, M.J.; Kwon, YJ.; et al. Macchilin A inhibits tumor growth and macrophage M2 polarization through the reduction of lactic acid. Cancers (Basel) 2019, 11. [Google Scholar]
- Jin H, He Y, Zhao P, Hu Y, Tao J, Chen J, Huang Y Targeting lipid metabolism to overcome EMT-associated drug resistance via integrin β/FAK pathway and tumor-associated macrophage repolarization using legumain activable delivery. Theranostics 2019, 9, 265–2378. [CrossRef] [PubMed]
- Chen, D.; Xie, J.; Fiskesund, R.; Dong, W.; Liang, X.; Lv, J.; Jin, X.; Liu, J.; Mo, S.; Zhang, T.; et al. Chloroquine modulates antitumor response by resetting tumor-associated macrophages toward M1 phenotype. Nat Commun 2018, 9, 873. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Chen, X.; Lin, L.; Yuan, Q.; Zeng Zu, X.; Shen, Y. The emerging role of metabolism in the crosstalk between breast cancer cells and tumor-associated macrophages. Int J Biol Sci 2023, 9, 4915–4930. [Google Scholar] [CrossRef]
- Tharp, K.M.; Kersten, K.; Maller, O.; Timblin, G.A.; Stashko, C.; Canale, F.P.; Menjivar, R.E.; Hayward, M.K.; Berestjuk, I.; Ten Hoeve, J.; et al. Tumor associated macrophages restrict CD8+ T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment. Nat Cancer 2024, 5, 1045–1062. [Google Scholar] [CrossRef]
- Wang, L.; Guo, W.; Guo, Z.; Yu, J.; Tan, J.; Simons, D.L.; Hu, K.; Liu, X.; Zhou, Q.; Zheng, Y.; et al. PD-L1 expressing tumor-associated macrophages are immunostimulatory and associate with good clinical outcome in human breast cancer. Cell Rep Med 2024, 5, 101420. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumors. Nat Rev Immunol 2012, 12, 253–268. [Google Scholar] [CrossRef]
- Hao, Z.; Li, R.; Wang, Y.; Li, S.; Hong, Z.; Han, Z. Landscape of myeloid derived suppressor cell in tumor immunotherapy. Biomark Res 2021, 9, 77. [Google Scholar] [CrossRef]
- Jin, S.; Yang, Z.; Hao, X.; Tang, W.; Ma, W.; Zong, H. Roles of HMGB1 in regulating myeloid-derived suppressor cells in the tumor microenvironment. Biomark Res 2020, 8, 21. [Google Scholar] [CrossRef]
- Nefedova, Y.; Nagaraj, S.; Rosenbauer, A.; Muro-Cacho, C.; Sebti, S.M.; Gabrilovich, D.I. Regulation of dendritic cell differentiation and antitumor response in cancer by pharmacological-selective inhibition of the janus-activate kinase/signal transducers and activators of transcription 3 pathway. Cancer Res 2005, 65, 9525–9535. [Google Scholar] [CrossRef]
- Apolloni, E.; Bronte, V.; Mazzoni, A.; Serfinin, P.; Cabrelle, A.; Segal, D.; Ypung, H.A.; Zanovello, P. Immortalized myeloid suppressor cells trigger apoptosis in antigen-activated lymphocytes. J Immunol 2000, 165, 6723–6730. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, T.; Zhu, W.; Xie, S.; Zhao, Z.; Feng, B.; Guo, H.; Yang, R. Targeting MDSC for immune check-point blockade in cancer immunotherapy: current progress and new prospects. Clin Med Insights Oncol 2017, 15, 11795549211035540. [Google Scholar] [CrossRef] [PubMed]
- Grover, A.; Sanseviero, E.; Timosenko, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells: a propitious road to clinic. Cancer Discov 2021, 11, 2693–2706. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, T.; Liu, J.; Tai, J.; Wang, B.; Chen, Z.; Quan, Z. Pan-cancer analysis reveals tumor-associated macrophage communication in the tumor microenvironment. Exp Hematol Oncol 2021, 10, 31. [Google Scholar] [CrossRef]
- Qui, Y.; Chen, T.; Hu, R.; Zhu, R.; Li, C.; Ruan, Y.; Xie, X.; Li, Y. Next frontier in tumor immunotherapy: macrophage mediated immune evasion. Biomark Res 2021, 9, 72. [Google Scholar]
- Tumino, N.; Weber, G.; Besi, F.; Del Bufalo, F.; Bertaina, V.; Paci, P.; Quatrini, L.; Antonucci, L.; Sinibaldi, M.; Quintarelli, C.; et al. Polymorphonuclear myeloid-derived suppressor cells impair the anti-tumor efficacy of GD2.CART-cells in patients with neuroblastoma. J Hematol Oncol 2021, 14, 191. [Google Scholar] [CrossRef]
- Rivera Vargs, T.; Apetoh, L. Can immunogenic chemotherapies relieve cancer cell resistance to immune check point inhibitors. Front Immunol 2019, 10, 1181. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody conjugate: the “biological missile” for targeted cancer therapy. Signal Target Ther 2022, 7, 93. [Google Scholar] [CrossRef]
- Fultang, J.; Panetti, S.; Ng, M.; Collins, P.; Graef, S.; Rizkalla, N.; Booth, S.; Lenton, R.; Noyvert, B.; Shannon-Lowe, C.; et al. MDSC targeting with gemtuzumab ozogamicin restores T-cell immunity and immunotherapy against cancers. EBioMed 2019, 47, 235–246. [Google Scholar] [CrossRef]
- Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich. All-trans retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 2003, 63, 4441–4449.
- Bauer, R.; Udonta, F.; Wroblewski, M.; Ben-Batalla, I.; Santos, I.M.; Taverna, T.; Kuhlencord, M.; Gensch, V.; Pasler, S.; Vinckier, S.; et al. Blockage of myeloid-derived suppressor cells expansion with all-trans retinoic acid increases the efficacy of antiangiogenic therapy. Cancer Res 2018, 78, 320–3230. [Google Scholar] [CrossRef]
- Michels, T.; Shirin, G.V.; Naiditch, H.; Sevko, A.; Umansky, V.; Shurin, M.R. Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner. J Immunotoxicol 2012, 9, 292–300. [Google Scholar] [CrossRef]
- Serafini, P.; Menellsckel, K.; Kelso, M.; Noonan, K.; Caslifano, J.; Koch, W.; Dolcetti, L.; Bronte, V.; Borrello, I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med 2006, 203, 2691–2702. [Google Scholar] [CrossRef]
- Orillion, A.; Hashimoto, A.; Damayanti, N.; Shen, L.; Adelaiye-Ogala, R.; Arisa, S.; Chintala, S.; Ordentlich, P.; Kao, C.; Elzey, B.; et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the effect of PD-1 inhibition in murine models of lung and renal carcinoma. Clin Cancer Res 2017, 23, 5187–5201. [Google Scholar] [CrossRef] [PubMed]
- Christmas, B.J.; Rafie, C.I.; Hopkins, A.C.; Scott, B.A.; Ma, H.S.; Cruz, K.A.; Woolman, S.; Armstrong, T.D.; Connolly, R.M.; Azad, NA.; et al. Entinostat converts immume-resistant breast and pancreatic cancers into checkpoint- responsive tumors by reprogramming tumor-infiltrating MDSCs. Cancer Immunol Res 2018, 6, 1561–1577. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Kohanbash, G.; Fellows-Mayle, W.; Hamilton, R.L.; Komohara, Y.; Decker, S.A.; Ohlfest, J.R.; Okada, H. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res 2011, 71, 2664–2674. [Google Scholar] [CrossRef]
- Proia, T.A.; Singh, M.; Woessner, R.; Carnealli, L.; Bommakanti, G.; Magiera, L.; Srinivasan, S.; Grosskurth, S.; Collins, M.; Womack, C.; et al. STAT3 antisense oligonucleotide remodels the suppressive tumor microenvironment to enhance immune activation in combination with anti PD-L1. Clin Cancer Res 2020, 26, 6335–6349. [Google Scholar] [CrossRef]
- Ko, J.S.; Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J.; et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 2009, 15, 2148–2157. [CrossRef] [PubMed]
- Zhang, X.; Liang, Q.; Cao, Y.; Yang, T.; An, M.; Liu, Z.; Yang, J.; Liu, Y. Dual depletion of myeloid derived suppressor ells and tumor cells with self-assembled gemcitabine-celecoxib nano-twin drug for cancer chemoimmunotherapy. J Nanotech 2024, 23, 319. [Google Scholar]
- Cortesi, L.; Rugo, H.S.; Jackisch, C. An overview of PARP inhibitors for the treatment of breast cancer. Target Oncol 2021, 16, 255–282. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for metastatic breast cancer in patients with germline BRCA mutation. N Eng J Med 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Goncalves, A.; Lee, K.H.; Fehrenbacher, L.; Yeruhalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Eng J Med 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Tung, N.; Garber, J.E. PARP inhibition in breast cancer: progress made and future hopes. NPJ Breast Cancer 2022, 8, 47. [Google Scholar] [CrossRef]
- De, K.; Jana, M.; Chowdhury, B.; Calaf, G.M.; Roy, D. Role of PARP inhibitors: A new hope for breast cancer therapy. Int J Mol Sci 2025, 26, 2773. [Google Scholar] [CrossRef]
- Tutt, A.N.J.; Garger, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmana, J.; et al. Adjuvant olaparib for patients with BRCA1 or BRCA 2 mutated breast cancer. N Eng J Med 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, X.; Zhao, Z.; Lu, W.; Guo, Q.; Wang, S.; You, J.; Zhang, Y.; Liu, L. Risk of pneumonitis in cancer patients treated with PARP inhibitors: a meta-analysis randomized controlled trials and a pharmacovigilance study of the FAERS database. Gyncol Oncol 2021, 162, 496–505. [Google Scholar] [CrossRef]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; van der Burg, E.; Nygren, A.O.H.; Zander, S.A.L.; Derkson, P.W.B.; de Bruin, M.; Zevenhoven, J.; Lau, J.; et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef]
- Pettitt, S.J.; Krastev, D.B.; Brandsma, I.; Drean, A.; Song, F.; Aleksandrov, R.; Harrell, M.I.; Menon, M.; Brough, R.; Campbell, J.; et al. Genome wide and high density CRISPR-Cas9 screens identify point mutations in PARP1 causing PARP inhibitor resistance. Nat Commun 2018, 9, 1849. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.; Jin, D.; Zhang, Z.; Shen, K.; Yang, J.; Chen, H.; Zhao, X.; Yang, L.; Lu, H. PARP inhibitor resistance in breast and gynaecological cancer: resistance mechanisms and combination therapy. Front Pharmacol 2022, 13, 967633. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.K.; Cheney, E.M.; Hartl, C.A.; Pantelidou, C.; Oliwa, M.; Castrillon, J.A.; Lin, J.R.; Hurst, K.E.; Taveira, M.O.; Johnson, NT.; et al. Targeting immunosuppressive macrophages overcomes PARP inhibitor resistance in BRCA1-associated triple-negative breast cancer. Nat Cancer 2021, 2, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhu, L.; Chen, J. Current advances and challenges in CAR-T therapy for solid tumors. Tumor associated antigens and the tumor microenvironment. Exp. Hematol. Oncol. 2023, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- George, P.; Dasyam, N.; Giunti, G.; Mester, B.; Bauer, E.; Andrews, B.; Perera, T.; Ostapowicz, T.; Frampton, C.; Li, P. Third generation anti CD19 chimeric antigen receptor T-cells incorporating a TRL2 domain for relapsed or refractory B-cell lymphoma: A phase I clinical trial protocol (ENABLE). BMJ Open 2020, 10, e034629. [Google Scholar] [CrossRef] [PubMed]
- Roselli, E.; Boucher, J.C.; Li, G.; Kotani, H.; Spitler, K.; Reid, K.; Cervantes, E.V.; Bulliard, Y.; Tu, N.; Lee, S.B. 4-1BB optimized CD28 co-stimulation enhances function of human mono-specific and bi-specific third-generation CAR T cells. J. Immunother. Cancer 2021, 9, e003354. [Google Scholar] [CrossRef]
- Ramos, C.A.; Rouce, R.; Robertson, C.S.; Reyna, A.; Narala, N.; Vys, G.; Mehta, B.; Zhang, H.; Dakhova, O.; Carrum, G. In vivo fate and activity of second versus third generation CD19 specific CAR-T cells in B cell non-Hodgkins lymphomas. Mol. Ther. 2018, 26, 2727–2737. [Google Scholar] [CrossRef]
- Erber, R.; Kailayangiri, S.; Hueber, H.; Ruebner, M.; Hartmann, A.; Haberle, L.; Meyer, J.; Volkl, S.; Mackensen, A.; Landgraf, L.; et al. Variable expression of the diganglioside GD2 in breast cancer molecular subtypes. Cancers (Basel) 2021, 13, 5577. [Google Scholar] [CrossRef]
- Seitz, C.M.; Scroeder, S.; Knopf, P.; Krahl, A.S.; Hau, J.; Schleicher, S.; Martella, M. Quintanilla-Martinez L, Kneilling M, Pichler B et al. GD2 targeted chimeric antigen receptor T-cells prevent metastasis formation by elimination of breast cancer stem-like cells. Oncoimmunology 2020, 9, 1683345. [Google Scholar] [CrossRef]
- Bajor, M.; Graczyk-Jarznka, A.; Marhelava, K.; Burdzinska, A.; Muchowicz, A.; Gorel, A.; Zhylko, A.; Soroczynska, K.; Retecki, K.; Krawczyk M.; et al. J Immunother Cancer 2022, 10, e002500.
- Fu, W.; Lei, C.; Liu, S.; Cui, Y.; Wang, C.; Qian, K.; Li, T.; Shen, Y.; Lin, F.; et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun 2019, 10, 4355. [Google Scholar] [CrossRef]
- Li, P.; Yang, L.; Li, T.; Bin, S.; Sun, B.; Huang, Y.; Yang, K.; Shan, D.; Gu, H.; Li, H. The third generation anti-HER2 chimeric antigen receptor mouse T-cells alone or together with anti-PD1 antibody inhibits the growth of mouse breast tumor cells expressing HER-2 in vitro and in immune competent mice. Front Oncol 2020, 10, 1143. [Google Scholar] [CrossRef]
- Duro-Sanchez, S.; Alonso, M.R.; Arribas, J. Immunotherapies against HER2 positive breast cancer. Cancer (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Niu, Z.; Wu, J.; Zhoa, Q.; Zhang, J.; Zhang, P.; Yang, Y. CAR-based immunotherapy for breast cancer: peculiarities, ongoing investigations and future strategies. Front Imunol 2024, 15, 1385571. [Google Scholar] [CrossRef] [PubMed]
- Rivas, E.I.; Linares, J.; Zwick, M.; Gomez-Llonin, A.; Guiu, M.; Labernadie, A.; Badia-Ramentol, J.; Llado, A.; Bardia, L.; Perez-Nunez, I.; et al. Targeted immunotherapy against distinct cancer-associated-fibroblasts overcomes treatment resistance in refractory HER2+ breast tumors. Nat Commun 2022, 13, 5310. [Google Scholar] [CrossRef]
- Das, S.; Valton, J.; Duchateau, P.; Poirot, L. Stromal depletion by TALEN-editied universal hipoimmunogenic FAP-CAR-T immunotherapy. Front Immunol 2023, 14, 1172681. [Google Scholar] [CrossRef]
- Nalawade, S.A.; Shafer, P.; Bajgain, P.; McKenna, M.K.; Ali, A.; Kelly, L.; Joubert, J.; Gottschalk, S.; Watanabe, N.; Leen, A.; et al. Selectively targeting myeloid-derived suppressor cells through TRAIL receptor 2 to enhance the efficacy of CAR T cell therapy for treatment of breast cancer. J Immunother Cancer 2021, 9, e003237. [Google Scholar] [CrossRef]
- Sun, R.; Luo, H.; Su, J.; Di, S.; Sun, R.; Chen, M.; Jiang, H.; et al. Olaparib suppresses MDSC recruitment via SDF1alpha/CXCR4 to improve the anti-tumor effect of CAR-T cells on breast cancer in mice. Mol Ther 2021, 29, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Zhu, M.; Pan, Z.; Sun, R.; Chen, M.; Jiang, H.; Shi, B. Luo H, Li Z. Adjuvant of poly LC improves antitumor effects of CAR-T cells. Front Oncol 2019, 9, 241. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, R.; Wu, X.; Zhang, M.; Jin, T. PD-L1 mediates triple-negative breast cancer evolution via the regulation of TAM/M2 polarization. Int J Oncol 2022, 61. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.; Porter, D. Cytokine release syndrome with chimeric antigen receptor T-cell therapy. Biol Blood Marrow Transplant 2018, 12, 756. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Z.; Ren, Z.; Li, Y. Reactions related to CAR-T cell therapy. Front Immunol 2021, 12, 663201. [Google Scholar] [CrossRef]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse effect following the administration of T-cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Gumber, D.; Wang, L.D. Improving CAR-T immunotherapy: overcoming the challenges of T-cell exhaustion. EBioMedicine 2022, 77, 103941. [Google Scholar] [CrossRef]
- Zhong, H.; Lai, Y.; Zhang, R.; Daoud, A.; Feng, Q.; Zhou, J.; Shang, J. Low dose cyclophosphamide modulates tumor microenvironment by TGF-ß signalling pathway. Int J Mol Sci 2020, 21, 957. [Google Scholar] [CrossRef]
- Jallouk, A.P.; Paravastu, S.; Weilbaecher, K.; Aft, R.L. Long-term outcome of (neo)adjuvant zoledronic acid therapy in locally advanced breast cancer. Breast Cancer Res Treat 2021, 187, 135–144. [Google Scholar] [CrossRef]
- Liu, M.; Qian, S.; Wu, J.; Xiao, J.; Zeng, X. The effects of neoadjuvant zoledronic acid in breast cancer patients: A meta-analysis of randomized controlled trials. Asian J Surg 2023, 46, 4124–4130. [Google Scholar] [CrossRef]
- Powles, T.; Paterson, A.; McCloskey, E.; Schein, P.; Scheffler, B.; Tidy, A.; Ashley, S.; Smith, I.; Ottestad, L.; Kanis, J. Reduction in bone relapse and improved survival with oral clondronate for adjuvant treatment of operable breast cancer (ISRCTN83688026). Breast Cancer Res 2006, 8, R13. [Google Scholar] [CrossRef] [PubMed]
- Diel, I.J.; Solomayer, E.F.; Costa, S.D.; Gollan, C.; Goerner, R.; Wallweiner, D.; Kaufmann, M.; Bastert, G. Reduction in new metastases in breast cancer adjuvant clodronate treatment. N Eng J Med 1998, 339, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.G.; Lucas, P.C.; Anderson, S.J.; Mamounas, E.P.; Brufsky, A.; Baez-Diaz, L.; King, K.M.; Lad, T.; Robidoux, A.; Finigan, M.; et al. MAF amplification and adjuvant clodronate outcomes in early-stage breast cancer in NSABP B-34 and potential impact on clinical practice. JNCI Cancer Spectr 2021, 28, 5. [Google Scholar] [CrossRef] [PubMed]
- Eisen, A.; Somerfeld, M.R.; Accordine, M.K.; Blanchette, P.S.; Clemons, M.J.; Dhesy-Thind, S.; Dillmon, M.S.; D´Oronzo, S.; Fletcher, G.G.; Frank, ES.; et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: ASCO-OH (CCO) guideline update. J Clin Oncol 2022, 40, 787–800. [Google Scholar] [CrossRef]
- Senaratne, S.G.; Pirianov, G.; Mansi, J.L.; Arnett, T.R.; Colston, K.W. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer 2000, 82, 1459–1468. [Google Scholar] [CrossRef]
- Borutaite, V. Mitochondria as decision-makers in cell death. Environ Mol Mutagen 2010, 51, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Formigue, O.; Lagneaux, L.; Body, J.J. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res 2000, 15, 2211–2221. [Google Scholar] [CrossRef]
- Boissier, S.; Ferreras, O.; Peyuhaud, O.; Magnetto, S.; Ebetino, F.H.; Colombel, M.; Delmas, P.; Delaisse, J.M.; Clezardin, P. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastasis. Cancer Res 2000, 60, 2949–2954. [Google Scholar] [PubMed]
- Denoyelle, C.; Hong, L.; Vannier, J.P.; Soria, J.; Soria, C. New insights into the action of bisphosphonate zoledronic acid in breast cancer cells by dual Rho-dependent and independent effects. Br J Cancer 2003, 88, 1631–1640. [Google Scholar] [CrossRef]
- Kimachi, K.; Kajiya, H.; Nakayama, S.; Ikebe, T.; Okabe, K. Zoledronic acid inhibits RANK expression and migration of osteoclast precursors during osteoclastogenesis. Naunyn-Schmiedeberg´s Arch of Pharmacology 2011, 383, 297–308. [Google Scholar] [CrossRef]
- Weber, M.; Homm, A.; Muller, S.; Frey, S.; Amann, K.; Ries, J.; Geppert, C.; Preidl, R.; Most, T.; Kammerer, PW.; et al. Zoledronate causes a systemic shift if macrophage polarization towards M1 in vivo. Int J Mol Sci 2021, 22, 1323. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.L.; Holen, I. Tumour macrophages as potential targets of bisphosphonates. J Transl Med 2011, 9, 177. [Google Scholar] [CrossRef]
- Zang, X.; Zhang, X.; Hu, H.; Qiao, M.; Zhao, X.; Deng, Y.; Chen, D. Targeted delivery of zoledronate to tumor-associated macrophages for cancer immunotherapy. Mol Pharm 2019, 16, 2249–2258. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, B.; Shen, Y.; Ren, J.; Chen, M.; Jiang, Y.; Wu, H.; Dai, W.; Zhang, H.; Wang, X.; et al. Bisphosphonate-mineralized nano-IFN gamma suppresses residual tumor growth caused by incomplete radiofrequency ablation through metabolically remodelling tumor-associated macrophages. Theranostics 2025, 15, 1057–1076. [Google Scholar] [CrossRef]
- Rahimian, L.; Khandani, B.K.; Nemati, M.; Hoseini-Shahrestanak, S.; Aminizadeh, N.; Jafarzedeh, A. Reduced expression of natural killer cell related activating receptors by peripheral blood mononuclear cells from patients with breast cancer and their improvement by zoledronic acid. Asian Pac J Cancer Prev. 2022, 23, 1661–1669. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.H.; Chen, S.C.; Chen, C.Y.; Lin, T.M. Zoledronic acid blocks the interaction between breast cancer cells and regulatory T-cells. BMC Cancer 2019, 19, 176. [Google Scholar] [CrossRef]
- Wang, S.; Huang, M.; Chen, M.; Sun, Z.; Jiao, Y.; Ye, G.; Pan, J.; Ye, W.; Zhao, J.; Zhang, D. Zoledronic acid and thymosin alpha1 elicit antumor immunity against prostate cancer by enhancing tumor inflammation and cytotoxic T cells. J Immunother Cancer 2023, 11, e006381. [Google Scholar] [CrossRef]
- Aschariyasakulchai, P.; Sakunrangsit, N.; Chokyakorn, S.; Suksanong, C.; Ketchart, W. Anticancer effect of zoledronic acid in endocrine-resistant breast cancer cells via HER-2 signalling. Biomed Pharmacother. 2024, 171, 116142. [Google Scholar]
- Li, X.Y.; Lin, Y.C.; Huang, W.L.; Hong, Q.C.; Chen, J.Y.; You, Y.; Li, W.B. Zoledronic acid inhibits proliferation and impairs migration and invasion through down regulation of VEGF and MMP expression in human nasopharyngeal carcinoma cells. Med Oncol 2012, 29, 714–720. [Google Scholar] [CrossRef]
- Dedes, P.G.; Gialelli, C.; Tsonis, A.I.; Kanakis, I.; Theocharis, A.D.; Kletsas, D.; Tzanakakis, G.N.; Karamanos, N.K. Expression of matrix macromolecules and functional properties of breast cancer cells are modulated by the bisphosphonate zoledronic acid. Biochem Biophys Acta 2012, 20, 1926.1939. [Google Scholar] [CrossRef] [PubMed]
- Fane, M.; Weeraratna, A.T. How the aging microenvironment influences tumour progression. Nat Rev Cancer 2019, 20, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Aunan, J.R.; Cho, W.C.; Soreide, K. The biology of aging and cancer: a brief overview of shared and divergent molecular hallmarks. Aging Dis 2017, 8, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Panwar, P.; Lamour, G.; MacKenzie, N.C.W.; Yang, H.; Ko, F.; Li, H.; Bromme, D. Changes in structural-mechanical properties and degradability of collagen during aging-associated modifications. J Biol Chem 2015, 290, 23291–23306. [Google Scholar] [CrossRef]
- Liu, H.; Wang Chen, S.C.; Chen, C.Y.; Lo, J.L.; Lin, T.M. Immune modulation of CD4+ CD25+ regulatory T cells by zoledronic acid. BMC Immunol 2016, 17, 45. [Google Scholar] [CrossRef]
- Sarhan, D.; Leijonhufvud, C.; Murray, S.; Witt k Seitz, C.; Wallerius, M.; Xie, H.; Ullen, A.; Harmenberg, U.; Lidbrink, E.; et al. Zoledronic acid inhibits NFAT and IL-2 signalling pathways in regulatory T-cells and diminishes their suppressive function in patients with metastatic cancer. Oncoimmunology 2017, 6, e1338238. [Google Scholar] [CrossRef]
- Rouhrazi, H.; Turgan, N.; Oktem, G. Zoledronic acid overcomes chemoresistance by sensitizing cancer stem cells to apoptosis. Biotech Histochem 2018, 93, 77–88. [Google Scholar] [CrossRef]
- Jackson, C.; Freeman, A.L.J.; Szlamka, Z.; Spiegelhalter, D.J. The adverse effects of bisphosphonates in breast cancer: A systemic review and network meta-analysis. PLoS One 2021, 16, e246441. [Google Scholar] [CrossRef]
- Petitprez, F.; Meylan, M.; de Reynies, A.; Sautes-Fridman, C.; Fridman, W.H. The tumor microenvironment in the response to immune check point blockage therapies. Front Oncol 2020, 11, 784. [Google Scholar]
- Alharbi, M.; Roy, A.M.; Krishnan, J.; Kalinski, P.; Yao, S.; Gandhi, S. Targeting the tumor microenvironment to improve clinical outcomes in triple negative breast cancer patients and bridge the current disparity gap. Front Immunol 2024, 15, 1428118. [Google Scholar] [CrossRef]
- Ribeiro, R.; Carvalho, M.J.; Goncalves, J.; Moreira, J.N. Immunotherapy in triple-negative breast ancer: insights into tumor immune landscape and therapeutic opportunities. Front Med Biosci 2022, 9, 903065. [Google Scholar]
- Song, F.; Tarantino, P.; Garrido-Castro, A.; Lynce, F.; Tolaney, S.M.; Schlam, I. Immunotherapy for early-stage triple negative breast cancer: Is earlier better? Cur Oncol Rep 2024, 26, 21–33. [Google Scholar] [CrossRef]
- Dixon-Duglas, J.; Loi, S. Immunotherapy in early-stage-triple-negative breast cancer: Where are we now and where are we headed? Curr Treat Optyions Oncol 2023, 24, 1004–1020. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Miura, K.; Tamori, S.; Akimoto, K. Identification of a gene expression signature to predict the risk of early recurrence and the degree of immune cell infiltration in triple-negative breast cancer. Camcer Genomics Proteomics 2024, 21, 316–326. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Z.; Shi, C.; Feng, M.; Feng, X.; Liu, T. The prognostic marker KRT81 is involved in suppressing CD8+ T cells and predicts immunotherapy response for triple-negative breast cancer. Cancer Biol Ther 2024, 25, 2355705. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, C.W. Epigenetic modulations in triple-negative breast cancer: therapeutic implications for tumor microenvironment. Pharmacol Res 2024, 204, 107205. [Google Scholar] [CrossRef]
- Mehta, A.K.; Cheney, E.M.; Hartl, C.A.; Pantelidou, C.; Oliwa, M.; Castrillon, J.A.; Lin, J.R.; Hurst, K.E.; Taveira, M.O.; Johnson, N.T.; et al. Targeting immunosuppressive macrophages overcomes PARP inhibitor resistance in BRCA1-associated triple-negative breast cancer. Nat Cancer 2021, 2, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Xu, Z.; Yao, K.; Zheng, B.; Zhang, Y.; Wang, X.; Zhang, T.; Lu, X.; Hu, H.; You, B.; et al. Osteoclast cancer cell metabolic cross-talk confers PARP inhibitor resistance in bone metastatic breast cancer. Cancer Res 2024, 84, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.P. Treatment of minimal residual disease in breast cancer: a longitudinal case study. Cureus 2017, 9, e1521. [Google Scholar] [CrossRef] [PubMed]
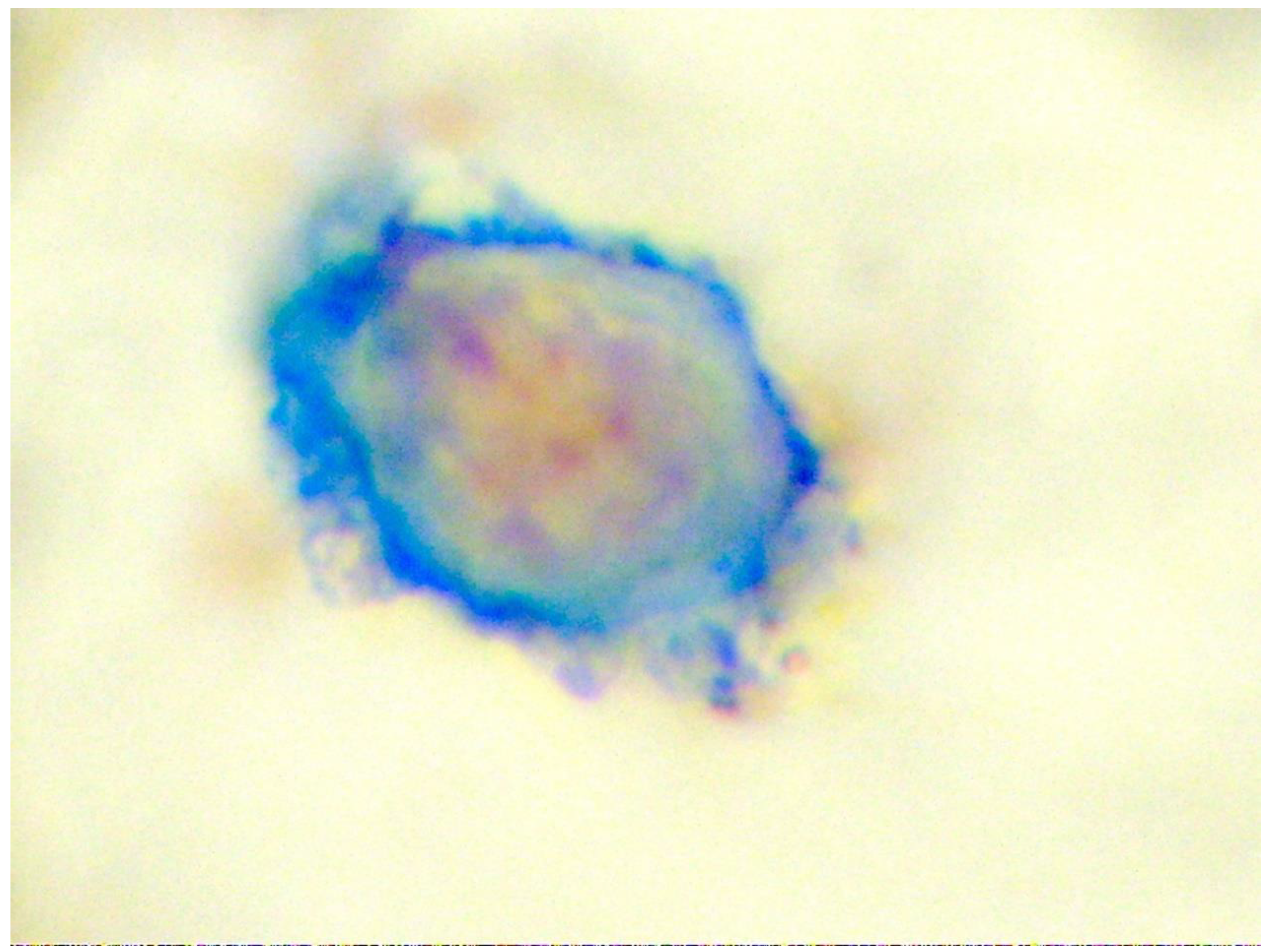
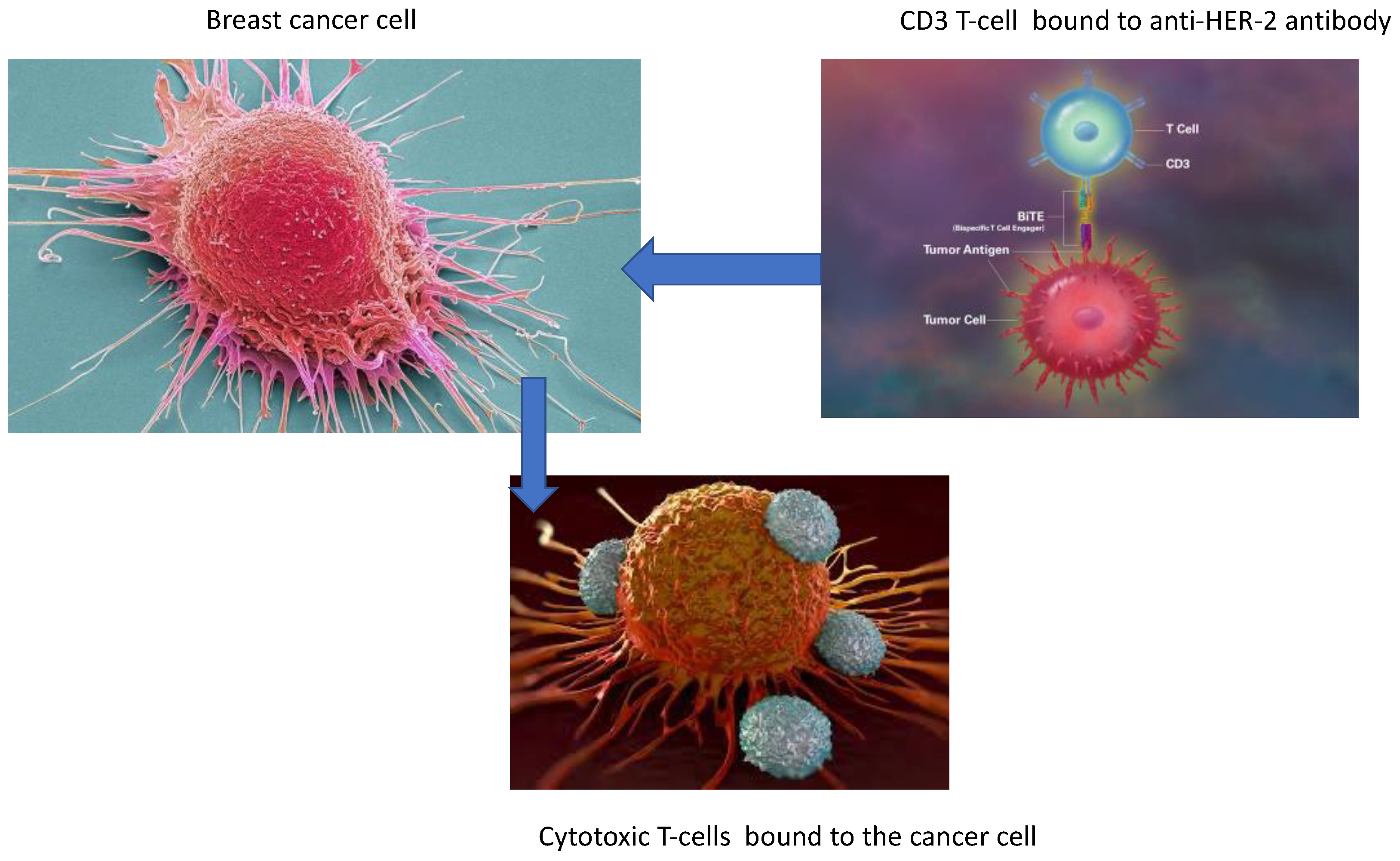
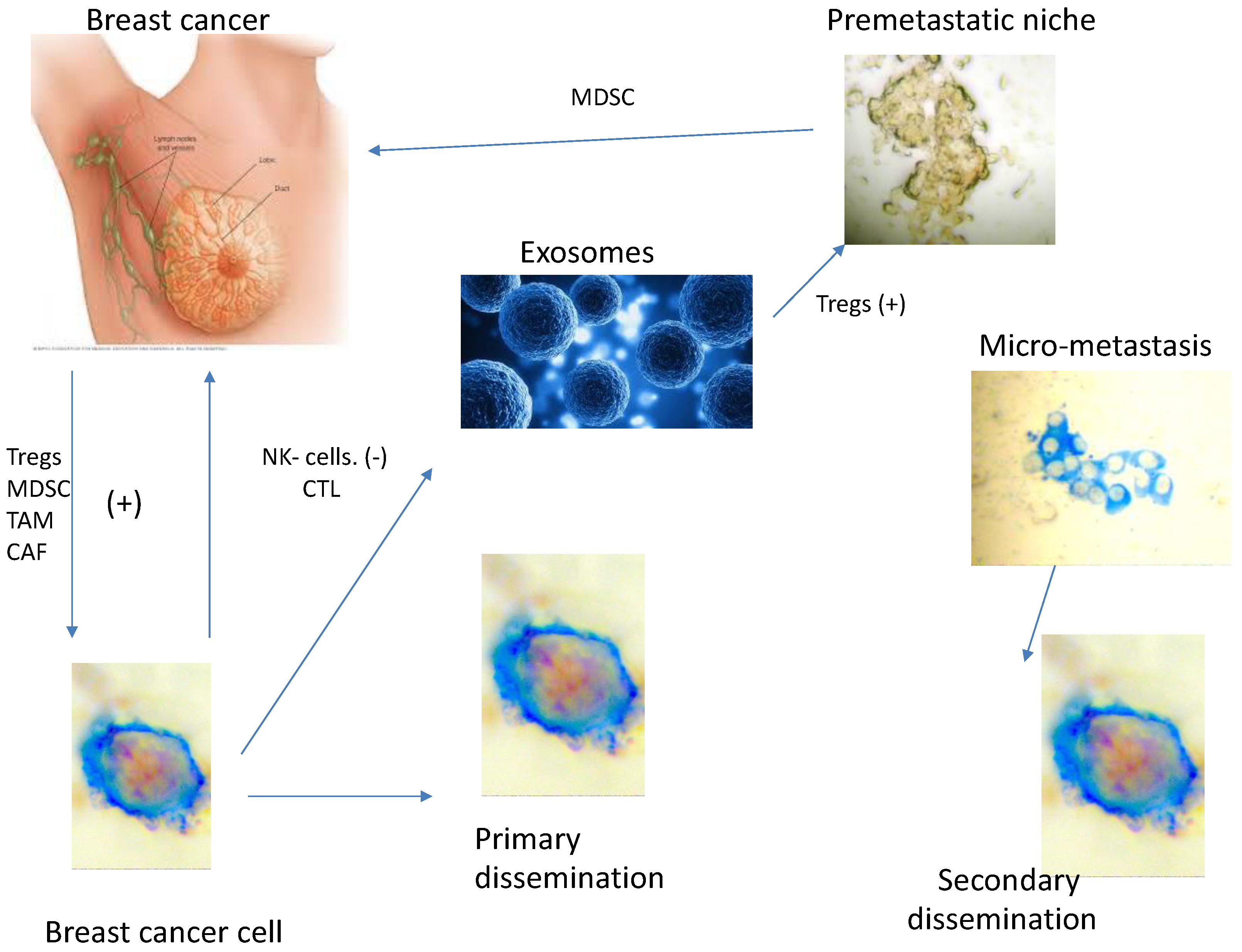

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



