GO Prepared
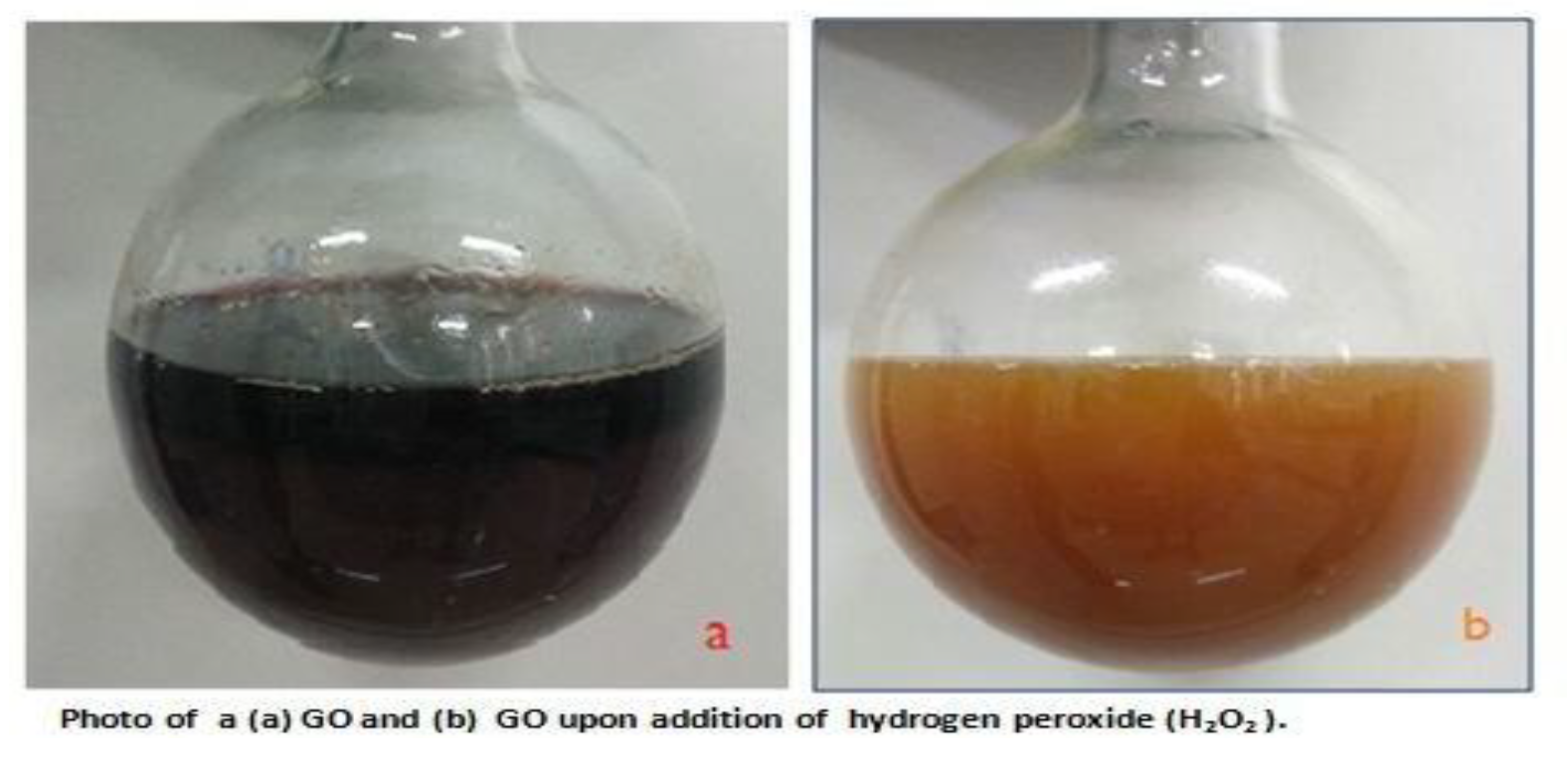
The photo
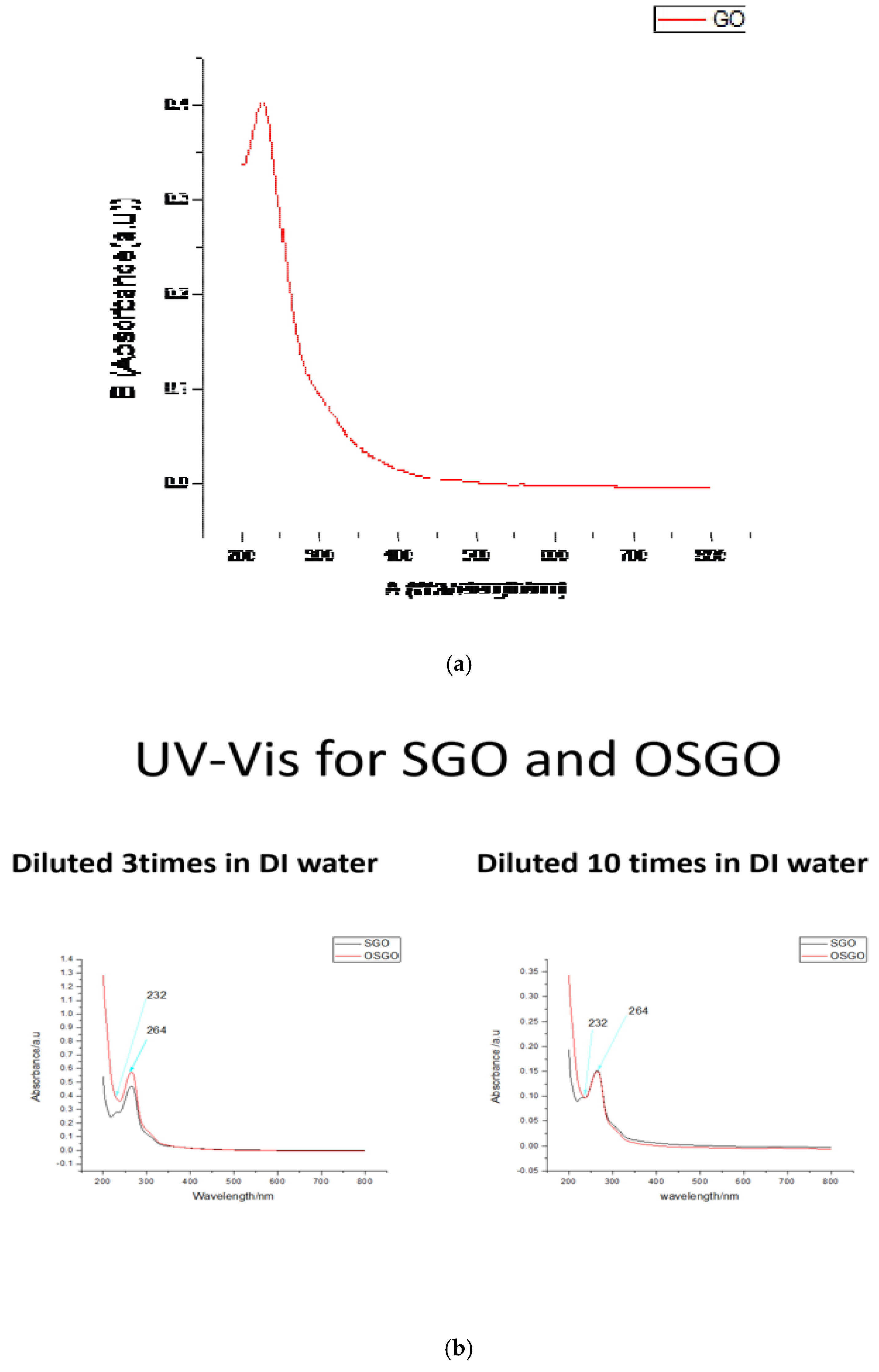
Figure 1a shows the graphene oxide which was prepared, a homogeneous solution with no precipitating particles at the bottom showing a fully conversion of graphite, the solution appears as a dark brown in colour, upon addition of hydrogen peroxide (
Figure 1b) the solution turned to brownish colour with no any particles precipitating, GO is highly hydrophilic, it attracted water upon drying, for storage the GO was suspended in DI water for later use
The UV-Vis of GO yields a distinct peak at ca.230nm, which has been assigned to various oxygen functionalities on the graphene-oxide surface[Gao, W., et al 2014] The UV-Vis for SGO and OSGO yields a peak at 232 and 264. After Ozonation a noticeable shift of
UV absorption were detected for SGO. Indicating a higher degree of oxidation for OSGO as compared to SGO.
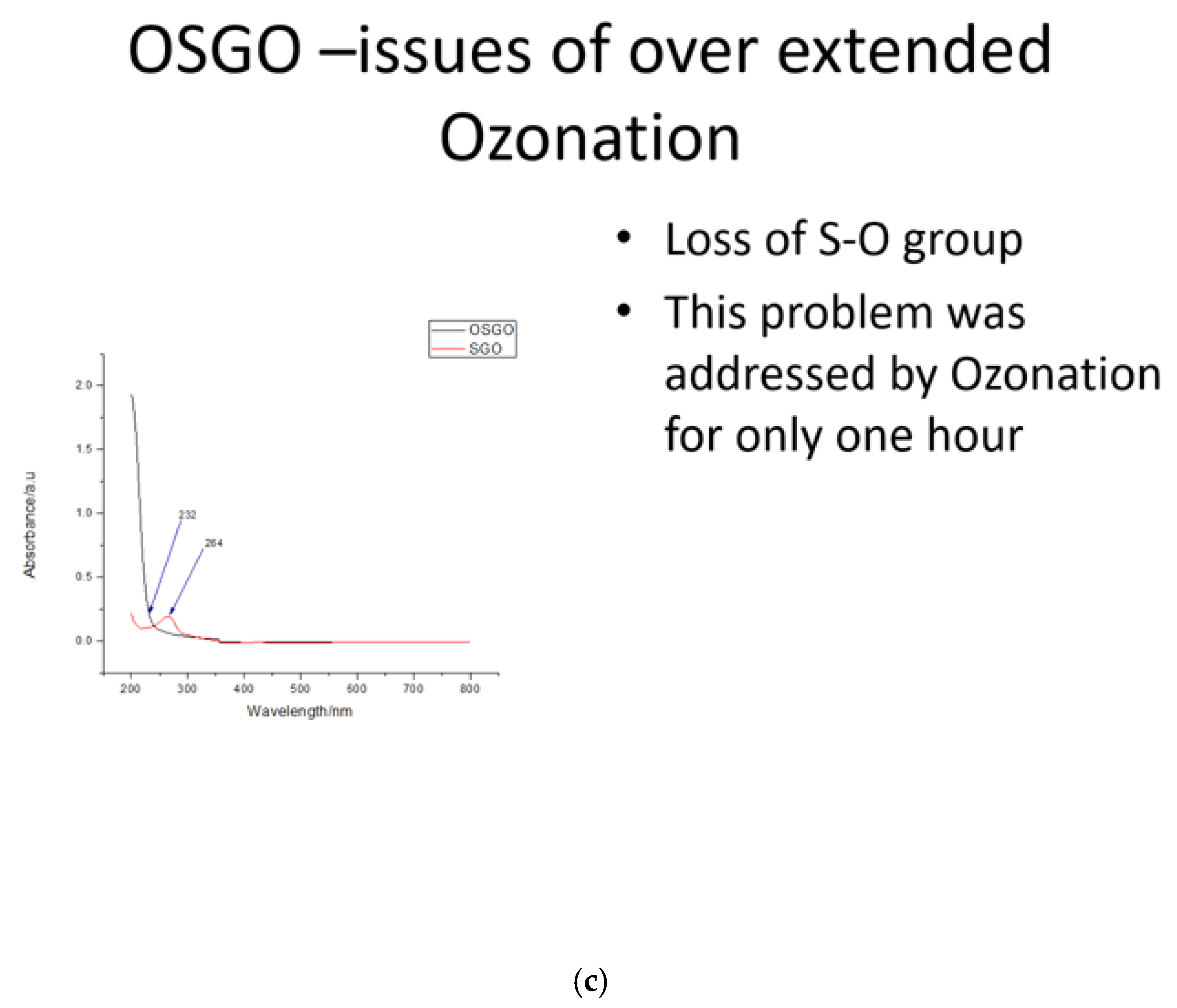
It was observed that there was a loss of S-O group (
Figure 2c)when the ozonation was extended for over 10 hours, hence for the best results it is recommended tp ozonate for one hour only.
Figure 2.
(a) UV-Vis for GO. (b) UV-Vis for SGO and OSGO. (c)
Figure 2.
(a) UV-Vis for GO. (b) UV-Vis for SGO and OSGO. (c)
Figure 3.
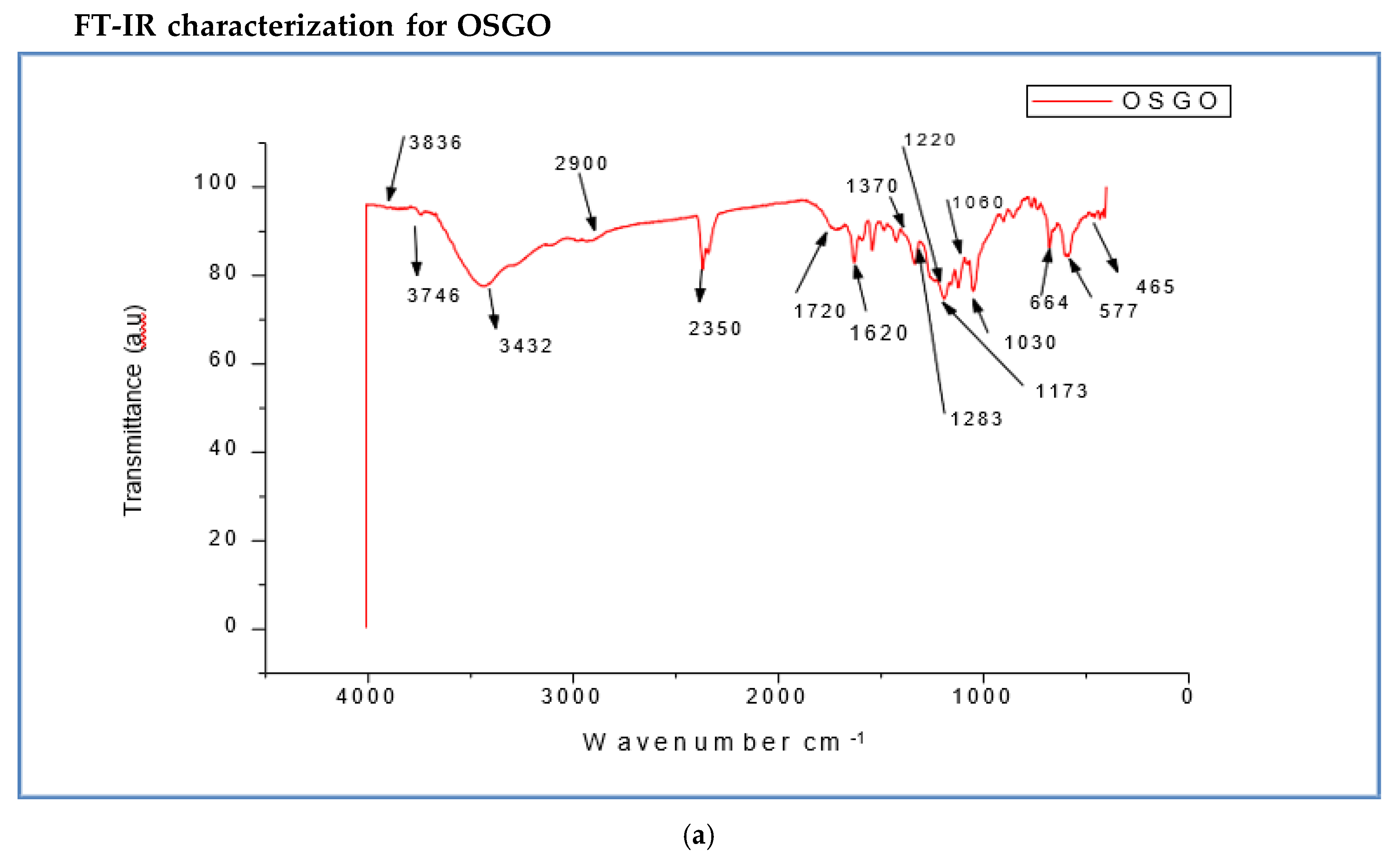
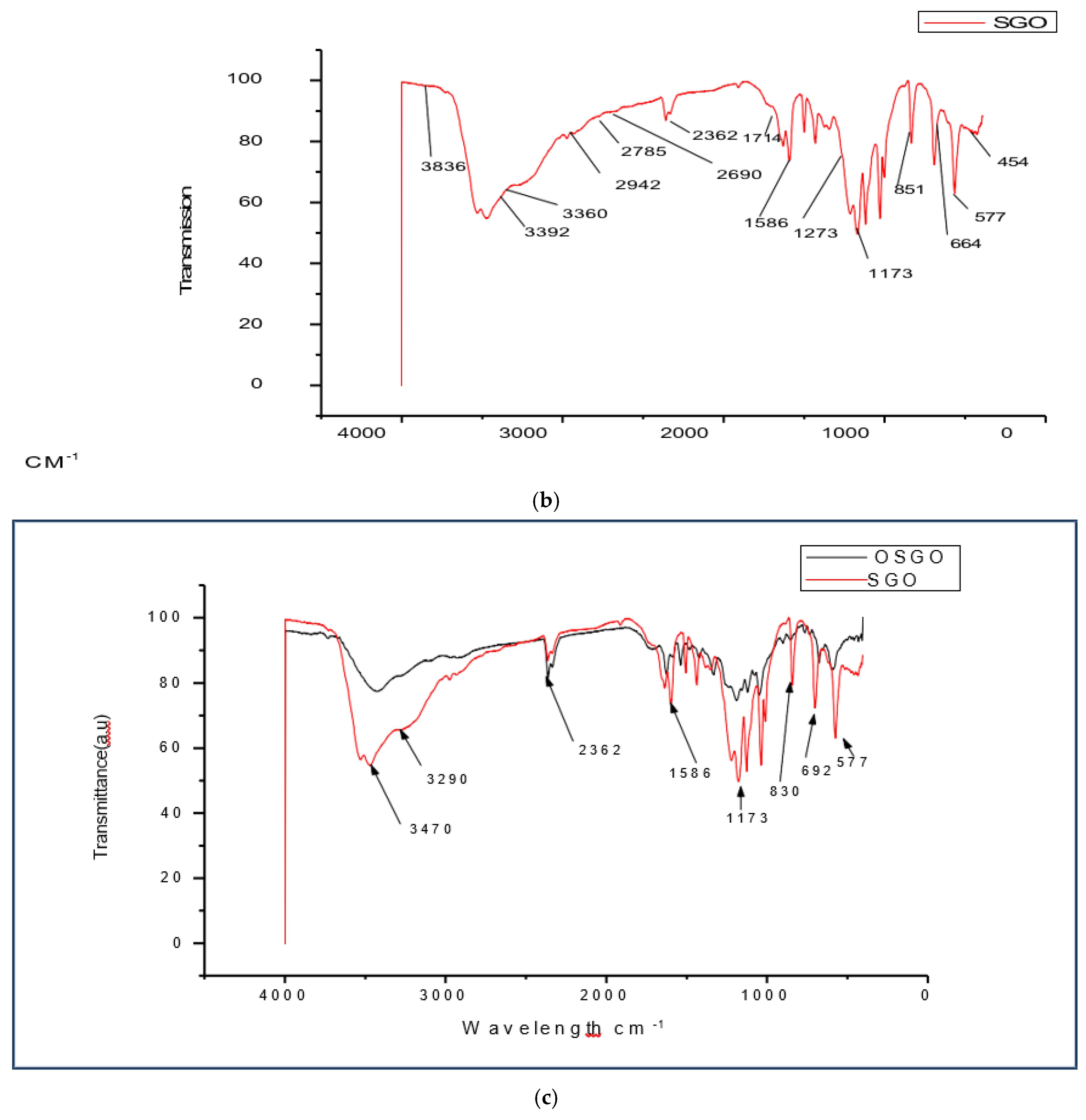
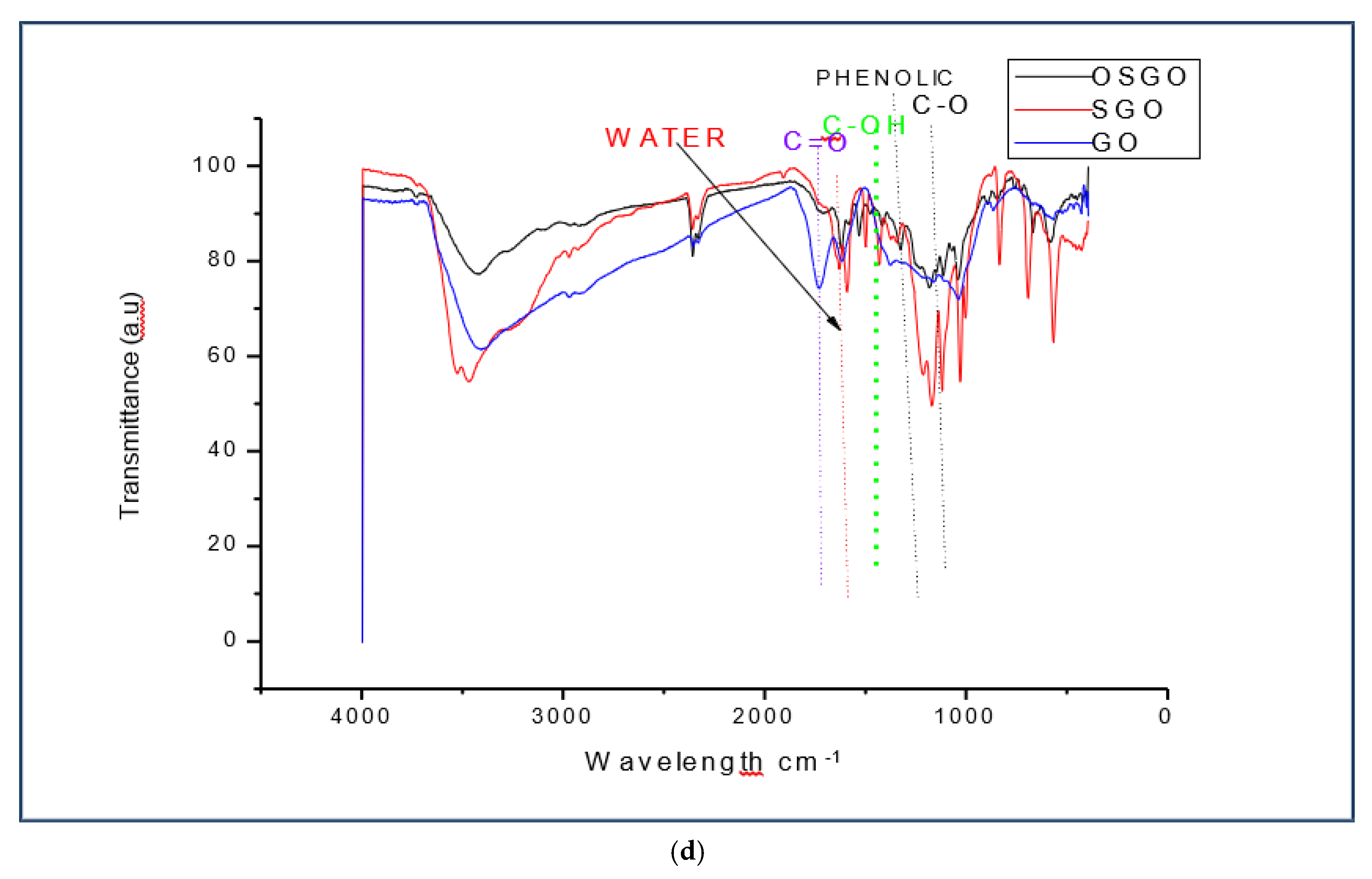
(a) FT-IR spectra for OSGO. (b) FT-IR spectra for SGO. (c) FT-IR spectra for OSGO and SGO. (d) FT-IR spectra for GO, SGO and OSGO.
Figure 3.
(a) FT-IR spectra for OSGO. (b) FT-IR spectra for SGO. (c) FT-IR spectra for OSGO and SGO. (d) FT-IR spectra for GO, SGO and OSGO.
The difference between FTIR spectra for GO and SGO/OSGO is additional of a new functional group 1173 which attributes the absorption of sulfonic acid group (-SO3H). The three spectra has overlapping bands centered around 1060 cm-1(C-O), 1220cm-1 (phenolic),1370cm-1 (O-H bending in tertiary alcohols), 1620 cm-1 (O-H bending in water), and 1720 cm-1( C=O) the stretching frequency around3432 cm-1 and 3360cm-1 (O-H hydrogen bond),3392cm-1, 3290cm-1,2942 cm-1,2785 cm-1,2690 cm-1, O-H stretching (acidic group). There is also a functional group around 2350 cm-1 and 2360 cm-1in SGO and OSGO which may be attributed to absorption of CO2. There is notable increased in transmittance (a.u.) in OSGO as compared to SGO and GO.
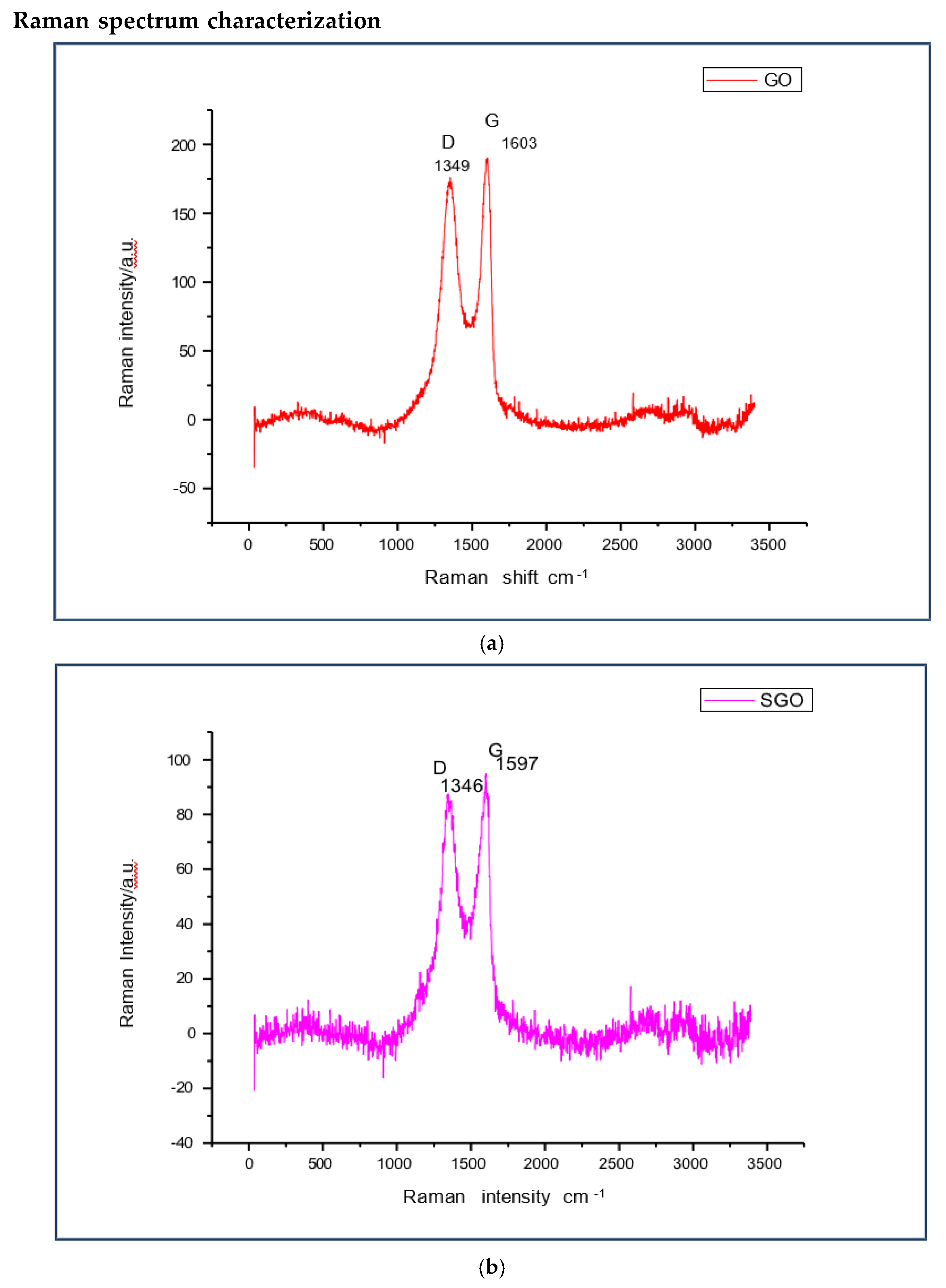
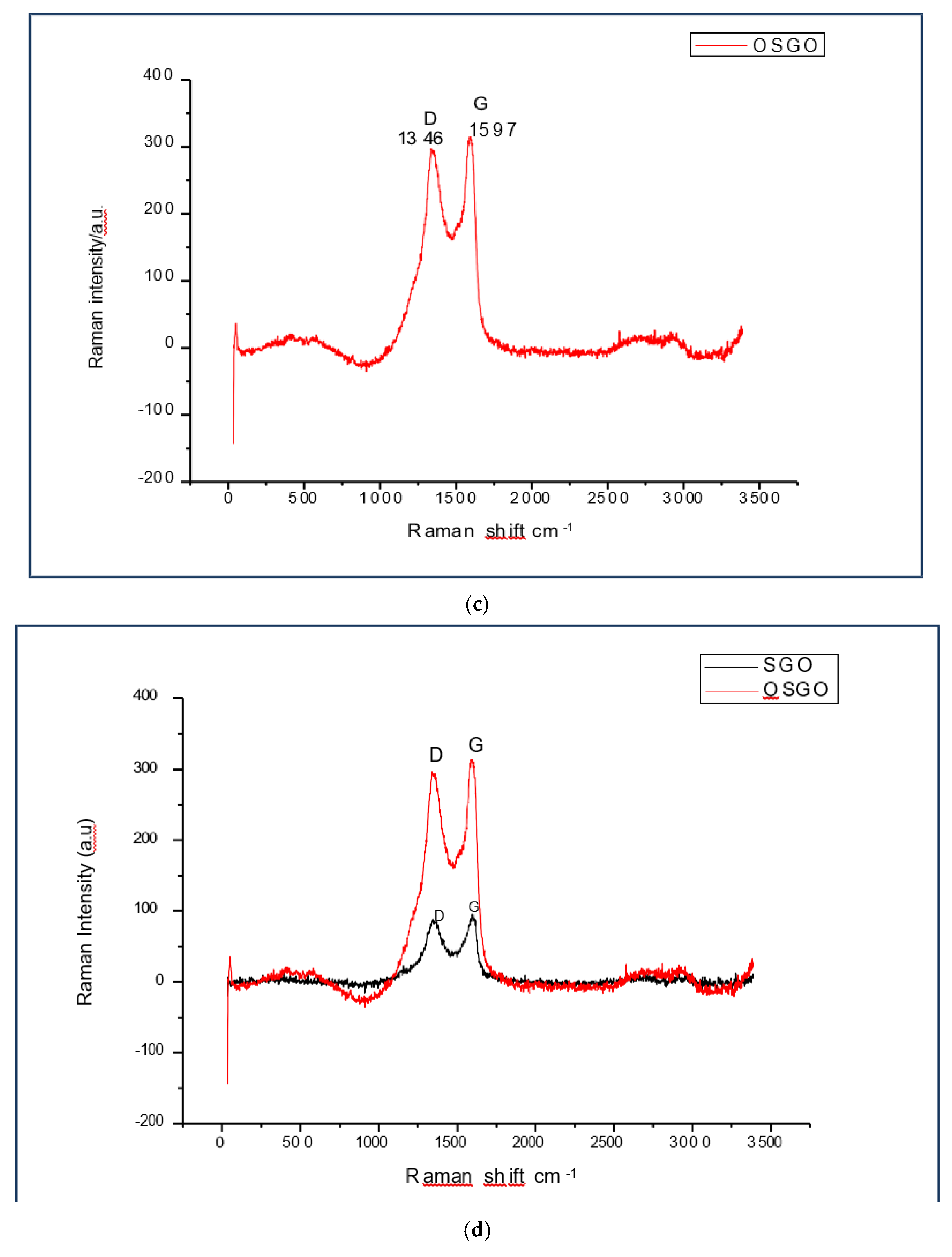
The Raman spectra shows the transformation of GO to SGO and SGO to OSGO. The ratio of the peak height ID/IG of GO(1349cm
-1,1603cm
-1),SGO (1346cm
-1,1597cm
- 1)increased significantly, while the peaks for both SGO and OSGO appears at 1346cm
-1 and 1597cm
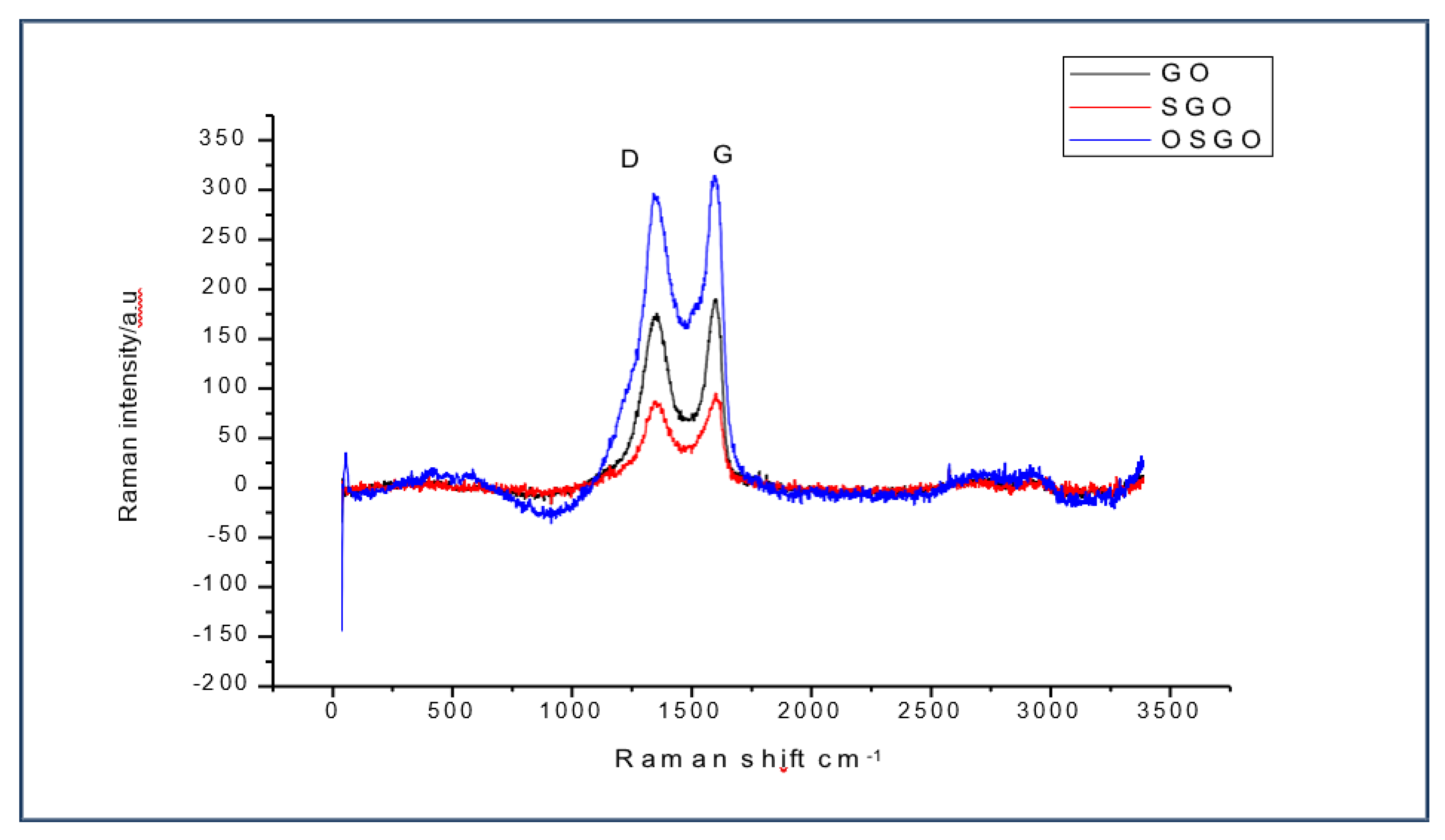
-1. There is increased Raman intensity in OSGO as compared to both SGO and GO
Figure 4d.
Figure 4.
(a) Raman spectra for GO, showing D peak at 1349 cm-1 and G peak at 1603 cm-1. (b) Raman spectra for SGO, showing D peak at 1346 cm-1 and G peak at 1597 cm-1. (c) Raman spectra for OSGO, showing D peak at 1346 cm-1 and G peak at 1597 cm-1, the peaks appear on the same as in SGO, with the difference only in Raman intensity (a.u). (d) Raman spectra for SGO and OSGO, showing D peak at 1346 cm-1 and G peak at 1597 cm-1, the peaks appear on the same as in SGO, with the difference only in Raman intensity (a.u).
Figure 4.
(a) Raman spectra for GO, showing D peak at 1349 cm-1 and G peak at 1603 cm-1. (b) Raman spectra for SGO, showing D peak at 1346 cm-1 and G peak at 1597 cm-1. (c) Raman spectra for OSGO, showing D peak at 1346 cm-1 and G peak at 1597 cm-1, the peaks appear on the same as in SGO, with the difference only in Raman intensity (a.u). (d) Raman spectra for SGO and OSGO, showing D peak at 1346 cm-1 and G peak at 1597 cm-1, the peaks appear on the same as in SGO, with the difference only in Raman intensity (a.u).
Figure 5.
(d) Raman spectra for GO, SGO and OSGO, showing the shift in raman intensity.
Figure 5.
(d) Raman spectra for GO, SGO and OSGO, showing the shift in raman intensity.
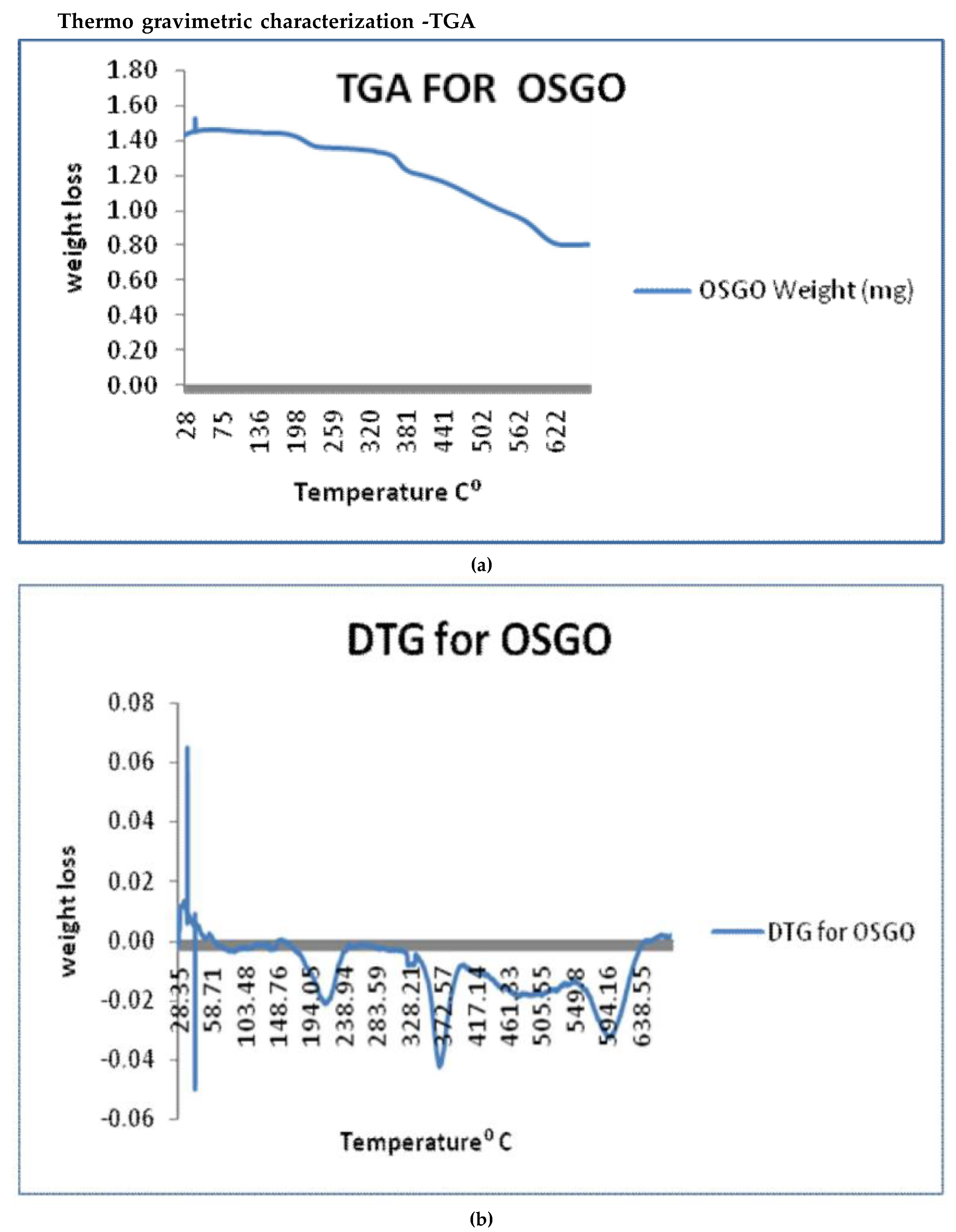
The TGA thermal curve was displayed a weight loss (mg) versus temperature. Based on the weight loss change as a function of temperature, each TGA curve can be divided into various regions, the first initial mass loss region occurred at 30°C (1.41 mg) to 150°C (1.40mg), from 150°C to 260°C (1.23mg). Region two which indicates major weight loss occurred from 260°C to around 340°C (0.81mg) the third region starts from 340 °C (0.81mg) to 490°C (0.66mg), another major weight loss up to temperature of 560°C (0.46mg), the last region stabilized up to 677°C (0.39mg). Weight loss below 100°C is attributed to the absorbed water. Loss of oxygen+containing functionalities such as CO and CO2 is the reason for the second weight loss between 150°C to 220°C. The weight loss at 258°C is attributed to decomposition of sulfonic acid group. Weight loss from 220°C to 400°C is due to weakening of van der Waals forces between the GO layers .
Figure 5b showing derivative of thermo gravimetric analysis, the DTG has various regions which agree with the TGA, with a major weight loss around 194⁰C, 372⁰C and 595⁰C.
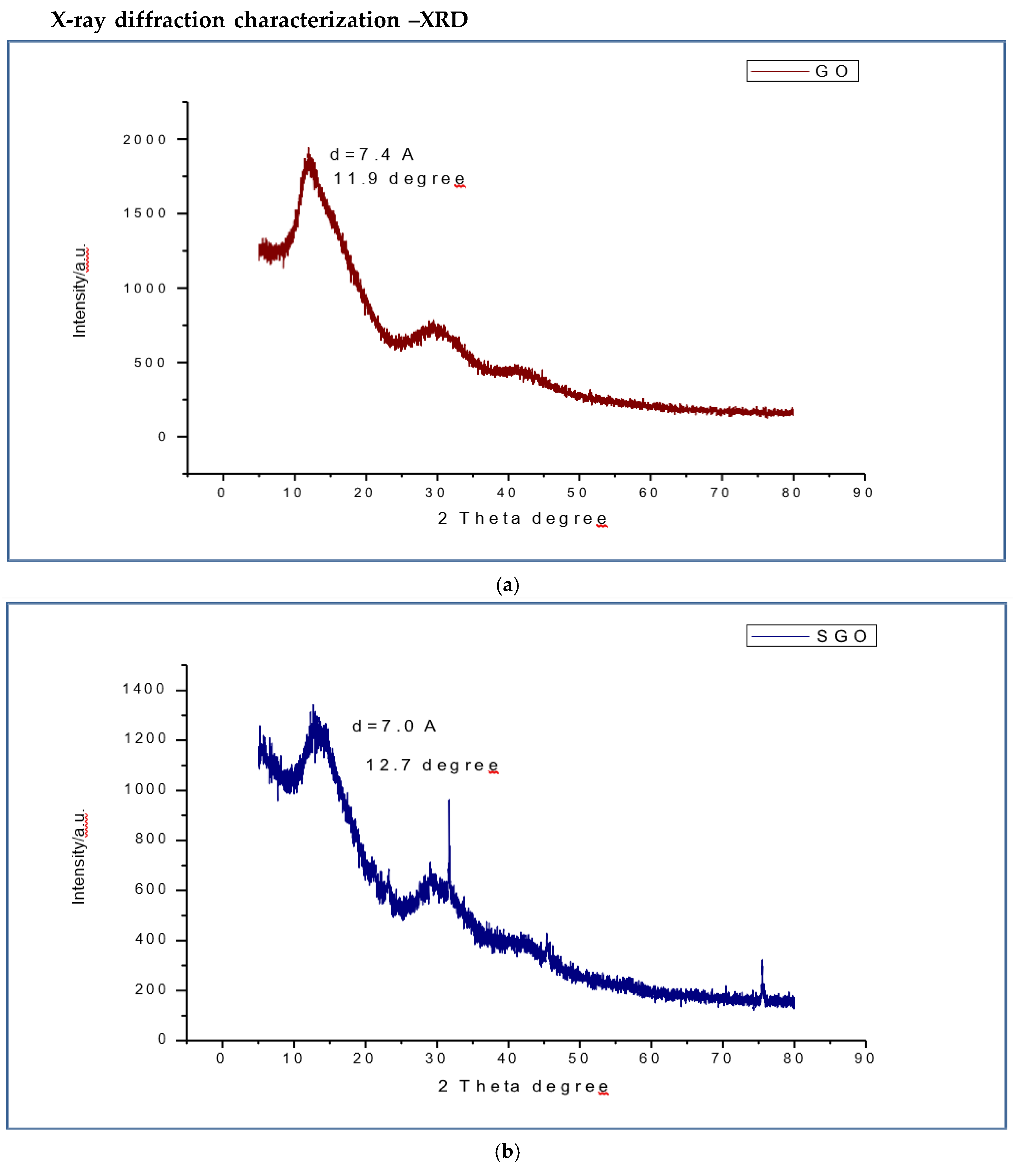
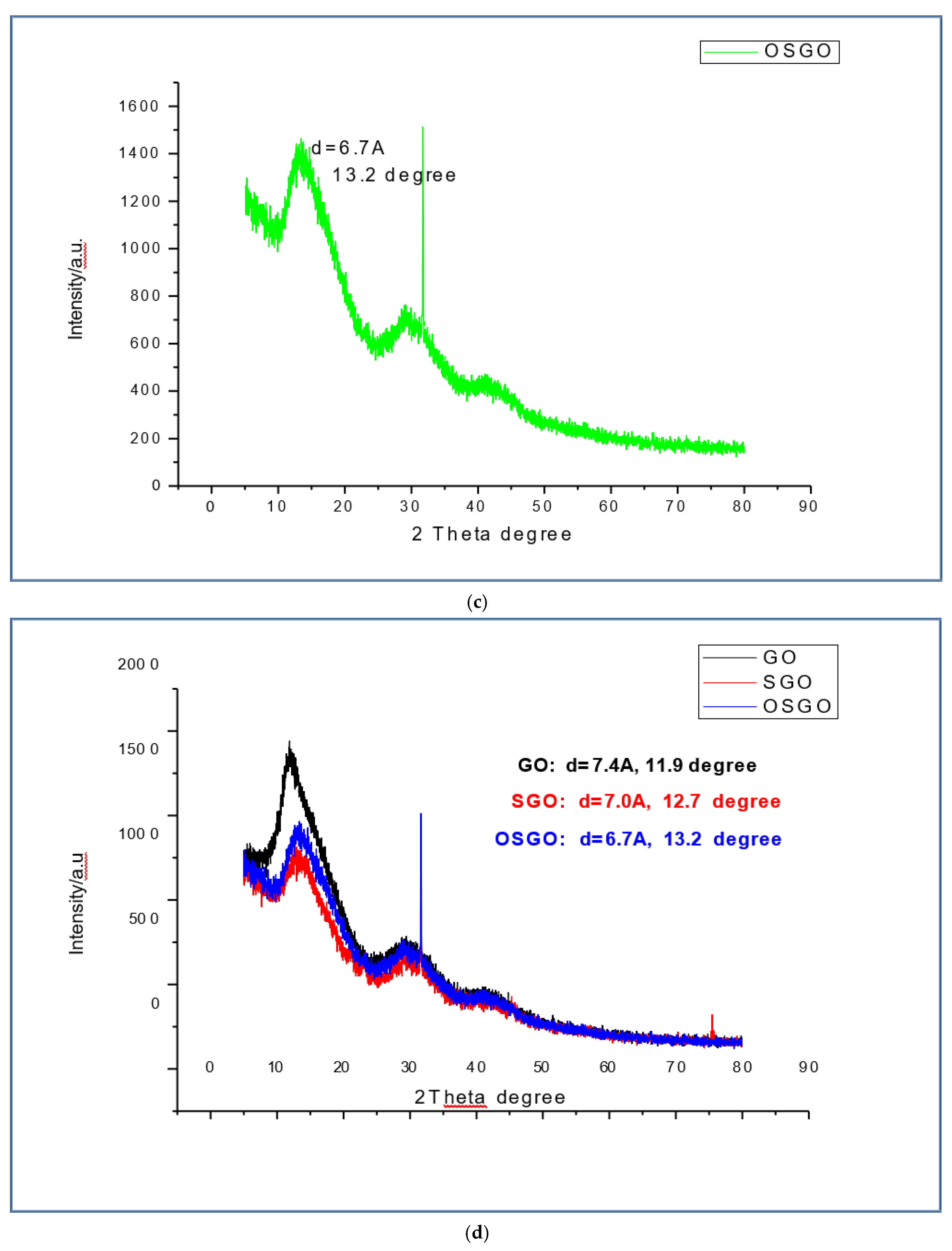
XRD for the GO(
Figure 6a) has the diffraction angle at 2 theta degree and interplanarspacing values as 11.9 degree and 7.4 Å respectively where as SGO(
Figure 6b) peak is observed at 12.7 degree and 7.0 Å, the OSGO (
Figure 6c)peak was at 13.2 degree and 6.7 Å. The interplanar spacing is caused by the partial restacking through partial restacking π−π interaction and the removal of oxygen functional groups after sulfornation [138] the shifting of SGO and OSGO film peak towards the higher 2 theta degree as compared to GO is due to addition of sulfonic acid group in SGO and OSGO (Ravikumar and K. Scottm 2012, Gahlot, S., et al., 2014) oxidation may have also contributed to shift towards 2 theta degree, a notable observation is decrease in interplanar spacing is noted in OSGO as compared to SGO and GO, where the OSGO is the least.
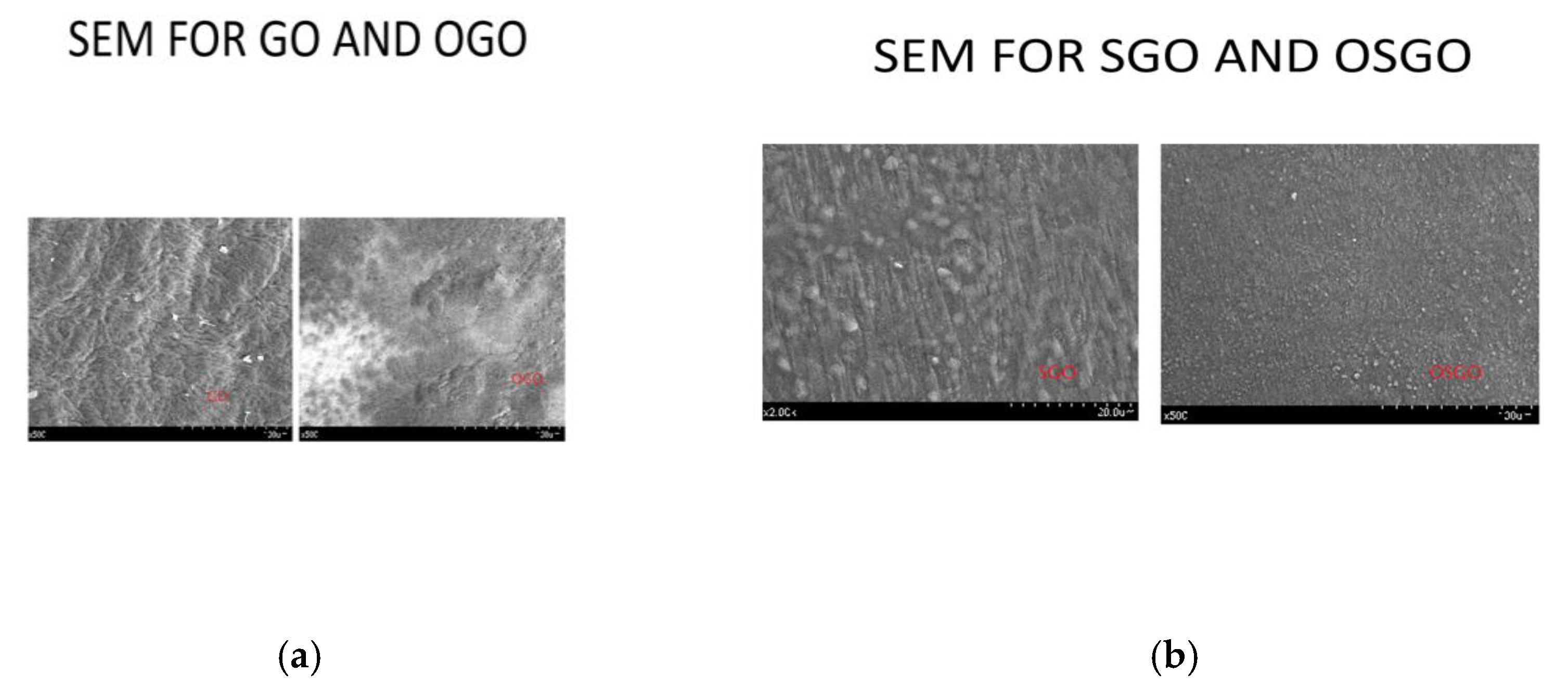
The scanning electron microscope images (
Figure 7 a,b) shows complex matrix with no pores on the surface which may be a good barrier against oxygen in proton exchange membranes