1. Introduction
Histologically, the adrenal medulla primarily comprises chromaffin cells, whereas smaller cell populations in the adrenal medulla consist of ganglion and sustentacular cells [
1]. Sustentaculoma of the adrenal medulla is an extremely rare tumor, first reported by Lau et al. in 2006 [
2]. It is characterized by differentiation into sustentacular cells and shows positivity for S-100 protein, CD56 (NCAM), and vimentin on immunohistochemical staining [
2].
We present a rare case of sustentaculoma occurring in the left adrenal gland of a young Japanese man in whom we performed histological, immunohistochemical, and ultrastructural examinations. The patient was followed for 16 years post-surgery without recurrence or metastasis and remains in good health. We present this case, accompanied by relevant literature for reference.
2. Detailed Case Description
During a routine check-up of a 39-year-old Japanese man, a mass was detected in his left adrenal gland by ultrasound. Thereafter, he visited the urology outpatient clinic at Shimada Municipal Hospital in Shimada, Shizuoka, Japan. His serum cortisol level was 7.5 μg/dL (normal range: 4.0–18.5 μg/dL); aldosterone, 70.0 pg/mL (normal range: 30–160 pg/mL); renin activity, 1.0 ng/mL/hr (normal range: 0.1–2.0 ng/mL/hr); epinephrine, 0.09 ng/mL (typical value: <0.1 ng/mL); and norepinephrine, 0.28 ng/mL (normal range: 0.1–0.5 ng/mL). No hormones showed abnormal elevation. There were no signs of hyperglycemia or hypertension, and his medical and family histories revealed no significant findings.
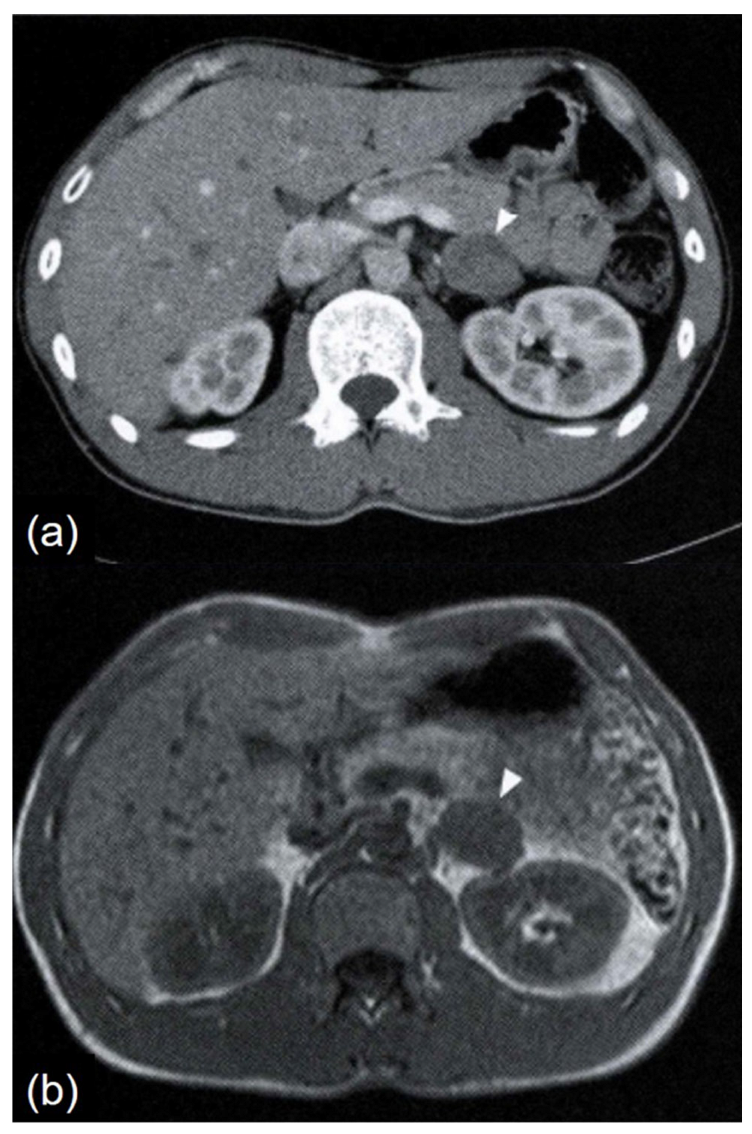
Abdominal computed tomography (CT) and magnetic resonance imaging (MRI) revealed a well-defined, smoothly bordered left adrenal tumor measuring 3.3 × 2.3 cm. The tumor appeared as a soft tissue density on plain CT, with no evidence of calcification. On contrast-enhanced CT, it showed slight heterogeneous enhancement (
Figure 1a). On T1-weighted MRI images, the tumor appeared iso-intense to skeletal muscle (
Figure 1b). It was mildly hyperintense compared to skeletal muscle on T2-weighted images, with no signal loss on out-of-phase imaging. No findings suggested fat content. Contrast-enhanced MRI revealed uniform moderate enhancement.
123I-metaiodobenzylguanidine scintigraphy showed no abnormal accumulation. Laparoscopic left adrenalectomy was subsequently performed.
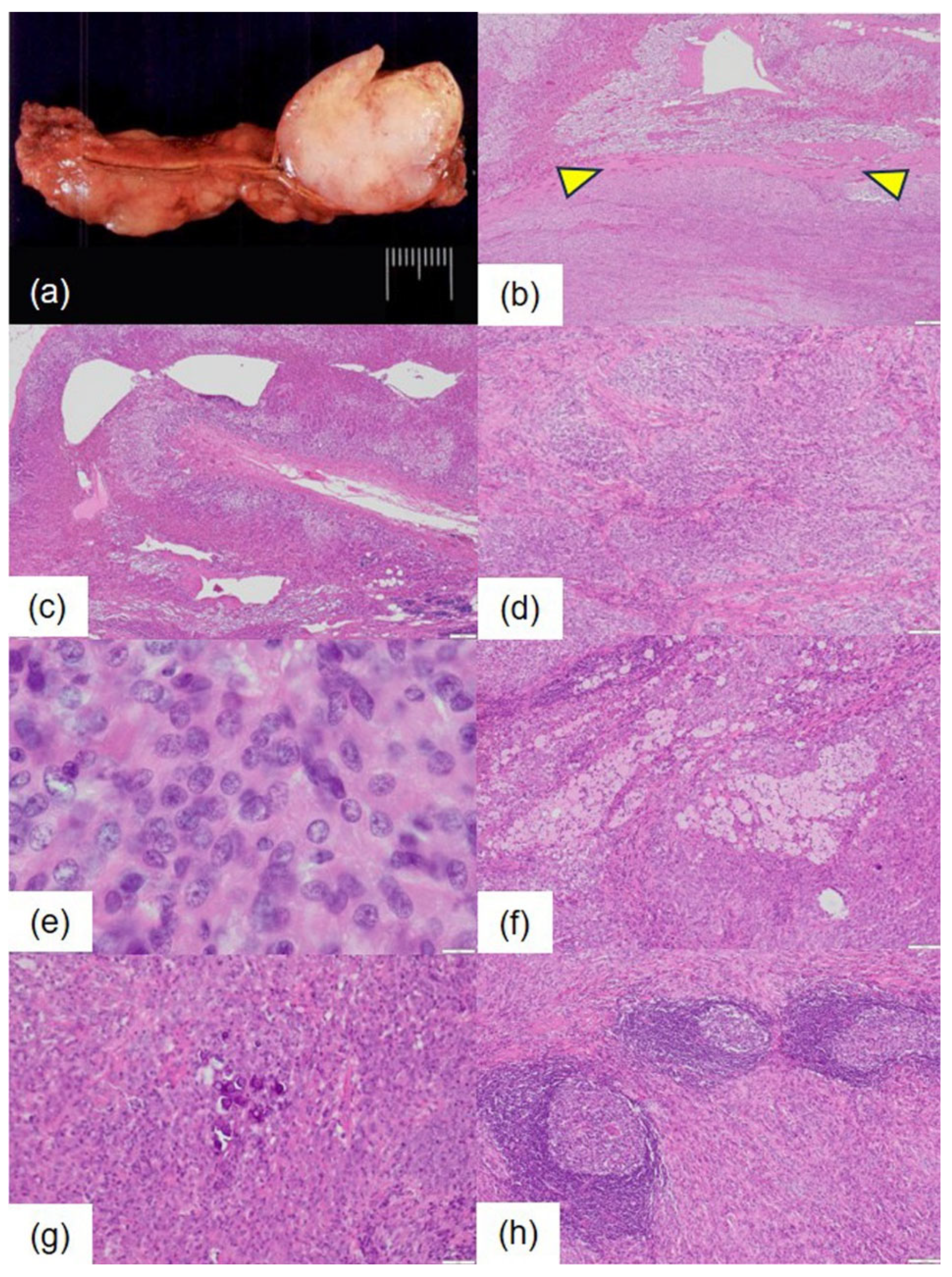
Macroscopically, the extracted left adrenal specimen measured 6.5 × 3.8 × 2.5 cm and weighed 33 g. A well-defined, solid mass measuring 3.5 × 2.7 × 2.5 cm was noted in the adrenal medulla. The cut surface of the tumor appeared pale, resembling fish flesh, with some yellowish areas present (
Figure 2a).
Microscopically, the tumor was well-demarcated from the uninvolved adrenal gland at low-power magnification and was encapsulated by a thin fibrous capsule (
Figure 2b). The non-tumorous adrenal tissue showed abundant lipids with no signs of atrophy or other changes (
Figure 2c).
Histologically, the tumor cells formed solid nests of varying sizes and irregular shapes, accompanied by a proliferation of collagen fibers in the stroma (
Figure 2d). No glandular, ductal, or papillary structures, nor Zellballen patterns, were observed. Additionally, no rosette-like or fascicular arrangements were noted. The tumor cells showed indistinct cell boundaries and eosinophilic cytoplasm (
Figure 2e). The nuclei were primarily round and uniform in size, but some displayed nuclear grooves. Cell density varied, revealing areas of high cellular density and focal nuclear stacking (
Figure 2e). No pleomorphic nuclei or atypical multinucleated cells were identified. Mitoses were rare, with zero to one mitotic figure observed per 10 high-power fields. The stroma contained occasional clusters of foam cells (
Figure 2f) and deposits of psammoma bodies (
Figure 2g). Lymphoid follicle formation was also noted, characterized by the infiltration of small lymphocytes and plasma cells, with Russell bodies in some areas (
Figure 2h). No hemorrhage or necrosis was detected.
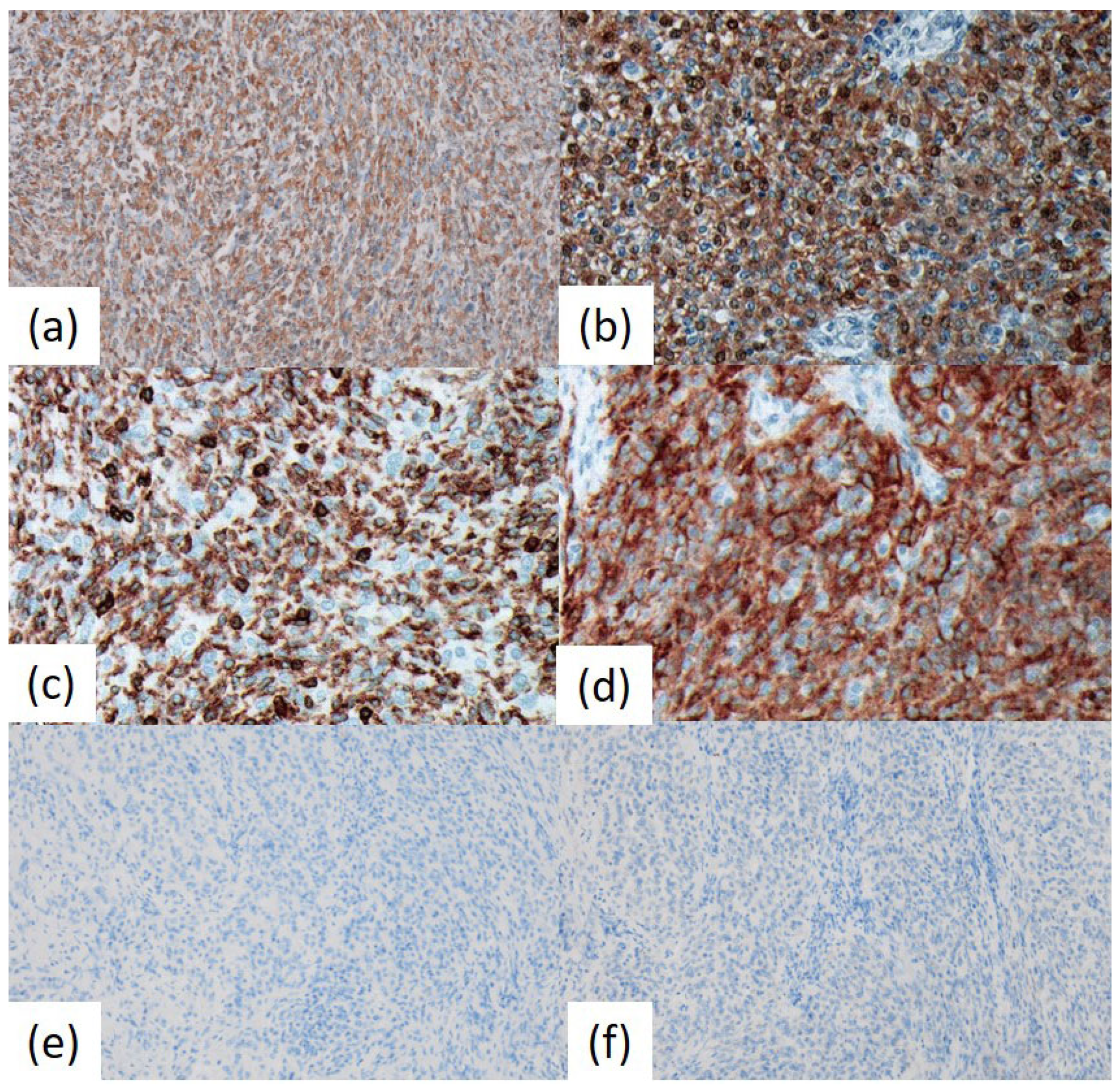
Immunohistochemical analysis revealed that the tumor cells were positive for vimentin, S-100 protein, and CD56 (
Figure 3). Approximately half of the tumor cells exhibited positivity for CD45 (LCA; B220; T200), whereas about 30% were positive for CD68 (KP-1). A few tumor cells were positive for EMA. In contrast, chromogranin A (CGA), synaptophysin, melanosome (HMB-45), glial fibrillary acidic protein (GFAP), TTF-1 (8G7G3/1), podoplanin (D2-40), CD20, CD3, CD79a, CD138, CD21, CD35, and keratin (AE1/AE3) showed negative results. The Ki-67 (MIB1) labeling index was 1.0%.
As previously reported [
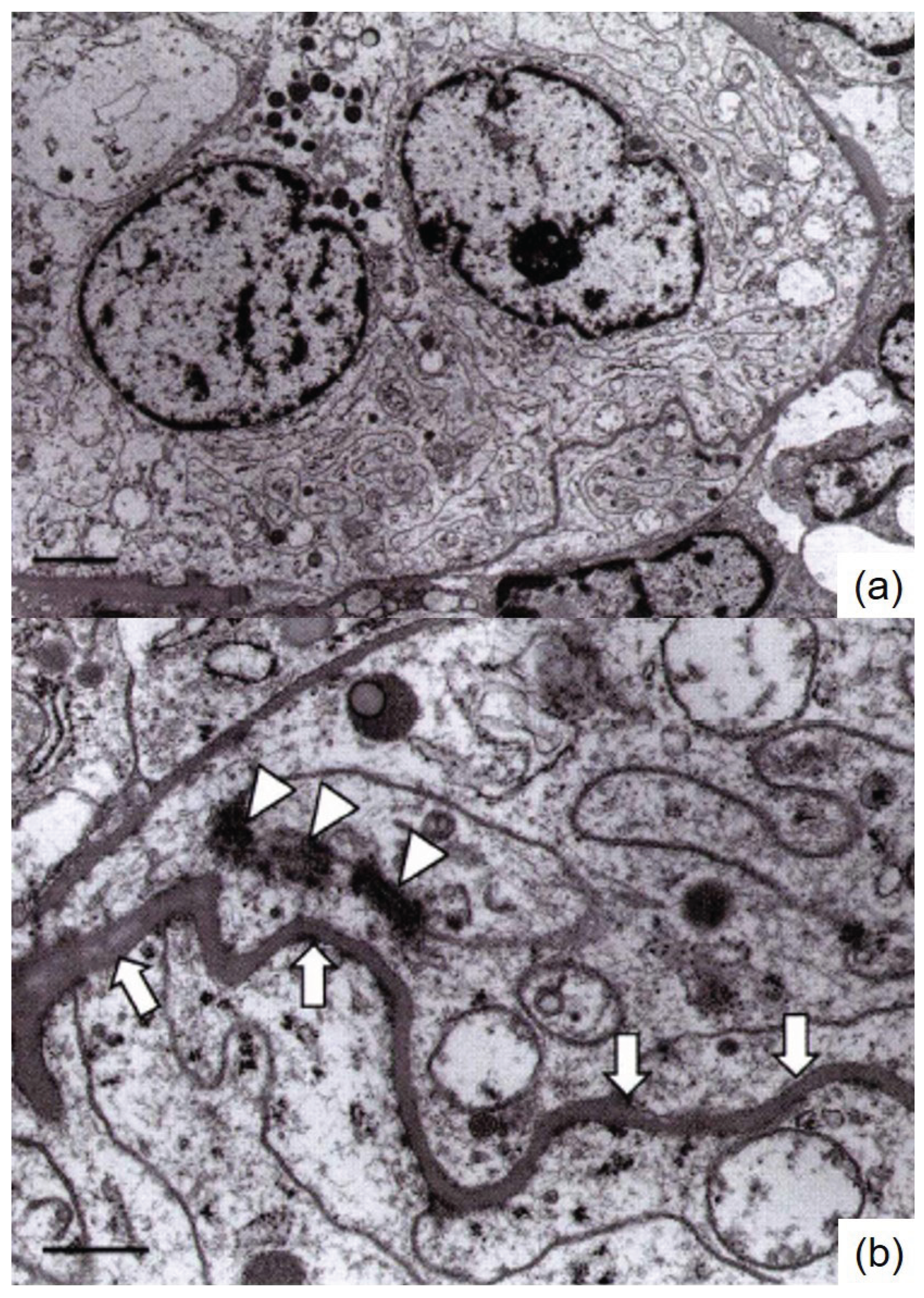
3], routine paraffin blocks were processed for ultrastructural observation, and an ultrastructural analysis was conducted. The tumor cells showed numerous spherical mitochondria, rough endoplasmic reticulum, and finger-like projections known as pseudomesaxons. A few desmosome-like structures were noted among the tumor cells. Neither chromaffin granules nor secretory granules were identified (
Figure 4).
Based on these findings, the diagnosis of primary adrenal medullary sustentaculoma was made. As of 16 years post-surgery, after which time contact with the patient was lost, no recurrence or metastasis was observed. Whether the patient is still under outpatient follow-up is unknown.
3. Discussion
The sustentacular cells of the adrenal medulla are spindle-shaped or stellate cells that test positive for S-100 protein [
1]. These cells originate from the neural crest, with a small number present in normal tissues [
2], and support the chromaffin cells of the adrenal medulla and the chief cells of the paraganglia.
However, they cannot be identified in H&E-stained specimens, making S-100 protein immunostaining necessary for their identification [
1,
2]. In addition to S-100 protein, these cells also strongly express vimentin and CD56. A tumor indicating differentiation into adrenal-derived sustentacular cells, termed sustentaculoma, was reported by Lau et al. in 2006 [
2].
Furthermore, sustentacular cells are recognized as components of tumors such as pheochromocytoma, neuroblastoma, ganglioneuroblastoma, and ganglioneuroma [
2,
4]. Differential diagnoses for sustentaculoma include pheochromocytoma and cellular schwannoma. Both sustentaculoma and pheochromocytoma are vimentin positive; however, sustentaculoma does not exhibit the Zellballen pattern. It is S-100 protein positive and CGA negative, and ultrastructurally, it presents pseudomesaxons while lacking chromaffin or secretory granules, thus distinguishing it from pheochromocytoma (
Table 1).
CGA, chromogranin A; GFAP, glial fibrillary acidic protein.
Both sustentaculoma and cellular schwannoma are positive for S-100 protein, vimentin, and CD56 but negative for CGA. Pseudomesaxons are observed under electron microscopy. However, in sustentaculoma, the Antoni A-type tissue pattern found in schwannoma and the basal lamina formed between individual tumor cells are absent, distinguishing it from cellular schwannoma. Therefore, it is possible to histologically differentiate sustentaculoma from both pheochromocytoma and cellular schwannoma (
Table 1).
Although extremely rare, the differential diagnosis also includes perineurioma. Adrenal primary perineurioma is an uncommon condition, first reported by Rampisela and Donner in 2009 [
5]. It is distinguished by a distinctive yellow-brown cut surface color and the histological presence of elongated, spindle-shaped cells along with negative S-100 protein results on immunohistochemical staining. Furthermore, electron microscopy reveals numerous pinocytic vesicles, setting this case apart.
In the Lau et al. case [
2], the tumor was CD45 negative. Furthermore, our investigation has revealed no reports of sustentacular cells being CD45 positive. However, approximately half of the tumor in the present case showed CD45 positivity. At this time, the significance of this positive finding remains unclear, and further accumulation of cases is necessary.
Ultrastructurally, numerous pseudomesaxons, or finger-like projections, were observed in the intertwined tumor cells. This finding was also reported in the Lau et al. study [
2]. It is believed that this observation indicates that sustentaculoma shows Schwann cell-like differentiation. As the macroscopic, histological, immunohistochemical, and ultrastructural findings in the present patient closely resemble those reported by Lau et al. in 2006 [
2], we thus believe these findings support the diagnosis of sustentaculoma.
This study has significant limitations as it is based on a single case report from one institution in Japan. Additional clinicopathological analyses, including multicenter studies, are necessary to clarify the etiology and pathophysiology of this rare condition. Making definitive statements about prognosis based solely on this case would be difficult. Reports from other countries, cultures, and institutions are anticipated.
4. Conclusions
We present a very rare case of primary adrenal medullary sustentaculoma. The tumor showed well-defined borders, and no cellular atypia indicative of malignancy was observed, and the Ki-67 labeling index was low. Furthermore, 16 years after surgery, no recurrence or metastasis has been noted. The CD45-positive finding in this case is unique as it is the first reported case in Japan and, to our knowledge, the second reported worldwide. Although further accumulation of cases is necessary, we believe it is highly likely that this is a benign tumor.
Author Contributions
Conceptualization, M.T.; Validation, M.T. and S.F.; Investigation, M.T. and S.F.; Resources, M.T.; Data Curation, M.T.; Writing—Original Draft Preparation, S.F.; Writing—Review and Editing, S.F.; Supervision, S.F.; Project Administration, M.T. and S.F. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki (1975) and was approved by the Ethics Committee of Shimada General Medical Center (protocol code: R6-9; date of approval: March 19, 2025).
Informed Consent Statement
Informed consent was not obtained from the patient included in this study because the surgery was performed 16 years ago, and he is no longer in contact with us.
Consent for Publication: Consent for publication was not obtained from the patient in this study because the surgery was performed 16 years ago, and we can no longer contact the patient.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Naoki Ooishi and Mizuki Naruse (Division of Pathology and Oral Pathology, Shimada General Medical Center, Shimada, Shizuoka, Japan) for helpful technical support with the immunohistochemical staining; Kohei Watanabe (Special Reference Laboratories, Hamura, Tokyo, Japan) for his skillful technical assistance with the electron microscopic examination; and Hiroko Tina Tajima (St. Marianna University School of Medicine, Kawasaki, Kanagawa, Japan) for kindly reviewing and editing the English manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CT |
Computed tomography |
| MRI |
Magnetic resonance image |
| CGA |
Chromogranin A |
| GFAP |
Glial fibrillary acidic protein |
References
- Carney, J.A. Adrenal. In: Histology for Pathologists, 5th ed.; Mills S.E., Ed.; Wolters Kluwer: Philadelphia, USA, 2020; pp. 1235–1242.
- Lau, S.K.; Romansky, S.G.; Weiss, L.M. Sustentaculoma report of a case of a distinctive neoplasm of the adrenal medulla. Am. J. Surg. Pathol. 2006, 30, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, Y. Electron microscopic study using formalin-fixed, paraffin-embedded material, with special reference to observation of microbial organisms and endocrine granules. Acta Histochem. Cytochem. 2018, 51, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Mete, O.; Asa, S.L.; Gill, A.J.; Kimura, N.; de Krijger, RR.; Tischler, A. Overview of the 2022 WHO Classification of Paragangliomas and Pheochromocytomas. Endocr. Pathol. 2022, 33, 90–114. [Google Scholar] [CrossRef]
- Rampisela, D.; Donner, L.R. Perineurioma of the adrenal gland. Ultrastruct. Pathol. 2009, 33, 165–168. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).