Submitted:
10 August 2025
Posted:
13 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
2.1. Data Source
2.2. Cohort Definition and Selection Criteria
2.3. Outcome Variables (End Points)
2.4. Statistical Analysis
2.5. Ethical Consideration
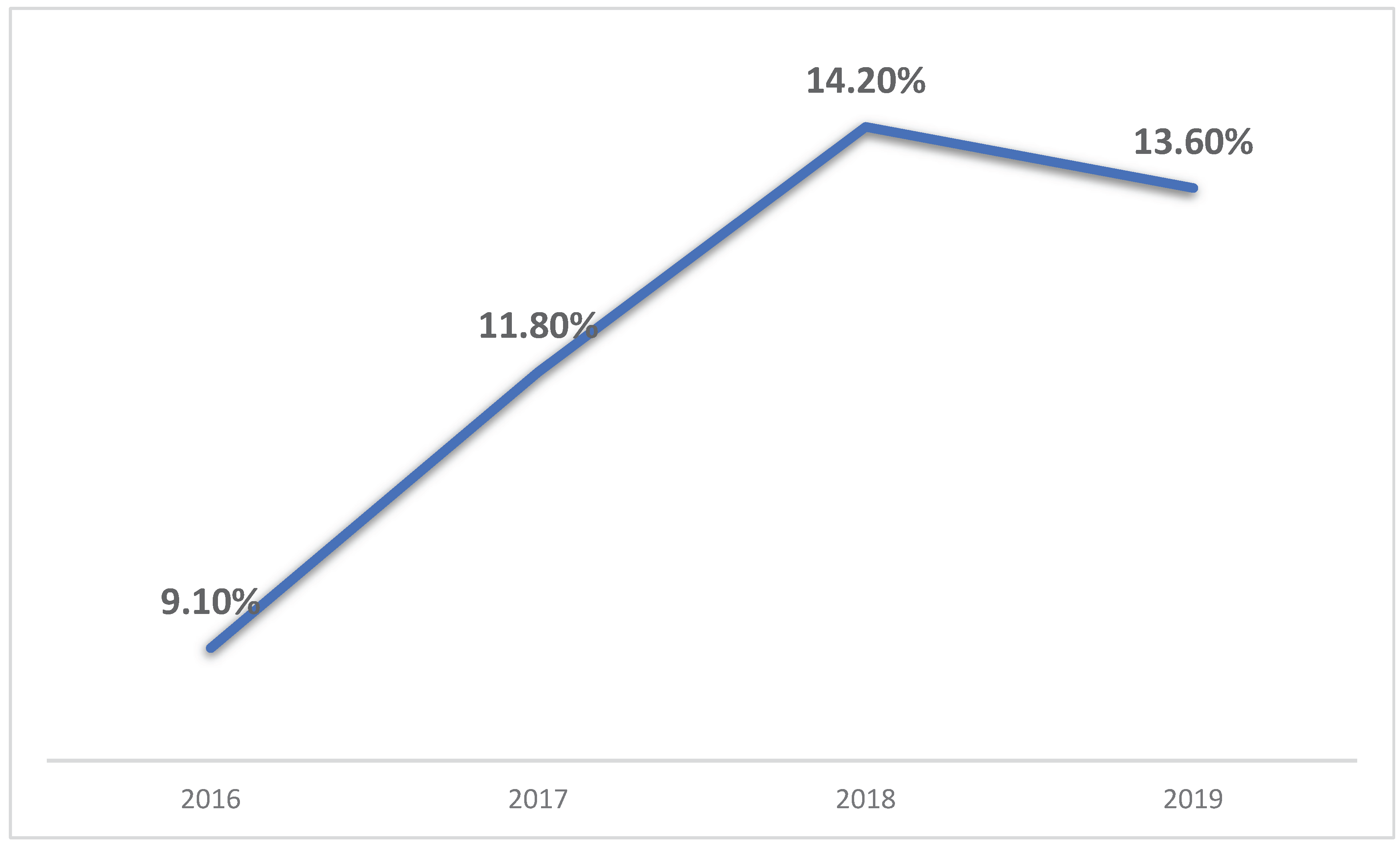
3. Results
4. Discussion
5. Conclusions
List of Abbreviations (A-Z)
| ACDF: | Anterior Cervical Discetomy and Fusion |
| CDA: | Cervical Disc Arthroplasty |
| HCUP: | Healthcare Cost and Utilization Project |
| ICD-10: | International Classification of Diseases, 10th Revision |
| NIS: | Nationwide Inpatient Sample |
| SPSS: | Statistical Package for the Social Sciences |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safiri S, Kolahi AA, Hoy D, et al. Global, regional, and national burden of neck pain in the general population, 1990-2017: systematic analysis of the Global Burden of Disease Study 2017. BMJ. Published online March 26, 2020:m791.
- Yang L, Chen J, Yang C, et al. Cervical Intervertebral Disc Degeneration Contributes to Dizziness: A Clinical and Immunohistochemical Study. World Neurosurgery. 2018;119:e686-e693. [CrossRef]
- Cohen SP, Hooten WM. Advances in the diagnosis and management of neck pain. BMJ. Published online August 14, 2017:j3221. [CrossRef]
- Mesregah MK, Repajic M, Mgbam P, Fresquez Z, Wang JC, Buser Z. Trends and patterns of cervical degenerative disc disease: an analysis of magnetic resonance imaging of 1300 symptomatic patients. Eur Spine J. 2022;31(10):2675-2683. [CrossRef]
- Brinjikji W, Luetmer PH, Comstock B, et al. Systematic Literature Review of Imaging Features of Spinal Degeneration in Asymptomatic Populations. AJNR Am J Neuroradiol. 2015;36(4):811-816. [CrossRef]
- Suzuki A, Daubs MD, Hayashi T, et al. Patterns of Cervical Disc Degeneration: Analysis of Magnetic Resonance Imaging of Over 1000 Symptomatic Subjects. Global Spine Journal. 2018;8(3):254-259. [CrossRef]
- Fejer R, Kyvik KO, Hartvigsen J. The prevalence of neck pain in the world population: a systematic critical review of the literature. Eur Spine J. 2006;15(6):834-848. [CrossRef]
- Genebra CVDS, Maciel NM, Bento TPF, Simeão SFAP, Vitta AD. Prevalence and factors associated with neck pain: a population-based study. Brazilian Journal of Physical Therapy. 2017;21(4):274-280. [CrossRef]
- Woods BI, Hilibrand AS. Cervical Radiculopathy: Epidemiology, Etiology, Diagnosis, and Treatment. Journal of Spinal Disorders & Techniques. 2015;28(5):E251-E259.
- Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine. 2015;40(12):E675-E693.
- Northover JR, Wild JB, Braybrooke J, Blanco J. The epidemiology of cervical spondylotic myelopathy. Skeletal Radiol. 2012;41(12):1543-1546.
- Ostrov PB, Reddy AK, Ryoo JS, Behbahani M, Mehta AI. Anterior Cervical Discectomy and Fusion Versus Cervical Disc Arthroplasty: A Comparison of National Trends and Outcomes. World Neurosurgery. 2022;160:e96-e110. [CrossRef]
- Sasso WR, Smucker JD, Sasso MP, Sasso RC. Long-term Clinical Outcomes of Cervical Disc Arthroplasty: A Prospective, Randomized, Controlled Trial. Spine. 2017;42(4):209-216.
- Murrey D, Janssen M, Delamarter R, et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. The Spine Journal. 2009;9(4):275-286. [CrossRef]
- Lavelle WF, Riew KD, Levi AD, Florman JE. Ten-year Outcomes of Cervical Disc Replacement With the BRYAN Cervical Disc: Results From a Prospective, Randomized, Controlled Clinical Trial. Spine. 2019;44(9):601-608.
- Burkus JK, Traynelis VC, Haid RW, Mummaneni PV. Clinical and radiographic analysis of an artificial cervical disc: 7-year follow-up from the Prestige prospective randomized controlled clinical trial: Clinical article. SPI. 2014;21(4):516-528. [CrossRef]
- Hisey MS, Zigler JE, Jackson R, et al. Prospective, Randomized Comparison of One-level Mobi-C Cervical Total Disc Replacement vs. Anterior Cervical Discectomy and Fusion: Results at 5-year Follow-up. Int J Spine Surg. 2016;10:10. [CrossRef]
- Nunley PD, Hisey M, Smith M, Stone MB. Cervical Disc Arthroplasty vs Anterior Cervical Discectomy and Fusion at 10 Years: Results From a Prospective, Randomized Clinical Trial at 3 Sites. Int J Spine Surg. 2023;17(2):230-240. [CrossRef]
- Saifi C, Fein AW, Cazzulino A, et al. Trends in resource utilization and rate of cervical disc arthroplasty and anterior cervical discectomy and fusion throughout the United States from 2006 to 2013. The Spine Journal. 2018;18(6):1022-1029. [CrossRef]
- Lu Y, McAnany SJ, Hecht AC, Cho SK, Qureshi SA. Utilization Trends of Cervical Artificial Disc Replacement After FDA Approval Compared With Anterior Cervical Fusion: Adoption of New Technology. Spine. 2014;39(3):249-255. [CrossRef]
- Nesterenko SO, Riley LH, Skolasky RL. Anterior Cervical Discectomy and Fusion Versus Cervical Disc Arthroplasty: Current State and Trends in Treatment for Cervical Disc Pathology. Spine. 2012;37(17):1470-1474.
- Kumar C, Dietz N, Sharma M, Wang D, Ugiliweneza B, Boakye M. Long-Term Comparison of Health Care Utilization and Reoperation Rates in Patients Undergoing Cervical Disc Arthroplasty and Anterior Cervical Discectomy and Fusion for Cervical Degenerative Disc Disease. World Neurosurgery. 2020;134:e855-e865. [CrossRef]
- Chen CM, Yang JJ, Wu CC. Cervical Disc Arthroplasty (CDA) versus Anterior Cervical Discectomy and Fusion (ACDF) for Two-Level Cervical Disc Degenerative Disease: An Updated Systematic Review and Meta-Analysis. J Clin Med. 2024 May 29;13(11):3203. [CrossRef]
- Demetriades AK, Ringel F, Meyer B (2014) Cervical disc arthroplasty: a critical review and appraisal of the latest available evidence. Adv Tech Stand Neurosurg 41:107–129.
- Fay LY, Huang WC, Tsai TY et al (2014) Differences between arthroplasty and anterior cervical fusion in two-level cervical degenerative disc disease. Eur Spine J 23(3):627–634.
- Rao, MJ., Nie, SP., Xiao, BW. et al. Cervical disc arthroplasty versus anterior cervical discectomy and fusion for treatment of symptomatic cervical disc disease: a meta-analysis of randomized controlled trials. Arch Orthop Trauma Surg 135, 19–28 (2015). [CrossRef]
- Ratnasamy PP, Rudisill KE, Maloy GC, Grauer JN. Cervical Disc Arthroplasty Usage Has Leveled Out From 2010 to 2021. Spine (Phila Pa 1976). 2023 Oct 15;48(20):E342-E348. [CrossRef]
- Singh M, Balmaceno-Criss M, Anderson G, Parhar K, Daher M, Gregorczyk J, Liu J, McDonald CL, Diebo BG, Daniels AH. Anterior cervical discectomy and fusion versus cervical disc arthroplasty: an epidemiological review of 433,660 surgical patients from 2011 to 2021. Spine J. 2024 Aug;24(8):1342-1351. [CrossRef]
- Nunley PD, Kerr EJ 3rd, Cavanaugh DA, Utter PA, Campbell PG, Wadhwa R, Frank KA, Marshall KE, Stone MB. Adjacent Segment Pathology After Treatment With Cervical Disc Arthroplasty or Anterior Cervical Discectomy and Fusion, Part 1: Radiographic Results at 7-Year Follow-Up. Int J Spine Surg. 2020 Jun 30;14(3):269-277.
- Xu S, Liang Y, Zhu Z, Qian Y, Liu H. Adjacent segment degeneration or disease after cervical total disc replacement: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2018 Oct 3;13(1):244. [CrossRef]
- Qureshi SA, Koehler SM, Lu Y, Cho S, Hecht AC. Utilization trends of cervical artificial disc replacement during the FDA investigational device exemption clinical trials compared to anterior cervical fusion. J Clin Neurosci. 2013 Dec;20(12):1723-6. [CrossRef]
- Lu Y, McAnany SJ, Hecht AC, Cho SK, Qureshi SA. Utilization trends of cervical artificial disc replacement after FDA approval compared with anterior cervical fusion: adoption of new technology. Spine (Phila Pa 1976). 2014 Feb 1;39(3):249-55. [CrossRef]
- Coric D, Cassis J, Carew JD, Boltes MO. Prospective study of cervical arthroplasty in 98 patients involved in 1 of 3 separate investigational device exemption studies from a single investigational site with a minimum 2-year follow-up. Clinical article. J Neurosurg Spine. 2010 Dec;13(6):715-21. [CrossRef]
- Zou S, Gao J, Xu B, Lu X, Han Y, Meng H. Anterior cervical discectomy and fusion (ACDF) versus cervical disc arthroplasty (CDA) for two contiguous levels cervical disc degenerative disease: a meta-analysis of randomized controlled trials. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2016. Epub 2016/06/18. [CrossRef]
- Heller JG, Sasso RC, Papadopoulos SM, Anderson PA, Fessler RG, Hacker RJ, Coric D, Cauthen JC, Riew DK. Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: clinical and radiographic results of a randomized, controlled, clinical trial. Spine (Phila Pa 1976).
- 36 Anderson PA, Sasso RC, Riew KD. Comparison of adverse events between the Bryan artificial cervical disc and anterior cervical arthrodesis. Spine (Phila Pa 1976). 2008 May 20;33(12):1305-12. [CrossRef]
- Quinto ES Jr, Paisner ND, Huish EG Jr, Senegor M. Ten-Year Outcomes of Cervical Disc Arthroplasty Versus Anterior Cervical Discectomy and Fusion : A Systematic Review With Meta-Analysis. Spine (Phila Pa 1976). 2024 Apr 1;49(7):463-469. [CrossRef]
- Abdelmalek G, Uppal H, Coban D, Patel N, Changoor S, Sahai N, Sinha K, Hwang K, Emami A. Is cervical disc arthroplasty an effective treatment option for patients with cervical spondylotic myelopathy? A matched cohort analysis compared to anterior cervical discectomy and fusion. Spine J. 2024 Nov 27:S1529-9430(24)01156-2. [CrossRef]
- Nunna RS, Ryoo JS, Ostrov PB, Patel S, Godolias P, Daher Z, Price R, Chapman JR, Oskouian RJ. Single-level cervical disc replacement (CDR) versus anterior cervical discectomy and fusion (ACDF): A Nationwide matched analysis of complications, 30- and 90-day readmission rates, and cost. World Neurosurg X. 2023 Oct 18;21:100242. [CrossRef]
- Leibold A, Glener S, Sarikonda A, Sami A, Self DM, Quraishi D, Ali DM, Isch EL, Heller J, Jallo J, Prasad SK, Sharan A, Vaccaro AR, Harrop J, Sivaganesan A. What is the True Cost of Motion Preservation? A Time-Driven Activity-Based Cost Analysis of Anterior Cervical Discectomy and Fusion versus Disc Replacement. World Neurosurg. 2024 Dec;192:e506-e512. [CrossRef]
- Du JY, Shafi K, Blackburn CW, Kim HJ, Iyer S, Qureshi S, Marcus RE, Albert TJ. Trends in costs, reimbursements, and surgeon payments for cervical disc arthroplasty cost of care from 2009 to 2019. J Neurosurg Spine. 2023 Jul 14;39(5):690-699. [CrossRef]
- Heijdra Suasnabar JM, Vleggeert-Lankamp CLA, Goedmakers CMW, de Vries F, Arts MP, van den Akker-van Marle ME. Cost effectiveness of implanting a prosthesis after anterior cervical discectomy for radiculopathy: results of the NECK randomized controlled trial. Spine J. 2023 Jun;23(6):851-858.
- Denaro V, Papalia R, Denaro L, Di Martino A, Maffulli N. Cervical spinal disc replacement. J Bone Joint Surg Br. 2009 Jun;91(6):713-9. [CrossRef]
- Bydon, M., Michalopoulos, G. D., Alvi, M. A., Goyal, A., & Abode-Iyamah, K. (2021). Cervical Total Disc Replacement: Food and Drug Administration-Approved Devices. Neurosurgery clinics of North America, 32(4), 425–435.
- Alves Ó. L. (2021). Cervical Total Disc Replacement: Expanded Indications. Neurosurgery clinics of North America, 32(4), 437–448. [CrossRef]
- Patel, N., Abdelmalek, G., Coban, D., Changoor, S., Sinha, K., Hwang, K., & Emami, A. (2024). Should patient eligibility criteria for cervical disc arthroplasty (CDA) be expanded? A retrospective cohort analysis of relatively contraindicated patients undergoing CDA. The spine journal : official journal of the North American Spine Society, 24(2), 210–218. [CrossRef]
- Auerbach, J. D., Jones, K. J., Fras, C. I., Balderston, J. R., Rushton, S. A., & Chin, K. R. (2008). The prevalence of indications and contraindications to cervical total disc replacement. The spine journal : official journal of the North American Spine Society, 8(5), 711–716. [CrossRef]
- Tu, T. H., Lee, C. Y., Kuo, C. H., Wu, J. C., Chang, H. K., Fay, L. Y., Huang, W. C., & Cheng, H. (2019). Cervical disc arthroplasty for less-mobile discs. Journal of neurosurgery. Spine, 31(3), 310–316. [CrossRef]
| Category | ICD 10 CODES |
|---|---|
| Cervical Disc Arthroplasty (CDA) | 0RR30JZ, 0RR20JZ |
| Anterior Cervical Discectomy and Fusion (ACDF) | 0RG10A0, 0RG10A1, 0RG10A4, 0RG10J0, 0RG10J1, 0RG10J4 |
| Heart Failure | I5021, I5031, I5033, I5041, I5043 |
| Acute Kidney Injury | N170, N171, N172, N178, N179 |
| Acute Coronary Artery Disease | I2101, I2102, I2109, I211, I2119, I2111, I212, I2129, I213, I214, I219 |
| Stroke | I60, I61, I62, I63, I650, I688, O873, O2250, O2251, O2252 |
| Pulmonary Edema | J810, J811, I501 |
| Hypertension | I10(start with) |
| Blood Loss Anemia | D62 (start with) |
| Pneumonia | J189, J159, J22 |
| Pulmonary Embolism | I2602, I2609, I2692, I2699 |
| DVT | I82401, I82402, I82403, I82409, I82411, I82412, I82413, I82419, I82421, I82422, I82423, I82429 |
| Dyslipidemia | E78(start with) |
| Obstructive Sleep Apnea | G473 |
| Chronic Anemia | D64(start with) |
| Alcohol Abuse History | F10 |
| Osteoporosis | M81, M82 |
| Mental Disorders | F (start with) |
| Parkinson Disease | G20 (start with) |
| Type 2 Diabetes Mellitus | E11 (start with) |
| Chronic Kidney Disease | N18 (start with) |
| Congestive Heart Failure | I500, I501, I509 |
| Chronic Lung Disease | J44 (start with) |
| History of Myocardial Infarction | I252 |
| Peripheral Vascular Disease | I73 (start with) |
| History of Cerebrovascular Accident (CVA) | Z8673, I69 (start with) |
| Dementia | F03 (start with) |
| Peptic Ulcer Disease | K25-K28 |
| Hemiplegia | G81 |
| Neoplasms | C (start with) |
| Neoplasms of Lymphoid and Hematopoietic Tissue | C81-C96 |
| Parameter | ACDF | CDA | Significance |
|---|---|---|---|
| Total Surgeries (%) | 85,584 | 11,415 | - |
| Average Age (y) | 55.6 | 47.2 | P<0.001 |
| Female (%) | 51.7 | 52.5 | P=0.108 |
| Primary expected payer - Medicare (%) | 33.9 | 10.7 | P<0.001 |
| Primary expected payer - Medicaid (%) | 10.7 | 9.7 | |
| Pimary expected payer - private including HMO (%) | 44.5 | 64.9 | |
| Primary expected payer - self-pay (%) | 1.2 | 1.3 | |
| Primary expected payer - no charge (%) | 0.1 | 0 | |
| Primary expected payer - other (%) | 9.6 | 13.3 |
| Parameter | ACDF (n=85,584) | CDA (n=11,415) | Significance |
|---|---|---|---|
| Hypertension (%) | 43.7 | 25.1 | P<0.001 |
| Dyslipidemia (%) | 30 | 17.3 | P<0.001 |
| Obstructive Sleep Apnea (%) | 9.5 | 6.9 | P<0.001 |
| Chronic Anemia (%) | 2.3 | 1.8 | P<0.001 |
| Alcohol Abuse (%) | 1.2 | 0.8 | P<0.001 |
| Osteoporosis (%) | 2.3 | 0.9 | P<0.001 |
| Parkinson Disease (%) | 0.5 | 0.1 | P<0.001 |
| Alzheimer Disease (%) | 0.1 | 0 | P=0.698 |
| Chronic Kidney Disease (%) | 3.8 | 1.1 | P<0.001 |
| Congestive Heart Failure (%) | 0.9 | 0.1 | P<0.001 |
| Chronic Lung Disease (%) | 8 | 3.2 | P<0.001 |
| Diabetes Mellitus (%) | 19.5 | 9.6 | P<0.001 |
| IBD (%) | 0.5 | 0.3 | P<0.001 |
| Liver Disease (%) | 1.1 | 0.7 | P<0.001 |
| Obesity (%) | 18.5 | 15.6 | P<0.001 |
| Fibromyalgia (%) | 3.8 | 3 | P<0.001 |
| Disorders of Thyroid (%) | 11.9 | 9.4 | P<0.001 |
| History of Myocardial Infarction (%) | 2.9 | 0.7 | P<0.001 |
| Peripheral Vascular Disease (%) | 1.3 | 0.6 | P<0.001 |
| History of Cerebrovascular Accident (%) | 3.9 | 1.3 | P<0.001 |
| Dementia (%) | 0.2 | 0.2 | P<0.001 |
| Neoplasms (%) | 0.8 | 0.3 | P<0.001 |
| Neoplasms of Lymphoid and Hematopoietic Tissue (%) | 0.3 | 0.1 | P<0.001 |
| Parameter | ACDF (n=11,415) | CDA (n=11,415) | Significance |
|---|---|---|---|
| Average Age (y) | 47.3 | 47.2 | P=0.36 |
| Female (%) | 52.1 | 52.5 | P=0.62 |
| Primary expected payer - Medicare (%) | 11.6 | 11.3 | P=0.41 |
| Primary expected payer - Medicaid (%) | 9.7 | 9.7 | |
| Primary expected payer - private including HMO (%) | 64.5 | 64.9 | |
| Primary expected payer - self-pay (%) | 1.5 | 1.3 | |
| Primary expected payer - no charge (%) | 0 | 0 | |
| Primary expected payer - other (%) | 12.7 | 12.7 | |
| Hypertension (%) | 24.7 | 25.1 | P=0.59 |
| Dyslipidemia (%) | 17.2 | 17.3 | P=0.93 |
| Obstructive Sleep Apnea (%) | 6.4 | 6.9 | P=0.05 |
| Chronic Anemia (%) | 1.6 | 1.8 | P=0.43 |
| Alcohol Abuse (%) | 0.7 | 0.8 | P=0.33 |
| Osteoporosis (%) | 0.9 | 0.9 | P=0.52 |
| Parkinson Disease (%) | 0 | 0.1 | P=0.20 |
| Alzheimer Disease (%) | 0 | 0 | P=1 |
| Chronic Kidney Disease (%) | 1 | 1.1 | P=0.51 |
| Congestive Heart Failure (%) | 0.1 | 0 | P=0.06 |
| Chronic Lung Disease (%) | 2.9 | 3.2 | P=0.09 |
| Diabetes Mellitus (%) | 8.9 | 9.6 | P=0.05 |
| Inflammatory Bowel Disease (%) | 0.4 | 0.3 | P=0.23 |
| Liver Disease (%) | 0.5 | 0.7 | P=0.19 |
| Obesity (%) | 16.4 | 15.6 | P=0.09 |
| Fibromyalgia (%) | 2.7 | 3 | P=0.11 |
| History of Myocardial Infarction (%) | 0.6 | 0.6 | P=0.18 |
| Peripheral Vascular Disease (%) | 0.6 | 0.6 | P=0.35 |
| History of Cerebrovascular Accident (%) | 1.3 | 1.3 | P=0.42 |
| Neoplasms (%) | 0.4 | 0.3 | P=0.09 |
| Neoplasms of Lymphoid and Hematopoietic Tissue (%) | 0.1 | 0.1 | P=1 |
| ACDF (n=11,415) | CDA (n=11,415) | Significance | |
|---|---|---|---|
| Length of stay mean in days | 1.39 (Std. deviation 1.52) | 1.32 (Std. deviation 1.27) | P<0.001 |
| Total charges mean in $ | 58,472 (Std. deviation 41703) | 82,431 (Std. deviation 53105) | P<0.001 |
| Parameter | ACDF (n=11,415) | CDA (n=11,415) |
Significance | Odds Ratio | Odds Ratio 95% Confidence |
|---|---|---|---|---|---|
| Dysphagia (%) | 4.90% | 3.60% | P<0.001 | 0.724 | 0.63 - 0.82 |
| Blood Loss Anemia (%) | 1.00% | 0.80% | P=0.17 | 0.825 | 0.62 - 1.08 |
| Cervical spinal cord injury (%) | 0.17% | 0.30% | P=0.04 | 1.752 | 1.01 - 3.03 |
| UTI (%) | 0.22% | 0.39% | P=0.02 | 1.803 | 1.1 - 2.94 |
| Acute Renal Failure (%) | 0.21% | 0.26% | P=0.50 | 1.201 | 0.7 - 2.04 |
| Pneumonia (%) | 0.17% | 0.17% | P=1.00 | 1.000 | 0.53 - 1.86 |
| Blood transfusion (%) | 0.13% | 0.00% | P=0.01 | 0.500 | 0.17 - 1.46 |
| Venous Thromboembolism (%) | 0.13% | 0.04% | P=0.03 | 0.308 | 0.12 - 0.75 |
| Pulmonary Edema (%) | 0.04% | 0.04% | P=1.00 | 1.000 | 0.28 - 3.45 |
| Ileus (%) | 0.08% | 0.17% | P=0.07 | 2.002 | 0.93 - 4.27 |
| Feeding Tube (%) | 0.08% | 0.00% | P=0.01 | 0.5 | 0.17 - 1.46 |
| Dural tear (%) | 0.04% | 0.04% | P=1.00 | 1.000 | 0.28 - 3.45 |
| Sepsis (%) | 0.04% | 0.00% | P=0.03 | 0.500 | 0.49 - 0.5 |
| Pulmonary Embolism (%) | 0.04% | 0.00% | P=0.03 | 0.500 | 0.49 - 0.5 |
| Mortality (%) | 0.00% | 0.00% | P=1.00 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





