1. Introduction
In the past thirty years, many clinical, epidemiological, biological and experimental studies have highlighted the impact of environmental pollution on human health. Specifically, environmental Endocrine Disrupting Chemicals (EDCs) have emerged as key players in a global health, social, economic, legal, and ethical scandal. According to the World Health Organization, an EDC is “an exogenous substance or mixture of substances that alters the function(s) of the endocrine system and consequently causes adverse effects in an intact organism, or its progeny, or (sub)populations.” These chemicals, mainly pesticides, but also plastics (bisphenols, phthalates), solvents (polychlorinated biphenyls), perfluorinated compounds (perfluorooctanoic acid), parabens (contained in cosmetics), heavy metals, dioxins and many others, contaminate indoor and outdoor air, drinking and irrigation water, and food.
EDCs were initially described as substances that disrupt the androgen/estrogen balance; however, they also alter the thyroid function (particularly during fetal life), metabolic balance (mainly by accumulating in the adipose tissue), immune defenses, microbiota activity, cell growth, oxidative stress levels, and neurodevelopment at vulnerable periods during fetal life, childhood, adolescence and adulthood [
1,
2]. The human nervous system is a potential target of residential, professional, and personal environmental pollution [
3]. The potential effects of such exposure are multiple: intellectual disability, neurodevelopmental disorders (motor coordination and learning) [
4,
5], attention and memory problems, autism spectrum disorders, and neurodegenerative diseases. Importantly, the consequences of contamination by EDCs during fetal life may become apparent only in adulthood. Moreover, the risk of transgenerational transmission of the effects of environmental pollution jeopardizes also future generations. In addition, EDCs are key factors in the increased incidence of chronic diseases, which represent a major health challenge and require the development of population-level protection and prevention strategies.
It is a real challenge for researchers and physicians to measure the clinical consequences of EDCs that have been upgraded from endocrine disruptors to endocrine-metabolic disruptors and endocrine-metabolic-neurological disruptors [
6]. The exposome concept is now used to describe and study the lifetime exposure of an individual to EDCs and their effects [
3,
4,
5].
It is estimated that currently there are nearly 100,000 chemical substances/EDCs. Their mechanism of action is mediated by binding to cell membrane/nuclear receptors and then by influencing the transcription of target genes. EDCs can also interact with receptors that modify intracellular signaling with many different biological effects [
7]. EDCs do not follow the classic law of toxicology: there is no threshold effect and the dose does not make the poison. Their effects can be potentiated, leading to a “cocktail” effect. As they can accumulate in adipose tissue for months or even years, their effects may manifest many years after the actual exposure. Therefore, the consequences of fetal exposure to an EDC may be observed in adulthood, as illustrated by the concept of the fetal origin of adult diseases [
8].
The purpose of this review was to highlight EDC harmfulness and their mechanism of action, using the example of children exposed in utero to synthetic sex hormones (estrogens and progestogens).
2. Material and Methods
The PubMed and Google Scholar databases were screened to identify studies (in English and French) published from 2000 to 2024 using the following keywords: endocrine disrupting chemicals (EDCs), thyroid and sex hormones, estrogens, progestins, psychosis and estrogens, epigenetic, multigenerational effects.
Data on children exposed in utero to synthetic sex hormones were extracted from the HHORAGES-France patient cohort. The HHORAGES-France association includes ~1,300 families (more than 2,000 children exposed in utero) and is registered on the INSERM epidemiological portal (the French National Institute of Medical Research) and on AVIESAN (the French National Alliance for Life and Health Sciences; epidemiologie-france.aviesan.fr). The French National Commission for Information Technology and Liberties (CNIL) approved the collection of the testimonies and medical questionnaires of patients in the HHORAGES-France Association (CNIL: J B/EM/DC042793, N° 1006460). As data were deidentified, the informed consent of individual subjects was not required.
3. Results
3.1. EDC Specific Features
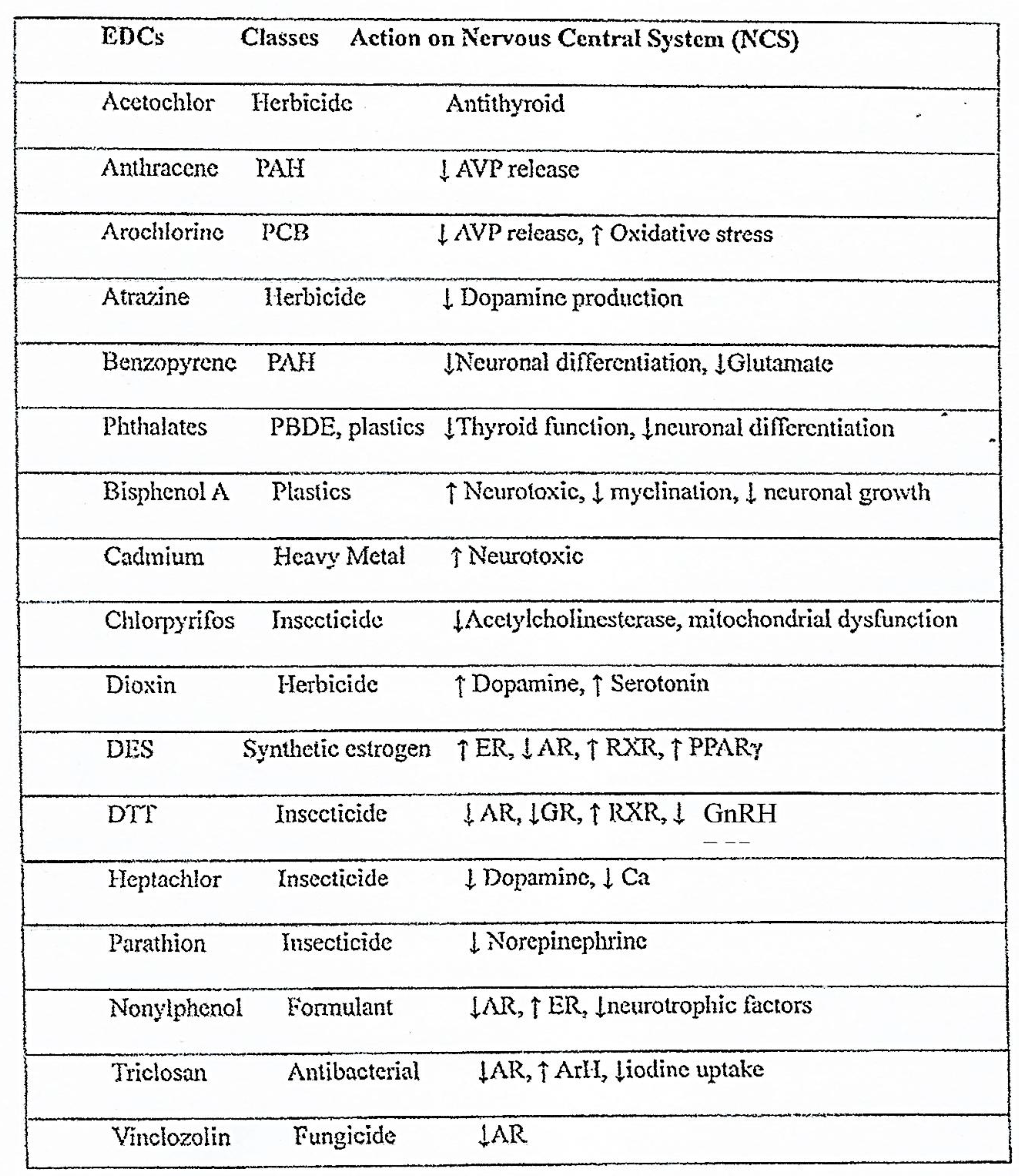
EDC mechanisms of action make scientific research and the establishment of a regulatory framework difficult. Indeed, EDCs can act through genetic (modulation of the transcription of target genes) and epigenetic (alteration of DNA and/or RNA methylation, histone modifications, chromatin structure and non-coding RNA functions) mechanisms, as summarized in
Figure 1. Therefore, EDCs can modulate target genes by affecting their transcriptional activation and also through epigenetic mechanisms that are involved in the multi-and transgenerational transmission of their deleterious effects. The most commonly studied epigenetic mechanism is DNA or RNA methylation. In addition, recent works highlighted the importance of microRNAs in the transgenerational transmission of the deleterious effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin [
9], a potent herbicide (
Table 1).
3.2. Hormones and Neuronal Development
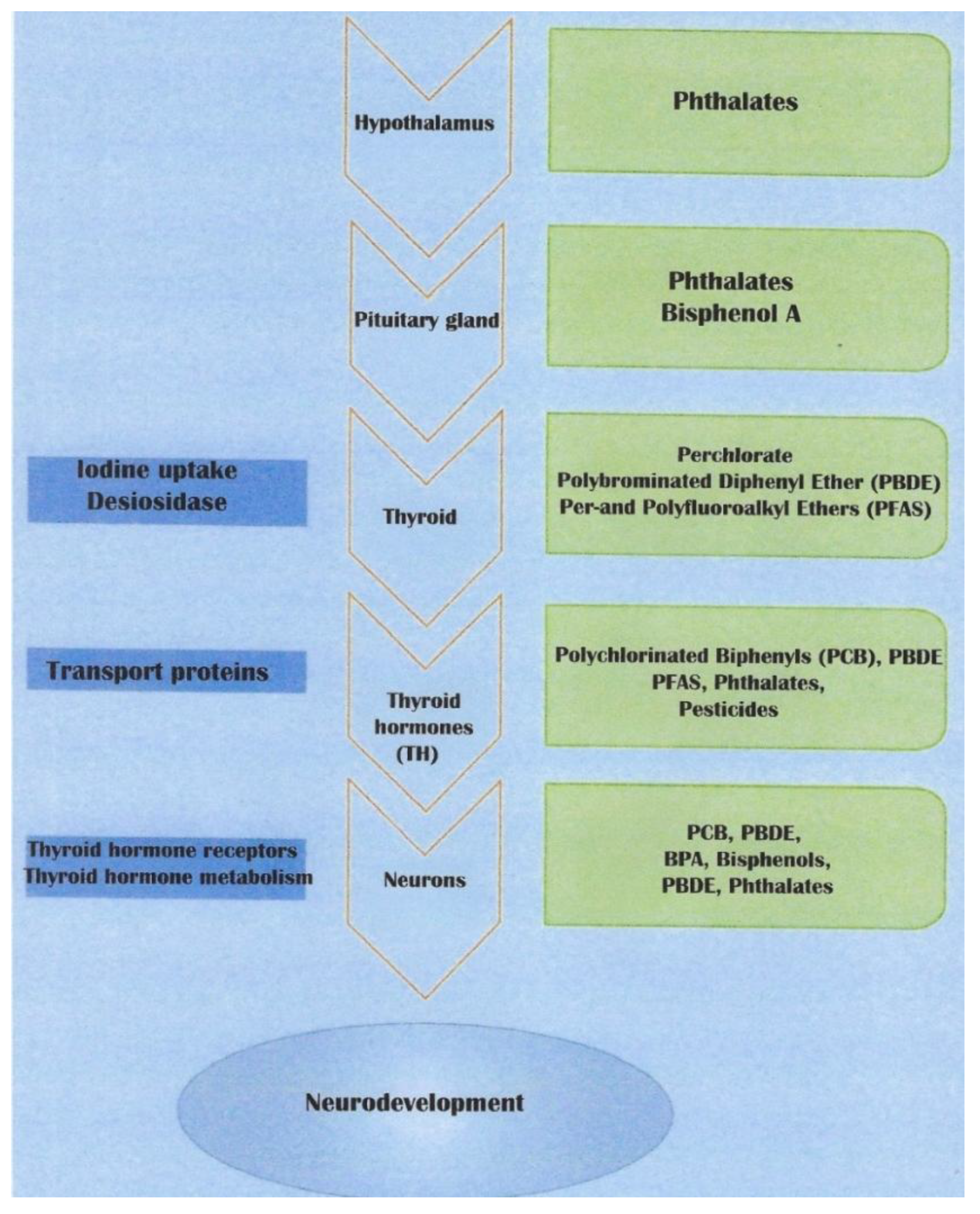
Thyroid hormones are essential for neurogenesis, neuronal migration, neuron and glial cell differentiation, and myelination. They also promote oligodendrocyte maturation. Therefore, all thyroid function alterations caused by exposure to environmental chemicals, particularly during fetal life, will affect neurodevelopment. The clinical consequences will appear at birth but also in childhood or adulthood [
10] (
Figure 2).
3.3. EDCs and Neurodevelopmental Abnormalities
The spectrum of the clinical consequences of environmental pollution continues to expand and includes abnormalities of fetal growth and neurodevelopment, immune function, reproduction and nervous system as well as metabolic disorders and cancers (endocrine-dependent and non-endocrine-dependent) [
11]. In
Table 1 are listed the main EDCs with effects on the central nervous system and their mechanism of action. One of the latest reports by Public Health France shows that in 2020 only 5 effects linked to environmental EDC were identified and monitored and that their number increased to 21 in 2024 and other 61 are currently assessed [
12]
Table 1.
Class and action of the main EDCs in the central nervous system. PAH: Polycyclic Aromatic Hydrocarbons, PCB: Polychlorinated biphenyl, AVP: Arginine Vasopressin, PBDE: Polybrominated diphenyl ethers, ER: Estrogen receptor, AR: Androgen receptor, RXR: Retinoid X receptor, PPARγ: Peroxisome proliferator-activated receptor γ, GR: Glucocorticoid receptor, GnRH: Gonadotropin-Releasing Hormone, ArH: Appetite-regulating hormones. From Sultan et al, Perturbateurs Endocriniens et Neurodéveloppement, LNE, 2025, XXIX, 3,
Figure 2, Courtesy of LNE [
2].
Table 1.
Class and action of the main EDCs in the central nervous system. PAH: Polycyclic Aromatic Hydrocarbons, PCB: Polychlorinated biphenyl, AVP: Arginine Vasopressin, PBDE: Polybrominated diphenyl ethers, ER: Estrogen receptor, AR: Androgen receptor, RXR: Retinoid X receptor, PPARγ: Peroxisome proliferator-activated receptor γ, GR: Glucocorticoid receptor, GnRH: Gonadotropin-Releasing Hormone, ArH: Appetite-regulating hormones. From Sultan et al, Perturbateurs Endocriniens et Neurodéveloppement, LNE, 2025, XXIX, 3,
Figure 2, Courtesy of LNE [
2].
3.4. Impact of Synthetic Sex Hormones: The HHORAGES-France Cohort
3.4.1. Sex Steroids
Sex steroids (androgens, estrogens) are key factors in the sexual differentiation of the brain. They play a role in the proliferation and migration of neurons, in neuronal differentiation, and in synaptic plasticity. Sex steroids also regulate the expression of Brain-Derived Neurotrophic Factor (BDNF), a neurotrophin implicated in brain development and functioning [
13]. Besides their role in establishing the gonadotropic axis, initiated during fetal life, the secretion of sex steroids is reactivated at the onset of puberty, and they are involved in learning and memorization (spatial memory). They are also instrumental in the production of gonadotropins and in the modulation of the Gonadotropin-Releasing Hormone (GnRH)-kisspeptin system [
14]. Therefore, chemicals with estrogenic or antiandrogenic activity can activate or repress the gonadotropic axis and affect reproductive functions. During fetal life, androgens are also involved in the structure and function of brain centers that play an important role in the establishment of male sexual identity [
15]. Consequently, any antiandrogenic chemical substance may potentially be implicated in gender identity disorders in boys [
16].
3.4.2. The Particular Case of Progestogens
Progestogens (also known as progestins) are a class of steroid hormones that play an important role in brain development, especially progesterone. According to [
17,
18] and as quoted in [
19], progesterone contributes to shaping the central nervous system structure and function (neurodevelopment, neurogenesis, and cognition) throughout life. Progesterone exerts powerful effects on the brain, such as regulation of neurogenesis, astroglial and synaptic plasticity, development of neuronal cell types, such as Purkinje cells and oligodendrocytes, as well as myelinization. Progesterone also exerts a significant influence on the activity of several neurotransmitters involved in the pathophysiology of psychosis, including the dopaminergic, glutamatergic and GABAergic systems. Importantly, progesterone can be converted to dihydroprogesterone and then allopregnanolone (or iso-pregnanolone) [
20], potent ligands of the GABA-A receptor. Progesterone elicits its effects by binding to the nuclear progesterone receptors and then modulating gene transcription (genomic mechanism) and also through non-genomic mechanisms by affecting signal transduction pathways. Preclinical studies have suggested that steroids might be involved in the pathophysiology of psychosis[
19].
3.4.3. Psychiatric Disorders in a Cohort of Patients Exposed in Utero to Synthetic Sex Hormones
The HHORAGES-France association recorded the testimonies of more than 1,300 families in which the mothers were treated with diethylstilbestrol (DES) or other estrogens, such as 17-α-ethinyl estradiol (EE) and/or progestins during pregnancy, (i.e. >2,000 children exposed
in utero). Different psychiatric disorders [
19,
21,
22] such as bipolar disorder, schizophrenia, Major Depression Disorder (MDD), anxiety, behavioral disorders, suicide attempts and suicides, were diagnosed in these children (
Table 2). Some of these children exposed
in utero also had somatic disorders [
23], including genital malformations, sterility or cancers [
24].
Similarly, in 2010, a study on the large Nurses’ Health Study II cohort (76,240 women among whom 1,612 were exposed to DES
in utero) showed that the risk of MDD was higher in women exposed
in utero to DES than in non-exposed controls (20%
vs 16%) [
25]. Unfortunately, the authors focused only on MDD, whereas the HHORAGES cohort revealed the presence also of psychotic disorders in children exposed
in utero to synthetic estrogens and progestogens. It has been reported that DES and EE promote the hypermethylation of genes involved in neurodevelopment [
26]. In 2017, Rivollier and colleagues (St Anne Hospital, Paris, France) analyzed the methylation variations of 411,947 CpG sites (DNA regions often present in promoters, where a cytosine is followed by a guanine residue) in 75 siblings from 31 families of the HHORAGES-France cohort. In these “informative families”, controls were older siblings who were not exposed and did not present any pathology. The authors found hypermethylation at differentially methylated regions in the
ZFP57 gene and the promoter of the
ADAM TS9 gene in the group of DES/EE-exposed children with psychosis compared with those without psychosis. The
ZFP57 gene, located on chromosome 6, is a transcriptional regulator of many genes implicated in neurodevelopment.
ADAM TS9 is involved in the control of organ shape, particularly in the development and function of the reproductive organs (which are often abnormal in children exposed
in utero to DES) and the central nervous system. The same year, Verdoux, et al. [
27] reported that in a sample of 2,566 DES daughters (i.e. exposed
in utero) and 2,967 controls (non-exposed women), DES daughters had consulted mental health specialists more often (1.7 times) than controls. This supports the hypothesis previously proposed by Verdoux (2001) [
28] of a link between
in utero exposure to estrogens and increased risk of psychiatric disorders.
3.4.4. Multigenerational Effect
The epigenetic mechanism highlighted by Rivollier et al using data from the HHORAGES cohort [
26] suggests a higher risk of neurodevelopment alterations also in the future generations. In 2018, Kioumourzoglou et al observed cognitive disorders, such as Attention Deficit Hyperactivity Disorders (ADHD), in the children of DES-exposed women of the Nurses’ Health Study II cohort (47,540 participants) [
29]. In this epidemiological study, the authors followed the participants (exposed
in utero; F1), their mothers (F0; to whom DES was prescribed) and the participants’live-born children (F2).
In utero exposure to DES was associated with higher risk of ADHD in the F2 generation: 7.7%
vs 5.2%. They concluded that exposure to this endocrine disruptor during pregnancy may be associated with multigenerational neurodevelopmental deficits. More recently, analysis of HHORAGES data highlighted bipolar-type disorders in the second (F2) and third (F3) generation of children from an informative family as well as autistic-type disorders in grandsons and also in one great-grandson [[
30],
Figure 1, [
31],
Table 3]. Similarly, the prevalence of psychiatric disorders (severe depression and bipolar disorders, schizophrenia, behavioral disorders and aggression) was increased in children from the HHORAGES cohort whose mothers had been treated with synthetic progestins alone during pregnancy [
32,
33]. It should be noted that progestins induce neuronal activation of the GABAergic system, which contributes to the development of psychological disorders [
17,
18,
34]. In a Danish cohort,
in utero exposure to estrogens and/or progestogen increased the risk of autism spectrum disorders [
35,
36]. Moreover, Yao’s group demonstrated in rats that prenatal exposure to the progestogen levonorgestrel induces autism-like behavior in the offspring through estrogen receptor beta suppression in the amygdala [
37]. Later, in a large epidemiological study, the same team demonstrated that in humans, prenatal exposure to progestin is associated with increased risk of autism [
38].
3.5. Gender Dysphoria/Incongruence
In a recent work, we investigated the impact of fetal exposure to DES on the acquisition of gender identity in boys [
16]. The prevalence of gender dysphoria/incongruence was increased in XY children/adolescents whose mothers had been treated with DES during pregnancy. In a group of 250 XY individuals exposed to DES
in utero from the HHORAGES-France cohort, 1.58% had gender dysphoria (
vs ~1 in 16,000 in the French general population). This original work contributes to strengthening the hypothesis that EDCs might alter the action of fetal androgens on the development of gender identity (and/or reproductive system) in XY children and adolescents. Therefore, EDCs could represent a risk factor of gender dysphoria [
39]. Similarly, in 2020, Troisi et al. documented the association of prenatal DES exposure with sexual orientation and gender identity [
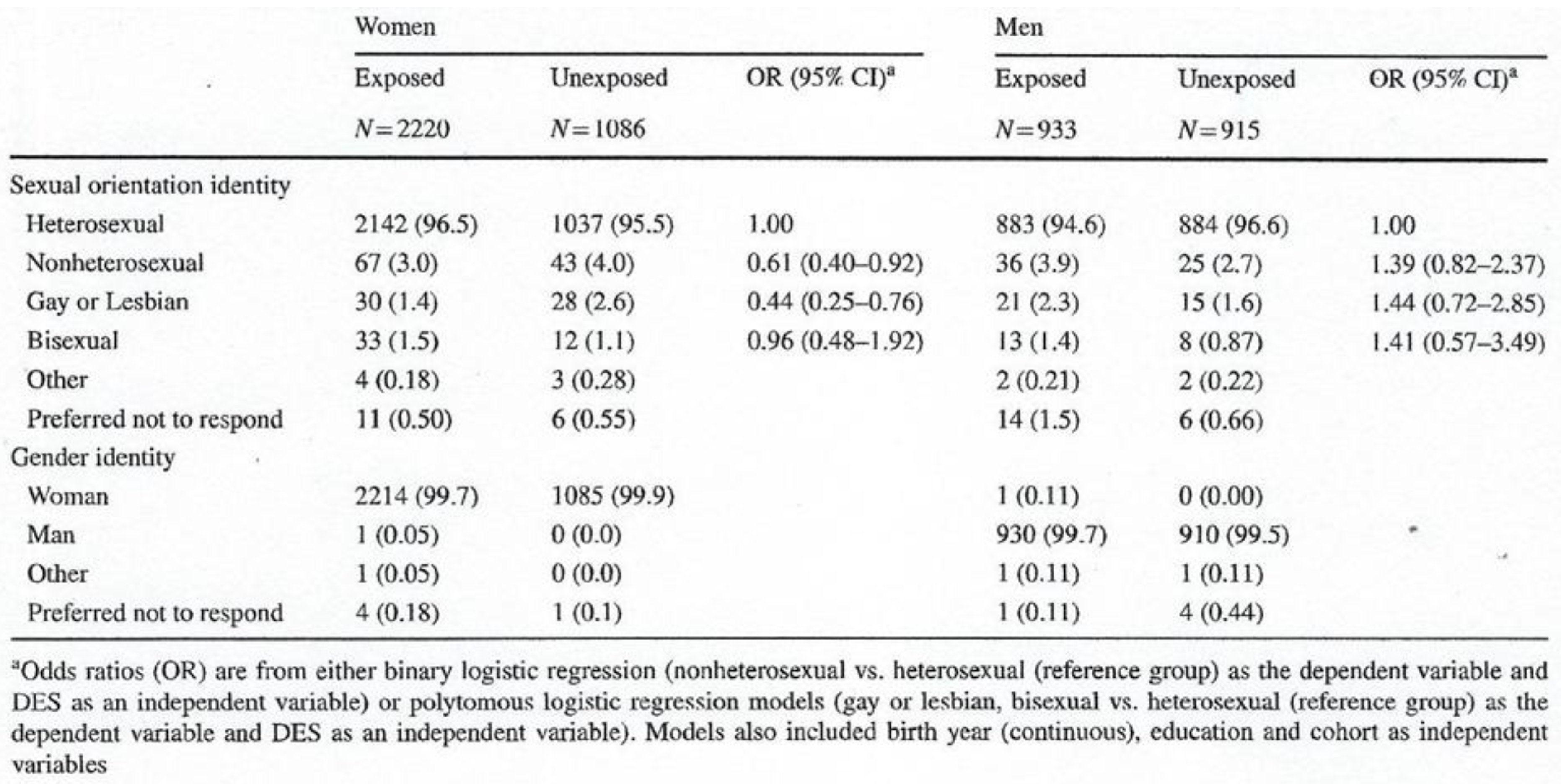
40] using data from five US cohorts: 3,306 women (2,220 exposed and 1,086 unexposed) and 1,848 men (933 exposed and 915 unexposed). They concluded that in men, exposure
in utero to DES increases the risk of reporting a non-heterosexual identity (odds ratio, OR =1.4 [95% CI 0.82–2.4]), gay identity (OR = 1.4 [95% CI 0.72–2.85]) and bisexual identity (OR=1.4 [95% CI 0.57–3.5]) (
Table 3). Overall, women exposed to DES were less likely to report being homosexual, bisexual or non-heterosexual.
Table 3.
Sexual orientation and gender identity in women and men in utero exposed or unexposed to DES. From Troisi et al. Gender Identity and Sexual Orientation Identity in Women and Men Prenatally Exposed to Diethylstilbestrol.
Arch Sex Behav. 2020; 49(2): 447-454 [
40]. Courtesy of Springer.
Table 3.
Sexual orientation and gender identity in women and men in utero exposed or unexposed to DES. From Troisi et al. Gender Identity and Sexual Orientation Identity in Women and Men Prenatally Exposed to Diethylstilbestrol.
Arch Sex Behav. 2020; 49(2): 447-454 [
40]. Courtesy of Springer.
These results confirm in men the hypotheses developed by Haney et al (1984) [
41] and Adamson et al., (2008) [
42]. These authors observed in rodents that DES suppresses testicular testosterone production. Other EDCs such as bisphenol A (BPA) and bisphenol analogues [
43] also might alter the role of fetal androgens in the development of gender identity in XY children and adolescents.
4. Discussion and Conclusion
Besides the endocrine system, EDCs can exert multiple influences on the neural system and behavior, mainly during development [
43,
44]. EDCs act as neurohormones, neuromodulators, neurotransmitters and/or neurotrophic factors. Recent experimental studies strongly suggest that EDCs significantly affect brain development and function [
45]. They can influence neural cell differentiation, proliferation and migration and also synaptic formation and activity. For example, in rats, prenatal exposure to bisphenol A affects the dendritic spine density of hippocampal neurons more in males and females [
46]. In rats, exposure to environmental chemicals impairs learning and memory and alter neuromorphology and neurotransmission [
47]. These effects were reported also across generations.
The fetal brain is especially vulnerable to EDCs because they can affect critical developmental steps, particularly neurogenesis, neuronal migration, neuron differentiation, myelinization and synaptogenesis.
EDCs may disrupt the neural system structure and function by modifying the local hormonal balance or by interacting with steroid hormone metabolism (high estrogenic activity, antiandrogen activity) [
48]. Therefore, they can be considered as neurotoxicants, neuro-endocrine disruptors and neuro-disruptors. The xenoestrogen DES, is considered THE model EDC [
49] with both psychiatric and somatic effects, and multi- and trans-generational activity. Currently, the descendants of women treated with DES represent more than 50 million people worldwide [
39] to whom must be added all people who are exposed to other EDC mixtures. Therefore, it can be estimated that the neurodevelopment of hundreds of millions of people is at risk of being altered by the EDCs present in the environment. Their action actually implies
de facto a multigenerational effect through their effects on the exposed fetus germ cells, but very likely also a transgenerational effect [
48,
50].
A “One Health” scientific approach should be used to study the EDC dangers to biodiversity and human health. The aim of the One Health approach is to integrate human, animal and environmental health to better anticipate and manage health crises. Indeed, several hundred molecules still escape health risk assessment [
51]. Much research and clinical expertise are necessary, particularly in the area of the interaction between brain and EDCs [
52,
53].
In conclusion, the DES experience may have a heuristic value in understanding the effects of xenoestrogens on neurodevelopment and psychiatric disorders [
54]. DES and neuro-disrupting chemicals affect the brain functions by altering neuronal communication, damaging the brain structure, inducing oxidative stress and impairing the blood brain barrier [
55]. They can induce cognitive decline, memory loss, reduced concentration and neurological disorders, such as Alzheimer’s and Parkinson’s diseases as well behavioral disorders and mood changes, such as anxiety, depression, aggressivity, and suicide [
56]. In humans, DES and other neuro-EDCs are key actors in neurodevelopment and neuropsychological disorders [
54,
55].
Author Contributions
Conceptualization, L.G., M.-O.S.-G. and C.S.; methodology, M.-O.S.-G. and C.S.; formal analysis, C.S.; investigation, M.-O.S.-G. and C.S.; data curation, C.S.; writing—original draft preparation, writing—review and editing, M.-O.S.-G. and C.S.; supervision, C.S.; project administration, M.-O.S.-G., C.S. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
M.-O.S.-G. is a researcher and President of the HHORAGES-France Association and a mother concerned with DES and other synthetic hormones. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. HHORAGES-France Association is financed exclusively by subscriptions and donations.
Acknowledgments
This work could not continue without the daily ongoing support of the HHORAGES-France families and board, especially Mauricette P. †, Yette B. and Aimée I. HHORAGES-France, a patient association, is registered at the Epidemiological Portal of French Health Databases INSERM (French National Institute for Medical Research) and AVIESAN (National Alliance for Life Sciences and Health) (epidemiologiefrance.aviesan.fr), The authors also thank Elisabetta Andermarcher for corrections and relevant suggestions for the manuscript.
References
- Özel F. ; Rüegg, J. Exposure to endocrine-disrupting chemicals and implications for neurodevelopment. Dev Med Child Neurol 2023, 65(8), 1005-11. [CrossRef]
- Sultan, C. ; Gaspari, L. ; Paris, F. ; Soyer-Gobillard M.O. Perturbateurs endocriniens et neurodéveloppement. La Lettre du Neurologue 2025, XXIV, 3, 64-67.
- Reis, J. ; Buguet, A. ; Román, G. C. ; Spencer, P.S.. Environmental neurology: concepts and short history of an interdisciplinary approach to etiology, treatment and prevention. J Neurol Sci 2023, 454 :120861. [CrossRef]
- Cediel-Ulloa, A . ; Lupu, D. L. ; Johansson, Y. ; Hinojosa, M. ; Özel, F. ; Rüegg, J. Impact of endocrine disrupting chemicals on neurodevelopment: the need for better testing strategies for endocrine disruption-induced developmental neurotoxicity. Expert Rev Endocrinol Metab 2022, 17(2), 131-41. Epub 2022 Mar 7. [CrossRef]
- Galbiati, V. ; Buoso, E. ; d’Emmanuele di Villa Bianca R. ; Di Paola, R. ; Morroni, F. ; Nocentini, G. ; Racchi, M. ; Viviani, B. ; Corsini, E. Immune and nervous systems interaction in endocrine disruptors toxicity: the case of atrazine. Front Toxicol 2021, 3, 649024. eCollection 2021. [CrossRef]
- Seralini, G.E.; Jungers, G. Endocrine disruptors also function as nervous disruptors and can be renamed endocrine and nervous disruptors (ENDs). Toxicol Rep 2021, 8 :1538-57. [CrossRef]
- Combarnous, Y; Nguyen, TMD. Comparative Overview of the Mechanisms of Action of Hormones and Endocrine Disruptor Compounds. Toxics 2019, 7(1) :5. [CrossRef] [PubMed] [PubMed Central]
- Skogen, JC; Overland, S. The fetal origins of adult disease: a narrative review of the epidemiological literature. JRSM Short Rep. 2012, 3(8):59. Epub 2012 Aug 22. [CrossRef] [PubMed] [PubMed Central]
- Gaspari, L.; Haouzi, D.; Gennetier, A.; Granes, G.; Soler, A.; Sultan, C.; Paris, F.; Hamamah, S. Transgenerational Transmission of 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) Effects in Human Granulosa Cells: The Role of MicroRNAs. Int J Mol Sci 2024, 25(2), 1144. [CrossRef]
- Demeneix, B.; Slama, R. Endocrine Disruptors: From Scientific Evidence to Human Health Protection. Brussels: Policy Department for Citizens’ Rights and Constitutional Affairs, Directorate General for Internal Policies of the Union; 2019. Available at: https:// www.europ arl.europa.eu/RegDa ta/etude s/STUD/2019/60886 6/ IPOL_STU (2019) 608866_EN. Pdf.
- Belfiore, A; Perks, CM. Grand challenges in cancer endocrinology: endocrine related cancers, an expanding concept. Front Endocrinol (Lausanne) 2013, 4 :141. [CrossRef]
- Haroche, Q. Perturbateurs endocriniens : santé publique France élargit son dispositif. 2024, https://www.jim.fr/viewarticle/perturbateurs-endocriniens-santé-publique-france-2024a10000yr.
- de Assis, GG; de Sousa, MBC; Murawska-Ciałowicz, E. Sex Steroids and Brain-Derived Neurotrophic Factor Interactions in the Nervous System: A Comprehensive Review of Scientific Data. Int J Mol Sci 2025, 26(6):2532. [CrossRef]
- Goodman, RL; Herbison, AE; Lehman, MN; Navarro, VM. Neuroendocrine control of gonadotropin-releasing hormone: Pulsatile and surge modes of secretion. J Neuroendocrinol 2022, 34(5): e13094. [CrossRef]
- Li, F; Xing, X; Jin, Q; Wang, XM; Dai, P; Han, M; Shi, H; Zhang, Z; Shao, X; Peng, Y; Zhu, Y; Xu, J; Li, D; Chen, Y; Wu, W; Wang, Q; Yu, C; Chen, L; Bai, F; Gao, D. Sex differences orchestrated by androgens at single-cell resolution. Nature 2024, 631(8019): E6. [CrossRef] [PubMed]
- Gaspari, L.; Soyer-Gobillard, M.-O.; Kerlin, S.; Paris, F.; Sultan, Ch. Early female transgender identity after prenatal exposure to DES: Report from a French national diethylstilbestrol (DES) cohort. J. of Xenobiotics 2024, 14, 166–175. [CrossRef]
- Schumacher, M. ; Mattern, C. ; Ghoumari, A. ; Oudinet, J.P. ; Liere, P. ; Labombarda, F. ; Sitruk-Ware, R. ; De Nicola, A. F. ; Guennoun, R. Revisiting the role of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors. Prog Neurobiol 2014, 113, 6–39.
- Melcangi, R.C. ; Giatti, S. ; Calabrese, D. ; Pesaresi , M. ; Cermenati , G. ; Mitro, N. ; Viviani, B. ; Garcia-Segura, L. M. ; Caruso, D. Levels and actions of progesterone and its metabolites in the nervous system during physiological and pathological conditions. Prog Neurobiol 2014, 113, 56–69.
- Soyer-Gobillard, M.O.; Puillandre, M.; Paris, F.; Gaspari, L.; Courtet, Ph.; Sultan, C. Neurodevelopmental disorders in children exposed in utero to progestin treatment: Study of a cohort of 115 children from the HHORAGES Association. Gynecol Endocrinol 2019, 35(3), 247–250. [CrossRef]
- Zhu, M.; Brinton, R. How Progestin, a synthetic female hormone could affect the brain. Atlantic 2012, 1–16. https://www.theatlantic. com/health/archive/2012/01/how-progestin-a-synthetic-female-hormone-could-affect-the-brain/251299.
- Soyer-Gobillard, M.O.; Sultan, C. Behavioral and Somatic Disorders in Children exposed in utero to Synthetic Hormones: a Testimony-Case Study in a French Family Troop. 2012, In: “State of the Art of Therapeutic Endocrinology”, Editor Dr. Sameh Magdeldin, Niigata University, Japan. InTech, pp. 67-86. Doi : 10.5772/48637.
- Soyer-Gobillard, M.O.; Paris, F.; Gaspari, L.; Courtet, P.; Sultan, C. Association between fetal DES-exposure and psychiatric disorders in adolescence / adulthood: Evidence from a French cohort of 1,002 prenatally exposed children. Gynecol Endocrinol 2016, 32, 25–29. [CrossRef].
- Tournaire, M.; Epelboin, S.; Devouche, E.; Viot, G.; Le Bidois, J.; Cabau, A.; Dunbavand, A.; Levadou, A. Adverse health effects in children of women exposed in utero to diethylstilbestrol (DES). Therapie 2016, 71, 395-404. [CrossRef]
- Tournaire, M.; Devouche, E.; Espié, M.; Asselain, B.; Levadou, A.; Cabau, A.; Dunbavand, A.; Grosclaude, P.; Epelboin, S. Cancer Risk in women exposed to Diethylstilbestrol in utero. Therapie 2015, 70(5), 433-41. [CrossRef]
- O’Reilly, E.J.; Mirzaei, F.; Forman, M.R.; Ascherio, A. Diethylstilbestrol exposure in utero and depression in women. Am J Epidemiol 2010, 171(8) : 876-882. [CrossRef]
- Rivollier, F. ; Chaumette, B. ; Bendjemaa, N. ; Chayet, M. ; Millet, B. ; Jaafari, N. ; Barhdadi, A. ; Lemieux Perreault, L.P. ; Provost, S. ; Dubé, M.P. ; et al. Methylomic changes in individuals with psychosis, prenatally exposed to endocrine disrupting compounds: Lessons from diethylstilbestrol. PLoS ONE 2017, 12, e0174783.
- Verdoux, H.; Devouche, E.; Tournaire, M.; Levadou, A. Impact of prenatal exposure to diethylstilbestrol (DES) on psychological outcome: a national survey of DES daughters and unexposed controls. Arch Womens Ment Health 2017, 20(3), 389-395. [CrossRef]
- Verdoux, H. Does prenatal exposure to diethylstilbestrol (DES) have psychiatric consequences? Annales Medico Psychologiques 2000, 158, 105-117.
- Kioumourtzoglou, M.-A.; Coull, B.A.; O’Reilly, E.J.; Ascherio, A.; Weisskopf, M.G. Association of exposure to diethylstilbestrol during pregnancy with multigenerational neurodevelopmental deficits. JAMA Pediatr 2018, 172, 670–677.
- Soyer-Gobillard, M-O.; Gaspari, L.; Paris, F.; Kalfa, N.; Hamamah, S.; Courtet, Ph.; Sultan, Ch. Prenatal contamination by DES and multigenerational transmission of psychiatric disorders in an informative family. Int J Environ Res Public Health 2021, 18, 9965. [CrossRef]
- Soyer-Gobillard, M.O.; Gaspari, L.; Courtet, Ph.; Sultan, Ch. Diethylstilbestrol and Autism. Frontiers in Endocrinology 2022, 13: 1034959. [CrossRef]
- Soyer-Gobillard, M.-O.; Gaspari, L.; Yao, P.; Sultan, Ch. Prenatal exposure to progestins: impact on neurodevelopment of the child. (25 pp., 2 Figs., 5 Tables). In: C. Martin, V.R. Preedy & R. Rajendram (Eds), Factors affecting Neurodevelopment, Academic Press London/Elsevier Inc. 2021, 34, pp. 395-408. ISBN : 978-0-12-818371-7 (Set) ISBN : 978-0-12-817986-4.
- Soyer-Gobillard, M.-O.; Gaspari, L.; Sultan, Ch. Evidence for link between mental disorders and in utero exposure to synthetic hormones: a long and crucial history. In: Psychopathology: An international and Interdisciplinary perspective. Ed. Robert Woolfolk, Lesley Allen, Federico Durbano & Floriana Irtelli. IntechOpen, London, 2020, pp. 7-22. [CrossRef]
- Zhu, M.; Brinton, R. How Progestin, a synthetic female hormone could affect the brain. Atlantic 2012, 1–16. https://www.theatlantic. com/health/archive/2012/01/how-progestin-a-synthetic-female-hormone-could-affect-the-brain/251299.
- Baron-Cohen, S.; Auyeung, B.; Nørgaard-Pedersen, B.; Hougaard, DM.; Abdallah, MW.; Melgaard, L.; Cohen, AS.; Chakrabarti, B.; Ruta, L.; Lombardo, M.V. Elevated fetal steroidogenic activity in autism. Mol Psychiatry 2015, 20(3), 369-76. [CrossRef]
- Baron-Cohen, S.; Tsompanidis, A.; Auyeung, B.; Nørgaard-Pedersen, B.; Hougaard, DM.; Abdallah, M.; Cohen, A.; Pohl, A. Foetal oestrogens and autism. Mol Psychiatry 2020, 25(11), 2970-2978. [CrossRef]
- Zou, Y. ; Lu, Q. ; Zheng, D. ; Chu, Z. ; Liu, Z. ; Chen, H. ; Ruan, Q. ; Ge, X. ; Zhang, Z. ; Wang, X.; Lou, W. ; Huang, Y. ; Wang, Y. ; Huang, X. ; Liu, Z. ; Xie, W. ; Zhou, Y. ; Yao, P. Prenatal levonorgestrel exposure induces autism-like behavior in offspring through ERβ suppression in the amygdala. Mol Autism 2017, 8, 46. [CrossRef]
- Li, L.; Li, M.; Lu, J.; Ge, X.; Xie, W.; Wang, Z.; Li, X.; Li, C.; Wang, X.; Han, Y.; Wang, Y.; Zhong, L.; Xiang, W.; Huang, X.; Chen, H.; Yao, P. Prenatal Progestin Exposure Is Associated with Autism Spectrum Disorders. Front Psychiatry 2018, 9, 611. [CrossRef]
- Soyer-Gobillard, M.-O.; Gaspari, L.; Sultan, Ch. Gender identity disorders: a legacy of fetal exposition to Diethylstilbestrol, an Endocrine Disruptor Chemical. Medical Research Archives 2025, 13 (3), 11pp. ISSN 2375-1924. [CrossRef]
- Troisi, R.; Palmer, J.R.; Hatch, E.E.; Strohsnitter, W.C.; Huo, D.; Hyer, M.; Fredriksen-Goldsen, K.I.; Hoover, R.; Titus, L. Gender Identity and Sexual Orientation Identity in Women and Men Prenatally Exposed to Diethylstilbestrol. Arch Sex Behav 2020, 49(2), 447-454. [CrossRef]
- Haney, AF.; Newbold, RR.; McLachlan, JA. Prenatal diethylstilbestrol exposure in the mouse: Effects on ovarian histology and steroidogenesis in vitro. Biology of Reproduction 1984, 30: 471-478.
- Adamson, NA.; Brokken, LJ.; Paranko, J. et al. In vivo and in vitro effects of flutamide and diethylstilbestrol on fetal testicular steroidogenesis in the rat. Reproductive Toxicology 2008, 25: 76-83. Doi:10.1016.
- Shaw, R.; Kamath, A.S.; Chaube, R. An overview of neurobehavioral and developmental toxicity induced by xenoestrogens. Discov Toxicol 2025, 2, 10https: //doi.org/10.1007/s44339-025-00025-x.
- Patisaul, HB. Endocrine disrupting chemicals (EDCs) and the neuroendocrine system: Beyond estrogen, androgen, and thyroid. Adv Pharmacol 2021, 92:101-150. doi: . Epub 2021 Jul 1. [CrossRef] [PubMed]
- Vaudry, H.; Ubuka, T.; Soma, KK.; Tsutsui, K. Editorial: Recent Progress and Perspectives in Neurosteroid Research. Front Endocrinol (Lausanne) 2022 Jul27; 13:951990. [CrossRef] [PubMed] [PubMed Central]
- Li, C.; Sang, C.; Zhang, S.; Zhang, S.; Gao, H. Effects of bisphenol A and bisphenol analogs on the nervous system. Chin Med J (Engl) 2023 Feb 5;136(3):295-304. [CrossRef] [PubMed] [PubMed Central]
- Reddy, V.; McCarthy, M.; Raval, AP. Xenoestrogens impact brain estrogen receptor signaling during the female lifespan: A precursor to neurological disease? Neurobiol Dis 2022, 163:105596. Epub 2021 Dec 20. [CrossRef] [PubMed]
- Reichard, J.; Zimmer-Bensch, G. The Epigenome in Neurodevelopmental Disorders. Front Neurosci 2021 Nov 3; 15:776809. [CrossRef] [PubMed] [PubMed Central]
- Robotti, S. The heritable legacy of diethylstilbestrol: a bellwether for endocrine disruption in humans. Biol Reprod 2021, 105(3), 687-689. [CrossRef]
- Soyer-Gobillard, M.-O.; Gaspari, L.; Sultan, Ch. Is in utero exposure to female xenohormones harming the neurodevelopment of children and grand-children? A French national experience and other lessons from the past. World Journal of Pharmaceutical Sciences and Research 2025, 4 (2), 08-19. [CrossRef]
- Zhou, Y; Frutos, R; Bennis, I; Wakimoto, MD. One Health governance: theory, practice and ethics. Sci One Health 2024, 3:100089. [CrossRef] [PubMed] [PubMed Central]
- Ting, X.; Daqiang, Y. The unlocking neurobehavioral effects of environmental endocrine-disrupting chemicals. Current Opinion in Endocrine and Metabolic Research 2019, 7, 9-13. [CrossRef]
- Cardenas-Iniguez, C.; Burnor, E.; Herting, MM. Neurotoxicants, the Developing Brain and Mental Health. Biol Psychiatry Glob Open Sci 2022, 2(3):223-232. Epub 2022 May 23. [CrossRef] [PubMed] [PubMed Central]
- Sultan, Ch.; Gaspari, L.; Soyer-Gobillard, M.-O. DES (Diethylstilbestrol): A prototype for evaluating Xenoestrogen action, a clinical experimental model for analyzing early, late and multigenerational effects of fetal contamination by Endocrine disruptors. In: Fetal Exposition to Sex Hormones: Their Nature and Impact on Human Health. Soyer-Gobillard & Sultan Eds., CSP Cambridge (UK), 2025, pp. XVII-XVIII. ISBN: 978-1-0364-4997-1; ISBN (Ebook): 978-1-0364-4998-8.
- Charlier, TD. Neuroendocrine Disrupters. Neuroendocrinology 2024, 114(2):107-110. Epub 2023 Dec 10. [CrossRef] [PubMed]
- Skovlund, C.W.; Morch, L.S.; Kessing, L.V. Association of Hormonal contraception with suicide attempts and Suicides. Am J Psychiatry 2017, 175(4):336-342. doi: 10.1176/appi.ajp.2017.17060616. Epub 2017 Nov 17.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).