Submitted:
10 September 2025
Posted:
11 September 2025
You are already at the latest version
Abstract
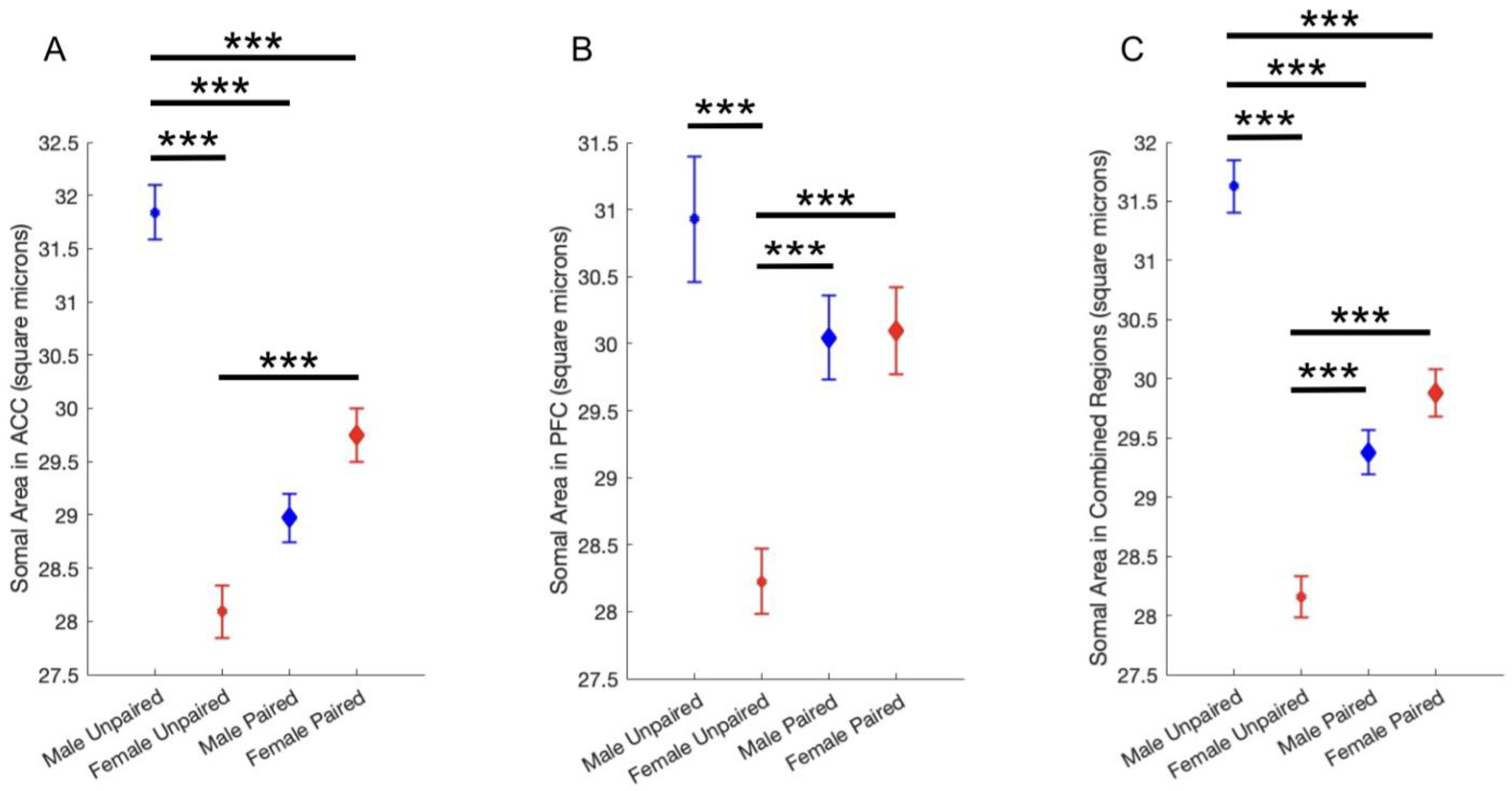
Microtus ochrogaster, monogamous prairie voles, are a translational animal model for studying monogamy and pair-bonding. Microglia, the resident immune cells of the brain, are one of several cell types still poorly understood in non-classical animal models including prairie voles. Microglia are known to play mechanistic roles in mediating social behaviors using inflammatory signaling, but the relationship between microglia reactivity and pair bonding has not yet been investigated. The present study first developed a robust protocol for quantitative histological visualization of microglia in Microtus ochrogaster. Second, it investigated differences in microglia morphology, a reliable index of microglia reactivity and function, in pair-bonded vs. unpaired voles. Sections containing prefrontal cortex (PFC) and anterior cingulate cortex (ACC) were stained for Ionized calcium-binding adaptor molecule I (Iba1) using immunohistochemistry (IHC). IHC results provided evidence for the successful use of murine histological protocols in prairie voles. Quantification results revealed a sexually dimorphic effect of pair-bonding on microglia: somas were significantly larger in pair-bonded vs. unpaired females, and somas were significantly smaller in pair-bonded vs. unpaired males. Additionally, somas were significantly larger in unpaired males than females, with larger somas indicating higher microglia reactivity. While conclusions are limited due to the small sample size, results provide novel characterization of microglia morphology in the frontal cortex and elucidate how pair-bonding may influence microglia function in a sexually dimorphic manner.
Keywords:
1. Introduction
2. Results
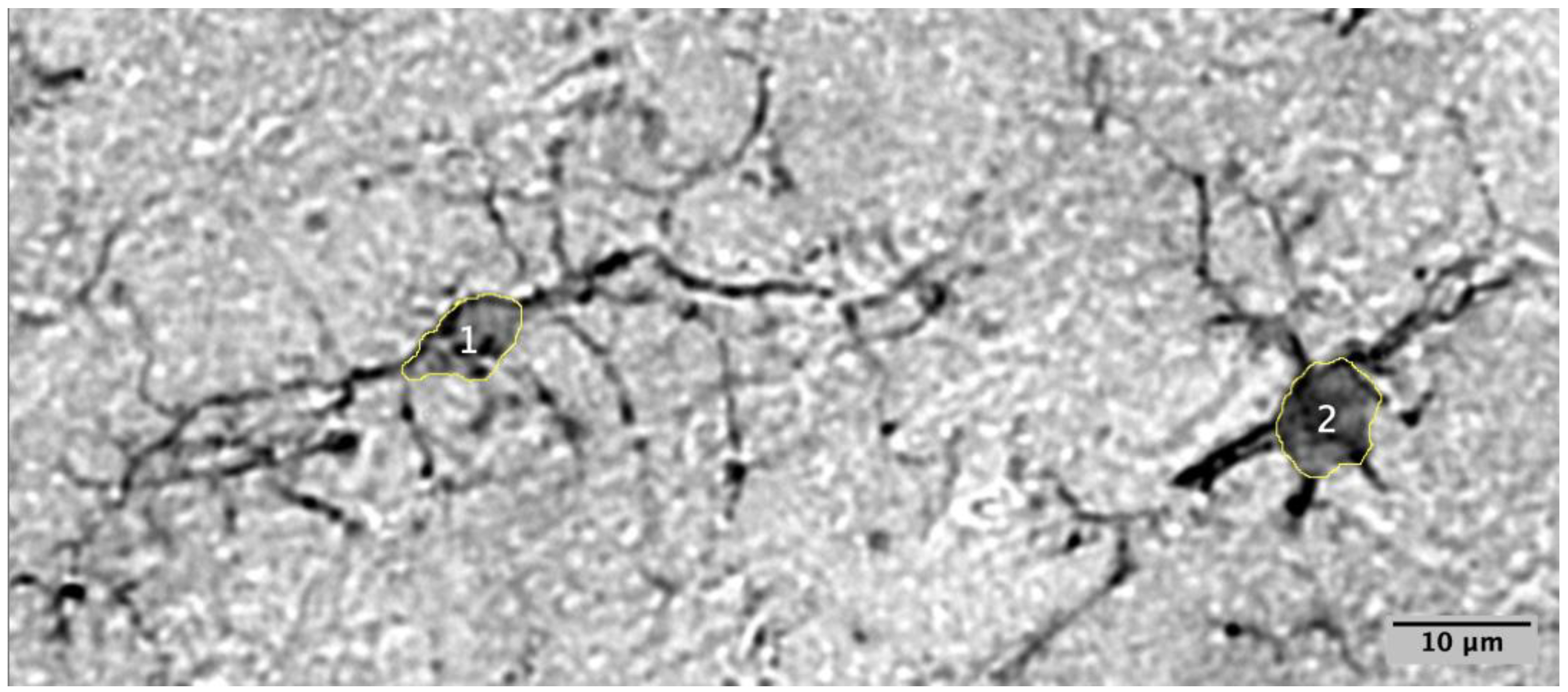
2.1. Characterizing Microglia in Prairie Voles
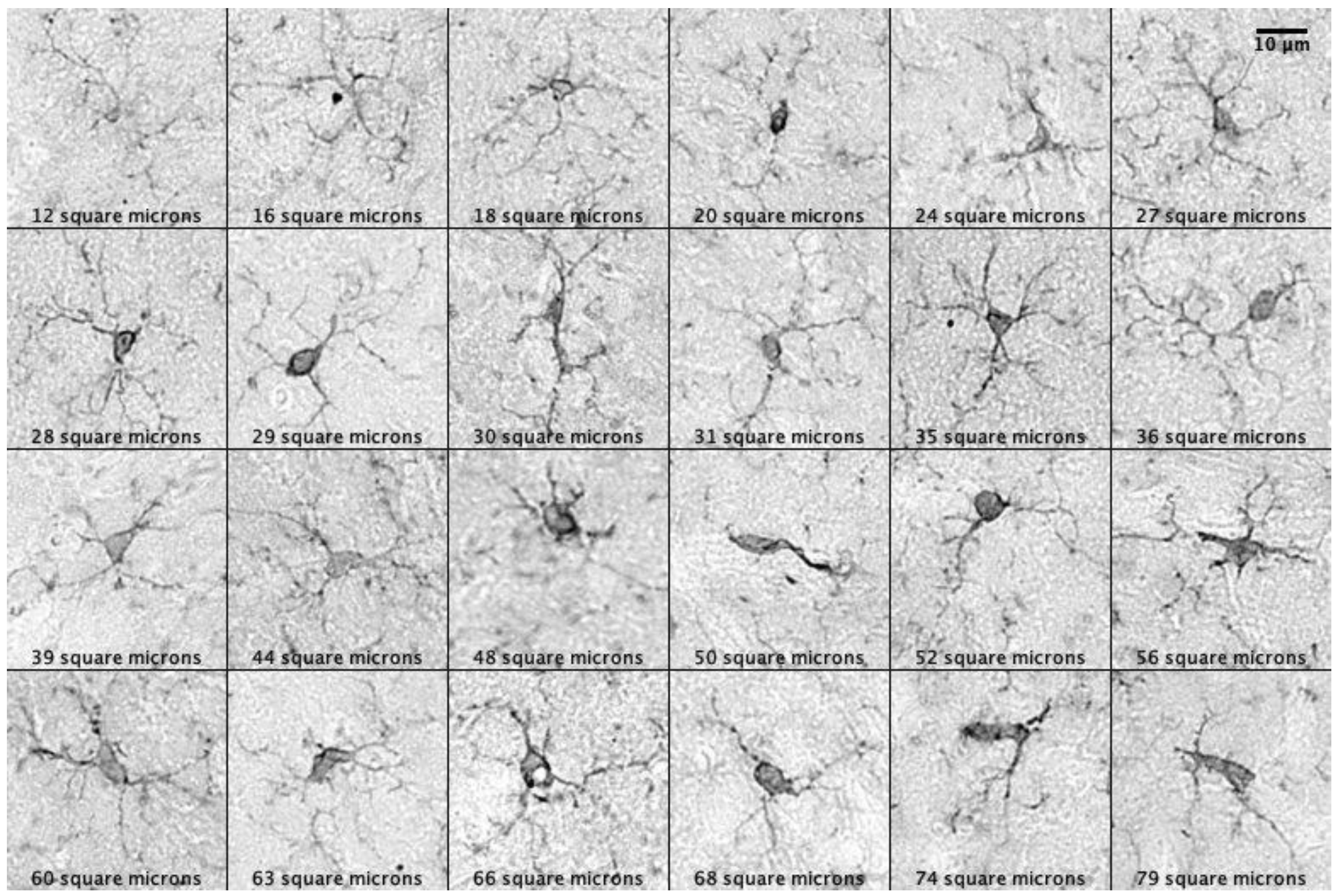
2.2. Evaluating the Normality of Microglia Soma Size Distributions in Prairie Voles
2.3. The Effect of Pair Bonding on Microglia Morphology
3. Discussion
4. Materials and Methods
4.1. Animal Care
4.1. Tissue Fixation and Sectioning
4.2. Immunohistochemistry
4.3. Imaging and Quantification
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFC | Prefrontal Cortex |
| ACC | Anterior Cingulate Cortex |
| Iba1 | Ionized calcium-binding adaptor molecule I |
| IHC | Immunohistochemistry |
| NAc | Nucleus Accumbens |
| AG | Amygdala |
| mm2 | Square millimeters |
| μm2 | Square microns (micrometers) |
| Q-Q | Quartile-quartile |
| s | Skewness |
| k | Kurtosis |
| ANOVA | Analysis of Variance |
| PVN | Paraventricular Nucleus |
| PBS | Phosphate Buffered Saline |
| PFA | Paraformaldehyde |
| TE | Tris-EDTA |
| HRP | Horseradish Peroxidase |
| DAB | Diaminobenzidine |
| ROI | Region of Interest |
References
- Young, K.A. , et al., The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol, 2011. 32(1): p. 53-69. [CrossRef]
- Aragona, B.J. and Z. Wang, The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. ILAR J, 2004. 45(1): p. 35-45. [CrossRef]
- Gunnar, M.R. , Quality of early care and buffering of neuroendocrine stress reactions: potential effects on the developing human brain. Prev Med, 1998. 27(2): p. 208-11. [CrossRef]
- Ditzen, B. , et al., Adult attachment and social support interact to reduce psychological but not cortisol responses to stress. J Psychosom Res, 2008. 64(5): p. 479-86. [CrossRef]
- Badanes, L.S. Dmitrieva, and S.E. Watamura, Understanding Cortisol Reactivity across the Day at Child Care: The Potential Buffering Role of Secure Attachments to Caregivers. Early Child Res Q, 2012. 27(1): p. 156-165. [CrossRef]
- Hiura, L.C. and Z.R. Donaldson, Prairie vole pair bonding and plasticity of the social brain. Trends Neurosci, 2023. 46(4): p. 260-262. [CrossRef]
- McGraw, L.A. and L.J. Young, The prairie vole: an emerging model organism for understanding the social brain. Trends Neurosci, 2010. 33(2): p. 103-9. [CrossRef]
- Loth, M.K. , et al., Lentiviral CRISPRa/i in the adult Prairie Vole Brain: Modulating Neuronal Gene Expression Without DNA Cleavage. bioRxiv, 2025. [CrossRef]
- Donaldson, Z.R. , et al., Production of germline transgenic prairie voles (Microtus ochrogaster) using lentiviral vectors. Biol Reprod, 2009. 81(6): p. 1189-95. [CrossRef]
- Keebaugh, A.C. , et al., Identification of variables contributing to superovulation efficiency for production of transgenic prairie voles (Microtus ochrogaster). Reprod Biol Endocrinol, 2012. 10: p. 54. [CrossRef]
- Horie, K. and K. Nishimori, CRISPR/Cas9-Mediated Genetic Engineering to Generate a Disease Model Prairie Vole, Based on Species-Optimized Assisted Reproductive Technology. Methods Mol Biol, 2022. 2384: p. 139-152. [CrossRef]
- McGraw, L.A. , et al., Development of genomic resources for the prairie vole (Microtus ochrogaster): construction of a BAC library and vole-mouse comparative cytogenetic map. BMC Genomics, 2010. 11: p. 70. [CrossRef]
- Horie, K. , et al., Investigation of Oxtr-expressing Neurons Projecting to Nucleus Accumbens using Oxtr-ires-Cre Knock-in prairie Voles (Microtus ochrogaster). Neuroscience, 2020. 448: p. 312-324. [CrossRef]
- Li, Q. and B.A. Barres, Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol, 2018. 18(4): p. 225-242. [CrossRef]
- Sierra, A. C. Paolicelli, and H. Kettenmann, Cien Anos de Microglia: Milestones in a Century of Microglial Research. Trends Neurosci, 2019. 42(11): p. 778-792. [CrossRef]
- Umpierre, A.D. and L.J. Wu, Microglia Research in the 100th Year Since Its Discovery. Neurosci Bull, 2020. 36(3): p. 303-306. [CrossRef]
- Paolicelli, R.C. , et al., Microglia states and nomenclature: A field at its crossroads. Neuron, 2022. 110(21): p. 3458-3483. [CrossRef]
- Rebuli, M.E. , et al., Sex differences in microglial colonization and vulnerabilities to endocrine disruption in the social brain. Gen Comp Endocrinol, 2016. 238: p. 39-46. [CrossRef]
- Loth, M.K. and Z.R. Donaldson, Oxytocin, Dopamine, and Opioid Interactions Underlying Pair Bonding: Highlighting a Potential Role for Microglia. Endocrinology, 2021. 162(2). [CrossRef]
- Donovan, M. , et al., Social isolation alters behavior, the gut-immune-brain axis, and neurochemical circuits in male and female prairie voles. Neurobiol Stress, 2020. 13: p. 100278. [CrossRef]
- Donovan, M.L. , et al., Post-weaning Social Isolation in Male and Female Prairie Voles: Impacts on Central and Peripheral Immune System. Front Behav Neurosci, 2021. 15: p. 802569. [CrossRef]
- Pohl, T.T. , et al., Microglia react to partner loss in a sex- and brain site-specific manner in prairie voles. Brain Behav Immun, 2021. 96: p. 168-186. [CrossRef]
- Marinello, W.P. , et al., Effects of developmental exposure to FireMaster(R) 550 (FM 550) on microglia density, reactivity and morphology in a prosocial animal model. Neurotoxicology, 2022. 91: p. 140-154. [CrossRef]
- Leyh, J. , et al., Classification of Microglial Morphological Phenotypes Using Machine Learning. Front Cell Neurosci, 2021. 15: p. 701673. [CrossRef]
- Madry, C. , et al., Microglial Ramification, Surveillance, and Interleukin-1beta Release Are Regulated by the Two-Pore Domain K(+) Channel THIK-1. Neuron, 2018. 97(2): p. 299-312 e6. [CrossRef]
- Shamay-Tsoory, S.G. Z. Marton-Alper, and A. Markus, Post-interaction neuroplasticity of inter-brain networks underlies the development of social relationship. iScience, 2024. 27(2): p. 108796. [CrossRef]
- Tay, T.L. , et al., Microglia Gone Rogue: Impacts on Psychiatric Disorders across the Lifespan. Front Mol Neurosci, 2017. 10: p. 421. [CrossRef]
- Kim, H.J. , et al., Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol Psychiatry, 2017. 22(11): p. 1576-1584. [CrossRef]
- Piirainen, S. , et al., Microglia contribute to social behavioral adaptation to chronic stress. Glia, 2021. 69(10): p. 2459-2473. [CrossRef]
- Nelson, L.H. and K.M. Lenz, Microglia depletion in early life programs persistent changes in social, mood-related, and locomotor behavior in male and female rats. Behav Brain Res, 2017. 316: p. 279-293. [CrossRef]
- Walum, H. and L.J. Young, The neural mechanisms and circuitry of the pair bond. Nat Rev Neurosci, 2018. 19(11): p. 643-654. [CrossRef]
- Eisenberger, N.I. , Meta-analytic evidence for the role of the anterior cingulate cortex in social pain. Soc Cogn Affect Neurosci, 2015. 10(1): p. 1-2. [CrossRef]
- Lai, C.H. , Promising Neuroimaging Biomarkers in Depression. Psychiatry Investig, 2019. 16(9): p. 662-670. [CrossRef]
- Salgado, S. and M.G. Kaplitt, The Nucleus Accumbens: A Comprehensive Review. Stereotact Funct Neurosurg, 2015. 93(2): p. 75-93. [CrossRef]
- Mihara, T. , et al., Amygdala activity associated with social choice in mice. Behav Brain Res, 2017. 332: p. 84-89. [CrossRef]
- Barko, K. , et al., Brain region- and sex-specific transcriptional profiles of microglia. Front Psychiatry, 2022. 13: p. 945548. [CrossRef]
- Bollinger, J.L. M. Bergeon Burns, and C.L. Wellman, Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun, 2016. 52: p. 88-97. [CrossRef]
- Wolf, S.A. W. Boddeke, and H. Kettenmann, Microglia in Physiology and Disease. Annu Rev Physiol, 2017. 79: p. 619-643. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




