Submitted:
30 July 2025
Posted:
31 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Characteristics of TRP Channels
3. Physiological Role and Expression of TRP Channels in Melanocytes
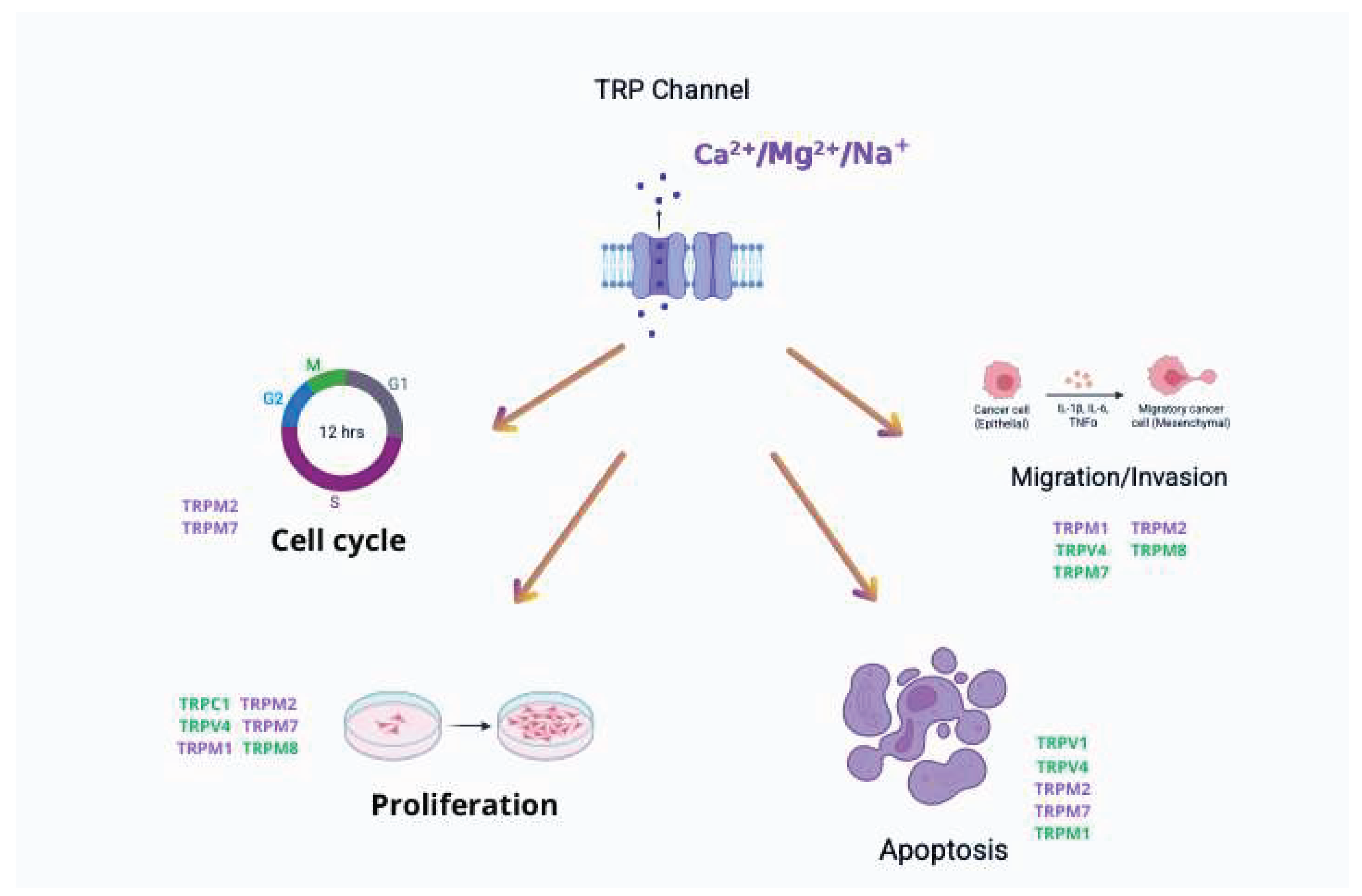
4. TRP Channels and Melanoma Development and Progression
5. TRP Channels as Potential Therapeutic Targets
6. Current Limitations and Future Directions in TRP Channel-Targeted Melanoma Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TRP | Transient Receptor Potential |
| TRPM | Transient Receptor Potential Melastatin |
| TRPV | Transient Receptor Potential Vanilloid |
| TRPC | Transient Receptor Potential Canonical |
| TRPA | Transient Receptor Potential Ankyrin |
| TRPP | Transient Receptor Potential Polycystin |
| TRPML | Transient Receptor Potential Mucolipin |
| ROS | Reactive Oxygen Species |
| EMT | Epithelial-Mesenchymal Transition |
| MITF | Microphthalmia-associated Transcription Factor |
| miR-211 | microRNA-211 |
| KCNMA1 | Potassium Calcium-Activated Channel Subfamily M Alpha 1 |
References
- Heistein, J.B.; Acharya, U.; Mukkamalla, S.K.R. Malignant Melanoma. StatPearls. Treasure Island (FL): StatPearls Publishing; 2025; [cited 2025 Jul 12].Available from: http://www.ncbi.nlm.nih.gov/books/NBK470409/.
- Naik, P.P. Cutaneous Malignant Melanoma: A Review of Early Diagnosis and Management. World J Oncol. 2021, 12, 7–19. [Google Scholar] [CrossRef]
- Ahmed, B.; Qadir, M.I.; Ghafoor, S. Malignant Melanoma: Skin Cancer-Diagnosis, Prevention, and Treatment. Crit Rev Eukaryot Gene Expr. 2020, 30, 291–297. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, Y.; Liu, Q.; Zhang, H.; Jia, L.; Chai, Y.; et al. Global trends in melanoma burden: A comprehensive analysis from the Global Burden of Disease Study, 1990-2021. J Am Acad Dermatol. 2025, 92, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pethő, Z.; Najder, K.; Bulk, E.; Schwab, A. Mechanosensitive ion channels push cancer progression. Cell Calcium. 2019, 80, 79–90. [Google Scholar] [CrossRef]
- Marini, M.; Titiz, M.; Souza Monteiro De Araújo, D.; Geppetti, P.; Nassini, R.; De Logu, F. TRP Channels in Cancer: Signaling Mechanisms and Translational Approaches. Biomolecules. 2023, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.-C.; Lee, C.-H. TRP channels in skin: from physiological implications to clinical significances. BIOPHYSICS. 2015, 11, 17–24. [Google Scholar] [CrossRef]
- Dhennin-Duthille, I.; Gautier, M.; Faouzi, M.; Guilbert, A.; Brevet, M.; Vaudry, D.; et al. High Expression of Transient Receptor Potential Channels in Human Breast Cancer Epithelial Cells and Tissues: Correlation with Pathological Parameters. Cell Physiol Biochem. 2011, 28, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Santoni, G.; Farfariello, V. TRP Channels and Cancer: New Targets for Diagnosis and Chemotherapy. Endocr Metab Immune Disord - Drug Targets. 2011, 11, 54–67. [Google Scholar] [CrossRef]
- Bai, S.; Wei, Y.; Liu, R.; Chen, Y.; Ma, W.; Wang, M.; et al. The role of transient receptor potential channels in metastasis. Biomed Pharmacother. 2023, 158, 114074. [Google Scholar] [CrossRef]
- Chen, J.; Luan, Y.; Yu, R.; Zhang, Z.; Zhang, J.; Wang, W. Transient receptor potential (TRP) channels, promising potential diagnostic and therapeutic tools for cancer. Biosci Trends. 2014, 8, 1–10. [Google Scholar] [CrossRef]
- Bödding, M. TRP proteins and cancer. Cell Signal. 2007, 19, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Shapovalov, G.; Ritaine, A.; Skryma, R.; Prevarskaya, N. Role of TRP ion channels in cancer and tumorigenesis. Semin Immunopathol. 2016, 38, 357–369. [Google Scholar] [CrossRef]
- Vrenken, K.S.; Jalink, K.; Van Leeuwen, F.N.; Middelbeek, J. Beyond ion-conduction: Channel-dependent and -independent roles of TRP channels during development and tissue homeostasis. Biochim Biophys Acta BBA - Mol Cell Res. 2016, 1863, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Tian, W.; Li, B.; Chen, W.; Zhang, W.; Xie, Y.; et al. Transient Receptor Potential channels, TRPV1 and TRPA1 in melanocytes synergize UV-dependent and UV-independent melanogenesis. Br J Pharmacol. 2021, 178, 4646–4662. [Google Scholar] [CrossRef] [PubMed]
- Shoji, K.F.; Bayet, E.; Leverrier-Penna, S.; Le Devedec, D.; Mallavialle, A.; Marionneau-Lambot, S.; et al. The mechanosensitive TRPV2 calcium channel promotes human melanoma invasiveness and metastatic potential. EMBO Rep. 2023, 24, e55069. [Google Scholar] [CrossRef]
- Du, W.; Gu, M.; Hu, M.; Pinchi, P.; Chen, W.; Ryan, M.; et al. Lysosomal Zn2+ release triggers rapid, mitochondria-mediated, non-apoptotic cell death in metastatic melanoma. Cell Rep. 2021, 37, 109848. [Google Scholar] [CrossRef]
- Huang, J.; Korsunsky, A.; Yazdani, M.; Chen, J. Targeting TRP channels: recent advances in structure, ligand binding, and molecular mechanisms. Front Mol Neurosci. 2024, 16, 1334370. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Zhang, L.; Barritt, G. TRP channels in cancer. Biochim Biophys Acta BBA - Mol Basis Dis. 2007, 1772, 937–946. [Google Scholar] [CrossRef]
- Wong, K.K.; Banham, A.H.; Yaacob, N.S.; Nur Husna, S.M. The oncogenic roles of TRPM ion channels in cancer. J Cell Physiol. 2019, 234, 14556–14573. [Google Scholar] [CrossRef]
- Huang, Y.; Anderle, P.; Bussey, K.J.; Barbacioru, C.; Shankavaram, U.; Dai, Z.; et al. Membrane transporters and channels: role of the transportome in cancer chemosensitivity and chemoresistance. Cancer Res. 2004, 64, 4294–4301. [Google Scholar] [CrossRef]
- Yang, P.; Feng, J.; Luo, J.; Madison, M.; Hu, H. A Critical Role for TRP Channels in the Skin. In: Emir TLR, editor. Neurobiology of TRP Channels., 1st ed. Boca Raton : CRC Press, 2017.: CRC Press; 2017; pp 95–111.
- Wiley. Special Issue:Themed Section: The pharmacology of TRP channels. Br J Pharmacol. 2014, 171. [Google Scholar] [CrossRef]
- Guo, H.; Carlson, J.A.; Slominski, A. Role of TRPM in melanocytes and melanoma. Exp Dermatol. 2012, 21, 650–654. [Google Scholar] [CrossRef]
- Hu, Q.; Yi, W.; Su, M.; Jiang, S.; Xu, S.; Lei, T. Induction of retinal-dependent calcium influx in human melanocytes by UVA or UVB radiation contributes to the stimulation of melanosome transfer. Cell Prolif. 2017, 50. [Google Scholar] [CrossRef]
- Devi, S.; Markandeya, Y.; Maddodi, N.; Dhingra, A.; Vardi, N.; Balijepalli, R.C.; et al. Metabotropic glutamate receptor 6 signaling enhances TRPM1 calcium channel function and increases melanin content in human melanocytes. Pigment Cell Melanoma Res. 2013, 26, 348–356. [Google Scholar] [CrossRef]
- Oancea, E.; Vriens, J.; Brauchi, S.; Jun, J.; Splawski, I.; Clapham, D.E. TRPM1 Forms Ion Channels Associated with Melanin Content in Melanocytes. Sci Signal. [CrossRef]
- Springer Netherlands TRPM1: New Trends for an Old, T.R.P. Advances in Experimental Medicine and Biology. Dordrecht;
- Yee, N. Role of TRPM7 in Cancer: Potential as Molecular Biomarker and Therapeutic Target. Pharmaceuticals. 2017, 10, 39. [Google Scholar] [CrossRef]
- Monteilh-Zoller, M.K.; Hermosura, M.C.; Nadler, M.J.S.; Scharenberg, A.M.; Penner, R.; Fleig, A. TRPM7 Provides an Ion Channel Mechanism for Cellular Entry of Trace Metal Ions. J Gen Physiol. 2003, 121, 49–60. [Google Scholar] [CrossRef]
- Yogi, A.; Callera, G.E.; Antunes, T.T.; Tostes, R.C.; Touyz, R.M. Transient Receptor Potential Melastatin 7 (TRPM7) Cation Channels, Magnesium and the Vascular System in Hypertension. Circ, J. 2011, 75, 237–245. [Google Scholar] [CrossRef]
- Hayes, P.; Meadows, H.J.; Gunthorpe, M.J.; Harries, M.H.; Duckworth, M.D.; Cairns, W.; et al. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000, 88, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, H.; Ugawa, S.; Ueda, T.; Morita, A.; Shimada, S. TRPM8 activation suppresses cellular viability in human melanoma. Am J Physiol-Cell Physiol. 2008, 295, C296–301. [Google Scholar] [CrossRef] [PubMed]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell. 2017, 8, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Julius, D. TRP Channels and Pain. Annu Rev Cell Dev Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Woo, J.H.; Nam, D.Y.; Kim, H.J.; Hong, P.T.L.; Kim, W.K.; Nam, J.H. Nootkatol prevents ultraviolet radiation-induced photoaging via ORAI1 and TRPV1 inhibition in melanocytes and keratinocytes. Korean J Physiol Pharmacol. 2021, 25, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.-Y.; Park, S.-Y.; Jo, J.Y.; Kang, G.; Park, J.B.; Kim, J.-G.; et al. Endogenous expression of TRPV1 channel in cultured human melanocytes. J Dermatol Sci. 2009, 56, 128–130. [Google Scholar] [CrossRef]
- Boudaka, A.; Al-Yazeedi, M.; Al-Lawati, I. Role of Transient Receptor Potential Vanilloid 4 Channel in Skin Physiology and Pathology. Sultan Qaboos Univ Med, J. 2020, 20, e138–e146. [Google Scholar] [CrossRef]
- Fusi, C.; Materazzi, S.; Minocci, D.; Maio, V.; Oranges, T.; Massi, D.; et al. Transient Receptor Potential Vanilloid 4 (TRPV4) Is Downregulated in Keratinocytes in Human Non-Melanoma Skin Cancer. J Invest Dermatol. 2014, 134, 2408–2417. [Google Scholar] [CrossRef]
- Elsevier. The role of TRPV4 channels in cutaneous epithelia. Current Topics in Membranes. 2022; pp 139–54.
- Olivan-Viguera, A.; Garcia-Otin, A.L.; Lozano-Gerona, J.; Abarca-Lachen, E.; Garcia-Malinis, A.J.; Hamilton, K.L.; et al. Pharmacological activation of TRPV4 produces immediate cell damage and induction of apoptosis in human melanoma cells and HaCaT keratinocytes. PLOS ONE. 2018, 13, e0190307. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Umemura, M.; Nakakaji, R.; Tanaka, R.; Sato, I.; Nagasako, A.; et al. Transient receptor potential cation 3 channel regulates melanoma proliferation and migration. J Physiol Sci. 2017, 67, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Margue, C.; Philippidou, D.; Reinsbach, S.E.; Schmitt, M.; Behrmann, I.; Kreis, S. New Target Genes of MITF-Induced microRNA-211 Contribute to Melanoma Cell Invasion. PLoS ONE. 2013, 8, e73473. [Google Scholar] [CrossRef]
- Boyle, G.M.; Woods, S.L.; Bonazzi, V.F.; Stark, M.S.; Hacker, E.; Aoude, L.G.; et al. Melanoma cell invasiveness is regulated by miR-211 suppression of the BRN2 transcription factor. Pigment Cell Melanoma Res. 2011, 24, 525–537. [Google Scholar] [CrossRef]
- Mazar, J.; DeYoung, K.; Khaitan, D.; Meister, E.; Almodovar, A.; Goydos, J.; et al. The Regulation of miRNA-211 Expression and Its Role in Melanoma Cell Invasiveness. PLoS ONE. 2010, 5, e13779. [Google Scholar] [CrossRef]
- Hantute-Ghesquier, A.; Haustrate, A.; Prevarskaya, N.; Lehen’kyi, V. TRPM Family Channels in Cancer. Pharmaceuticals. 2018, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Luanpitpong, S.; Rodboon, N.; Samart, P.; Vinayanuwattikun, C.; Klamkhlai, S.; Chanvorachote, P.; et al. A novel TRPM7/O-GlcNAc axis mediates tumour cell motility and metastasis by stabilising c-Myc and caveolin-1 in lung carcinoma. Br J Cancer. 2020, 123, 1289–1301. [Google Scholar] [CrossRef]
- Egawa, M.; Schmücker, E.; Grimm, C.; Gudermann, T.; Chubanov, V. Expression Profiling Identified TRPM7 and HER2 as Potential Targets for the Combined Treatment of Cancer Cells. Cells. 2024, 13, 1801. [Google Scholar] [CrossRef]
- Xing, Y.; Wei, X.; Wang, M.; Liu, Y.; Sui, Z.; Wang, X.; et al. Stimulating TRPM7 suppresses cancer cell proliferation and metastasis by inhibiting autophagy. Cancer Lett. 2022, 525, 179–197. [Google Scholar] [CrossRef]
- Kijpornyongpan, T.; Sereemaspun, A.; Chanchao, C. Dose-Dependent Cytotoxic Effects of Menthol on Human Malignant Melanoma A-375 Cells: Correlation with TRPM8 Transcript Expression. Asian Pac J Cancer Prev. 2014, 15, 1551–1556. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, H.; Wei, Z.; Wang, X.; Shen, P.; Wang, S.; et al. TRPM8: a potential target for cancer treatment. J Cancer Res Clin Oncol. 2016, 142, 1871–1881. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, W.; Ma, J.; Xu, P.; Zhang, W.; Guo, S.; et al. Downregulated TRPV1 Expression Contributes to Melanoma Growth via the Calcineurin-ATF3-p53 Pathway. J Invest Dermatol. 2018, 138, 2205–2215. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zheng, J.; Wu, T.; He, Y.; Guo, J.; Xu, J.; et al. Activation of TRPV4 Induces Exocytosis and Ferroptosis in Human Melanoma Cells. Int J Mol Sci. 2022, 23, 4146. [Google Scholar] [CrossRef] [PubMed]
- Elzamzamy, O.M.; Penner, R.; Hazlehurst, L.A. The Role of TRPC1 in Modulating Cancer Progression. Cells. 2020, 9, 388. [Google Scholar] [CrossRef]
- Foster, H.M.; Carle, M.N.; Jira, L.R.; Koh, D.W. TRPM2 Channels: A Potential Therapeutic Target in Melanoma? Int J Mol Sci. 2023, 24, 10437. [Google Scholar] [CrossRef]
- Miller, B.A. TRPM2 in Cancer. Cell Calcium. 2019, 80, 8–17. [Google Scholar] [CrossRef]
- Syed Mortadza, S.A.; Wang, L.; Li, D.; Jiang, L.-H. TRPM2 Channel-Mediated ROS-Sensitive Ca2+ Signaling Mechanisms in Immune Cells. Front Immunol. [CrossRef]
- Yamamoto, S.; Shimizu, S. Targeting TRPM2 in ROS-Coupled Diseases. Pharmaceuticals. 2016, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Maliougina, M.; El Hiani, Y. TRPM2: bridging calcium and ROS signaling pathways—implications for human diseases. Front Physiol. 2023. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, M.; Li, S.; Zhang, X.; Zhang, R.; Lyu, H.; et al. TRPM channels in human cancers: regulatory mechanism and therapeutic prospects. Biomark Res. [CrossRef]
- Zhong, T.; Zhang, W.; Guo, H.; Pan, X.; Chen, X.; He, Q.; et al. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm Sin, B. 2022, 12, 1761–1780. [Google Scholar] [CrossRef]
- Köles L, Ribiczey P, Szebeni A, Kádár K, Zelles T, Zsembery, Á. The Role of TRPM7 in Oncogenesis. Int J Mol Sci. 2024, 25, 719. [Google Scholar] [CrossRef] [PubMed]
- Zierler, S.; Yao, G.; Zhang, Z.; Kuo, W.C.; Pörzgen, P.; Penner, R.; et al. Waixenicin A Inhibits Cell Proliferation through Magnesium-dependent Block of Transient Receptor Potential Melastatin 7 (TRPM7) Channels. J Biol Chem. 2011, 286, 39328–39335. [Google Scholar] [CrossRef]
- Erin, N.; Szallasi, A. Carcinogenesis and Metastasis: Focus on TRPV1-Positive Neurons and Immune Cells. Biomolecules. 2023, 13, 983. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Chiang, C.; Xiao, T.; Chen, Y.; Zhao, Y.; et al. The Impact of TRPV1 on Cancer Pathogenesis and Therapy: A Systematic Review. Int J Biol Sci. 2021, 17, 2034–2049. [Google Scholar] [CrossRef]
- Liu, Y.; Mikrani, R.; He, Y.; Faran Ashraf Baig, M.M.; Abbas, M.; Naveed, M.; et al. TRPM8 channels: A review of distribution and clinical role. Eur J Pharmacol. 2020, 882, 173312. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yu, X.; Yang, J.; Lu, L.; Hua, N.; Duan, X.; et al. TRPM2 regulates cell cycle through the Ca2+-CaM-CaMKII signaling pathway to promote HCC. Hepatol Commun. 2023, 7, e0101. [Google Scholar] [CrossRef]
- Ciaglia, T.; Vestuto, V.; Bertamino, A.; González-Muñiz, R.; Gómez-Monterrey, I. On the modulation of TRPM channels: Current perspectives and anticancer therapeutic implications. Front Oncol. 2023, 12, 1065935. [Google Scholar] [CrossRef]
- Zhong, T.; Zhang, W.; Guo, H.; Pan, X.; Chen, X.; He, Q.; et al. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm Sin, B. 2022, 12, 1761–1780. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




