Submitted:
30 July 2025
Posted:
31 July 2025
You are already at the latest version
Abstract
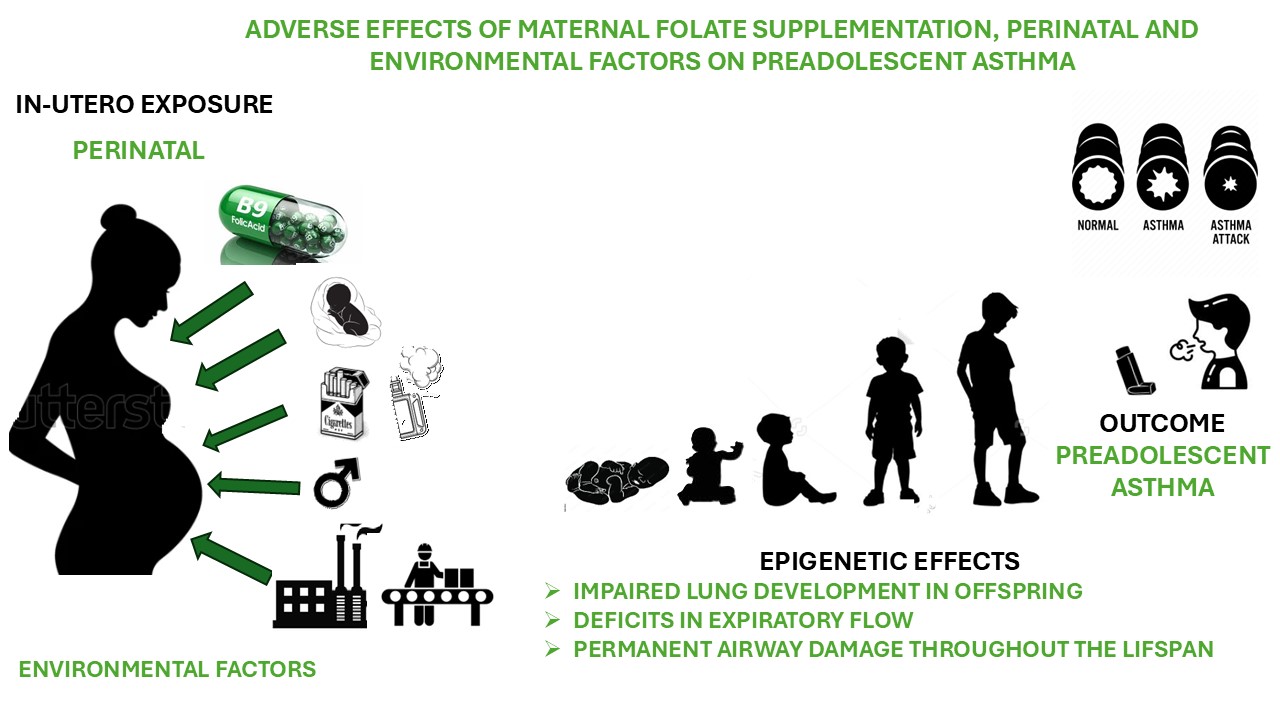
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Ethical Considerations
2.3. Data Collection
2.4. Asthma Status
2.5. Maternal Folic Acid Supplementation
2.6. Statistical Analysis
3. Results
4. Discussion
4.1. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGA | Appropriate for gestational age |
| 95%CI | 95% Confidence Interval |
| HGS | Healthy Growth Study |
| ISAAC | International Study on Asthma and Allergy in Childhood |
| IQR | Interquartile range |
| LGA | Large for gestational age |
| OR | Odds ratio |
| SGA | Small for gestational age |
| WHO | World Health Organization |
References
- Yuan L, Tao J, Wang J, She W, Zou Y, Li R, Ma Y, Sun C, Bi S, Wei S, et al: Global, regional, national burden of asthma from 1990 to 2021, with projections of incidence to 2050: a systematic analysis of the global burden of disease study 2021. eClinical Medicine 2025, 80.
- Global Strategy for Asthma Management and Prevention [https://ginasthma.org/2024-report/].
- Serebrisky D, Wiznia A: Pediatric Asthma: A Global Epidemic. Ann Glob Health 2019, 85:6. [CrossRef]
- Plaza-González S, Zabala-Baños MdC, Astasio-Picado Á, Jurado-Palomo J: Psychological and Sociocultural Determinants in Childhood Asthma Disease: Impact on Quality of Life. Internat J Environ Res Public Health 2022, 19:2652. [CrossRef]
- Holden KA, Hawcutt DB, Sinha IP: Socioeconomic determinants of outcomes in childhood asthma. Paediatr Respir Revs 2025. S1526-0542(25)00028-4. [CrossRef]
- Mallol J: Childhood asthma in developing countries. Low income aspects and related matters. Allergolog. Immunopathol 2000, 28:283-286.
- Asher MI, García-Marcos L, Pearce NE, Strachan DP: Trends in worldwide asthma prevalence. Eur Respir J 2020, 56. [CrossRef]
- Karachaliou F, Vlachopapadopoulou E, Psaltopoulou T, Manios Y, Koutsouki D, Bogdanis G, Carayianni V, Sergentanis T, Hatzakis A, Michalacos S: Prevalence of asthma symptoms and association with obesity, sedentary lifestyle and sociodemographic factors: data from the Hellenic National Action Plan for the assessment, prevention and treatment of childhood obesity (MIS301205). J Asthma 2020, 57:55-61. [CrossRef]
- Konstantakopoulou O KD, Economou C and Charalambous G Barriers in Access to Pharmaceutical Care in Greece: The Case Study of the Out-of-Hospital Management of Patients With Acute Asthma. Front Public Health 2018, 6:199. [CrossRef]
- Johnson SB, Spin P, Connolly F, Stein M, Cheng TL, K. C: Asthma and Attendance in Urban Schools. Prev Chronic Dis 2019, 16:190074. [CrossRef]
- Toyran M, Yagmur IT, Guvenir H, Haci IA, Bahceci S, Batmaz SB, Topal OY, Celik IK, Karaatmaca B, Misirlioglu ED, et al: Asthma control affects school absence, achievement and quality of school life: a multicenter study. Allergolog Immunopathol. 2020, 48:545-552. [CrossRef]
- Reiter J, Ramagopal M, Gileles-Hillel A, Forno E: Sleep disorders in children with asthma. Pediatr Pulmonol 2022, 57:1851-1859. [CrossRef]
- Perry R, Braileanu G, Palmer T, Stevens P: The Economic Burden of Pediatric Asthma in the United States: Literature Review of Current Evidence. Pharmacoeconomics 2019, 37:155-167. [CrossRef]
- Boulieri A, Hansell A, Blangiardo M: Investigating trends in asthma and COPD through multiple data sources: A small area study. Spat Spatiotemporal Epidemiol 2016, 19:28-36. [CrossRef]
- Halterman JS, Auinger P, Conn KM, Lynch K, Yoos HL, Szilagyi PG: Inadequate Therapy and Poor Symptom Control among Children with Asthma: Findings from a Multistate Sample. Ambulatory Pediatr 2007, 7:153-159. [CrossRef]
- Dubaybo BA: The Care of Asthma Patients in Communities with Limited Resources. Res Rep Trop Med 2021, 12:33-38. [CrossRef]
- Barker DJ: The developmental origins of adult disease. J Am Coll Nutr 2004, 23:588S-595S. [CrossRef]
- Warner JO: The early life origins of asthma and related allergic disorders. Arch Dis Child 2004, 89:97-102. [CrossRef]
- Gomez JL: Epigenetics in Asthma. Curr Allergy Asthma Rep 2019, 19:56. [CrossRef]
- Tareke AA, Melak EG, Mengistu BK, Hussen J, Molla A: Association between maternal dietary diversity during pregnancy and birth outcomes: evidence from a systematic review and meta-analysis. BMC Nutrition 2024, 10:151. [CrossRef]
- Chia A-R, Chen L-W, Lai JS, Wong CH, Neelakantan N, Van Dam RM, Chong MF-F: Maternal dietary patterns and birth outcomes: a systematic review and meta-analysis. Adv Nutr 2019, 10:685-695. [CrossRef]
- Chen L-W, Lyons B, Navarro P, Shivappa N, Mehegan J, Murrin CM, Hébert JR, Kelleher CC, Phillips CM: Maternal dietary inflammatory potential and quality are associated with offspring asthma risk over 10-year follow-up: the Lifeways Cross-Generation Cohort Study. Am J Clin Nutr 2020, 111:440-447. [CrossRef]
- De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, Rayco-Solon P: Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database of Systematic Reviews 2015. [CrossRef]
- Li W, Xu B, Cao Y, Shao Y, Wu W, Zhou J, Tan X, Wu X, Kong J, Hu C, et al: Association of maternal folate intake during pregnancy with infant asthma risk. Sci Rep 2019, 9:8347. [CrossRef]
- Yang F, Zhu J, Wang Z, Wang L, Tan T, Sun L: Relationship between maternal folic acid supplementation during pregnancy and risk of childhood asthma: Systematic review and dose-response meta-analysis. Front Pediatr 2022, Volume 10 - 2022. [CrossRef]
- Moschonis G, Tanagra S, Vandorou A, Kyriakou AE, Dede V, Siatitsa PE, Koumpitski A, Androutsos O, Grammatikaki E, Kantilafti M, et al: Social, economic and demographic correlates of overweight and obesity in primary-school children: preliminary data from the Healthy Growth Study. Public Health Nutr 2010, 13:1693-1700. [CrossRef]
- Hellenic National Statistical Service of Greece [https://www.statistics.gr/en/home/].
- Asher M, Keil U, Anderson H, Beasley R, Crane J, Martinez F, Mitchell E, Pearce N, Sibbald B, Stewart A: International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995, 8:483-491. [CrossRef]
- Beasley R, Semprini A, Mitchell EA: Risk factors for asthma: is prevention possible? Lancet 2015, 386:1075-1085. [CrossRef]
- Rosenquist NA, Richards M, Ferber JR, Li DK, Ryu SY, Burkin H, Strickland MJ, Darrow LA: Prepregnancy body mass index and risk of childhood asthma. Allergy 2023, 78:1234-1244. [CrossRef]
- Dumas O, Arroyo AC, Faridi MK, James K, Hsu S, Powe C, Camargo CA, Jr.: Cohort Study of Maternal Gestational Weight Gain, Gestational Diabetes, and Childhood Asthma. Nutrients 2022, 14:5188. [CrossRef]
- Shah R, Newcomb DC: Sex Bias in Asthma Prevalence and Pathogenesis. Front Immunol 2018, Volume 9 - 2018. [CrossRef]
- Leps C, Carson C, Quigley MA: Gestational age at birth and wheezing trajectories at 3-11 years. Arch Dis Child 2018, 103:1138-1144. [CrossRef]
- Zacharasiewicz A: Maternal smoking in pregnancy and its influence on childhood asthma. ERJ Open Research 2016, 2:00042-02016. [CrossRef]
- Zhang GQ, Özuygur Ermis SS, Rådinger M, Bossios A, Kankaanranta H, Nwaru B: Sex Disparities in Asthma Development and Clinical Outcomes: Implications for Treatment Strategies. J Asthma Allergy 2022, 15:231-247. [CrossRef]
- Vink NM, Postma DS, Schouten JP, Rosmalen JG, Boezen HM: Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents' Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol 2010, 126:498-504.e491-496. [CrossRef]
- Miyake K, Kushima M, Shinohara R, Horiuchi S, Otawa S, Akiyama Y, Ooka T, Kojima R, Yokomichi H, Yamagata Z, et al: Maternal smoking status before and during pregnancy and bronchial asthma at 3 years of age: a prospective cohort study. Sci Reps 2023, 13:3234. [CrossRef]
- Gambadauro A, Galletta F, Andrenacci B, Foti Randazzese S, Patria MF, Manti S: Impact of E-Cigarettes on Fetal and Neonatal Lung Development: The Influence of Oxidative Stress and Inflammation. Antioxidants 2025, 14:262. [CrossRef]
- Hayashi T, Adachi Y, Hasegawa K, Morimoto M: Less sensitivity for late airway inflammation in males than females in BALB/c mice. Scand J Immunol 2003, 57:562-567. [CrossRef]
- Papamichael MM, Katsardis C: Chapter 18 - Nutrient intake, epigenetics, and asthma. In Epigenetics in Human Disease (Third Edition). Edited by Tollefsbol T: Academic Press; 2024: 677-716. [CrossRef]
- Barker DJ: The origins of the developmental origins theory. J Intern Med 2007, 261:412-417. [CrossRef]
- Graham IM, O'Callaghan P: Vitamins, homocysteine and cardiovascular risk. Cardiovasc Drugs Ther 2002, 16:383-389. [CrossRef]
- Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, Bailey N, Potts EN, Whitehead G, Brass DM, Schwartz DA: In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest 2008, 118:3462-3469. [CrossRef]
- Baccarelli A, Rusconi F, Bollati V, Catelan D, Accetta G, Hou L, Barbone F, Bertazzi PA, Biggeri A: Nasal cell DNA methylation, inflammation, lung function and wheezing in children with asthma. Epigenomics 2012, 4:91-100. [CrossRef]
- Malmhäll C, Alawieh S, Lu Y, Sjöstrand M, Bossios A, Eldh M, Rådinger M: MicroRNA-155 is essential for T(H)2-mediated allergen-induced eosinophilic inflammation in the lung. J Allergy Clin Immunol 2014, 133:1429-1438, 1438.e1421-1427. [CrossRef]
- Martinez-Nunez RT, Louafi F, Sanchez-Elsner T: The interleukin 13 (IL-13) pathway in human macrophages is modulated by microRNA-155 via direct targeting of interleukin 13 receptor alpha1 (IL13Ralpha1). J Biol Chem 2011, 286:1786-1794. [CrossRef]
- Mayoral RJ, Deho L, Rusca N, Bartonicek N, Saini HK, Enright AJ, Monticelli S: MiR-221 influences effector functions and actin cytoskeleton in mast cells. PLoS One 2011, 6:e26133. [CrossRef]
- Zhang J, Ma C, Yang A, Zhang R, Gong J, Mo F: Is preterm birth associated with asthma among children from birth to 17 years old? -A study based on 2011-2012 US National Survey of Children’s Health. Ital J Pediatr 2018, 44:151. [CrossRef]
- Wang J, Zhang Z, Chen O: What is the impact of birth weight corrected for gestational age on later onset asthma: a meta-analysis. Allergy Asthma Clin Immunol 2022, 18:1. [CrossRef]
- Zhong Z, Chen M, Dai S, Wang Y, Yao J, Shentu H, Huang J, Yu C, Zhang H, Wang T, Ren W: Association of cesarean section with asthma in children/adolescents: a systematic review and meta-analysis based on cohort studies. BMC Pediatr 2023, 23:571. [CrossRef]
- Neuman Å, Hohmann C, Orsini N, Pershagen G, Eller E, Kjaer HF, Gehring U, Granell R, Henderson J, Heinrich J, et al: Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med 2012, 186:1037-1043. [CrossRef]
- Bianco-Miotto T, Craig JM, Gasser YP, van Dijk SJ, Ozanne SE: Epigenetics and DOHaD: from basics to birth and beyond. J Dev Orig Health Dis 2017, 8:513-519. [CrossRef]
- Mallol J, Crane J, von Mutius E, Odhiambo J, Keil U, Stewart A: The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: A global synthesis. Allergolog Immunopathol 2013, 41:73-85. [CrossRef]
- Ellwood P, Asher MI, García-Marcos L, Williams H, Keil U, Robertson C, Nagel G: Do fast foods cause asthma, rhinoconjunctivitis and eczema? Global findings from the International Study of Asthma and Allergies in Childhood (ISAAC) phase three. Thorax 2013, 68:351-360. [CrossRef]
- Dadvand P, Villanueva CM, Font-Ribera L, Martinez D, Basagaña X, Belmonte J, Vrijheid M, Gražulevičienė R, Kogevinas M, Nieuwenhuijsen MJ: Risks and Benefits of Green Spaces for Children: A Cross-Sectional Study of Associations with Sedentary Behavior, Obesity, Asthma, and Allergy. Environ Health Perspectives 2014, 122:1329-1335. [CrossRef]
- Abate BB, Kumsa H, Kibret GA, Wodaynew T, Habtie TE, Kassa M, Munie MA, Temesgen D, Tilahun BD, Merchaw A, et al: Preconception Folic Acid and Multivitamin Supplementation for the Prevention of Neural Tube Defect: An Umbrella Review of Systematic Review and Meta-analysis. Neuroepidemiology 2024:1-14. [CrossRef]
- Adgent MA, Vereen S, McCullough A, Jones SH, Torstenson E, Velez Edwards DR, Hartmann KE, Carroll KN: Periconceptional folic acid supplementation and child asthma: a Right From the Start follow-up study. J Matern Fetal Neonatal Med 2022, 35:10232-10238. [CrossRef]
- Parr CL, Magnus MC, Karlstad Ø, Haugen M, Refsum H, Ueland PM, McCann A, Nafstad P, Håberg SE, Nystad W, London SJ: Maternal Folate Intake during Pregnancy and Childhood Asthma in a Population-based Cohort. Am J Respir Crit Care Med 2017, 195:221-228. [CrossRef]
- Clark NM, Dodge JA, Thomas LJ, Andridge RR, Awad D, Paton JY: Asthma in 10- to 13-year-olds: challenges at a time of transition. Clin Pediatr 2010, 49:931-937. [CrossRef]
- Emmanuel M, BR. B: Tanner Stages. In StatPearls [Internet]. Treasure Island (FL), USA: StatPearls Publishing; 2022.
- IOM: Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, DC USA: National Academy; 1998.
- Wang X, Cheng Z: Cross-Sectional Studies: Strengths, Weaknesses, and Recommendations. Chest 2020, 158:S65-S71. [CrossRef]
- Kuphanga D: Questionnaires in Research: Their Role, Advantages, and Main Aspects.2024.
- Katsardis CV, Alexandraki S, Paraskakis E: Chapter 2: Spirometry in children 6-16 years old In Paediatric Pulmonary Function Testing Indications and Interpretation. Edited by Katsardis Ch, Koumbourlis A, Anthracopoulos M & Paraskakis E. New York, USA NOVA Biomedical.; 2015: 15–42.
| Characteristics. | Pre-adolescent asthma | |||
|---|---|---|---|---|
| No n (%) | Yes n (%) | P-value | ||
| Childhood | ||||
| Age (years), n (Mean ± S.D) | 1876 (11.2 ± 0.7) | 450 (11.1 ±0.6) | 0.040b | |
| Child Sex, n (%) | Males | 892 (47.4) | 263 (58.3) | < 0.001a |
| Females | 989 (52.6) | 188 (41.7) | ||
| Socio-economic Level of School (SEL), n (%) | Lower | 498 (26.5) | 106 (23.6) | 0.017a |
| Medium | 638 (34.0) | 133 (29.6) | ||
| Higher | 743 (39.5) | 211 (46.8) | ||
| Mothers educational level, n (%) | Primary | 127 (7.4) | 24 (5.9) | 0.47a |
| Secondary | 845 (49.3) | 211 (51.6) | ||
| Tertiary | 742 (43.3) | 174 (42.5) | ||
|
Maternal age (years) [Median, (min, max) IQR] |
[40 (26, 58) IQR: 32] | [40(28, 58) IQR: 30] | 0.002c | |
| Environmental | ||||
| Neighborhood has parks areas for exercise, n (%) | Agree | 694 (46.6) | 175 (48.1) | 0.60a |
| Disagree | 797 (53.5) | 189 (51.9) | ||
| Neighborhood too much traffic, n (%) | Agree | 1062 (68.9) | 236 (64.5) | 0.042a |
| Disagree | 456 (31.1) | 130 (35.5) | ||
| Perinatal | ||||
| Gestational diabetes n (%) | Yes | 41(2.2) | 17(3.8) | 0.047a |
| No/I don’t know | 1836(97.8) | 428(96.2) | ||
| Mode of delivery, n (%) | Normal Birth | 1366 (72.6) | 299 (66.3) | 0.008a |
| Caesarean | 515 (27.4) | 152 (33.7) | ||
| Gestational age, n (%) | < 37 weeks | 339 (18.0) | 99 (21.9) | 0.05a |
| ≥ 37 weeks | 1542 (82.0) | 352 (78.1) | ||
| Weight categories for gestational age, n (%) | AGA | 1528 (81.2) | 347 (76.9) | 0.12a |
| SGA | 219 (11.6) | 65 (14.4) | ||
| LGA | 134 (7.1) | 39 (6.7) | ||
| Exclusive breastfeeding, n (%) | Not Exclusive | 1724 (91.6) | 418 (92.7) | 0.47a |
| Exclusive | 157 (8.4) | 33 (7.3) | ||
| Maternal folic acid intake during pregnancy | ||||
| Trimester 1, n (%) | No | 1594 (84.7) | 365 (81.1) | 0.059a |
| Yes | 287 (15.3) | 85 (18.9) | ||
| Trimester 2, n (%) | No | 1487 (79.1) | 337 (74.9) | 0.054a |
| Yes | 394 (20.9) | 113 (25.1) | ||
| Trimester 3, n (%) | No | 1513 (80.4) | 344 (76.4) | 0.059a |
| Yes | 368 (19.6) | 106 (23.6) | ||
| Mother smoking during pregnancy | No | 1578 (83.9) | 381 (84.5) | 0.76a |
| Yes | 303 (16.1) | 70 (15.5) | ||
| Passive smoking during pregnancy, n (%) | No | 1408 (74.9) | 317 (70.3) | 0.047a |
| Yes | 473 (25.1) | 134 (29.7) | ||
| Maternal Folic acid Intake (Yes). | Pre-adolescent asthma | |||||
|---|---|---|---|---|---|---|
| n | cOR (95%CI), P-value | n | aOR (95%CI), Padj* | |||
| 1ST Trimester | 372 | 1.29(0.99, 1.69), 0.059 | 322 | 1.32(0.99, 1.76), p=0.063 | ||
| 2nd Trimester | 507 | 1.27(0.99, 1.61), 0.055 | 445 | 1.30(0.99, 1.68), p=0.051 | ||
| 3rd Trimester | 474 | 1.27(0.99, 1.62), 0.059 | 411 | 1.34(1.03, 1.75), p=0.030 | ||
| Stratified analysis by child sex | ||||||
| cOR (95%CI), P-value | aOR (95%CI), Padj* | |||||
| Strata→ | n | Male | Female | Male | Female | |
| 1ST Trimester | 2066 | 1.42(1.00, 2.01), 0.050 | 1.11(0.73, 1.70), 0.62 | 1.57(1.07, 2.29), 0.018 | 1.00(0.62, 1.61), 0.99 | |
| 2nd Trimester | 2066 | 1.36(0.99, 1.86), 0.053 | 1.09(0,74, 1.59), 0.67 | 1.50(1.07, 2.11), 0.018 | 1.01(0.66, 1.54), 0.96 | |
| 3rd Trimester | 2066 | 1.29(0.93, 1.77), 0.12 | 1.18(0.80, 1.73), 0.41 | 1.36(0.96, 1.93) 0.09 | 1.27(0.84, 1.93), 0.26 | |
| . | Pre-adolescent Asthma | ||||||
|---|---|---|---|---|---|---|---|
| cOR (95%CI), P-value | aOR (95%CI), Padj* | ||||||
| By Gestational age | |||||||
| Maternal Folic acid Intake (Yes) | n | Gestational Age < 37 weeks | Gestational Age ≥ 37 weeks | Gestational Age < 37 weeks | Gestational Age ≥ 37 weeks | ||
| 1st Trimester | 2066 | 1.25(0.69, 2.27), 0.45 | 1.31(0.97,1.76), 0.08 | 1.46(0.77, 2.77), 0.25 | 1.29(0.94, 1,80), 0.12 | ||
| 2nd Trimester | 2066 | 1.12(0.65, 1.92), 0.69 | 1.31(1.00, 1.71), 0.048 | 1.25(0.69, 2.25), 0.45 | 1.33(0.99, 1.77), 0.058 | ||
| 3rd Trimester | 2066 | 1.44(0.83, 2.47), 0.19 | 1.24(0.94,1.63), 0.13 | 1.82(1.01, 3.27), 0.046 | 1.28(0.95, 1.73), 0.11 | ||
| By weight for age | |||||||
| cOR (95%CI), P-value | aOR (95%CI), Padj* | ||||||
| Maternal Folic acid Intake (Yes) | n | Weight for age =AGA | Weight for age =SGA | Weight for age =LGA | Weight for age=AGA | Weight for age = SGA | Weight for age=LGA |
| 1st Trimester | 2066 | 1.39(1.03, 1.88), 0.029 | 0.87(0.40, 185), 0.71 | 1.21(0.44, 3.30), 0.71 | 1.41(1.02, 1.95), 0.036 | 1.02(0.45, 2.34), 0.96 | 0.85(0.24, 2.99), 0.80 |
| 2nd Trimester | 2066 | 1.24(0.94, 1.63), 0.12 | 1.14(0.61, 2.13), 0.68 | 1.81(0.77, 4.27), 0.17 | 1.24(0.92, 1.68), 0.15 | 1.41(0.72, 2.76), 0.32 | 1.84(0.65, 5.20), 0.25 |
| 3rd Trimester | 2066 | 1.20(0.90, 1.59), 0.21 | 1.36(0.73, 2.53), 0.33 | 1.72(0.74, 4.03), 0.21 | 1.24(0.92, 1.69), 0.16 | 1.76(0.90, 3.42), 0.09 | 1.37(0.47, 3.95), 0.56 |
| . | Pre-adolescent Asthma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| cOR (95%CI), P-value | aOR (95%CI), Padj* | ||||||||||
| Maternal smoking | |||||||||||
| Maternal Folic acid Intake (Yes) | n | Maternal Smoking = No |
Maternal smoking = Yes | Maternal Smoking = No | Maternal Smoking = Yes | ||||||
| 1st Trimester | 2066 | 1.31(0.97, 1.75), 0.073 | 1.23(0.64, 2.39), 0.53 | 1.37(1.00, 1.88), 0.050 | 1.12(0.52, 2.40), 0.77 | ||||||
| 2nd Trimester | 2066 | 1.35(1.04, 1.75), 0.025 | 0.92(0.51, 1.69), 0.80 | 1.44(1.08, 1.91), 0.012 | 0.80(0.40, 1.57), 0.51 | ||||||
| 3rd Trimester | 2066 | 1.36(1.03, 1.77), 0.027 | 0.91(0.48, 1.70), 0.76 | 1.52(1.14, 2.02), 0.005 | 0.80(0.40, 1.61), 0.53 | ||||||
| School economic level | |||||||||||
| cOR (95%CI), P-value | aOR (95%CI), Padj* | ||||||||||
| Maternal Folic acid Intake (Yes) | n | SEL = Low | SEL= Medium | SEL = High | SEL = Low | SEL = Medium |
SEL = High |
||||
| 1st Trimester | 2066 | 1.84(1.05, 3.21), 0.034 | 1.02(0.61, 1.70), 0.95 | 1.23(0.84, 1.80), 0.29 | 1.94 (1.07, 3.55), 0.030 | 1.03(0.59, 1.81), 0.92 | 1.26 (0.83, 1.93), 0.27 | ||||
| 2nd Trimester | 2066 | 1.68(1.03,2.77), 0.039 | 1.10(0.70, 1.72), 0.69 | 1.17(0.83,1.66), 0.37 | 1.87 (1.10, 3.19), 0.021 | 1.11(0.68, 1.83), 0.67 | 1.17(0.80, 1.71), 0.42 | ||||
| 3rd Trimester | 2066 | 1.73(1.04,2.85), 0.034 | 1.01(0.64, 1.61), 0.95 | 1.22(0.86,1.75), 0.27 | 2.02 (1.17, 3.49), 0.011 | 1.10(0.66, 1.82), 0.72 | 1.29(0.88, 1.91), 0.19 | ||||
| Neighborhood parks | |||||||||||
| Maternal Folic acid Intake (Yes) | n | Neighborhood parks = Agree | Neighborhood parks = Disagree | Neighborhood parks = Agree | Neighborhood parks = Disagree | ||||||
| cOR (95%CI), P-value | aOR (95%CI), Padj* | ||||||||||
| 1st Trimester | 1664 | 1.15(0.75, 1.77), 0.511 | 1.61(1.08, 2.40), 0.020 | 1.21(0.76, 1.93), 0.413 | 1.60(1.04, 2.49), 0.034 | ||||||
| 2nd Trimester | 1664 | 1.41(0.97, 2.05), 0.069 | 1.18(0.82, 1.72), 0.369 | 1.41(0.94, 2.12), 0.098 | 1.24(0.84, 1.85), 0.282 | ||||||
| 3rd Trimester | 1664 | 1.52(1.04, 2.23), 0.031 | 1.09(0.74, 1.59), 0.673 | 1.52(1.00, 2.30), 0.047 | 1.18(0.78, 1.79), 0.422 | ||||||
| Neighborhood traffic | |||||||||||
| Maternal Folic acid Intake (Yes) | n | Neighborhood traffic = Agree | Neighborhood traffic = Disagree | Neighborhood traffic = Agree | Neighborhood traffic = Disagree | ||||||
| cOR (95%CI), P-value | aOR (95%CI), Padj* | ||||||||||
| 1st Trimester | 1694 | 1.28(0.89, 1.84), 0.187 | 1.57(0.96, 2.56), 0.073 | 1.32(0.89, 1.98), 0.172 | 1.55(0.92, 2.63), 0.103 | ||||||
| 2nd Trimester | 1694 | 1.31(0.95, 1.81), 0.103 | 1.63(1.05, 2.52), 0.029 | 1.39(0.97, 1.98), 0.069 | 1.52(0.95,2.45), 0.081 | ||||||
| 3rd Trimester | 1694 | 1.28(0.92, 1.79), 0.143 | 1.74(1.11, 2.72), 0.016 | 1.37(0.96, 1.97), 0.086 | 1.73(1.07, 2.82), 0.026 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





