Submitted:
29 July 2025
Posted:
30 July 2025
You are already at the latest version
Abstract
Keywords:
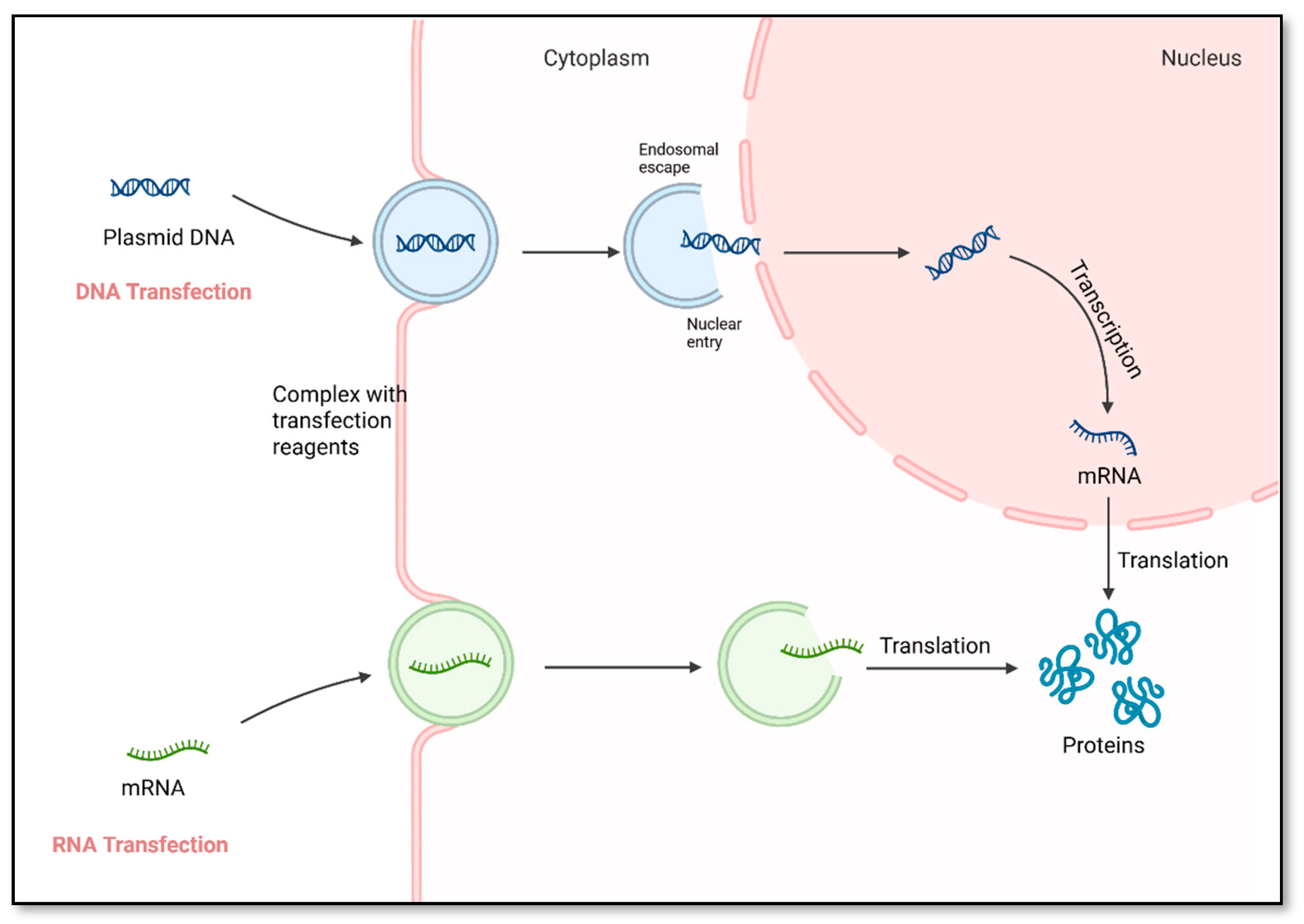
1. Introduction
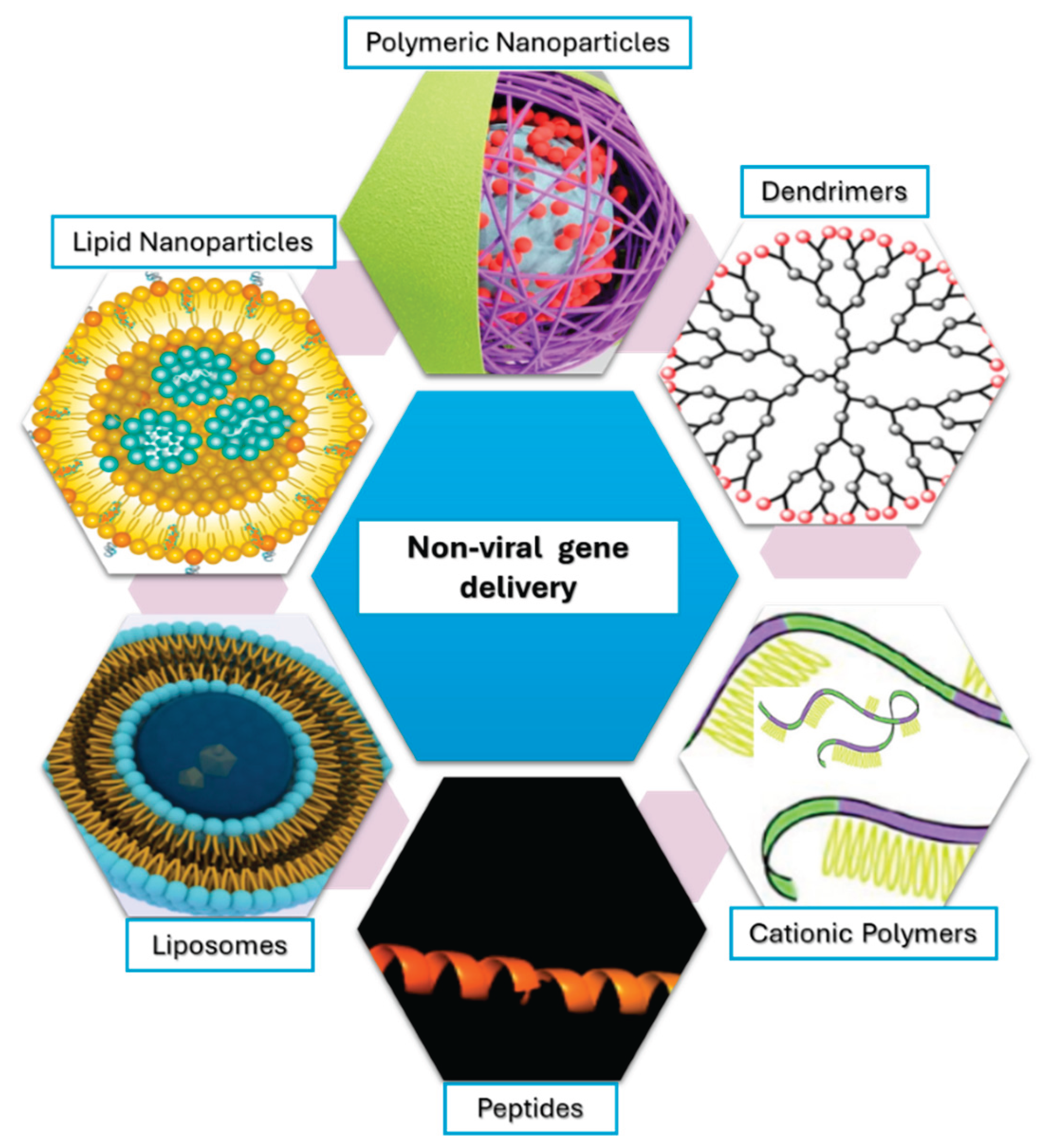
2. Non-Viral Gene Delivery Systems
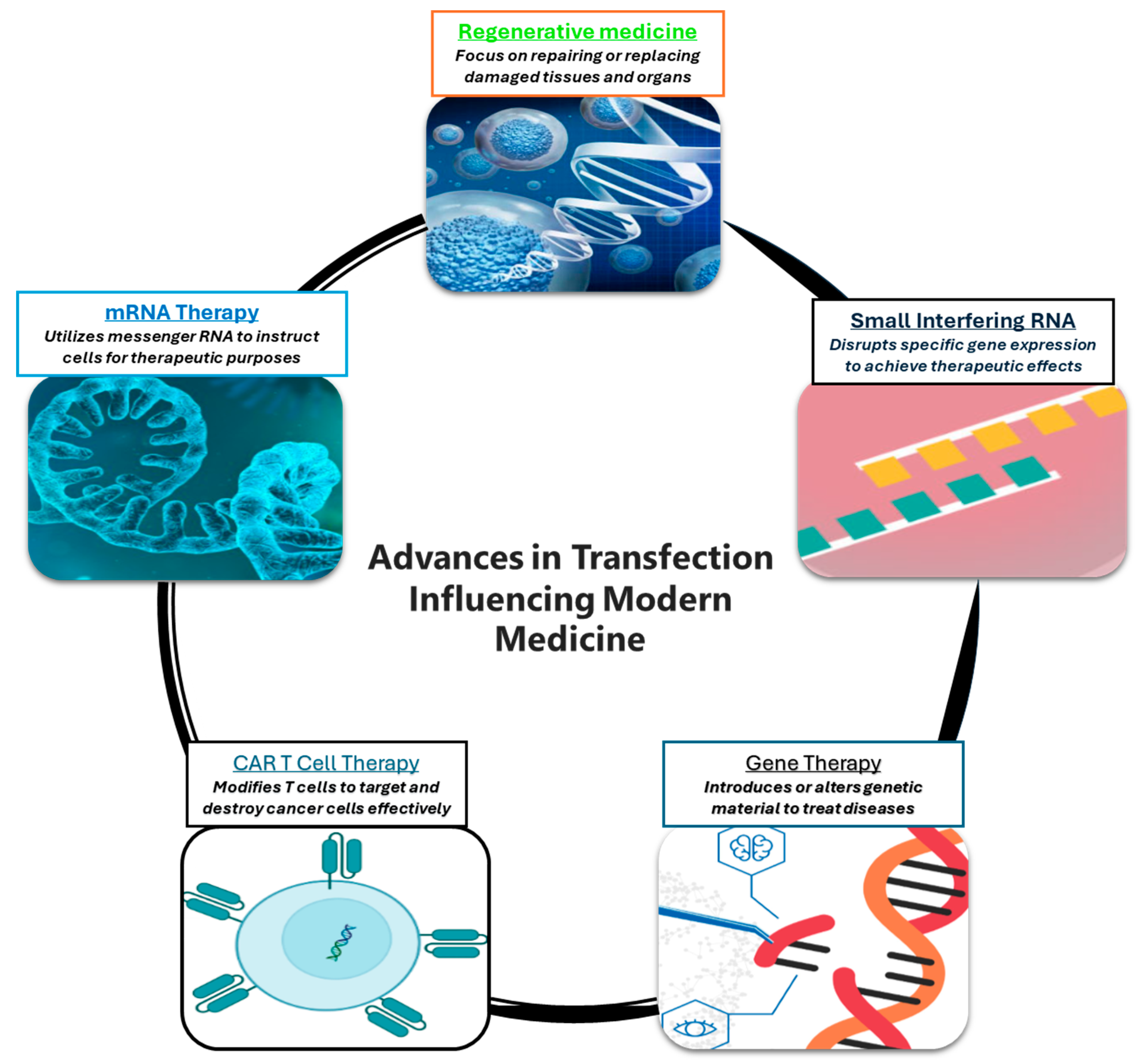
3. Advancing to Clinical Path Through Transfection
3.1. mRNA-Based Therapies
3.2. CAR-T Cell Therapy
3.3. Revolutionizing Gene Therapy
3.4. Small Interfering RNA and Antisense Oligonucleotide Therapies
3.5. Regenerative Medicine
| Category | Company | Drug | Description |
|---|---|---|---|
| mRNA-based Therapies | Moderna | mRNA-1273 | COVID-19 vaccine using lipid nanoparticles for delivery. |
| Pfizer-BioNTech | BNT162b2 | COVID-19 vaccine using lipid nanoparticles for delivery. | |
| Arcturus Therapeutics | ARCT-810 | mRNA therapy for Ornithine Transcarbamylase (OTC) deficiency using lipid nanoparticles. | |
| CureVac | CVnCoV | COVID-19 vaccine candidate using lipid nanoparticles. | |
| Translate Bio (Sanofi) | MRT5005 | mRNA therapy for cystic fibrosis using lipid nanoparticles. | |
| BioNTech | BNT111 | mRNA cancer immunotherapy using lipid nanoparticles. | |
| GenEdit | Various | Developing non-lipid, hydrophilic polymer nanoparticles for autoimmune diseases and other indications. | |
| CAR-T Therapies | Cellectis | UCART19 | Allogeneic CAR-T therapy for leukemia using TALEN gene editing technology. |
| Poseida Therapeutics | P-BCMA-101 | CAR-T therapy for multiple myeloma using piggyBac DNA Modification System. | |
| Precision BioSciences | PBCAR0191 | CAR-T therapy for B-cell malignancies using ARCUS genome editing technology. | |
| Sana Biotechnology | SC291 | CAR-T therapy for hematologic malignancies using fusogen technology for non-viral delivery. | |
| Allogene Therapeutics | ALLO-501 | Allogeneic CAR-T therapy for non-Hodgkin lymphoma using TALEN gene editing technology. | |
| Arcellx | CART-ddBCMA | CAR-T therapy for multiple myeloma using a novel synthetic binding scaffold. | |
| Gene therapy | ElevateBio | Various | Developing a broad portfolio of cell and gene therapies using non-viral delivery systems. |
| Tessera Therapeutics | Gene Writing™ | Pioneering Gene Writing technology to treat diseases at their source. | |
| Mediphage Bioceuticals | ministring DNA (msDNA) | Developing non-viral, safe, and redosable gene therapies using msDNA technology. | |
| Clearside Biomedical | CLS-AX | Developing therapies for chronic eye diseases using non-viral delivery methods. | |
| Code Biotherapeutics | 3DNA | Leveraging a non-viral multivalent synthetic DNA delivery platform for various genetic disorders. | |
| Mana.bio | Various | Using AI-based drug delivery platform for oligonucleotide therapies, including mRNA-based therapeutics. | |
| Nanoscope Therapeutics | MCO-010 | Developing gene therapies for vision impairment and blindness using non-viral delivery systems. | |
| Generation Bio | ceDNA | Developing non-viral genetic medicines with long-term efficacy and support for redosing. | |
| siRNA and ASO Therapies | Alnylam Pharmaceuticals | Onpattro (patisiran) | siRNA therapy for hereditary transthyretin-mediated amyloidosis using lipid nanoparticles. |
| Ionis Pharmaceuticals | Spinraza (nusinersen) | ASO therapy for spinal muscular atrophy. | |
| Arrowhead Pharmaceuticals | ARO-AAT | siRNA therapy for alpha-1 antitrypsin deficiency using TRiM™ platform. | |
| Dicerna Pharmaceuticals | DCR-PHXC | siRNA therapy for primary hyperoxaluria using GalXC™ platform. | |
| Wave Life Sciences | WVE-120101 | ASO therapy for Huntington's disease using stereopure oligonucleotides. | |
| Silence Therapeutics | SLN360 | siRNA therapy for cardiovascular disease using GalNAc conjugation. | |
| ProQR Therapeutics | Sepofarsen | ASO therapy for Leber congenital amaurosis 10 (LCA10). | |
| Arbutus Biopharma | AB-729 | siRNA therapy for chronic hepatitis B using GalNAc conjugation. | |
| OliX Pharmaceuticals | OLX101 | siRNA therapy for hypertrophic scars using asymmetric siRNA technology. | |
| DTx Pharma | FALCON platform drugs | Developing siRNA therapies using Fatty Acid Ligand Conjugated OligoNucleotide (FALCON) platform. | |
| Regenerative Medicine | Aspen Neuroscience | ANPD001 | Autologous iPSC-derived neuron replacement therapy for Parkinson’s Disease. |
| Nanoscope Therapeutics | MCO-010 | Gene therapy for vision impairment and blindness using non-viral delivery systems. | |
| Generation Bio | ceDNA | Developing non-viral genetic medicines with long-term efficacy and support for redosing. | |
| Tessera Therapeutics | Gene Writing™ | Pioneering Gene Writing technology to treat diseases at their source. | |
| Code Biotherapeutics | 3DNA | Leveraging a non-viral multivalent synthetic DNA delivery platform for various genetic disorders. | |
| Clearside Biomedical | CLS-AX | Developing therapies for chronic eye diseases using non-viral delivery methods. |
4. Key Challenges in Transfection
5. Transfection Technologies: Future Perspectives
6. Conclusion
Author Contributions
Funding
Ethics Approval and Consent to Participate
Consent to Participation
Availability of Data and Material
Competing Interest
Acknowledgments
Abbreviations
| AI | Artificial intelligence |
| ALL | Acute lymphoblastic leukemia |
| ASOs | Antisense Oligonucleotides |
| CAR-T | Chimeric antigen receptor- T |
| COVID-19 | Coronavirus disease 2019 |
| CPP | Cell penetrating peptides |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| DMD | Duchenne muscular dystrophy |
| DNA | Deoxy ribonucleic acid |
| EVs | Extracellular vesicles |
| HTS | High-throughput screening |
| HSC | Hematopoietic stem cell |
| LNPs | Lipid Nanoparticles |
| MOF | Metal-organic frameworks |
| mRNA | Messenger RNA |
| PBAEs | Poly(beta-amino esters) |
| PEI | Polyetherimide |
| PLGA | Poly (lactic-co-glycolic acid) |
| RNA | Ribonucleic acid |
| SB | Sleeping Beauty |
| SiRNA | Small interfering RNA |
| SMA | Spinal muscular atrophy |
References
- Wells-Holland, C; Elfick, A. Transfection reflections: fit-for-purpose delivery of nucleic acids. Nat. Rev. Mol. Cell. Biol. 2023, 24, 771–772.
- Fus-Kujawa, A; Prus, P; Bajdak-Rusinek, K; Teper, P; Gawron, K; Kowalczuk, A; Sieron, A.L. An Overview of Methods and Tools for Transfection of Eukaryotic Cells in vitro. Front. Bioeng. Biotechnol. 2021, 9, 701031. [CrossRef]
- Chong, Z.X; Yeap, S.K; Ho, W.Y. Transfection types, methods and strategies: A technical review. Peer J. 2021, 9, e11165.
- Rose, J.K. Optimization of transfection. Curr. Protoc. Cell Biol. 2003, Chapter 20, Unit 20.7.
- Liu, F; Su, R; Jiang, X; Wang, S; Mu, W; Chang, L. Advanced micro/nano-electroporation for gene therapy: recent advances and future outlook. Nanoscale. 2024, 16, 10500–21. [CrossRef]
- Campelo SN, Huang P-H, Buie CR, Davalos RV. Recent advancements in electroporation technologies: from bench to clinic. Annu. Rev. Biomed. Eng. 2023, 25, 77–100.
- Sharma, D; Arora, S; Singh, J; Layek, B. A review of the tortuous path of nonviral gene delivery and recent progress. Int. J. Biol. Macromol. 2021, 183, 2055-2073. [CrossRef]
- Dan, L; Kang-Zheng, L. Optimizing viral transduction in immune cell therapy manufacturing: key process design considerations. J. Transl. Med. 2025, 23(1), 501.
- Beta Lifesci. Understanding transfection and its role in modern cell biology. Frontier News. 2025, June 10.
- Feng, S; Li, Y; Tan, Z; Shen, S. Current landscape of metal-organic framework-mediated nucleic acid delivery and therapeutics. Int. J. Pharm. 2025, 672, 125295. [CrossRef]
- Lawson, H. D.; Nguyen, H. H.; Lee, K.J.; Wongsuwan, N.; Tupe, A.; Lu, M.; Arral, M. L.; Behre, A.; Ling, Z.; Whitehead, K. A.; Feinberg, A. W.; Ren, X.; Zheng, S.-Y. Synthetic Strategy for mRNA Encapsulation and Gene Delivery with Nanoscale Metal-Organic Frameworks. Adv. Funct. Mater. 2025, 35, 2404465.
- Kim, M.; Hwang, Y.; Lim, S.; Jang, H.-K.; Kim, H.-O. Advances in Nanoparticles as Non-Viral Vectors for Efficient Delivery of CRISPR/Cas9. Pharmaceutics 2024, 16, 1197.
- Wu, J.; Liang, J.; Zhang, Y.; Dong, C.; Tan, D.; Wang, H.; Zheng, Y.; He, Q. Strategic Advances in Targeted Delivery Carriers for Therapeutic Cancer Vaccines. Int. J. Mol. Sci. 2025, 26, 6879. [CrossRef]
- Sadiq, S.; Khan, S.; Khan, I.; Khan, A.; Humayun, M.; Wu, P.; Usman, M.; Khan, A.; Alanazi, A. F.; Bououdina, M. A Critical Review on Metal-Organic Frameworks (MOFs) Based Nanomaterials for Bio-Medical Applications: Designing, Recent Trends, Challenges, and Prospects. Heliyon 2024, 10(3), e25521.
- Desai, N.; Rana, D.; Salave, S.; Benival, D.; Khunt, D.; Prajapati, B. G. Achieving Endo/Lysosomal Escape Using Smart Nanosystems for Efficient Cellular Delivery. Molecules 2024, 29(13), 3131.
- Gao, J.; Karp, J. M.; Langer, R.; Joshi, N. The Future of Drug Delivery. Chem. Mater. 2023, 35(2), 359–363.
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A Potential Biopolymer in Drug Delivery and Biomedical Applications. Pharmaceutics 2023, 15(4), 1313. [CrossRef]
- Albuquerque, T.; Faria, R.; Sousa, A.; Neves, A. R.; Queiroz, J. A.; Costa, D. Polymer Peptide Ternary Systems as a Tool to Improve the Properties of Plasmid DNA Vectors in Gene Delivery. J. Mol. Liq. 2020, 309, 113157.
- Yin, H.; Kanasty, R. L.; Eltoukhy, A. A.; Vegas, A. J.; Dorkin, J. R.; Anderson, D. G. Non-Viral Vectors for Gene-Based Therapy. Nat. Rev. Genet. 2014, 15(8), 541–555.
- Yip, B. H. Recent Advances in CRISPR/Cas9 Delivery Strategies. Biomolecules 2020, 10(6), 839.
- Thermo Fisher Scientific. Lipofectamine® CRISPRMAX™ Cas9 Transfection Reagent.
- Sigma-Aldrich. PEI Prime™ Linear Polyethylenimine for Gene Delivery.
- Thermo Fisher Scientific. Lipofectamine® 3000 Transfection Reagent.
- Promega Corporation. ViaFect™ Transfection Reagent.
- Promega Corporation. FuGENE® HD Transfection Reagent.
- Lipofectamine® RNAiMAX in Drug Discovery: Thermo Fisher Scientific.
- Horizon Discovery. DharmaFECT® 1 Transfection Reagent.
- Tretbar, U. S.; Rurik, J. G.; Rustad, E. H.; Sürün, D.; Köhl, U.; Olweus, J.; Buchholz, F.; Ivics, Z.; Fricke, S.; Blache, U. Non-Viral Vectors for Chimeric Antigen Receptor Immunotherapy. Nat. Rev. Methods Primers 2024, 4, 74. [CrossRef]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977.
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156.
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzás, E.I.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Portillo, H.A.D.; et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J. Extracell. Vesicles 2015, 4, 30087. [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763.
- Raguram, A.; Banskota, S.; Liu, D. R. Therapeutic In Vivo Delivery of Gene Editing Agents. Cell 2022, 185(15), 2806–2827. [CrossRef]
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging Non-Viral Vectors for Gene Delivery. J. Nanobiotechnol. 2023, 21(1), 272.
- Zhang, Y.; Wu, Z.-Y. Gene Therapy for Monogenic Disorders: Challenges, Strategies, and Perspectives. J. Genet. Genomics 2024, 51(2), 133–143. [CrossRef]
- Giorgioni, L.; Ambrosone, A.; Cometa, M. F.; Salvati, A. L.; Nisticò, R.; Magrelli, A. Revolutionizing CAR T-Cell Therapies: Innovations in Genetic Engineering and Manufacturing to Enhance Efficacy and Accessibility. Int. J. Mol. Sci. 2024, 25(19), 10365.
- Ghassemi, S.; Durgin, J. S.; Nunez-Cruz, S.; Patel, J.; Leferovich, J.; Pinzone, M.; Shen, F.; Cummins, K. D.; Plesa, G.; Cantu, V. A.; Reddy, S.; Bushman, F. D.; Gill, S. I.; O'Doherty, U.; O'Connor, R. S.; Milone, M. C. Rapid Manufacturing of Non-Activated Potent CAR T Cells. Nat. Biomed. Eng. 2022, 6(2), 118–128. [CrossRef]
- Metzloff, A. E.; Padilla, M. S.; Gong, N.; Billingsley, M. M.; Han, X.; Merolle, M.; Mai, D.; Figueroa-Espada, C. G.; Thatte, A. S.; Haley, R. M.; Mukalel, A. J.; Hamilton, A. G.; Alameh, M.-G.; Weissman, D.; Sheppard, N. C.; June, C. H.; Mitchell, M. J. Antigen Presenting Cell Mimetic Lipid Nanoparticles for Rapid mRNA CAR T Cell Cancer Immunotherapy. Adv. Mater. 2024, 36(26), 2313226.
- Magnani, C. F.; Gaipa, G.; Lussana, F.; Belotti, D.; Gritti, G.; Napolitano, S.; Matera, G.; Cabiati, B.; Buracchi, C.; Borleri, G.; Fazio, G.; Zaninelli, S.; Tettamanti, S.; Cesana, S.; Colombo, V.; Quaroni, M.; Cazzaniga, G.; Rovelli, A.; Biagi, E.; Galimberti, S.; Calabria, A.; Benedicenti, F.; Montini, E.; Ferrari, S.; Introna, M.; Balduzzi, A.; Valsecchi, M. G.; Dastoli, G.; Rambaldi, A.; Biondi, A. Sleeping Beauty-Engineered CAR T Cells Achieve Antileukemic Activity without Severe Toxicities. J. Clin. Invest. 2020, 130(11), 6021–6033. [CrossRef]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci. 2017, 38(4), 406–424.
- Yip, T.; Qi, X.; Yan, H.; Chang, Y. Therapeutic Applications of RNA Nanostructures. RSC Adv. 2024, 14(39), 28807–28821. [CrossRef]
- Han, X.; Gong, N.; Xue, L.; Billingsley, M. M.; El-Mayta, R.; Shepherd, S. J.; Alameh, M.-G.; Weissman, D.; Mitchell, M. J. Ligand-Tethered Lipid Nanoparticles for Targeted RNA Delivery to Treat Liver Fibrosis. Nat. Commun. 2023, 14(1), 75.
- Xiao, W.; Jiang, W.; Chen, Z.; Huang, Y.; Mao, J.; Zheng, W.; Hu, Y.; Shi, J. Advance in Peptide-Based Drug Development: Delivery Platforms, Therapeutics and Vaccines. Signal Transduct. Target. Ther. 2025, 10, 74. [CrossRef]
- Bui, T. A.; Mei, H.; Sang, R.; Ortega, D. G.; Deng, W. Advancements and Challenges in Developing In Vivo CAR T Cell Therapies for Cancer Treatment. EBioMedicine 2024, 106, 105266. [CrossRef]
- Vermes, K. FDA Approves Investigational New Drug Application for Ceramide NanoLiposome. Pharmacy Times 2017, January 14.
- Kubarek, D. FDA Approves Investigational New Drug Application for Ceramide NanoLiposome. Penn State News 2017, June 29.
- Arcturus Therapeutics. Arcturus' Pipeline of mRNA Medicines and Vaccines. Arcturus Therapeutics 2025.
- New Drug Approvals. ARCT-021 (LUNAR-COV19). New Drug Approvals 2021, June 26.
- Nanobiotix. Pipeline Overview—NBTXR3 in the Clinic. Nanobiotix 2025.
- Voss, M. H.; Hussain, A.; Vogelzang, N.; Lee, J. L.; Keam, B.; Rha, S. Y.; Vaishampayan, U.; Harris, W. B.; Richey, S.; Randall, J. M.; Shaffer, D.; Cohn, A.; Crowell, T.; Li, J.; Senderowicz, A.; Stone, E.; Figlin, R.; Motzer, R. J.; Haas, N. B.; Hutson, T. A Randomized Phase II Trial of CRLX101 in Combination with Bevacizumab versus Standard of Care in Patients with Advanced Renal Cell Carcinoma. Ann. Oncol. 2017, 28(11), 2754–2760. [CrossRef]
- Hamaguchi, T.; Tsuji, A.; Yamaguchi, K.; Takeda, K.; Uetake, H.; Esaki, T.; Amagai, K.; Sakai, D.; Baba, H.; Kimura, M.; Matsumura, Y.; Tsukamoto, T. A phase II study of NK012, a polymeric micelle formulation of SN-38, in unresectable, metastatic or recurrent colorectal cancer patients. Cancer Chemother. Pharmacol. 2018, 82(6), 1021–1029.
- Szota, M.; Szwedowicz, U.; Rembialkowska, N.; Janicka-Klos, A.; Doveiko, D.; Chen, Y.; Kulbacka, J.; Jachimska, B. Dendrimer Platforms for Targeted Doxorubicin Delivery—Physicochemical Properties in Context of Biological Responses. Int. J. Mol. Sci. 2024, 25(13), 7201.
- Innovation Pharmaceuticals Inc. Stages of Development. Available online: http://www.ipharminc.com/stages-of-development/ (accessed on 22 July 2025).
- National Cancer Institute. A Phase I Study of [177Lu]Lu-FF58 in Patients With Advanced Solid Tumors. Available online: https://www.cancer.gov/clinicaltrials/NCI-2024-03006 (accessed on 22 July 2025).
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.; Obaidi, H.; Quigley, A.; Fagan, A.; Barron, N.; Clynes, M. Mammalian Cell Culture for Production of Recombinant Proteins: A Review of the Critical Steps in Their Biomanufacturing. Biotechnol. Adv. 2020, 43(1), 107552.
- Vavilis, T.; Stamoula, E.; Ainatzoglou, A.; Sachinidis, A.; Lamprinou, M.; Dardalas, I.; Vizirianakis, I.S. mRNA in the Context of Protein Replacement Therapy. Pharmaceutics 2023, 15(1), 166. [CrossRef]
- Kashte, S.; Gulbake, A.; El-Amin III, S.F.; Gupta, A. COVID-19 Vaccines: Rapid Development, Implications, Challenges and Prospects. Hum. Cell 2021, 34(3), 711–733.
- Sayour, E.J.; Boczkowski, D.; Mitchell, D.A.; Nair, S.K. Cancer mRNA Vaccines: Clinical Advances and Future Opportunities. Nat. Rev. Clin. Oncol. 2024, 21(7), 489–500.
- Zeng, C.; Zhang, C.; Walker, P.G.; Dong, Y. Formulation and Delivery Technologies for mRNA Vaccines. In: Yu, D.; Petsch, B. (Eds.) mRNA Vaccines. Curr. Top. Microbiol. Immunol. 2020, 440, 71–110.
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of mRNA-Based Vaccines. Pharmaceutics 2020, 12(2), 102. [CrossRef]
- Qureischi, M.; Mohr, J.; Arellano-Viera, E.; Knudsen, S.E.; Vohidov, F.; Garitano-Trojaola, A. mRNA-Based Therapies: Pre-Clinical and Clinical Applications. Int. Rev. Cell Mol. Biol. 2022, 372, 1–54.
- Eralp, Y. Application of mRNA Technology in Cancer Therapeutics. Vaccines 2022, 10(8), 1262. [CrossRef]
- Hajj, K.A.; Whitehead, K.A. Tools for Translation: Non-Viral Materials for Therapeutic mRNA Delivery. Nat. Rev. Mater. 2017, 2, 17056.
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The Promise of mRNA Vaccines: A Biotech and Industrial Perspective. NPJ Vaccines 2020, 5, 11.
- Yang, W.; Mixich, L.; Boonstra, E.; Cabral, H. Polymer-Based mRNA Delivery Strategies for Advanced Therapies. Adv. Healthc. Mater. 2023, 12(15), e2202688.
- Mali, S. Delivery Systems for Gene Therapy. Indian J. Hum. Genet. 2013, 19(1), 3–8.
- Kim, T.K.; Eberwine, J.H. Mammalian Cell Transfection: The Present and the Future. Anal. Bioanal. Chem. 2010, 397(8), 3173–3178.
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; Mui, B.L.; Semple, S.C.; Tam, Y.K.; Ciufolini, M.; Witzigmann, D.; Kulkarni, J.A.; van der Meel, R.; Cullis, P.R. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nat. Nanotechnol. 2019, 14(12), 1084–1087. [CrossRef]
- Wei, T.; Cheng, Q.; Min, Y.-L.; Olson, E.N.; Siegwart, D.J. Systemic Nanoparticle Delivery of CRISPR-Cas9 Ribonucleoproteins for Effective Tissue Specific Genome Editing. Nat. Commun. 2020, 11, 3232.
- Kenjo, E.; Hozumi, H.; Makita, Y.; Iwabuchi, K.A.; Fujimoto, N.; Matsumoto, S.; Kimura, M.; Amano, Y.; Ifuku, M.; Naoe, Y.; Inukai, N.; Hotta, A. Low Immunogenicity of LNP Allows Repeated Administrations of CRISPR-Cas9 mRNA into Skeletal Muscle in Mice. Nat. Commun. 2021, 12, 7101. [CrossRef]
- Li, Y.; Tenchov, R.; Smoot, J.; Liu, C.; Watkins, S.; Zhou, Q. A Comprehensive Review of the Global Efforts on COVID-19 Vaccine Development. ACS Cent. Sci. 2021, 7(4), 512–533. [CrossRef]
- Zhang, X.; Zhao, W.; Nguyen, G.N.; Zhang, C.; Zeng, C.; Yan, J.; Du, S.; Hou, X.; Li, W.; Jiang, J.; Deng, B.; McComb, D.W.; Dorkin, R.; Shah, A.; Barrera, L.; Gregoire, F.; Singh, M.; Chen, D.; Sabatino, D.E.; Dong, Y. Functionalized Lipid-Like Nanoparticles for In Vivo mRNA Delivery and Base Editing. Sci. Adv. 2020, 6(34), eabc2315.
- Del Toro Runzer, C.; Anand, S.; Mota, C.; Moroni, L.; Plank, C.; Van Griensven, M.; Balmayor, E.R. Cellular Uptake of Modified mRNA Occurs via Caveolae-Mediated Endocytosis, Yielding High Protein Expression in Slow-Dividing Cells. Mol. Ther. Nucleic Acids 2023, 32, 960–979. [CrossRef]
- Cardarelli, F.; Digiacomo, L.; Marchini, C.; Amici, A.; Salomone, F.; Fiume, G.; Rossetta, A.; Gratton, E.; Pozzi, D.; Caracciolo, G. The Intracellular Trafficking Mechanism of Lipofectamine-Based Transfection Reagents and Its Implication for Gene Delivery. Sci. Rep. 2016, 6, 25879.
- Diorio, C.; Teachey, D.T.; Grupp, S.A. Allogeneic Chimeric Antigen Receptor Cell Therapies for Cancer: Progress Made and Remaining Roadblocks. Nat. Rev. Clin. Oncol. 2025, 22(1), 10–27.
- Maakaron, J.E.; Hu, M.; El-Jurdi, N. Chimeric Antigen Receptor T Cell Therapy for Cancer: Clinical Applications and Practical Considerations. BMJ 2022, 378, e068956.
- Cappell, K.M.; Kochenderfer, J.N. Long-Term Outcomes Following CAR T Cell Therapy: What We Know So Far. Nat. Rev. Clin. Oncol. 2023, 20(6), 359–371. [CrossRef]
- Jamour, P.; Jamali, A.; Langeroudi, A.G.; Sharafabad, B.E.; Abdoli, A. Comparing Chemical Transfection, Electroporation, and Lentiviral Vector Transduction to Achieve Optimal Transfection Conditions in the Vero Cell Line. BMC Mol. Cell Biol. 2024, 25(1), 15.
- Rahimmanesh, I.; Totonchi, M.; Khanahmad, H. The Challenging Nature of Primary T Lymphocytes for Transfection: Effect of Protamine Sulfate on the Transfection Efficiency of Chemical Transfection Reagents. Res. Pharm. Sci. 2020, 15(5), 437–446. [CrossRef]
- Balke-Want, H.; Keerthi, V.; Cadinanos-Garai, A.; Fowler, C.; Gkitsas, N.; Brown, A.K.; Tunuguntla, R.; Abou-El-Enein, M.; Feldman, S.A. Non-Viral Chimeric Antigen Receptor (CAR) T Cells Going Viral. Immuno-Oncol. Technol. 2023, 18, 100375.
- VanderBurgh JA, Corso TN, Levy SL. et al. Scalable continuous-flow electroporation platform enabling T cell transfection for cellular therapy manufacturing. Sci Rep. (2023) 13:6857. [CrossRef]
- Kitte, R.; Rabel, M.; Geczy, R.; Park, S.; Fricke, S.; Koehl, U.; Tretbar, U.S. Lipid nanoparticles outperform electroporation in mRNA-based CAR T cell engineering. Mol. Ther. Methods Clin. Dev. 2023, 31, 101139.
- Dhayalan, M.; Wang, W.; Riyaz, S.U.M.; Dinesh, R.A.; Shanmugam, J.; Irudayaraj, S.S.; Stalin, A.; Giri, J.; Mallik, S.; Hu, R. Advances in functional lipid nanoparticles: from drug delivery platforms to clinical applications. 3 Biotech 2024, 14(2), 57.
- Uslu, U.; June, C.H. Beyond the blood: expanding CAR T cell therapy to solid tumors. Nat. Biotechnol. 2024. [CrossRef]
- Giulimondi, F.; Digiacomo, L.; Renzi, S.; Cassone, C.; Pirrottina, A.; et al. Optimizing transfection efficiency in CAR-T cell manufacturing through multiple administrations of lipid-based nanoparticles. ACS Appl. Bio Mater. 2024, 7(6), 3746–3757.
- Bui, T.A.; Mei, H.; Sang, R.; Ortega, D.G.; Deng, W. Advances and challenges in developing in vivo CAR T cell therapies for cancer treatment. eBioMedicine 2024, 106, 105266.
- Li, J.; Chen, P.; Ma, W. The next frontier in immunotherapy: potential and challenges of CAR-macrophages. Exp. Hematol. Oncol. 2024, 13(1), 76. [CrossRef]
- Kohn, D.B.; Chen, Y.Y.; Spencer, M.J. Successes and challenges in clinical gene therapy. Gene Ther. 2023, 30(10–11), 738–746.
- Cring, M.R.; Sheffield, V.C. Gene therapy and gene correction: targets, progress, and challenges for treating human diseases. Gene Ther. 2022, 29(1–2), 3–12.
- Liu, F.; Li, R.; Zhu, Z.; Yang, Y.; Lu, F. Current developments of gene therapy in human diseases. MedComm 2024, 5(9), e645. [CrossRef]
- Konda, P.; Garinet, S.; Van Allen, E.M.; Viswanathan, S.R. Genome-guided discovery of cancer therapeutic targets. Cell Rep. 2023, 42(8), 112978.
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Zhai, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer stem cells: advances in knowledge and implications for cancer therapy. Signal Transduct. Target Ther. 2024, 9(1), 170.
- Zu, H.; Gao, D. Non-viral vectors in gene therapy: recent development, challenges, and prospects. AAPS J. 2021, 23, 78.
- Liu, B.; Zhou, H.; Tan, L.; et al. Exploring treatment options in cancer: tumor treatment strategies. Signal Transduct. Target Ther. 2024, 9, 175. [CrossRef]
- Wells-Holland, C.; Elfick, A. Transfection reflections: fit-for-purpose delivery of nucleic acids. Nat. Rev. Mol. Cell Biol. 2023, 24(11), 771–772.
- Hou, X.; Zaks, T.; Langer, R.; et al. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094.
- Wang, B.; Shen, B.; Xiang, W.; et al. Advances in the study of LNPs for mRNA delivery and clinical applications. Virus Genes 2024, 60, 577–591. [CrossRef]
- Chen, L.; Hong, W.; Ren, W.; et al. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target Ther. 2021, 6, 225.
- Hu, B.; Zhong, L.; Weng, Y.; et al. Therapeutic siRNA: state of the art. Signal Transduct. Target Ther. 2020, 5, 101. [CrossRef]
- Collotta, D.; Bertocchi, I.; Chiapello, E.; Collino, M. Antisense oligonucleotides: a novel frontier in pharmacological strategy. Front. Pharmacol. 2023, 14, 1304342.
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [CrossRef]
- Zhu, Y.; Zhu, L.; Wang, X.; et al. RNA-based therapeutics: an overview and prospectus. Cell Death Dis. 2022, 13, 644. [CrossRef]
- Alshaer, E.; Zureigat, H.; Karaki, A.A.; et al. siRNA: mechanism of action, challenges, and therapeutic approaches. Eur. J. Pharmacol. 2021, 905, 174178.
- Chemello, F.; Chai, A.C.; Li, H.; Rodriguez-Caycedo, C.; Sanchez-Ortiz, E.; Atmanli, A.; Mireault, A.A.; Liu, N.; Bassel-Duby, R.; Olson, E.N. Precise correction of Duchenne muscular dystrophy exon deletion mutations by base and prime editing. Sci. Adv. 2021, 7(18), eabg4910.
- McDowall, S.; Aung-Htut, M.; Wilton, S.; Li, D. Antisense oligonucleotides and their applications in rare neurological diseases. Front. Neurosci. 2024, 18, 1414658. [CrossRef]
- Sang, A.; Zhuo, S.; Bochanis, A.; et al. Mechanisms of action of the US Food and Drug Administration-approved antisense oligonucleotide drugs. BioDrugs 2024, 38, 511–526.
- Chen, S.; Heendeniya, S.N.; Le, B.T.; et al. Splice-modulating antisense oligonucleotides as therapeutics for inherited metabolic diseases. BioDrugs 2024, 38, 177–203. [CrossRef]
- Lim, K.H.; Han, Z.; Jeon, H.Y.; et al. Antisense oligonucleotide modulation of non-productive alternative splicing upregulates gene expression. Nat. Commun. 2020, 11, 3501. [CrossRef]
- Rinaldi, C.; Wood, M. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [CrossRef]
- Ponti, F.; Campolungo, M.; Melchiori, C.; Bono, N.; Candiani, G. Cationic lipids for gene delivery: many players, one goal. Chem. Phys. Lipids 2021, 235, 105032.
- Aartsma-Rus, A.; Krieg, A.M. FDA approves eteplirsen for Duchenne muscular dystrophy: the next chapter in the eteplirsen saga. Nucleic Acid Ther. 2017, 27(1), 1–3.
- Cullis, P.R.; Felgner, P.L. The 60-year evolution of lipid nanoparticles for nucleic acid delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722.
- Lei, J.; Qi, S.; Yu, X.; Gao, X.; Yang, K.; Zhang, X.; Cheng, M.; Bai, B.; Feng, Y.; Lu, M.; Wang, Y.; Li, H.; Yu, G. Development of mannosylated lipid nanoparticles for mRNA cancer vaccine with high antigen presentation efficiency and immunomodulatory capability. Angew. Chem. Int. Ed. Engl. 2024, 63(13), e202318515. [CrossRef]
- Haque, M.A.; Shrestha, A.; Mikelis, C.M.; Mattheolabakis, G. Comprehensive analysis of lipid nanoparticle formulation and preparation for RNA delivery. Int. J. Pharm. X 2024, 8, 100283.
- Liu, Y.; Huang, Y.; He, G.; Guo, C.; Dong, J.; Wu, L. Development of mRNA lipid nanoparticles: targeting and therapeutic aspects. Int. J. Mol. Sci. 2024, 25(18), 10166. [CrossRef]
- Lauffer, M.C.; van Roon-Mom, W.; Aartsma-Rus, A.; N = 1 Collaborative. Possibilities and limitations of antisense oligonucleotide therapies for the treatment of monogenic disorders. Commun. Med. (Lond.) 2024, 4(1), 6.
- Amiri, A.; Barreto, G.; Sathyapalan, T.; Sahebkar, A. siRNA therapeutics: future promise for neurodegenerative diseases. Curr. Neuropharmacol. 2021, 19(11), 1896–1911. [CrossRef]
- Baylot, V.; Le, T.K.; Taïeb, D.; et al. Between hope and reality: treatment of genetic diseases through nucleic acid-based drugs. Commun. Biol. 2024, 7, 489.
- Sun, J.; Roy, S. Gene-based therapies for neurodegenerative diseases. Nat. Neurosci. 2021, 24, 297–311. [CrossRef]
- García-González, N.; Gonçalves-Sánchez, J.; Gómez-Nieto, R.; Gonçalves-Estella, J.M.; López, D.E. Advances and challenges in gene therapy for neurodegenerative diseases: a systematic review. Int. J. Mol. Sci. 2024, 25(23), 12485. [CrossRef]
- Horch, R.E.; Kneser, U.; Polykandriotis, E.; Schmidt, V.J.; Sun, J.; Arkudas, A. Tissue engineering and regenerative medicine—where do we stand? J. Cell. Mol. Med. 2012, 16(6), 1157–1165.
- Aljabali, A.A.A.; Mohamed, E.-T.; Murtaza, M.T. Principles of CRISPR-Cas9 technology: advances in genome editing and emerging trends in drug delivery. J. Drug Deliv. Sci. Technol. 2024, 92, 105338.
- Li, Z.H.; Wang, J.; Xu, J.P.; Wang, J.; Yang, X. Recent advances in CRISPR-based genome editing technology and its applications in cardiovascular research. Mil. Med. Res. 2023, 10(1), 12.
- Moffat, J.; Komor, A.C.; Lum, L. Impact of CRISPR in cancer drug discovery. Science 2024, 386(6720), 378–379. [CrossRef]
- Sampogna, G.; Guraya, S.Y.; Forgione, A. Regenerative medicine: historical roots and potential strategies in modern medicine. J. Microsc. Ultrastruct. 2015, 3(3), 101–107.
- Yanez Arteta, M.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; Lindfors, L. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115(15), E3351–E3360.
- Xiong, Y.; Mi, B.B.; Shahbazi, M.A.; Xia, T.; Xiao, J. Microenvironment-responsive nanomedicines: a promising direction for tissue regeneration. Mil. Med. Res. 2024, 11(1), 69.
- Wang, C.; Pan, C.; Yong, H.; et al. Emerging non-viral vectors for gene delivery. J. Nanobiotechnol. 2023, 21, 272. [CrossRef]
- Zu, H.; Gao, D. Non-viral vectors in gene therapy: recent development, challenges, and prospects. AAPS J. 2021, 23, 78. [CrossRef]
- Buntz, B. 100 cell and gene therapy leaders to watch in 2025. Drug Discov. Dev. 2024.
- Adam, J. Eight biotech companies advancing the field of siRNA. Labiotech 2024.
- DelveInsight Business Research LLP. 80+ pharma companies unite to shape the future of RNA-based drugs. 2024.
- Buntz, B. 50 leading cell and gene therapy companies. Drug Discov. 2022.
- Knutsen, A. Nonviral platforms streamline gene therapy delivery. Genet. Eng. Biotechnol. News 2023, 43(9), 22–25. [CrossRef]
- Fu, Y.; Han, Z.; Cheng, W.; et al. Improvement strategies for transient gene expression in mammalian cells. Appl. Microbiol. Biotechnol. 2024, 108, 480.
- Zhao, Y.; Sampson, M.G.; Wen, X. Quantify and control reproducibility in high-throughput experiments. Nat. Methods 2020, 17, 1207–1213.
- Shin, H.; Park, S.-J.; Yim, Y.; Kim, J.; Choi, C.; Won, C.; Min, D.-H. Recent advances in RNA therapeutics and RNA delivery systems based on nanoparticles. Adv. Therap. 2018, 1, 1800065. [CrossRef]
- Rinoldi, C.; Zargarian, S.S.; Nakielski, P.; Li, X.; Liguori, A.; Petronella, F.; Presutti, D.; Wang, Q.; Costantini, M.; De Sio, L.; Gualandi, C.; Ding, B.; Pierini, F. Nanotechnology-assisted RNA delivery: from nucleic acid therapeutics to COVID-19 vaccines. Small Methods 2021, 5(9), e2100402.
- Cullis, P.R.; Felgner, P.L. The 60-year evolution of lipid nanoparticles for nucleic acid delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722. [CrossRef]
- Herrera-Barrera, M.; Renee, C.R.; Gautam, M.; et al. Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates. Sci. Adv. 2023, 9, eadd4623.
- Kwon, M.; Firestein, B.L. DNA transfection: calcium phosphate method. Methods Mol. Biol. 2013, 1018, 107–110.
- Jens, C.; Susanne, H.; Schenk, E.P.; Pascal, D.; Ali, D.; Jörg, H. Polyethyleneimine (PEI) in gene therapy: current status and clinical applications. J. Control Release 2023, 362, 667–691.
- Cai, X.; Dou, R.; Guo, C.; Tang, J.; Li, X.; Chen, J.; Zhang, J. Cationic polymers as transfection reagents for nucleic acid delivery. Pharmaceutics 2023, 15(5), 1502. [CrossRef]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in gene therapy: current status and clinical applications. J. Control Release 2023, 362, 667–691.
- Salameh, J.W.; Zhou, L.; Ward, S.M.; Santa Chalarca, C.F.; Emrick, T.; Figueiredo, M.L. Polymer-mediated gene therapy: recent advances and merging of delivery techniques. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 2, e1598. [CrossRef]
- Johnsen, K.B.; et al. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta 2014, 1846, 75–87. [CrossRef]
- O'Brien, K.; et al. Exosomes and their role in immune regulation and cancer. Semin. Cell Dev. Biol. 2020, 102, 55–63.
- Villemejane, J.; Mir, L.M. Physical methods of nucleic acid transfer: general concepts and applications. Br. J. Pharmacol. 2009, 157(2), 207–219.
- Huang, S.; Henderson, T.R.; Dojo Soeandy, C.; Lezhanska, A.; Henderson, J.T. An efficient low-cost means of biophysical gene transfection in primary cells. Sci. Rep. 2024, 14(1), 13179. [CrossRef]
- Napotnik, T.B.; Tamara, P.; Damijan, M. Cell death due to electroporation – a review. Bioelectrochemistry 2021, 141.
- Lonez, C.; Lensink, M.F.; Kleiren, E.; Vanderwinden, J.M.; Ruysschaert, J.M.; Vandenbranden, M. Fusogenic activity of cationic lipids and lipid shape distribution. Cell. Mol. Life Sci. 2010, 67(3), 483–494.
- Narum, S.; Deal, B.; Ogasawara, H.; Mancuso, J.N.; Zhang, J.; Salaita, K. An endosomal escape Trojan horse platform to improve cytosolic delivery of nucleic acids. ACS Nano 2024, 18(8), 6186–6201. [CrossRef]
- Pei, D.; Buyanova, M. Overcoming endosomal entrapment in drug delivery. Bioconjug. Chem. 2019, 30(2), 273–283.
- Cavalcanti, R.R.M.; Lira, R.B.; Riske, K.A. Membrane fusion biophysical analysis of fusogenic liposomes. Langmuir 2022, 38(34), 10430–10441.
- Grau, M.; Wagner, E. Strategies and mechanisms for endosomal escape of therapeutic nucleic acids. Curr. Opin. Chem. Biol. 2024, 81, 102506. [CrossRef]
- Gandek, T.B.; van der Koog, L.; Nagelkerke, A. A comparison of cellular uptake mechanisms, delivery efficacy, and intracellular fate between liposomes and extracellular vesicles. Adv. Healthc. Mater. 2023, 12(25), e2300319.
- Tran, V.A.; Thuan Le, V.; Doan, V.D.; Vo, G.N.L. Utilization of functionalized metal-organic framework nanoparticle as targeted drug delivery system for cancer therapy. Pharmaceutics 2023, 15(3), 931. [CrossRef]
- Alavi, S.E.; Alavi, S.F.; Koohi, M.; et al. Nanoparticle-integrated metal–organic frameworks: a revolution in next-generation drug delivery systems. J. Pharm. Investig. 2024, 54, 751–783.
- Raza, A.; Wu, W. Metal-organic frameworks in oral drug delivery. Asian J. Pharm. Sci. 2024, 19(5), 100951.
- Wang, Z.; Kelley, S.O. Microfluidic technologies for enhancing the potency, predictability and affordability of adoptive cell therapies. Nat. Biomed. Eng. 2025. [CrossRef]
- Hur, J.; Chung, A.J. Microfluidic and nanofluidic intracellular delivery. Adv. Sci. 2021, 8, 2004595.
- Loo, J.; Sicher, I.; Goff, A.; et al. Microfluidic transfection of mRNA into human primary lymphocytes and hematopoietic stem and progenitor cells using ultra-fast physical deformations. Sci. Rep. 2021, 11, 21407.
- Teo, C. Created in BioRender. BioRender.com 2025. https://BioRender.com/ludvsnm.
- Gupta, A.; Andresen, J.L.; Manan, R.S.; Langer, R. Nucleic acid delivery for therapeutic applications. Adv. Drug Deliv. Rev. 2021, 178, 113834. [CrossRef]
- Pavlov, R.V.; Akimov, S.A.; Dashinimaev, E.B.; Bashkirov, P.V. Boosting lipofection efficiency through enhanced membrane fusion mechanisms. Int. J. Mol. Sci. 2024, 25(24), 13540.
- Mollé, L.M.; Smyth, C.H.; Yuen, D.; Johnston, A.P.R. Nanoparticles for vaccine and gene therapy: Overcoming the barriers to nucleic acid delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1809. [CrossRef]
- Hosseini, S.A.; Kardani, A.; Yaghoobi, H. A comprehensive review of cancer therapies mediated by conjugated gold nanoparticles with nucleic acid. Int. J. Biol. Macromol. 2023, 253, 127184.
- Wang, C.; Pan, C.; Yong, H.; Wang, F.; Bo, T.; Zhao, Y.; Ma, B.; He, W.; Li, M. Emerging non-viral vectors for gene delivery: Progress and challenges. J. Nanobiotechnol. 2023, 21, 272.
- Kim, J.; Eygeris, Y.; Ryals, R.C.; Jozić, A.; Sahay, G. Strategies for non-viral vectors targeting organs beyond the liver. Nat. Nanotechnol. 2024, 19(4), 428–447. [CrossRef]
- Simonsen, J.B. Lipid nanoparticle-based strategies for extrahepatic delivery of nucleic acid therapies—challenges and opportunities. J. Control Release 2024, 370, 763–772.
- Lee, S.; Chen, L.; Zhang, X. Non-Viral Gene Delivery Systems for Safe and Targeted Regenerative Therapies. J. Genet. Eng. 2025.
- Mann, Z.; Sengar, M.; Verma, Y.K.; Rajalingam, R.; Raghav, P.K. Hematopoietic stem cell factors: their functional role in self-renewal and clinical aspects. Front. Cell Dev. Biol. 2022, 10, 664261.
- Lee, H.; Rho, W.Y.; Kim, Y.H.; Chang, H.; Jun, B.H. CRISPR-Cas9 gene therapy: non-viral delivery and stimuli-responsive nanoformulations. Molecules 2025, 30(3), 542. [CrossRef]
- Campelo, S.N.; Huang, P.H.; Buie, C.R.; Davalos, R.V. Recent advancements in electroporation technologies: from bench to clinic. Annu. Rev. Biomed. Eng. 2023, 25, 77–100.
- Qin, S.; Tang, X.; Chen, Y.; et al. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct. Target Ther. 2022, 7, 166.
- Lu, R.M.; Hsu, H.E.; Perez, S.J.L.P.; et al. Current landscape of mRNA technologies and delivery systems for new modality therapeutics. J. Biomed. Sci. 2024, 31, 89.
- Shen, G.; Liu, J.; Yang, H.; Xie, N.; Yang, Y. mRNA therapies: pioneering a new era in rare genetic disease treatment. J. Control Release 2024, 369, 696–721. [CrossRef]
- Iyer, V.R.; Kaduskar, B.D.; Moharir, S.C.; Mishra, R.K. mRNA biotherapeutics landscape for rare genetic disorders. J. Biosci. 2024, 49, 33.
- Wei, P.S.; Thota, N.; John, G.; Chang, E.; Lee, S.; Wang, Y.; Ma, Z.; Tsai, Y.H.; Mei, K.C. Enhancing RNA-lipid nanoparticle delivery: organ- and cell-specificity and barcoding strategies. J. Control Release 2024, 375, 366–388.
- Xu, X.; Xia, T. Recent advances in site-specific lipid nanoparticles for mRNA delivery. ACS Nanosci. Au 2023, 3(3), 192–203.
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 2019, 27(4), 710–728. [CrossRef]
- Shi, Y.; Shi, M.; Wang, Y.; You, J. Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications. Signal Transduct. Target Ther. 2024, 9(1), 322. [CrossRef]
- Pardi, N.; Krammer, F. mRNA vaccines for infectious diseases—advances, challenges and opportunities. Nat. Rev. Drug Discov. 2024, 23(11), 838–861.
- Liu, C.; Shi, Q.; Huang, X.; Koo, S.; Kong, N.; Tao, W. mRNA-based cancer therapeutics. Nat. Rev. Cancer 2023, 23(8), 526–543.
- Kazemian, P.; Yu, S.Y.; Thomson, S.B.; Birkenshaw, A.; Leavitt, B.R.; Ross, C.J.D. Lipid-nanoparticle-based delivery of CRISPR/Cas9 genome-editing components. Mol. Pharm. 2022, 19(6), 1669–1686.
- Kirian, R.D.; Steinman, D.; Jewell, C.M.; Zierden, H.C. Extracellular vesicles as carriers of mRNA: opportunities and challenges in diagnosis and treatment. Theranostics 2024, 14(5), 2265–2289.
- Cecchin, R.; Troyer, Z.; Witwer, K.; Morris, K.V. Extracellular vesicles: the next generation in gene therapy delivery. Mol. Ther. 2023, 31(5), 1225–1230. [CrossRef]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; et al. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12.
- Ezzat, K.; Andaloussi, S.E.; Zaghloul, E.M.; et al. PepFect14, a novel cell-penetrating peptide for oligonucleotide delivery in vivo. Nucleic Acids Res. 2011, 39(12), 2163–2171.
- McErlean, E.M.; McCrudden, C.M.; McBride, J.W.; et al. Rational design and characterisation of an amphipathic cell-penetrating peptide for non-viral gene delivery. Int. J. Pharm. 2021, 596. [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13(10), 759–780.
- Simeoni, F.; Morris, M.C.; Heitz, F.; Divita, G. Insight into the mechanism of the peptide-based gene delivery system MPG: implications for delivery of siRNA. Nucleic Acids Res. 2003, 31(11), 2717–2724.
- Crombez, L.; Aldrian-Herrada, G.; Konate, K.; Nguyen, Q.N.; McMaster, G.K.; Brasseur, R.; Heitz, F.; Divita, G. A new potent secondary amphipathic cell-penetrating peptide for siRNA delivery into mammalian cells. Mol. Ther. 2009, 17(1), 95–103.
- Tao, W.; Zeng, X.; Liu, T.; Xiao, Q.; Wang, M.; Pan, Q.; Zhu, X. Emerging concepts of artificial intelligence for rational design of drug delivery systems. Adv. Drug Deliv. Rev. 2023, 195, 114762.
- Zhang, Y.; Sun, C.; Zhao, Y. AI-powered prediction of nanocarrier performance for gene therapy. Nano Today 2023, 48, 101716.
- Moseman, J.; Cheng, L.; Mao, H.-Q. Machine learning helps predict efficient lipid nanoparticle design. Johns Hopkins University Press Release 2025, March.
- Wu, K.; Wang, Z.; Yang, X.; Chen, Y.; Han, Z.; Zhang, J.; Liu, L. T. TransMA: an explainable deep learning model for predicting properties of ionizable lipid nanoparticles in mRNA delivery. Preprint, 2024.
- Kim, M.J.; Lee, Y.; Kim, S.I.; Moon, H. AI-based integration of single-cell transcriptomics and nanocarrier engineering for targeted gene delivery. Small 2023, 19, e2207883.
- Lewoczko, E.; Dorsey, Z.; Zou, Y.; Chen, R.; et al. AI-GPT-driven design of novel lipid nanoparticles for targeted and safe mRNA-based cancer immunotherapy [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2025; Part 1. Cancer Res. 2025, 85(8_Suppl_1), Abstract nr 3761.
- Sharma, A.; Lysenko, A.; Jia, S.; et al. Advances in AI and machine learning for predictive medicine. J. Hum. Genet. 2024, 69, 487–497. [CrossRef]
- Singh, S.; Kumar, R.; Payra, S.; Singh, S.K. Artificial intelligence and machine learning in pharmacological research: bridging the gap between data and drug discovery. Cureus 2023, 15(8), e44359.
- Wu, K.; Wang, Z.; Yang, X.; Chen, Y.; Han, Z.; Zhang, J.; Liu, L. TransMA: An explainable multi-modal deep learning model for predicting properties of ionizable lipid nanoparticles in mRNA delivery. arXiv 2024, arXiv:2407.05736.
- Xu, Y.; Ma, S.; Cui, H.; Chen, J.; Xu, S.; Gong, F.; Golubovic, A.; Zhou, M.; Wang, K.C.; Varley, A.; Lu, R.X.Z.; Wang, B.; Li, B. AGILE platform: a deep learning powered approach to accelerate LNP development for mRNA delivery. Nat. Commun. 2024, 15, 6305. [CrossRef]
- Zhou, Y.; Wang, Z.; Wu, K.; Yang, X.; Zhang, J.; Liu, L. Lipid nanoparticle structure–activity relationships for mRNA delivery: a data-driven approach. J. Control Release 2024, 382, 1–12.
- Wu, K.; Yang, X.; Wang, Z.; Li, N.; Zhang, J.; Liu, L. Data-balanced transformer for accelerated ionizable lipid nanoparticles screening in mRNA delivery. Brief. Bioinform. 2024, 25(3), bbae186. [CrossRef]
| Description | Applications | Clinical Trials (ClinicalTrials.gov) |
References |
|---|---|---|---|
| Liposomes | Targeted cancer therapy-Solid Tumors | NanoLiposome- Phase 1 (NCT02834611 | Card Results | ClinicalTrials.gov) |
Keystone Nano [46,47] |
| Lipid Nanoparticles (LNPs) | mRNA-based enzyme replacement | ARCT-810- Phase 1 (NCT04416126 | Card Results | ClinicalTrials.gov) ARCT-032 -Phase 2 (NCT06747858 | Card Results | ClinicalTrials.gov) |
Arcturus Therapeutics [48,49] |
| Infectious disease vaccine-COVID-19 mRNA Vaccine | ARCT-021- Phase 2 (NCT04480957 | Card Results | ClinicalTrials.gov) |
||
| Polymeric Nanoparticles | Radiation enhancer for cancer | NBTXR3-Phase 1/2/3 (NCT05039632 | Card Results | ClinicalTrials.gov) |
Nanobiotix [50] |
| Tumor-targeted chemotherapy | CRLX101- Phase 1/2a (NCT02187302 | Card Results | ClinicalTrials.gov) |
Lumos Pharma [51] | |
| Nanoparticle drug delivery-Triple-negative Breast Cancer, Small Cell Lung Cancer | NK012-Phase 2 (NCT00951054 | Card Results | ClinicalTrials.gov) |
Nippon Kayaku [52] | |
| Dendrimers | Cancer therapy | DEP SN38: (Clinical Trials register - Search for DEP SN38) | StarPharma [53] |
| Cationic Polymers (e.g., PEI) | Antimicrobial therapy | Brilacidin- Phase 2a (NCT02052388 | Card Results | ClinicalTrials.gov) |
Innovation Pharmaceuticals [54] |
| Peptide | Metastatic tumors | 177Lu-Integrin-Phase 1 (Study Details | Study to Evaluate the Safety and Activity (Including Distribution) of 177Lu-3BP-227 in Subjects With Solid Tumours Expressing Neurotensin Receptor Type 1. | ClinicalTrials.gov) |
PeptiDream [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




