Genetic Basis: Molecular Genetic Framework of Cardiac Involvement in ALS
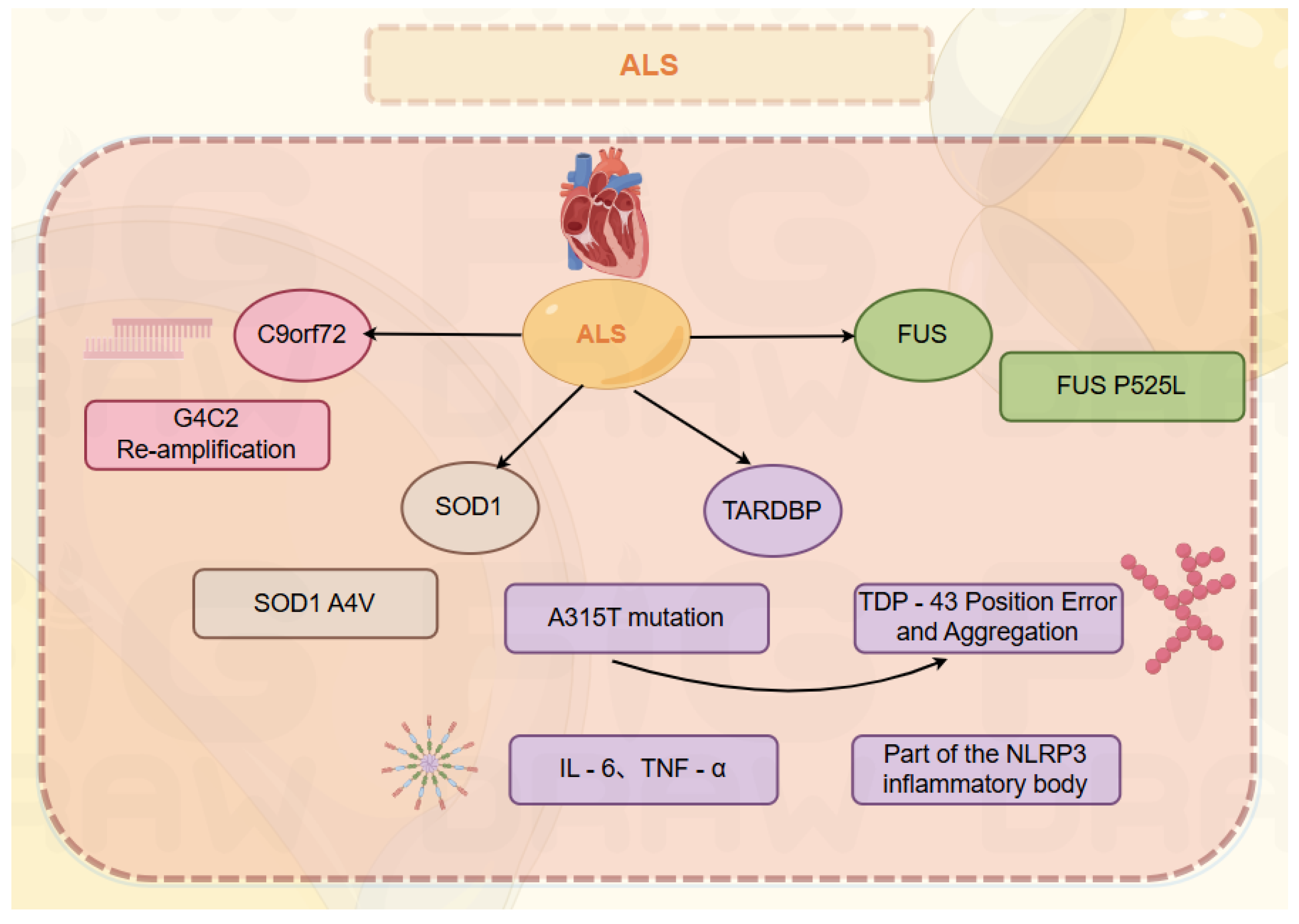
To date, over 50 pathogenic genes for ALS and more than 100 related gene variants (which increase disease susceptibility or influence clinical phenotypes) have been reported [
17]. Statistics indicate that approximately 20% of hereditary ALS cases are caused by SOD1 gene mutations [
3]. Hexanucleotide (G4C2) repeat expansions in the non-coding region of the C9orf72 gene are the most common pathogenic factor in familial ALS (fALS) patients in Europe. Patients carrying these mutations have a relatively higher risk of cardiac complications. Approximately half of familial ALS patients harbor this pathogenic G4C2 repeat expansion, which also occurs in 5%-10% of sporadic cases [
12]. Carriers of C9orf72 variants typically have an earlier onset and milder manifestations; they often present with cognitive impairment or behavioral deficits, predominantly bulbar onset, a family history, and poor prognosis [
16]. Due to differences in genetic backgrounds, the spectrum of ALS pathogenic genes varies across regions. In Europe, the most common pathogenic gene is C9orf72, followed by SOD1, TARDBP, and FUS [
18], whereas C9orf72 variants are less frequent in Asian patients, particularly in Chinese populations [
19]. Other genes associated with rapid disease progression include CAMTA1961, CX3CRI⁹⁷¹, COL19A1⁹⁸, ZNF512B⁹⁹, SOD1 A4V [
13], and FUS P525L [
14]. In China, the C9 genotype is rare, accounting for only ~0.3% of sporadic cases [
15]. In Asia, the most common pathogenic gene is SOD1, followed by FUS, C9orf72, and TARDBP [
18]; in China, SOD1 also has the highest mutation rate, accounting for ~25.60% of familial ALS and 1.60% of sporadic ALS [
19].
For sporadic ALS (sALS), despite its more complex pathogenesis, genetic factors are non-negligible. Studies have found that variations in the 9p21 chromosomal region are associated with myocardial fibrosis, suggesting that genetic susceptibility loci in sALS may impact cardiac health [
4].
To explore the relationship between ALS and myocardial fibrosis, we included a study on cardiomyopathy. Cardiomyopathy is a type of disease characterized by abnormal structure and function of the heart muscle, typically manifested as heart hypertrophy, dilation, or fibrosis, ultimately leading to heart failure, arrhythmia, and even sudden death. Based on the cause, cardiomyopathy can be classified as hereditary and acquired. In recent years, it has been discovered that certain neurodegenerative diseases (such as ALS) especially sALS may also be accompanied by myocardial damage.
In patients with amyotrophic lateral sclerosis (ALS), in addition to the typical degeneration of motor neurons, some cases also show cardiac dysfunction, such as arrhythmia, decreased myocardial contractility, and even heart failure. This phenomenon may be related to the abnormal aggregation of the TDP-43 protein pathology (the key pathological marker of ALS) in myocardial cells (
Figure 1). TDP-43 not only affects the RNA metabolism and mitochondrial function of neurons, but may also damage the energy supply and contractile ability of myocardial cells through similar mechanisms[
44], thereby inducing "ALS cardiomyopathy".
Regarding the TDP-43 gene (TARDBP), its polymorphisms play a key role in cardiac susceptibility. From the perspective of TDP-43’s genetic regulation and cardiac expression, transcriptional regulation of the TARDBP gene is complex; methylation of the SP1 binding site in the promoter region can affect TDP-43 expression levels in cardiomyocytes [
5]. A typical pathological feature in ALS patients is the mislocalization of TDP-43 from the nucleus to the cytoplasm, where it forms ubiquitinated aggregates [
30]. These aggregates are a hallmark of ALS and are present in most cases, regardless of genetic mutations [
31]. The exact mechanisms underlying TDP-43 mislocalization and aggregation remain under investigation. However, recent studies indicate that post-translational modifications—such as phosphorylation, ubiquitination, and cleavage—play important roles. For example, hyperphosphorylation of TDP-43 at specific serine residues (e.g., S409/S410) promotes its cytoplasmic localization and aggregation [
32].
Additionally, mutations in the TARDBP gene (which encodes TDP-43) can cause abnormal protein folding and aggregation. The A315T mutation—one of the most studied TARDBP mutations in ALS—disrupts TDP-43’s normal interactions with RNA and other proteins, leading to increased aggregation and toxicity [
33]. TDP-43’s role in regulating cardiac gene expression is disrupted in ALS. In cardiomyocytes, mislocalized and aggregated TDP-43 fails to perform its normal transcriptional and post-transcriptional regulatory functions, leading to dysregulated expression of genes critical for cardiac function. For instance, genes encoding proteins involved in cardiac contraction (e.g., troponin and myosin heavy chain) have been shown to be abnormally expressed in cardiomyocytes with TDP-43 abnormalities [
34]. A recent transcriptomic analysis of cardiac tissue from ALS patients with cardiomyopathy revealed significantly downregulated expression of genes related to calcium handling—essential for normal myocardial contraction and relaxation [
35]. Such gene dysregulation can ultimately impair cardiac contractility and contribute to cardiomyopathy development. The presence of abnormal TDP-43 in cardiac tissue triggers an inflammatory response. In the hearts of ALS patients with cardiomyopathy, levels of proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) are elevated [
36]. TDP-43 aggregates can activate innate immune pathways in cardiomyocytes and cardiac macrophages. For example, the NLRP3 inflammasome—a key component of the innate immune system—has been shown to be activated by TDP-43 aggregates in cardiac cells [
37]. The resulting chronic inflammation further damages cardiac tissue, impairs normal cardiac function, and promotes cardiomyopathy progression.
TCM Theories on Hereditary Cardiomyopathy in ALS
Based on clinical symptoms, ALS is classified under "flaccidity syndrome" in TCM. Early manifestations include local muscle weakness or atrophy, with rapid progression from onset to death. The London staging system—a simple and practical method for ALS staging [
6]—divides the disease course into 5 stages based on patients’ milestone clinical symptoms [
7]. Its criteria reflect the expansion of ALS lesions, which correlates with the evolution of disease location and progression in TCM. Studies have shown that spleen-kidney deficiency is the primary syndrome, present throughout ALS pathogenesis [
8]; liver-kidney deficiency is more common in stages 2-4, while insufficient pectoral qi is typical in stage 4b or 5 [
9].
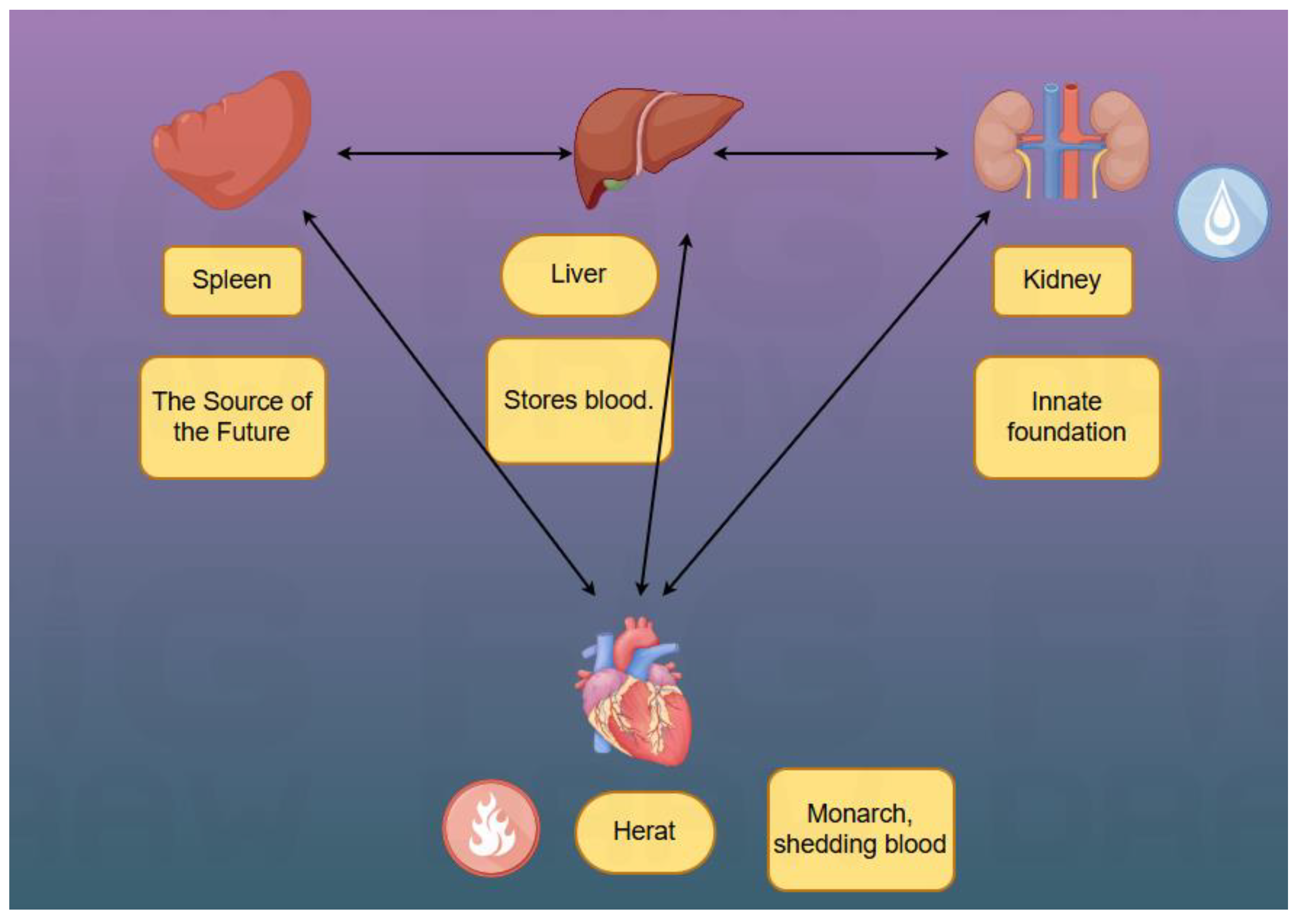
In TCM theory, the kidney is the "congenital foundation," and the spleen is the "acquired foundation." The "hereditary" nature of the disease is attributed to "congenital endowment deficiency"—i.e., deficient parental essence or inadequate fetal nourishment leads to insufficient congenital kidney essence and weak visceral function in offspring, forming the internal basis for disease onset. Su Wen · Jing Mai Bie Lun (Canon of Medicine: Treatise on Divergent Meridians) states: "Food qi enters the stomach; turbid qi returns to the heart, and nutrient essence flows into the vessels" and "the spleen governs transportation and transformation"—responsible for converting cereal essence into qi and blood, serving as the source of qi and blood production. "The heart governs the blood vessels" and relies on sufficient qi and blood for normal circulation and mental activity. If spleen deficiency impairs transportation, qi and blood production decreases, depriving the heart vessels of nourishment, potentially causing heart qi or blood deficiency, manifested as palpitations. Symptoms such as "palpitations", "chest obstruction", "breathing disorders" and "edema" are precisely the typical manifestations of cardiomyopathy in traditional Chinese medicine. Pi Wei Lun (Treatise on the Spleen and Stomach) notes: "Spleen-stomach deficiency leads to obstruction of the nine orifices and inability to move the limbs." The spleen governs muscles; spleen deficiency can indirectly cause poor circulation in heart vessels (
Figure 2).
The kidney belongs to "water" and the heart to "fire"—"heart-kidney interaction maintains balance between water and fire." Additionally, the kidney stores essence, which can transform into blood ("essence and blood share the same origin"). Deficient kidney essence reduces essence and blood production, depriving the heart of nourishment, leading to heart blood deficiency—manifested as palpitations, dizziness, and amnesia.
"The liver stores blood, and the heart circulates blood"; "the liver governs dispersion and regulates mental activity." Liver deficiency can affect the heart. Lin Zheng Zhi Nan Yi An (Guide to Clinical Practice) proposes: "The liver and kidney share the same origin and should be treated together," emphasizing the importance of regulating both. Liver-kidney yin deficiency generates internal deficient fire, burning blood vessels and causing heart blood stasis—manifested as palpitations, chest pain, and dark red tongue. Concurrent liver-kidney deficiency leads to disordered liver dispersion and impaired kidney qi reception, disrupting qi movement and further impairing the heart’s vessel-governing function—manifested as chest tightness, shortness of breath, and palpitations. The Jueyin Liver Meridian "runs behind the throat and enters the pharynx," so liver-kidney impairment may also cause speech difficulties and dysphagia. Key manifestations of liver-kidney deficiency syndrome include marked muscle disuse/atrophy, fasciculations, flaccid tendons, insomnia with dreams, emotional depression, red tongue with scanty coating, and thready rapid pulse [
10].
Su Wen · Wei Lun (Canon of Medicine: Treatise on Flaccidity) mentions "flaccid feet" and "tendon flaccidity," emphasizing their association with "deficiency of the five zang-organs." Cardiomyopathy falls under "palpitations" and "chest impediment" in TCM, with "disharmony of heart vessels" as the core. Despite different disease locations, "congenital endowment deficiency" causes "kidney-spleen-liver-heart" dysfunction, forming a complex syndrome of "deficiency (of essence, qi, blood, yin, yang) + stasis (qi stagnation and blood stasis)"—deficiency as the root and stasis as the branch—ultimately manifesting as concurrent "flaccidity syndrome" and "palpitations," consistent with the multi-system damage in "ALS hereditary cardiomyopathy."
A study on 77 ALS patients using EMG and TCM syndrome analysis found widespread neurogenic damage in all patients: 41 cases in the medullary segment, 77 in the cervical segment, 29 in the thoracic segment, and 64 in the lumbosacral segment. Sensory and nerve conduction velocities were roughly normal, with reduced compound muscle action potential amplitude as a common finding. Stratified by involved segments, syndromes were predominantly liver-kidney deficiency, spleen-stomach weakness, and collateral stasis. Among patients with thoracic segment neurogenic damage, liver-kidney deficiency (48.3%) and collateral stasis (27.6%) were primary; among those without, spleen-stomach weakness (39.6%) and liver-kidney deficiency (31.3%) predominated [
11].
Exploring Treatments for ALS Cardiomyopathy: Integrated Chinese-Western Medicine Strategies
Heart failure, as a major cause of death in cardiomyopathy, is usually the focus of treatment and prevention for heart-related diseases. The drug treatment for symptoms of heart failure mainly falls into the following two aspects: To improve and alleviate symptoms such as palpitations and chest tightness, clinical treatment usually employs diuretics (such as furosemide, spironolactone, etc.) and vasodilators (such as nitroglycerin) to alleviate symptoms and hemodynamics by reducing the cardiac volume load and preload [
45]. Oxidative stress can activate multiple signaling pathways and lead to organ fibrosis. Therefore, a class of antioxidants with anti-oxidative stress effects (such as N-acetylcysteine) is also often used as a potential drug. Animal experiments on hypertrophic cardiomyopathy have shown that N-acetylcysteine has the effect of reversing myocardial hypertrophy, myocardial fibrosis, and disordered cell arrangement, and preventing heart failure [
46]. The cornerstone drugs for improving the long-term prognosis of heart failure symptoms include renin-angiotensin system inhibitors, beta-blockers, and new SGLT2 inhibitors. When ALS patients have a rapid progression of the disease and develop life-threatening symptoms related to heart failure, these drugs may offer a glimmer of hope for the patients.
However, long-term use of diuretics may lead to electrolyte imbalance. Therefore, traditional Chinese medicine's syndrome differentiation and adjustment therapy holds significant importance for patients with stable-stage cardiomyopathy[
47]. In traditional Chinese medicine theory, the pathogenesis of cardiomyopathy is complex and involves both deficiency and excess. During clinical diagnosis and treatment, different treatments are given based on the different diagnostic conditions. The "Discourse on Regulating the Meridians" in "The Inner Classic" states: "When qi and blood are out of balance, various diseases will arise." The blood stasis caused by the depletion of heart qi is one of the main causes of cardiomyopathy. The "Difficult Classic" mentions: "Qi is the foundation of the human body." When qi is deficient, it is unable to carry blood, and blood stasis subsequently occurs; at the same time, the functions of qi and blood in protecting the human body are lost, and vitality is difficult to maintain. Therefore, from a traditional Chinese medicine perspective, the treatment of cardiomyopathy mainly focuses on nourishing qi and yin, promoting blood circulation, and relaxing meridians[
48].
In exploring treatments for ALS-related cardiac lesions, Western medicine and TCM-guided interventions exhibit distinct characteristics and synergistic potential. Current ALS treatments include medication [
39], targeted therapy, gene therapy, TCM herbal therapy, and acupuncture [
38]. FDA-approved drugs for ALS include riluzole, edaravone, dextromethorphan hydrobromide, and quinidine sulfate, which delay disease progression and extend survival. However, these drugs often have side effects and are costly [
20]. Western gene therapy focuses on key pathogenic mechanisms; gene silencing in such strategies inhibits harmful gene expression using antisense oligonucleotides (ASOs) or RNA interference [
21]. The first ASO approved for ALS marks a new era in treatment [
22]. ASO-based gene therapies for ALS target 3 specific gene mutations (SOD1, C9orf72, FUS) and 1 potential modifier of TDP-43 (ATXN2) [
23].
There is a new development: a combined cellular-gene therapy for ALS has been adopted.NurOwn is the first cell therapy for ALS. It uses adult stem cells from the patient's own bone marrow, which are expanded and subjected to exogenous intervention. These cells can secrete various neurotrophic factors (NTFs) in vitro. Then, they are injected intrathecally back into the patient's body to exert neuroprotective effects. It can also stimulate the growth of motor neurons and promote the re-distribution of nerve-muscle junction nerves. [
42] In a phase III clinical trial involving 196 patients, the safety and efficacy of repeated use of NurOwn in ALS patients were evaluated. The results showed that neither the primary nor the secondary endpoints reached statistical significance. However, in the cerebrospinal fluid of patients who received this treatment, there were significant improvements in markers related to neuroinflammation, neurodegeneration, and neuroprotection, and the levels of NTFs also increased. Moreover, the treatment was well tolerated [
43].
In TCM treatment of ALS, most herbs are warm, neutral, or slightly warm in nature, with sweet, bitter, and pungent flavors predominating. Su Wen · Xuan Ming Wu Qi (Canon of Medicine: Manifestations of the Five Qi) states: "Sour enters the liver, pungent enters the lung, bitter enters the heart, salty enters the kidney, sweet enters the spleen." These three flavors primarily act on the heart, spleen, and lung: sweet tonifies, harmonizes, and relieves; bitter purges and dries; pungent disperses and promotes circulation. Thus, ancient formulas for flaccidity syndrome focus on tonifying deficiency, clearing heat, promoting qi, and activating blood. In terms of meridian tropism, they mainly act on the liver, spleen, and kidney meridians: the liver governs tendons (liver heat causes tendon spasm; deficient nourishment leads to flaccidity); the Taiyin Spleen Meridian, rich in qi and blood, nourishes muscles/limbs (spleen deficiency reduces qi/blood, causing muscle atrophy); the kidney, the congenital foundation, governs bones and generates marrow (kidney deficiency leads to bone withering and marrow depletion, causing bone flaccidity). Ancient TCM formulas for flaccidity syndrome primarily use warm/neutral tonics, supplemented by cold herbs to eliminate pathogens, focusing on tonifying the liver, spleen, and kidney, clearing lung heat, and dispelling wind, cold, and dampness [
24]. Representative formulas include Si Junzi Decoction [
26], Buzhong Yiqi Decoction [
25], and Dihuang Yinzi (Rehmannia Decoction) [
27], which have shown clinical efficacy. In exploring the association between ALS and cardiac lesions, clinical manifestations of cardiac dysfunction and related assessment methods are critical. Diastolic dysfunction—an early detectable cardiac functional change—exhibits unique characteristics in ALS patients. The innovative integrated Chinese-Western medicine model aims to integrate Western precision gene therapy with TCM syndrome differentiation. By establishing a "gene-syndrome associated diagnosis and treatment" framework, it correlates mutations (e.g., TARDBP) with TCM syndromes. For example, patients with abnormal TDP-43 deposition due to specific gene mutations, presenting with "phlegm-stasis intertwinement," may receive gene editing or ASO therapy combined with phlegm-resolving and stasis-dispersing herbal formulas or monomers, achieving multi-level intervention from gene to systemic regulation, and providing more comprehensive, personalized solutions for ALS-related cardiac lesions. Additionally, with technological advancements, AI has entered ALS treatment. Researchers such as Pun FW utilized PandaOmics—a cloud-based platform integrating deep learning and AI—to analyze transcriptomic and proteomic data from central nervous system samples in public datasets, as well as direct iPSC-derived motor neurons (diMNs) from the Answer ALS project, identifying potential drug targets and predicting druggability [
29]. The study identified 17 high-confidence therapeutic targets for ALS, including ADRA2B, CYBB, and FLT1. Furthermore, in a c9ALS Drosophila model, 8 previously unreported genes (KCNB2, KCNS3, ADRA2B, NR3C1, P2RY14, PPP3CB, PTPRC, RARA) were validated; their inhibition significantly alleviated neurodegeneration, demonstrating AI’s potential to accelerate the discovery of new therapeutic targets [
28].
Conclusion
The genetic aspects of ALS cardiomyopathy have received relatively less research at present. The cause of cardiomyopathy due to genetic inheritance or mutation of the ALS gene still requires further exploration.ALS cardiomyopathy is closely related to TDP-43, and the underlying mechanism still needs to be explored..
TCM, starting from "flaccidity syndrome," has established a theoretical system based on visceral interactions.Among them, deficiency of yang in the spleen and kidney, deficiency of yin in the liver and kidney, and insufficiency of qi are the causes leading to ALS cardiomyopathy. All of these can lead to a deficiency of heart qi, resulting in chest tightness, palpitations and heart palpitations. And the yang energy gradually accumulates, eventually leading to a deficiency of yang in the heart, resulting in symptoms such as feeling cold and palpitations. The progression of ALS cardiomyopathy can lead to heart failure. Clinical studies have confirmed correlations between syndrome distribution and neurogenic damage segments, providing a basis for syndrome differentiation.
In treatment, Western medicine has advanced in drugs, supportive care, and gene therapy but faces challenges like side effects and high costs. The integrated Chinese-Western medicine model, by correlating genes with syndromes, holds promise for personalized, multi-level intervention. AI technology accelerates target discovery, opening new avenues for drug development. Future research should deepen genetic mechanism studies, optimize integrated treatment protocols, and advance AI applications to improve ALS diagnosis/treatment, enhance prognosis, overcome current bottlenecks, and provide more effective strategies for managing ALS and its complex manifestations like cardiac involvement.