Submitted:
18 July 2025
Posted:
22 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Ultra-Processed Foods
3. Impact of Ultra-Processed Foods on Gut Microbiota and Intestinal Homeostasis
4. Impact of Food Additives on Gut Microbiota
4.1. Emulsifiers
4.2. Non-Caloric Artificial Sweeteners
4.3. Maltodextrin
4.4. Carrageenan
4.5. Synthetic Colorants (Azo Dyes)
4.6. Nanoparticles and Microparticles
5. Dysbiosis and Modulation of the Intestinal Microbiota in IBD
5.1. Microbial Dysbiosis in IBD
5.2. Gut Microbiota Modulation in IBD
5.3. Dietary Patterns
5.4. Prebiotics
5.5. Probiotics
5.6. Symbiotic
5.7. Postbiotics
5.8. Fecal Microbiota Transplantation (FMT)
6. The Complex Relationship: UPF, Gut Microbiota, and IBD
7. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| CMC | Carboxymethylcellulose |
| CD | Crohn's disease |
| CDED | Crohn's disease Exclusion Diet |
| IBD | Inflammatory bowel diseases |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-18 | Interleukin-18 |
| LPS | Lipopolysaccharides |
| MASLD | Metabolic dysfunction-associated steatohepatitis |
| NAS | Non-caloric artificial sweeteners |
| NCDs | Non-communicable chronic diseases |
| NF-kB | Fator Nuclear Kappa B |
| SCFAs | Short-chain fatty acids |
| SFA | Saturated fatty acids |
| TIO2 | Titanium dioxide |
| TLR | Toll-like receptor |
| TNF-α | Tumor Necrosis Factor |
| UC | Ulcerative colitis |
| UPF | Ultra-processed foods |
References
- Ultra-processed foods and the nutrition transition: Global, regional and national trends, food systems transformations and political economy drivers - Baker - 2020 - Obesity Reviews - Wiley Online Library [Internet]. [citado 9 de julho de 2025]. Disponível em: https://onlinelibrary.wiley.com/doi/10.1111/obr.13126.
- Ultra-processed products are becoming dominant in the global food system - Monteiro - 2013 - Obesity Reviews - Wiley Online Library [Internet]. [citado 9 de julho de 2025]. Disponível em: https://onlinelibrary.wiley.com/doi/10.1111/obr.12107.
- Bernard Srour*, Melissa C Kordahi*, Erica Bonazzi*, Mélanie Deschasaux-Tanguy, Mathilde Touvier†, Benoit Chassaing. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol Hepatol. 8 de agosto de 2022;
- Jardim, M.Z.; Costa BVde, L.; Pessoa, M.C.; Duarte, C.K. Ultra-processed foods increase noncommunicable chronic disease risk. Nutrition Research. 1o de novembro de 2021, 95, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Pourmotabbed, A.; Talebi, S.; Mehrabani, S.; Bagheri, R.; Ghoreishy, S.M.; et al. The association of ultra-processed food consumption with adult inflammatory bowel disease risk: a systematic review and dose-response meta-analysis of 4 035 694 participants. Nutrition Reviews. 1o de julho de 2024, 82, 861–71. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.; Knudsen, A.; Arnesen, E.K.; Hatlebakk, J.G.; Sletten, I.S.; Fadnes, L.T. Diet, Food, and Nutritional Exposures and Inflammatory Bowel Disease or Progression of Disease: an Umbrella Review. Advances in Nutrition. 1o de maio de 2024, 15, 100219. [Google Scholar] [CrossRef] [PubMed]
- Lane, M.M.; Gamage, E.; Du, S.; Ashtree, D.N.; McGuinness, A.J.; Gauci, S.; et al. Ultra-processed food exposure and adverse health outcomes: umbrella review of epidemiological meta-analyses. BMJ. 28 de fevereiro de 2024, 384, e077310. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet. 23 de dezembro de 2017, 390, 2769–78. [Google Scholar]
- Magro, D.O.; Rossoni, C.; Saad-Hossne, R.; Santos, A. INTERACTION BETWEEN FOOD PYRAMID AND GUT MICROBIOTA. A NEW NUTRITIONAL APPROACH. Arq Gastroenterol. 12 de maio de 2023, 60, 132–6. [Google Scholar] [CrossRef] [PubMed]
- Racine, A.; Carbonnel, F.; Chan, S.S.M.; Hart, A.R.; Bueno-de-Mesquita, H.B.; Oldenburg, B.; et al. Dietary Patterns and Risk of Inflammatory Bowel Disease in Europe: Results from the EPIC Study. Inflammatory Bowel Diseases. 1o de fevereiro de 2016, 22, 345–54. [Google Scholar] [CrossRef] [PubMed]
- Louzada, M.L.D.C.; Gabe, K.T. Classificação de alimentos Nova: uma contribuição da epidemiologia brasileira. Rev bras epidemiol [Internet]. 2025 [citado 9 de julho de 2025];28. Disponível em: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1415-790X2025000100201&tlng=pt.
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. abril de 2019, 22, 936–41. [Google Scholar] [CrossRef] [PubMed]
- Hracs, L.; Windsor, J.W.; Gorospe, J.; Cummings, M.; Coward, S.; Buie, M.J.; et al. Global evolution of inflammatory bowel disease across epidemiologic stages. Nature. junho de 2025, 642, 458–66. [Google Scholar] [CrossRef] [PubMed]
- Tabela de Aditivos - ANVISA.
- Llavero-Valero, M.; Martín, J.E.S.; Martínez-González, M.A.; Basterra-Gortari, F.J.; Fuente-Arrillaga Cde la Bes-Rastrollo, M. Ultra-processed foods and type-2 diabetes risk in the SUN project: A prospective cohort study. Clinical Nutrition. 1o de maio de 2021, 40, 2817–24. [Google Scholar] [CrossRef] [PubMed]
- LaFata, E.M.; Allison, K.C.; Audrain-McGovern, J.; Forman, E.M. Ultra-Processed Food Addiction: A Research Update. Curr Obes Rep. 1o de junho de 2024, 13, 214–23. [Google Scholar] [CrossRef] [PubMed]
- Kendig, M.D.; Hasebe, K.; McCague, R.; Lee, F.; Leigh, S.J.; Arnold, R.; et al. Adolescent exposure to a solid high-fat, high-sugar 'cafeteria' diet leads to more pronounced changes in metabolic measures and gut microbiome composition than liquid sugar in female rats. Appetite. 1o de maio de 2022, 172, 105973. [Google Scholar] [CrossRef] [PubMed]
- Mignogna, C.; Costanzo, S.; Castelnuovo, A.D.; Ruggiero, E.; Shivappa, N.; Hebert, J.R.; et al. The inflammatory potential of the diet as a link between food processing and low-grade inflammation: An analysis on 21,315 participants to the Moli-sani study. Clinical Nutrition. 1o de outubro de 2022, 41, 2226–34. [Google Scholar] [CrossRef] [PubMed]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut. julho de 2021, 70, 1287–98. [Google Scholar] [CrossRef] [PubMed]
- Martínez Leo, E.E.; Segura Campos, M.R. Effect of ultra-processed diet on gut microbiota and thus its role in neurodegenerative diseases. Nutrition. 1o de março de 2020, 71, 110609. [Google Scholar] [CrossRef] [PubMed]
- Magro, D.O.; Kotze, P.G.; Martinez, C.A.R.; Camargo, M.G.; Guadagnini, D.; Calixto, A.R.; et al. Changes in serum levels of lipopolysaccharides and CD26 in patients with Crohn's disease. Intest Res. julho de 2017, 15, 352–7. [Google Scholar] [CrossRef] [PubMed]
- Hölttä, V.; Klemetti, P.; Sipponen, T.; Westerholm-Ormio, M.; Kociubinski, G.; Salo, H.; et al. IL-23/IL-17 immunity as a hallmark of Crohn's disease. Inflamm Bowel Dis. setembro de 2008, 14, 1175–84. [Google Scholar] [CrossRef] [PubMed]
- Candelli, M.; Franza, L.; Pignataro, G.; Ojetti, V.; Covino, M.; Piccioni, A.; et al. Interaction between Lipopolysaccharide and Gut Microbiota in Inflammatory Bowel Diseases. Int J Mol Sci. 10 de junho de 2021, 22, 6242. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Andreu-Sánchez, S.; Vogl, T.; Hu, S.; Vila, A.V.; Gacesa, R.; et al. Phage-display immunoprecipitation sequencing of the antibody epitope repertoire in inflammatory bowel disease reveals distinct antibody signatures. Immunity. 13 de junho de 2023, 56, 1393–1409.e6. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.N.; Duck, L.W.; Wu, J.; Rujani, M.; Thomes, P.G.; Elson, C.O.; et al. Crohn's Disease Patients Uniquely Contain Inflammatory Responses to Flagellin in a CD4 Effector Memory Subset. Inflammatory Bowel Diseases. 1o de dezembro de 2022, 28, 1893–903. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Bancil, A.S.; Lindsay, J.O.; Chassaing, B. Ultra-processed foods and food additives in gut health and disease. Nat Rev Gastroenterol Hepatol. junho de 2024, 21, 406–27. [Google Scholar] [CrossRef] [PubMed]
- Bancil, A.S.; Sandall, A.M.; Rossi, M.; Chassaing, B.; Lindsay, J.O.; Whelan, K. Food Additive Emulsifiers and Their Impact on Gut Microbiome, Permeability, and Inflammation: Mechanistic Insights in Inflammatory Bowel Disease. Journal of Crohn’s and Colitis. 1o de junho de 2021, 15, 1068–79. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Sandall, A.; Smith, L.; Rossi, M.; Whelan, K. Food additive emulsifiers: a review of their role in foods, legislation and classifications, presence in food supply, dietary exposure, and safety assessment. Nutrition Reviews. 1o de junho de 2021, 79, 726–41. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Pacifico, T.; Monteleone, G.; Laudisi, F. Impact of Western Diet and Ultra-Processed Food on the Intestinal Mucus Barrier. Biomedicines. 18 de julho de 2023, 11, 2015. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. outubro de 2014, 514, 181–6. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.R.; Tomas, J.; Brenner, C.; Sansonetti, P.J. Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie. 1o de outubro de 2017, 141, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Laudisi, F.; Di Fusco, D.; Dinallo, V.; Stolfi, C.; Di Grazia, A.; Marafini, I.; et al. The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress–Driven Mucus Depletion and Exacerbates Intestinal Inflammation. Cell Mol Gastroenterol Hepatol. 11 de setembro de 2018, 7, 457–73. [Google Scholar] [CrossRef] [PubMed]
- Pinget, G.; Tan, J.; Janac, B.; Kaakoush, N.O.; Angelatos, A.S.; O'Sullivan, J.; et al. Impact of the Food Additive Titanium Dioxide (E171) on Gut Microbiota-Host Interaction. Front Nutr. 14 de maio de 2019, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, L.; Catalan-Dibene, J.; Bongers, G.; Faith, J.J.; Suebsuwong, C.; et al. Food colorants metabolized by commensal bacteria promote colitis in mice with dysregulated expression of interleukin-23. Cell Metab. 6 de julho de 2021, 33, 1358–1371.e5. [Google Scholar] [CrossRef] [PubMed]
- Santos FSdos Mintem, G.C.; Oliveira IOde Horta, B.L.; Ramos, E.; Lopes, C.; et al. Consumption of ultra-processed foods and IL-6 in two cohorts from high- and middle-income countries. British Journal of Nutrition. maio de 2023, 129, 1552–62. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Compher, C.; Bonhomme, B.; Liu, Q.; Tian, Y.; Walters, W.; et al. Randomized Controlled-Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome. Gastroenterology. 1o de março de 2022, 162, 743–56. [Google Scholar] [CrossRef] [PubMed]
- Sieg, H.; Schaar, C.; Fouquet, N.; Böhmert, L.; Thünemann, A.F.; Braeuning, A. Particulate iron oxide food colorants (E 172) during artificial digestion and their uptake and impact on intestinal cells. Toxicology in Vitro. 1o de abril de 2024, 96, 105772. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Guo, X.; Thanuphol, P.; Ji, R.; Zhu, Z.; Wu, Y.; et al. Gut Microbiota-Mediated Degradation of Food-Grade Lambda-Carrageenan by Bacteroides xylanisolvens and Its Role in Inflammation. J Agric Food Chem. 19 de fevereiro de 2025, 73, 4288–98. [Google Scholar] [CrossRef] [PubMed]
- Elmén, L.; Zlamal, J.E.; Scott, D.A.; Lee, R.B.; Chen, D.J.; Colas, A.R.; et al. Dietary Emulsifier Sodium Stearoyl Lactylate Alters Gut Microbiota in vitro and Inhibits Bacterial Butyrate Producers. Front Microbiol [Internet]. 15 de maio de 2020 [citado 9 de julho de 2025];11. Disponível em: https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2020.00892/full.
- Chassaing, B.; Koren, O.; Goodrich, J.; Poole, A.; Srinivasan, S.; Ley, R.E.; et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 5 de março de 2015, 519, 92–6. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.L.; Keita, Å.V.; Duncan, S.H.; O'Kennedy, N.; Söderholm, J.D.; Rhodes, J.M.; et al. Translocation of Crohn's disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut. outubro de 2010, 59, 1331–9. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Ung, V.; Sydora, B.C.; Loening-Baucke, V.; Doerffel, Y.; Verstraelen, H.; et al. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm Bowel Dis. março de 2009, 15, 359–64. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Xu, X.; Qian, Z.; Cheng, H.; Shen, X.; Chen, S.; et al. Xanthan gum-assisted fabrication of stable emulsion-based oleogel structured with gelatin and proanthocyanidins. Food Hydrocolloids. 1o de junho de 2021, 115, 106596. [Google Scholar] [CrossRef]
- Thymann, T.; Møller, H.K.; Stoll, B.; Støy, A.C.F.; Buddington, R.K.; Bering, S.B.; et al. Carbohydrate maldigestion induces necrotizing enterocolitis in preterm pigs. Am J Physiol Gastrointest Liver Physiol. dezembro de 2009, 297, G1115–25. [Google Scholar] [CrossRef] [PubMed]
- Udo, T.; Mummaleti, G.; Mohan, A.; Singh, R.K.; Kong, F. Current and emerging applications of carrageenan in the food industry. Food Research International. 1o de novembro de 2023, 173, 113369. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Shani Levi, C.; Fahoum, L.; Ungar, Y.; Meyron-Holtz, E.G.; Shpigelman, A.; et al. Revisiting the carrageenan controversy: do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct. 1o de março de 2018, 9, 1344–52. [Google Scholar] [CrossRef] [PubMed]
- Brito AKde, B.; Cardoso, K.G.M.; Soares, S.D.; Chisté, R.C. CORANTES ARTIFICIAIS PERMITIDOS NO BRASIL: PRINCIPAIS CARACTERÍSTICAS E EFEITOS TOXICOLÓGICOS. Em Editora Científica Digital; 2021 [citado 9 de julho de 2025]. p. 428–44. Disponível em: https://www.editoracientifica.com.br/artigos/corantes-artificiais-permitidos-no-brasil-principais-caracteristicas-e-efeitos-toxicologicos.
- Vojdani, A.; Vojdani, C. Immune reactivity to food coloring. Altern Ther Health Med. 2015, 21 Suppl 1, 52–62. [Google Scholar] [PubMed]
- Elder, R.; Vancuren, S.J.; Botschner, A.J.; Josephy, P.D.; Allen-Vercoe, E. Metabolism of azo food dyes by bacterial members of the human gut microbiome. Anaerobe. 1o de outubro de 2023, 83, 102783. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Jung, S. Food colors caught red-handed. Cell Metabolism. 6 de julho de 2021, 33, 1267–9. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, C.D. Desenvolvimento e caracterização de nanopartículas poliméricas para encapsulação de L-asparaginase /. UFRJ,; 2018.
- em 27/07/2023 15h33 P em 27/07/2023 12h49 A. Agência Nacional de Vigilância Sanitária - Anvisa. [citado 9 de julho de 2025]. Nota: avaliação do aditivo alimentar dióxido de titânio. Disponível em: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2023/nota-avaliacao-do-aditivo-alimentar-dioxido-de-titanio.
- Joint FAO/WHO Expert Committee on Food Additives risk assessment of titanium dioxide risk released – background information [Internet]. [citado 9 de julho de 2025]. Disponível em: https://www.who.int/publications/m/item/jecfa-risk-assessment-of-titanium-dioxide-risk-released-background-information.
- Bettini, S.; Boutet-Robinet, E.; Cartier, C.; Coméra, C.; Gaultier, E.; Dupuy, J.; et al. Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci Rep. 20 de janeiro de 2017, 7, 40373. [Google Scholar] [CrossRef] [PubMed]
- Proquin, H.; Rodríguez-Ibarra, C.; Moonen, C.G.J.; Urrutia Ortega, I.M.; Briedé, J.J.; de Kok, T.M.; et al. Titanium dioxide food additive (E171) induces ROS formation and genotoxicity: contribution of micro and nano-sized fractions. Mutagenesis. 1o de janeiro de 2017, 32, 139–49. [Google Scholar] [CrossRef] [PubMed]
- Quaglio, A.E.V.; Grillo, T.G.; Oliveira, E.C.S.D.; Stasi, L.C.D.; Sassaki, L.Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World Journal of Gastroenterology. 14 de agosto de 2022, 28, 4053–60. [Google Scholar] [CrossRef] [PubMed]
- Giambra, V.; Pagliari, D.; Rio, P.; Totti, B.; Di Nunzio, C.; Bosi, A.; et al. Gut Microbiota, Inflammatory Bowel Disease, and Cancer: The Role of Guardians of Innate Immunity. Cells. 19 de novembro de 2023, 12, 2654. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.T.; Amos, G.C.A.; Murphy, A.R.J.; Murch, S.; Wellington, E.M.H.; Arasaradnam, R.P. Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog. 4 de janeiro de 2020, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; et al. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn's Disease. Digestion. 2016, 93, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int J Mol Sci. 23 de março de 2022, 23, 3464. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; et al. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front Immunol. 11 de março de 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; et al. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients. 21 de outubro de 2023, 15, 4466. [Google Scholar] [CrossRef] [PubMed]
- Hee Bvan der Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends in Microbiology. 1o de agosto de 2021, 29, 700–12. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells. 26 de agosto de 2020, 9, 1970. [Google Scholar] [CrossRef] [PubMed]
- De Preter, V.; Arijs, I.; Windey, K.; Vanhove, W.; Vermeire, S.; Schuit, F.; et al. Impaired butyrate oxidation in ulcerative colitis is due to decreased butyrate uptake and a defect in the oxidation pathway. Inflamm Bowel Dis. junho de 2012, 18, 1127–36. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Horn, P.; Wong, V.W.S.; Ratziu, V.; Bugianesi, E.; Francque, S.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). Journal of Hepatology. 1o de setembro de 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; et al. Definition and diagnostic criteria of clinical obesity. The Lancet Diabetes & Endocrinology. 1o de março de 2025, 13, 221–62. [Google Scholar]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; et al. The interplay between diet and the gut microbiome: implications for health and disease. Nat Rev Microbiol. novembro de 2024, 22, 671–86. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Van Hul, M. Gut microbiota in overweight and obesity: crosstalk with adipose tissue. Nat Rev Gastroenterol Hepatol. março de 2024, 21, 164–83. [Google Scholar] [CrossRef] [PubMed]
- Nesci, A.; Carnuccio, C.; Ruggieri, V.; D’Alessandro, A.; Di Giorgio, A.; Santoro, L.; et al. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of a Complex Relationship. Int J Mol Sci. 22 de maio de 2023, 24, 9087. [Google Scholar] [CrossRef] [PubMed]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota–Gut–Brain Axis in Psychiatric Disorders. Int J Mol Sci. 24 de setembro de 2022, 23, 11245. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.A.; Inniss, S.; Kumagai, T.; Rahman, F.Z.; Smith, A.M. The Role of Diet and Gut Microbiota in Regulating Gastrointestinal and Inflammatory Disease. Front Immunol. 5 de abril de 2022, 13, 866059. [Google Scholar] [CrossRef] [PubMed]
- Ferenc, K.; Sokal-Dembowska, A.; Helma, K.; Motyka, E.; Jarmakiewicz-Czaja, S.; Filip, R. Modulation of the Gut Microbiota by Nutrition and Its Relationship to Epigenetics. Int J Mol Sci. 19 de janeiro de 2024, 25, 1228. [Google Scholar] [CrossRef] [PubMed]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; et al. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients. 22 de dezembro de 2020, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients. fevereiro de 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- García-Gavilán, J.F.; Atzeni, A.; Babio, N.; Liang, L.; Belzer, C.; Vioque, J.; et al. Effect of 1-year lifestyle intervention with energy-reduced Mediterranean diet and physical activity promotion on the gut metabolome and microbiota: a randomized clinical trial. The American Journal of Clinical Nutrition. 1o de maio de 2024, 119, 1143–54. [Google Scholar] [CrossRef] [PubMed]
- Meslier, V.; Laiola, M.; Roager, H.M.; De Filippis, F.; Roume, H.; Quinquis, B.; et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. julho de 2020, 69, 1258–68. [Google Scholar] [CrossRef] [PubMed]
- Haskey, N.; Estaki, M.; Ye, J.; Shim, R.K.; Singh, S.; Dieleman, L.A.; et al. A Mediterranean Diet Pattern Improves Intestinal Inflammation Concomitant with Reshaping of the Bacteriome in Ulcerative Colitis: A Randomised Controlled Trial. J Crohns Colitis. 24 de abril de 2023, 17, 1569–78. [Google Scholar] [CrossRef] [PubMed]
- Godny, L.; Elial-Fatal, S.; Arrouasse, J.; Fischler, T.S.; Reshef, L.; Kutukov, Y.; et al. Mechanistic Implications of the Mediterranean Diet in Patients With Newly Diagnosed Crohn's Disease: Multiomic Results From a Prospective Cohort. Gastroenterology. 1o de maio de 2025, 168, 952–964.e2. [Google Scholar] [CrossRef] [PubMed]
- Hashash, J.G.; Elkins, J.; Lewis, J.D.; Binion, D.G. AGA Clinical Practice Update on Diet and Nutritional Therapies in Patients With Inflammatory Bowel Disease: Expert Review. Gastroenterology. 1o de março de 2024, 166, 521–32. [Google Scholar] [CrossRef] [PubMed]
- Erol Doğan, Ö.; Karaca Çelik, K.E.; Baş, M.; Alan, E.H.; Çağın, Y.F. Effects of Mediterranean Diet, Curcumin, and Resveratrol on Mild-to-Moderate Active Ulcerative Colitis: A Multicenter Randomized Clinical Trial. Nutrients. 16 de maio de 2024, 16, 1504. [Google Scholar] [CrossRef] [PubMed]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflammatory Bowel Diseases. 1o de janeiro de 2021, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Randeni, N.; Bordiga, M.; Xu, B. A Comprehensive Review of the Triangular Relationship among Diet–Gut Microbiota–Inflammation. Int J Mol Sci. 29 de agosto de 2024, 25, 9366. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. agosto de 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 6 de fevereiro de 2017, 8, 172–84. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clinical Nutrition. 1o de março de 2020, 39, 632–53. [Google Scholar] [CrossRef] [PubMed]
- Sinopoulou, V.; Gordon, M.; Gregory, V.; Saadeh, A.; Akobeng, A.K. Prebiotics for induction and maintenance of remission in ulcerative colitis - Sinopoulou, V - 2024 | Cochrane Library. [citado 9 de julho de 2025]; Disponível em: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD015084.pub2/full.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. agosto de 2014, 11, 506–14. [Google Scholar] [CrossRef] [PubMed]
- Iheozor-Ejiofor, Z.; Kaur, L.; Gordon, M.; Baines, P.A.; Sinopoulou, V.; Akobeng, A.K. Probiotics for maintenance of remission in ulcerative colitis - Iheozor-Ejiofor, Z - 2020 | Cochrane Library. [citado 9 de julho de 2025]; Disponível em: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD007443.pub3/full.
- Bischoff, S.C.; Bager, P.; Escher, J.; Forbes, A.; Hébuterne, X.; Hvas, C.L.; et al. ESPEN guideline on Clinical Nutrition in inflammatory bowel disease. Clinical Nutrition. março de 2023, 42, 352–79. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.N.; da Costa, A.L.; Jorge, E.N.; Paiano, V.F.; Camparoto, M.L.; Keller, R.; et al. Synbiotics improve clinical indicators of ulcerative colitis: systematic review with meta-analysis. Nutrition Reviews. 1o de fevereiro de 2022, 80, 157–64. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dhaneshwar, S. Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World Journal of Gastroenterology. 14 de abril de 2023, 29, 2078–100. [Google Scholar] [CrossRef] [PubMed]
- Lê, A.; Mantel, M.; Marchix, J.; Bodinier, M.; Jan, G.; Rolli-Derkinderen, M. Inflammatory bowel disease therapeutic strategies by modulation of the microbiota: how and when to introduce pre-, pro-, syn-, or postbiotics? American Journal of Physiology-Gastrointestinal and Liver Physiology. dezembro de 2022, 323, G523–53. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021, 18, 649–67. [Google Scholar] [CrossRef] [PubMed]
- Kavita Om, H.; Chand, U.; Kushawaha, P.K. Postbiotics: An alternative and innovative intervention for the therapy of inflammatory bowel disease. Microbiological Research. 1o de fevereiro de 2024, 279, 127550. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, P.; Wang, D.; Shen, S.; Wang, S.; Li, Y.; et al. Postbiotics in inflammatory bowel disease: efficacy, mechanism, and therapeutic implications. Journal of the Science of Food and Agriculture. 2025, 105, 721–34. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. abril de 2017, 66, 569–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wellens, J.; Kalla, R.; Fu, T.; Deng, M.; Zhang, H.; et al. Intake of Ultra-processed Foods Is Associated with an Increased Risk of Crohn's Disease: A Cross-sectional and Prospective Analysis of 187 154 Participants in the UK Biobank. J Crohns Colitis. 28 de outubro de 2022, 17, 535–52. [Google Scholar] [CrossRef] [PubMed]
- Peery, A.F.; Kelly, C.R.; Kao, D.; Vaughn, B.P.; Lebwohl, B.; Singh, S.; et al. AGA Clinical Practice Guideline on Fecal Microbiota–Based Therapies for Select Gastrointestinal Diseases. Gastroenterology. 1o de março de 2024, 166, 409–34. [Google Scholar] [CrossRef] [PubMed]
- Halkjær, S.I.; Lo, B.; Cold, F.; Højer Christensen, A.; Holster, S.; König, J.; et al. Fecal microbiota transplantation for the treatment of irritable bowel syndrome: A systematic review and meta-analysis. World J Gastroenterol. 28 de maio de 2023, 29, 3185–202. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.; Pandit, N.G.; Zaman, M.; Minkoff, N.Z.; Tanner-Smith, E.E.; Gomez-Duarte, O.G.; et al. Fecal transplantation for treatment of inflammatory bowel disease - Imdad, A - 2023 | Cochrane Library. [citado 9 de julho de 2025]; Disponível em: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012774.pub3/full.
- Yan, J.; Wang, L.; Gu, Y.; Hou, H.; Liu, T.; Ding, Y.; et al. Dietary Patterns and Gut Microbiota Changes in Inflammatory Bowel Disease: Current Insights and Future Challenges. Nutrients. 27 de setembro de 2022, 14, 4003. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Vaidean, G.; Parekh, N. Ultra-processed Foods and Cardiovascular Diseases: Potential Mechanisms of Action. Advances in Nutrition. 1o de setembro de 2021, 12, 1673–80. [Google Scholar] [CrossRef] [PubMed]
- Rondinella, D.; Raoul, P.C.; Valeriani, E.; Venturini, I.; Cintoni, M.; Severino, A.; et al. The Detrimental Impact of Ultra-Processed Foods on the Human Gut Microbiome and Gut Barrier. Nutrients. 28 de fevereiro de 2025, 17, 859. [Google Scholar] [CrossRef]
- Vissers, E.; Wellens, J.; Sabino, J. Ultra-processed foods as a possible culprit for the rising prevalence of inflammatory bowel diseases. Front Med (Lausanne). 7 de novembro de 2022, 9, 1058373. [Google Scholar] [CrossRef] [PubMed]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; Mateos, D.; Ugarriza, L.; Gómez, C.; et al. Oxidative Stress and Inflammatory Biomarkers Are Related to High Intake of Ultra-Processed Food in Old Adults with Metabolic Syndrome. Antioxidants (Basel). 31 de julho de 2023, 12, 1532. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, G.; Lou, P.; Zhang, M.; Yao, K.; Xiao, J.; et al. Excessive nucleic acid R-loops induce mitochondria-dependent epithelial cell necroptosis and drive spontaneous intestinal inflammation. Proc Natl Acad Sci U S A. 121, e2307395120.
- Dang, P.M.C.; Rolas, L.; El-Benna, J. The Dual Role of Reactive Oxygen Species-Generating Nicotinamide Adenine Dinucleotide Phosphate Oxidases in Gastrointestinal Inflammation and Therapeutic Perspectives. Antioxidants & Redox Signaling. 10 de agosto de 2020, 33, 354–73. [Google Scholar]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; et al. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens. 13 de agosto de 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Tristan Asensi, M.; Napoletano, A.; Sofi, F.; Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients. 22 de março de 2023, 15, 1546. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Song, R.; Liu, Y.; Wu, Z.; Zhang, X. Effects of ultra-processed foods on the microbiota-gut-brain axis: The bread-and-butter issue. Food Research International. 1o de maio de 2023, 167, 112730. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; et al. Association of ultra-processed food intake with risk of inflammatory bowel disease: prospective cohort study. BMJ. 15 de julho de 2021, 374, n1554. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Dong, C.; Casagrande, C.; Chan, S.S.M.; Huybrechts, I.; Nicolas, G.; et al. Food Processing and Risk of Crohn's Disease and Ulcerative Colitis: A European Prospective Cohort Study. Clinical Gastroenterology and Hepatology. 1o de junho de 2023, 21, 1607–1616.e6. [Google Scholar] [CrossRef] [PubMed]
- Preda, C.M.; Istratescu, D.; Nitescu, M.; Manuc, T.; Manuc, M.; Stroie, T.; et al. Diet Optimization in Inflammatory Bowel Disease: Impact on Disease Relapse and Inflammatory Markers. A 1-year Prospective Trial. Journal of Gastrointestinal and Liver Diseases. 29 de junho de 2024, 33, 184–93. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Li, F.; Zhang, D. Dietary fiber intake reduces risk of inflammatory bowel disease: result from a meta-analysis. Nutrition Research. 1o de setembro de 2015, 35, 753–8. [Google Scholar] [CrossRef] [PubMed]
- Sigall Boneh, R.; Westoby, C.; Oseran, I.; Sarbagili-Shabat, C.; Albenberg, L.G.; Lionetti, P.; et al. The Crohn's Disease Exclusion Diet: A Comprehensive Review of Evidence, Implementation Strategies, Practical Guidance, and Future Directions. Inflamm Bowel Dis. 18 de novembro de 2023, 30, 1888–902. [Google Scholar] [CrossRef] [PubMed]
- Strisciuglio, C.; Cenni, S.; Serra, M.R.; Dolce, P.; Martinelli, M.; Staiano, A.; et al. Effectiveness of Mediterranean Diet's Adherence in Children with Inflammatory Bowel Diseases. Nutrients. 20 de outubro de 2020, 12, 3206. [Google Scholar] [CrossRef] [PubMed]
- Martín-Masot, R.; Herrador-López, M.; Navas-López, V.M. Dietary Habit Modifications in Paediatric Patients after One Year of Treatment with the Crohn's Disease Exclusion Diet. Nutrients. 20 de janeiro de 2023, 15, 554. [Google Scholar] [CrossRef] [PubMed]
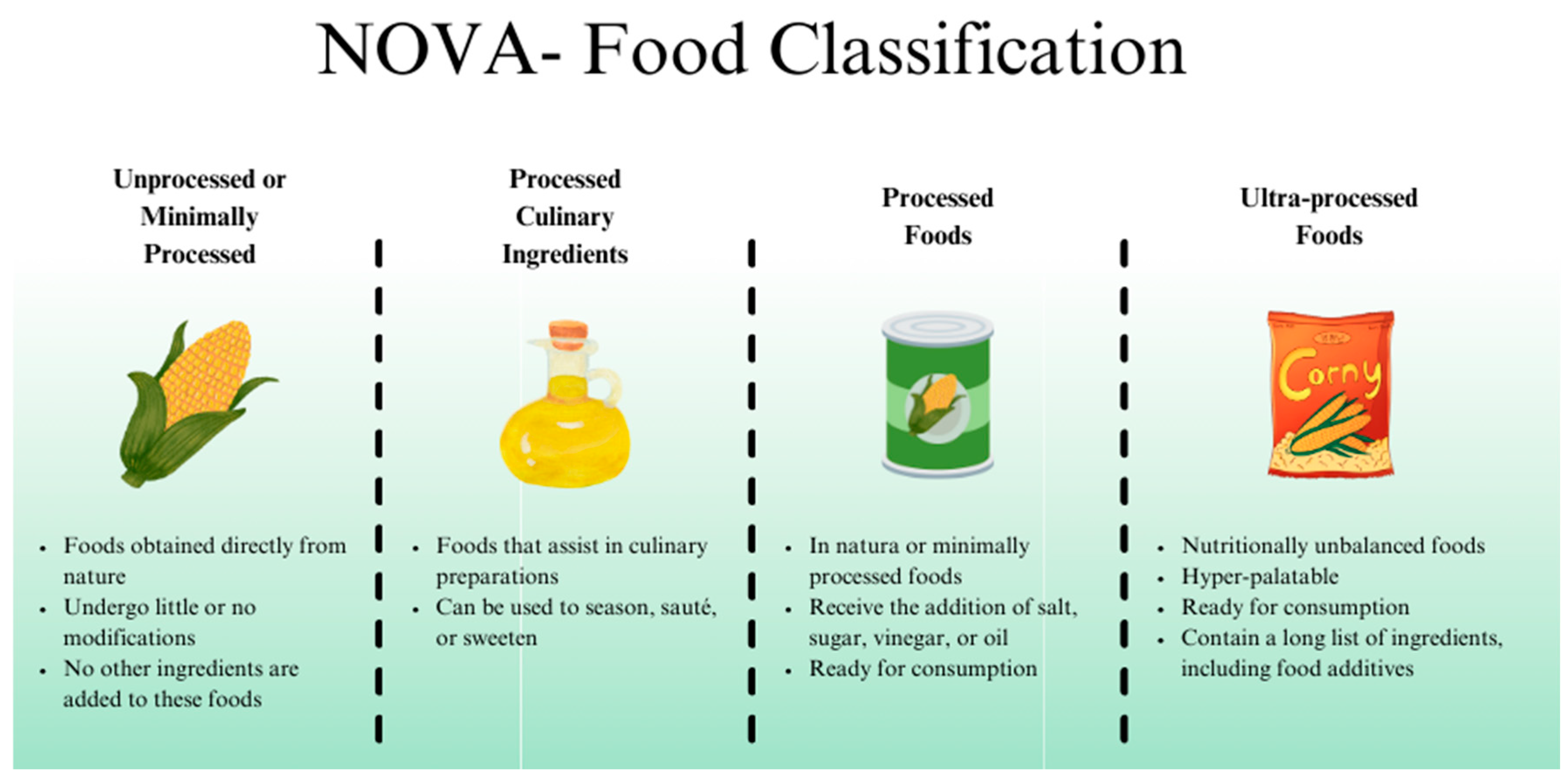
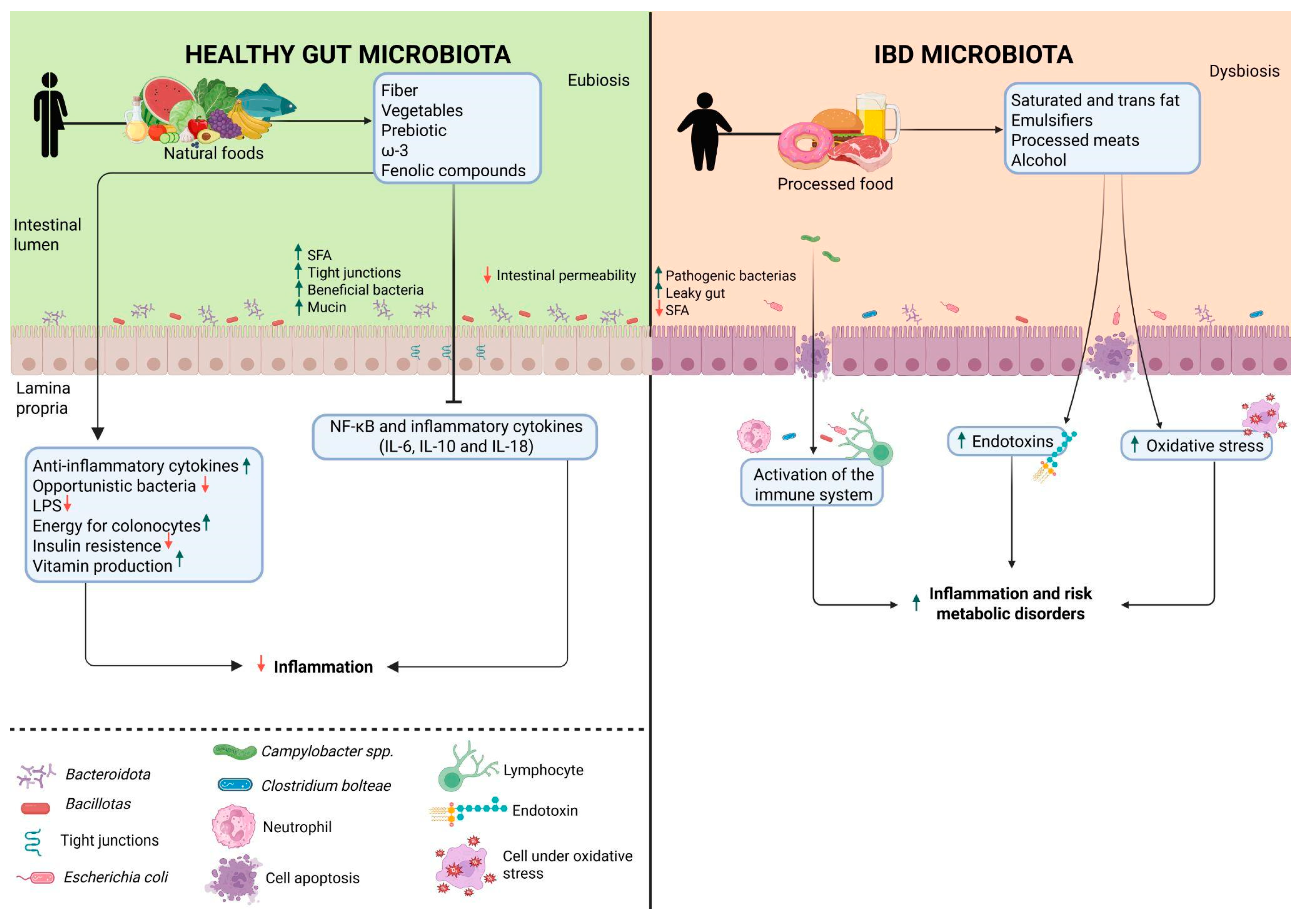
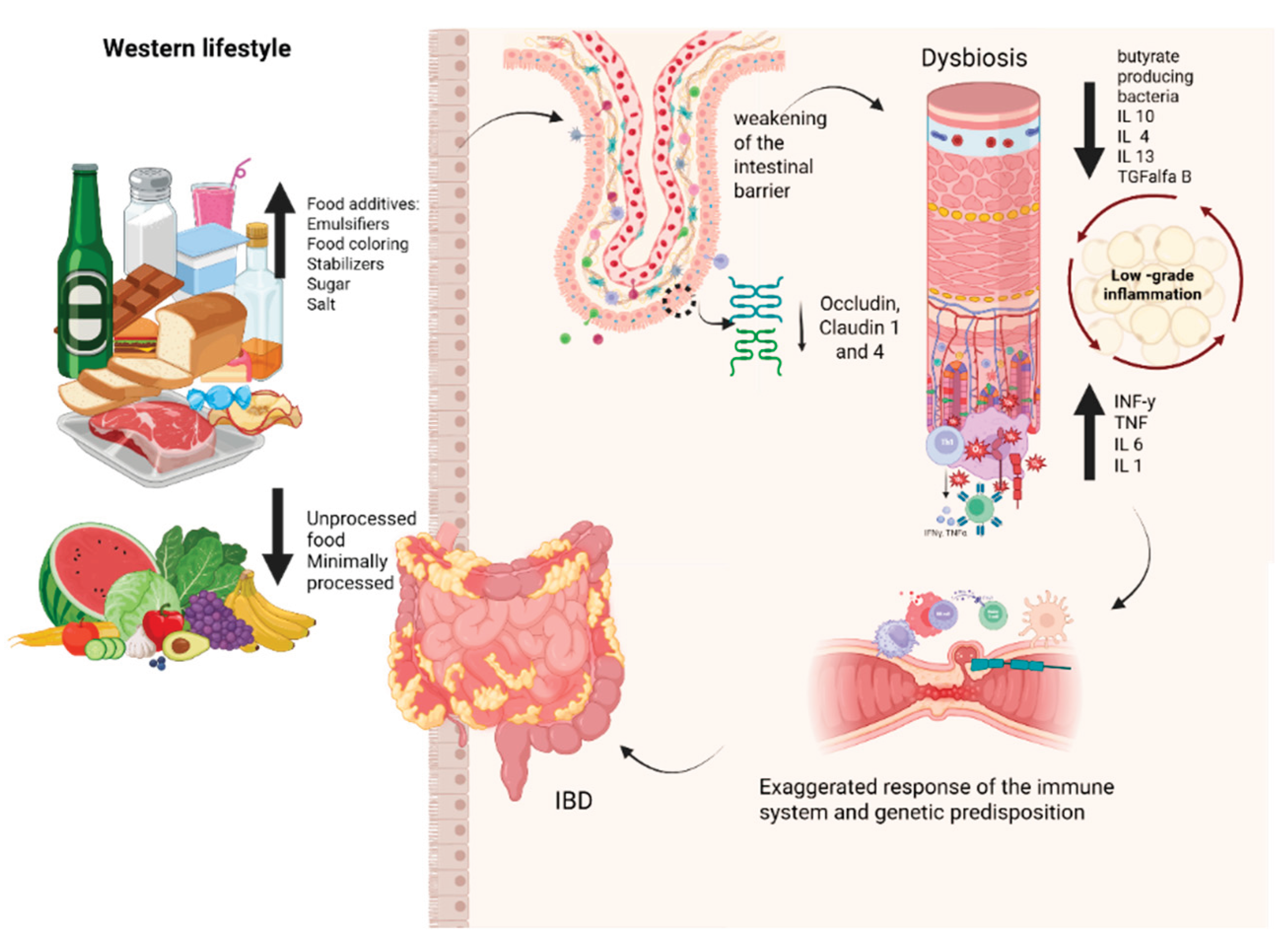
| Study (year) | Model | Additive(s) | Key Findings Related to Gut Microbiota/Health |
|---|---|---|---|
| Suez et al., 2014 [30] | Germ-free mice | Non-caloric artificial sweeteners (NAS) | Excessive consumption may promote glucose intolerance, dysbiosis, and metabolic alteration. |
| Araújo, 2017 [31] | Humans, ages 18-60 years | Carboxymethylcellulose (CMC) | Increased bacterial proliferation and infiltration, with an increase in Roseburia spp. and Lachnospiraceae bacterium species. |
| Laudisi, 2018 [32] | Mice | Maltodextrin | Decreased Muc-2 results in greater adhesion of pathogenic bacteria. |
| Pinget et al., 2019 [33] | Mice | Titanium Dioxide (TiO₂) | TiO₂ may impair intestinal homeostasis, increase inflammatory cytokine expression, and decrease crypt length. |
| He et al., 2021 [34] | Mice | Colorants Red 40 and Yellow 6 | It can intensify intestinal inflammation and induce colitis. |
| Silva, 2022 [35] | Wistar rats | Xanthan gum | Continuous consumption increases pro-inflammatory cytokines (TNF-α, IL-6, and IL-10) and alters intestinal barrier integrity. |
| Chassaing et al., 2022 [36] | Humans, 16 adults | Carboxymethylcellulose (CMC) | Alteration in gut microbiota composition and reduction of metabolites like SCFAs. |
| Sieg et al., 2024 [37] | In vitro | Iron oxide food colorants (E 172) | E 172 showed strong interaction with intestinal cells, though no toxic effects were observed. |
| Han et al., 2025 [38] | In vitro | Carrageenan | Degraded carrageenan generates pro-inflammatory cytokines, such as IL1-b and TNF-a, which are related to the development of IBD. |
| Phylum | Description | Reference |
|---|---|---|
| Bacteroidota and Bacillotas | It comprises 90% of the gut microbiota and is often reduced, potentially impairing the inflammatory response and short-chain fatty acid production. | Giambra et al. [57]; Santana et al. [60] |
| Proteobacteria | It typically increases, including opportunistic pathogens, such as Enterobacteriaceae and Burkholderiaceae, that can exacerbate inflammation. | Alam et al. [58] |
| Actinobacteria | In patients with Crohn's disease, they are increase, which influences dysbiosis and intestinal inflammation. | Takahashi et al. [59] |
| Increased in IBD | Decrease in IBD | ||
|---|---|---|---|
| Phylum | Species | Phylum | Species |
|
Proteobacteria |
E. coli Campylobacter spp. H. parainfluenzae E. corrodens |
Verrucomicrobia | A. muciniphila |
|
Bacteroidota |
B. fragilis |
Bacillota |
F. prausnitzii R. albus Eubacterium spp |
| Bacillota |
R. torques Ruminococcus spp. C. hathewayi C. bolteae R. gnavus |
||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





